Abstract
Objective
Peritoneal dissemination is difficult to diagnose by conventional imaging technologies. We aimed to construct a nomogram to predict peritoneal dissemination in gastric cancer (GC) patients.
Methods
We retrospectively analyzed 1,112 GC patients in Sun Yat-sen University Cancer Center between 2001 and 2010 as the development set and 474 patients from The Sixth Affiliated Hospital, Sun Yat-sen University between 2010 and 2016 as the validation set. The clinicopathological variables associated with gastric cancer with peritoneal dissemination (GCPD) were analyzed. We used logistic regression analysis to identify independent risk factors for peritoneal dissemination. Then, we constructed a nomogram for the prediction of GCPD and defined its predictive value with a receiver operating characteristic (ROC) curve. External validation was performed to validate the applicability of the nomogram.
Results
In total, 250 patients were histologically identified as having peritoneal dissemination. Logistic regression analysis demonstrated that age, sex, tumor location, tumor size, signet-ring cell carcinoma (SRCC), T stage, N stage and Borrmann classification IV (Borrmann IV) were independent risk factors for peritoneal dissemination. We constructed a nomogram consisting of these eight factors to predict GCPD and found an optimistic predictive capability, with a C-index of 0.791, an area under the curve (AUC) of 0.791, and a 95% confidence interval (95% CI) of 0.762−0.820. The results found in the external validation set were also promising.
Conclusions
We constructed a highly sensitive nomogram that can assist clinicians in the early diagnosis of GCPD and serve as a reference for optimizing clinical management strategies.
Keywords: Gastric cancer, peritoneal dissemination, nomogram
Introduction
Despite decreasing incidence and improvements in treatment in recent years, gastric cancer (GC) remains the fifth most common malignant tumor and the third leading cause of cancer-related deaths worldwide (1). Data have shown that the incidence of newly diagnosed GC in China is up to 30 per 100,000, accounting for approximately 40% of all cases in the world and leading to 294,000 cancer-related deaths, accounting for approximately 50% of the world’s GC deaths (2-4). The peritoneum and liver are the most common metastatic sites that lead to the high mortality of GC (5). Reports have indicated that the median survival time of patients with peritoneal dissemination is 3−6 months, with a dismal 5-year survival rate of less than 3% (6,7). Surgery is the only possible way to curatively treat GC; however, it does not benefit gastric cancer with peritoneal dissemination (GCPD) (8). Thus, to avoid unnecessary invasive harm from “open-and-close” surgery, the accurate staging of GC patients, especially the accurate diagnosis of peritoneal dissemination, is an essential component of precise personal therapy.
Traditional radiological imaging, such as computed tomography (CT) and magnetic resonance imaging (MRI), cannot accurately estimate the tumor burden, especially when peritoneal metastasis is suspected (9). Once visible manifestations such as the thickening of the peritoneal wall, the enhancement of the signals of nodules and the formation of an “omentum cake” have occurred, patients are no longer in the early stage of peritoneal dissemination (10). Koh et al. reported that the sensitivity of CT for the detection of peritoneal nodules less than 0.5 cm and less than 1.0 cm in diameter was only 11% and 25%−50%, respectively (11). Similarly, De Bree et al. also found that the sensitivity for detecting nodules larger than 5 cm was merely 59.3%−66.7% (12). In addition, the diagnosis of metastases violating the lesser sac, mesenteric root, left hemidiaphragm, and surface of the small bowel remains challenging (13). The disadvantages of MRI are the image artifacts caused by abdominal movement and its expensive cost. A double-blind, prospective study of 57 patients concluded that the assessment of GCPD by MRI was superior to that by CT, with greater interobserver agreement for lesser sac, liver surface, and right diaphragm diseases (14). Positron emission tomography/computed tomography (PET/CT) techniques have been favored by clinicians in recent years due to their sensitivity in detecting organ metastasis and possible systemic testing. Dromain et al. compared preoperative CT and PET/CT results with intraperitoneal findings and ultimately found that PET/CT failed to recognize peritoneal micronodules; for larger metastases, PET/CT also did not perform better than CT. The limitations of PET/CT include low spatial resolution, inaccurate location of foci, high false negative rates due to inconsistent FDG uptake by tumors or lesions, high costs, and the inability to accurately identify special types of GC, such as mucinous adenocarcinoma, which result in PET/CT being used only as a supplement to CT in the detection of distant organ metastases (15-17). Staging laparoscopy is the “gold standard” to detect occult peritoneal dissemination, but it is not psychologically accepted by many patients mainly because it has the disadvantages of extra operation, high costs and more complications (18). Therefore, because of the inherent limitations of all imaging detection methods, a more effective, noninvasive tool to detect peritoneal dissemination in GC patients is urgently needed.
The aim of our study was to analyze the clinicopathological and demographic parameters of GC patients, to establish a robust nomogram to predict peritoneal dissemination and to validate the nomogram’s predictive value both internally and externally to provide a superior guide for individual clinical treatment.
Materials and methods
Ethics statement
This study was conducted in accordance with the ethical standards of the World Medical Association Declaration of Helsinki and the Ethical Guidelines for Clinical Research. All extractions of information from the database were approved by The Sixth Affiliated Hospital, Sun Yat-sen University and The Sun Yat-sen University Cancer Center (SYSUCC). The study was approved by the Ethics Committee of The Sixth Affiliated Hospital, Sun Yat-sen University, and all informed consent forms were signed preoperatively.
Inclusion and exclusion criteria
The retrospective database that provided the information used to create the nomogram was derived from the SYSUCC medical records. The original database included a total of 1,377 patients, and the patients were selected according to the inclusion and exclusion criteria.
The inclusion criteria were as follows: 1) histopathologically confirmed diagnosis of primary gastric carcinoma; 2) no synchronous tumors; 3) no peritoneal metastasis preoperatively identified by CT or other imaging tools; and 4) fit for radical surgery.
The exclusion criteria were as follows: 1) had undergone surgery for GC; 2) positive peritoneal cytology but no peritoneal metastatic nodules were found during laparoscopic exploration; 3) had received neoadjuvant chemotherapy; or 4) major organ dysfunction such as heart, kidney or liver dysfunction.
Statistical analysis
We retrospectively collected clinicopathological parameters, including demographic statistics, tumor statistics and patient statistics, and selected sex, age, tumor location, tumor size, pathological type, Borrmann classification, carcinoembryonic antigen (CEA), and preoperative T and N stage for final analysis according to inclusion and exclusion criteria. Continuous variables with a normal distribution are presented as the mean [standard deviation (SD)]; non-normally distributed variables are reported as the median [interquartile range (IQR)]. The frequencies of the categorical variables were compared using the Pearson χ2 test or Fisher’s exact test, when appropriate.
For risk factor analysis, tumor locations were transformed into categorical variables from number 1 to 5 based on whether they were located in the proximal stomach, the middle stomach, the distal stomach, the entire stomach or the residual stomach. The cut-off value for age was obtained from a receiver operating characteristic (ROC) curve, as was the tumor size. T stage, N stage and tumor size were identified by preoperative contrast-enhanced CT scan. After patients underwent multidetector-row computed tomography (MDCT) examination, we reconstructed their three-dimensional (3D) imaging. Then, combining the different cross-sections and 3D imaging, we judged the T stage and N stage accordingly. In addition, we measured the length, width and height of the tumor at different levels, and we used the longest diameter of the tumor as the tumor size. Borrmann classification was identified by gastroscopy in the same way as tumor location. We incorporated all these parameters into univariate tests to detect risk factors of peritoneal dissemination. Then, factors significant in univariate analysis were entered in the stepwise logistic multivariate regression model. Finally, independent risk factors were obtained for nomogram construction. The probability of peritoneal dissemination was presented as odds ratios (ORs) and binary 95% confidence intervals (95% CIs). Two-sided P values less than 0.05 were considered statistically significant.
A nomogram based on the independent risk factors identified by the multivariate analysis was developed to identify patients at risk for peritoneal dissemination, and it graphically represents those risk factors, which can be used to calculate the risk of peritoneal dissemination for an individual patient by summing the points correlated with each risk factor. Discrimination was evaluated to test the power of the nomogram for distinguishing events from nonevents, as quantified by the C-index, which is equivalent to the area under the ROC curve (AUC). The ROC curve is a tool that can graphically recognize disease at arbitrary cut-off values. The AUC ranges from 0 to 1, with 1 indicating perfect concordance and 0.5 indicating no likelihood greater than chance. The closer the area is to 1, the better the discrimination. It is generally accepted that an AUC between 0.5 and 0.7 indicates a low predictive ability, between 0.7 and 0.9 indicates a moderate predictive accuracy and above 0.9 indicates a high predictive accuracy. Subsequently, we constructed a calibration plot to reduce the overfit bias of the nomogram. Finally, we performed 1,000 bootstrap replicates to internally validate the model and also externally validated it using the database from The Sixth Affiliated Hospital, Sun Yat-sen University (19).
All data were analyzed using the SPSS 20.0 statistical package (IBM corp., New York, USA) and R version 3.4.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
There were 1,377 patients in the development set from the original database, 265 of whom were excluded because of missing data, among whom 24 patients lacked information on tumor size and signet-ring cell carcinoma (SRCC), 45 lacked information on Borrmann classification IV (Borrmann IV), 119 lacked information on T stage and N stage, and the remaining 77 lacked CEA data. As a result, a total of 1,112 patients were included in the development set, with 862 (77.5%) of the patients identified as not having peritoneal metastasis and 250 (22.5%) identified as having peritoneal metastasis in the postoperative pathological examination.
The validation set used to validate the predictive accuracy of the nomogram was derived from The Sixth Affiliated Hospital, Sun Yat-sen University. The original database included a total of 615 patients, and the inclusion and exclusion criteria were the same as those for the development set. After we excluded some patients with missing data, a total of 474 patients were finally included in the validation set, with 409 (86.3%) of the patients identified as not having peritoneal metastasis and 65 (13.7%) of the patients identified as having one or more peritoneal metastases in the postoperative pathological examination.
The baseline demographic and clinicopathological characteristics of the patients are shown in Table 1 . The sex and age of the two groups were basically balanced. Comparing the two groups, the development set had a larger proportion of patients with tumors >5 cm that were in the T4, N3a or N3b stage, and tumors in the validation set were more likely to be located in the distal stomach, with an earlier stage of T3, N0 or N1, poor pathological type of Borrmann IV and SRCC. They were statistically significantly different.
1. Clinicopathological factors associated with peritoneal dissemination in patients with gastric cancer.
| Variables | n (%) | P | ||
| Total (N=1,586) | Development set (n=1,112) | Validation set (n=474) | ||
| SRCC, signet-ring cell carcinoma. | ||||
| Sex | 0.395 | |||
| Male | 1,060 (66.8) | 751 (67.5) | 309 (65.2) | |
| Female | 526 (33.2) | 361 (32.5) | 165 (34.8) | |
| Age (year) | 0.005 | |||
| <55 | 600 (37.8) | 446 (40.1) | 154 (32.5) | |
| ≥55 | 986 (62.2) | 666 (59.9) | 320 (67.5) | |
| Tumor location | 0.009 | |||
| Proximal | 577 (36.4) | 423 (38.1) | 154 (32.5) | |
| Middle | 317 (20.0) | 229 (20.6) | 88 (18.6) | |
| Distal | 636 (40.1) | 415 (37.3) | 221 (46.6) | |
| Entire | 20 (1.3) | 16 (1.4) | 4 (0.8) | |
| Residual | 36 (2.2) | 29 (2.6) | 7 (1.5) | |
| Tumor size (cm) | <0.001 | |||
| ≤5 | 1,067 (67.3) | 655 (58.9) | 412 (86.9) | |
| >5 | 519 (32.7) | 457 (41.1) | 62 (13.1) | |
| Pathological type | <0.001 | |||
| Non-SRCC | 1,287 (81.1) | 956 (86.0) | 331 (69.8) | |
| SRCC | 299 (18.9) | 156 (14.0) | 143 (30.2) | |
| Borrmann classification | <0.001 | |||
| Non-Borrmann IV | 1,420 (89.5) | 1,022 (91.9) | 398 (84.0) | |
| Borrmann IV | 166 (10.5) | 90 (8.1) | 76 (16.0) | |
| T stage | <0.001 | |||
| 1 | 118 (7.4) | 86 (7.7) | 32 (6.7) | |
| 2 | 174 (11.0) | 90 (8.1) | 84 (17.7) | |
| 3 | 321 (20.2) | 21 (1.9) | 300 (63.3) | |
| 4 | 973 (61.3) | 915 (82.3) | 58 (12.3) | |
| N stage | <0.001 | |||
| 0 | 407 (25.7) | 260 (23.4) | 147 (31.0) | |
| 1 | 307 (19.4) | 189 (17.0) | 118 (24.9) | |
| 2 | 340 (21.4) | 213 (19.1) | 127 (26.8) | |
| 3a | 420 (26.5) | 338 (30.4) | 82 (17.3) | |
| 3b | 112 (7.1) | 112 (10.1) | 0 (0) | |
| Peritoneal dissemination | <0.001 | |||
| No | 1,271 (80.1) | 862 (77.5) | 409 (86.3) | |
| Yes | 315 (19.9) | 250 (22.5) | 65 (13.7) | |
Univariate analysis of clinicopathological parameters associated with GCPD in development set
Of the 1,112 patients in the development set, we found that the risk factors associated with peritoneal dissemination were sex (P=0.001), age (P<0.001), tumor size (P<0.001), tumor location (P<0.001), SRCC (P<0.001), Borrmann IV (P<0.001), T stage (P=0.024) and N stage (P<0.001). The data are shown inTable 2 .
2. Clinicopathological factors associated with peritoneal dissemination in patients with gastric cancer (Development set, n=1,112).
| Variables | Peritoneal dissemination [n (%)] | Univariate analysis | Multivariable analysis | ||||
| Positive | Negative | P | OR (95% CI) | P | |||
| SRCC, signet-ring cell carcinoma; CEA, carcinoembryonic antigen; OR, odds ratio; 95% CI, 95% confidence interval. | |||||||
| Sex | 0.001 | 1.416 (1.019−1.967) | 0.038 | ||||
| Male | 146 (58.4) | 605 (70.2) | |||||
| Female | 104 (41.6) | 257 (29.8) | |||||
| Age (year) | <0.001 | 0.597 (0.434−0.821) | 0.002 | ||||
| <55 | 135 (54.0) | 311 (36.1) | |||||
| ≥55 | 115 (46.0) | 551 (63.9) | |||||
| Tumor location | <0.001 | 1.213 (1.034−1.424) | 0.018 | ||||
| Proximal | 57 (22.8) | 366 (42.5) | |||||
| Middle | 75 (30.0) | 154 (17.9) | |||||
| Distal | 113 (45.2) | 302 (35.0) | |||||
| Entire | 3 (1.2) | 13 (1.5) | |||||
| Residual | 2 (0.8) | 27 (3.1) | |||||
| Tumor size (cm) | <0.001 | 2.387 (1.718−3.317) | <0.001 | ||||
| ≤5 | 100 (40.0) | 555 (64.4) | |||||
| >5 | 150 (60.0) | 307 (35.6) | |||||
| Pathological type | <0.001 | 2.923 (1.968−4.341) | <0.001 | ||||
| SRCC | 76 (30.4) | 80 (9.3) | |||||
| Non-SRCC | 174 (69.6) | 782 (90.7) | |||||
| Borrmann classification | <0.001 | 3.210 (1.842−5.594) | <0.001 | ||||
| Borrmann IV | 47 (18.8) | 43 (5.0) | |||||
| Non-Borrmann IV | 203 (81.2) | 819 (95.0) | |||||
| CEA (ng/mL) | 0.093 | ||||||
| <5 | 200 (80.0) | 643 (74.6) | |||||
| ≥5 | 50 (20.0) | 219 (25.4) | |||||
| T stage | 0.024 | 1.592 (1.282−1.977) | <0.001 | ||||
| 1 | 12 (4.8) | 74 (8.6) | |||||
| 2 | 12 (4.8) | 78 (9.0) | |||||
| 3 | 5 (2.0) | 16 (1.9) | |||||
| 4 | 221 (88.4) | 694 (80.5) | |||||
| N stage | <0.001 | 1.461 (1.288−1.658) | <0.001 | ||||
| 0 | 16 (6.4) | 244 (28.3) | |||||
| 1 | 32 (12.8) | 157 (18.2) | |||||
| 2 | 42 (16.8) | 171 (19.8) | |||||
| 3a | 143 (57.2) | 195 (22.6) | |||||
| 3b | 17 (6.8) | 95 (11.0) | |||||
Multivariable analysis and nomogram for prediction of GCPD
Multivariable analysis showed that sex (P=0.038), age (P=0.002), tumor size (P<0.001), tumor location (P=0.018), SRCC (P<0.001), Borrmann IV (P<0.001), T stage (P<0.001) and N stage (P<0.001) were independent risk factors for GCPD. The outcomes are shown inTable 2 .
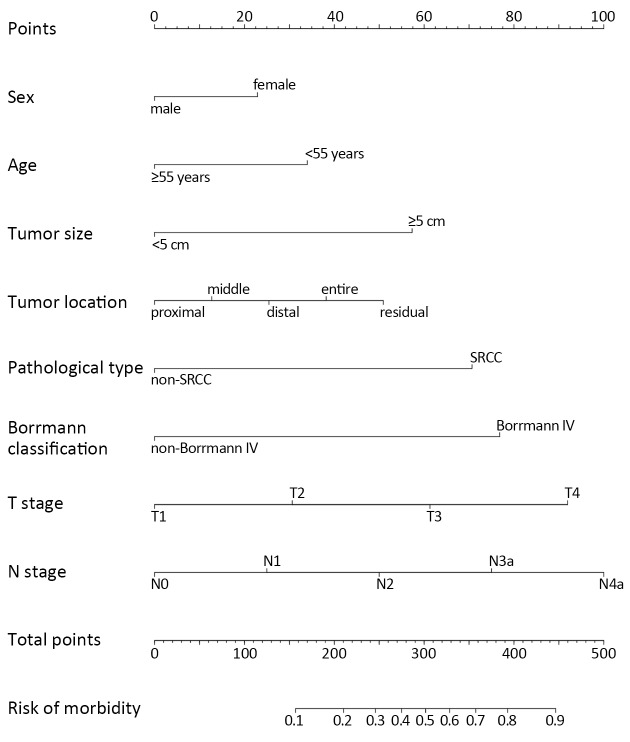
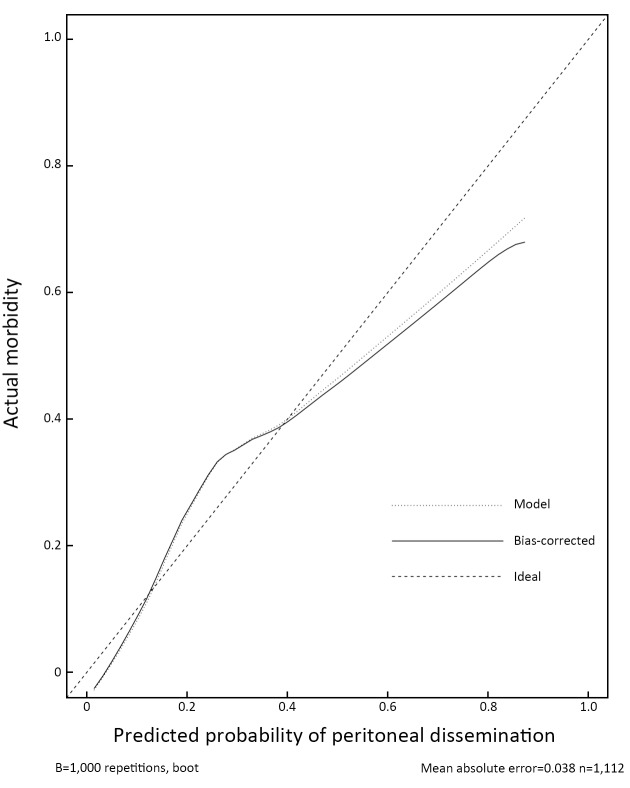
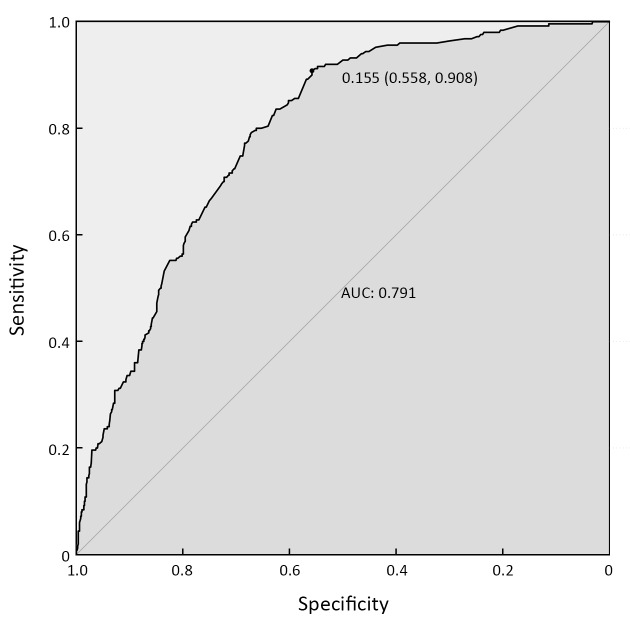
Thus, we integrated these eight factors into a nomogram for predicting peritoneal dissemination in GC patients. The nomogram included risk factors that may increase the possibility of peritoneal dissemination, as shown in Figure 1 . The total score of each patient is the sum of the points calculated by these eight risk factors. The corresponding value on the risk axis is the probability that the patient will develop peritoneal dissemination. Furthermore, we developed an internal calibration curve to assess the predictive accuracy of the nomogram and found that the C-index was 0.791, indicating a good fit and a cut-off value of 0.155 (Figure 2 ,3 ).
1. Total points are calculated by adding up the point value for each variable, which is determined by drawing a line straight upward to the points axis. Draw a line straight down to the risk axis to determine the possibility of peritoneal dissemination in patients with gastric cancer. SRCC, signet-ring cell carcinoma.
2. Calibration plot of predictive model from development set (n=1,112): predicted probability vs. actual morbidity. The 45-degree dotted line is the ideal prediction curve, indicating 100% predictive power. The apparent line represents the actual prediction ability of the nomogram; it fluctuates above and below the ideal curve, which indicates a medium prediction ability. The bias-corrected line is obtained after correcting overfitting of the apparent line. The closer they are, the less likely that overfitting is present.
3. Receiver operating characteristic (ROC) curve of predictive model from development set (n=1,112). AUC=0.791, 95% CI=0.762−0.820. The value of 0.155 (0.558, 0.908) represents the most optimal cut-off value of the nomogram to predict peritoneal dissemination. At this cut-off value, the sensitivity for predicting peritoneal dissemination was 90.8%, and the specificity was 55.8%. AUC, area under the curve; 95% CI, 95% confidence interval.
External validation of nomogram model with data from GC patients at The Sixth Affiliated Hospital, Sun Yat-sen University
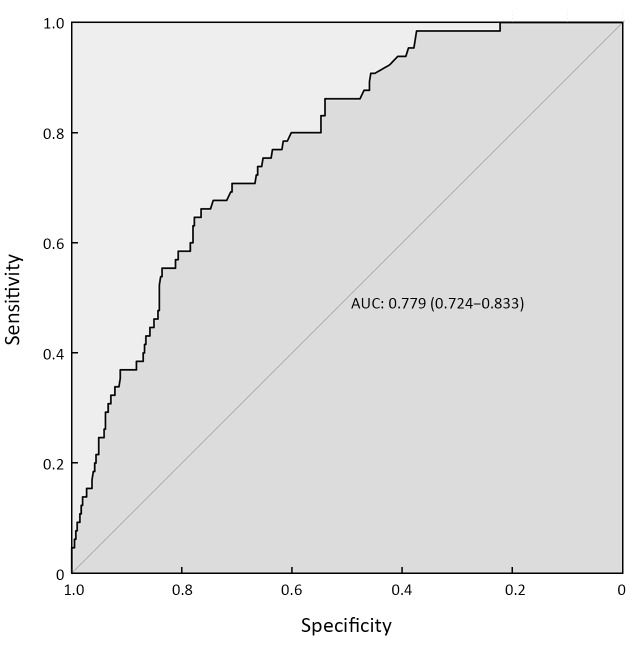
We established an external validation curve using a dataset that consisted of 474 GC patients from The Sixth Affiliated Hospital, Sun Yat-sen University to validate the predictive power of the nomogram. The C-index (AUC) was 0.779, and the 95% CI ranged from 0.724 to 0.833 (Figure 4 ).
4. Receiver operating characteristic (ROC) curve of predictive model from validation set (n=474). AUC=0.779, 95% CI=0.724−0.833. AUC, area under the curve; 95% CI, 95% confidence interval.
Discussion
Peritoneal dissemination is one of the most common metastases of GC and leads to poor prognosis in GC patients, while hyperthermic intraperitoneal chemotherapy (HIPEC) + cytoreductive surgery (CRS) may improve the prognosis accordingly (20-22). However, conventional imaging technologies cannot satisfy the current clinical need for the accurate preoperative diagnosis of peritoneal dissemination. A retrospective study revealed that approximately 23% of GC patients who were found to have peritoneal metastasis during surgery were clinically and radiologically misdiagnosed before surgery. Although molecular medical diagnostic techniques and laparoscopic staging skills have developed rapidly in recent years, it is still challenging to apply them in practice because of the high false positive rate, the lack of uniform standards and the relatively high cost (23-28). Our present study analyzed a high-volume database, established a risk model to preoperatively predict the possibility of peritoneal dissemination in GC patients, and finally developed a nomogram (AUC=0.791) to provide patients with tailored treatment.
Eight independent risk factors associated with peritoneal dissemination were used in our nomogram model, including age, sex, tumor location, tumor size, SRCC, Borrmann IV, T stage and N stage. Our risk prediction model revealed that patients with GC who were female, aged <55 years old and had a tumor size >5 cm, a tumor located in the distal, entire, or residual stomach, a pathological type of SRCC, Borrmann IV GC, or more advanced T and N stages were more likely to develop peritoneal dissemination than others. We constructed a discrimination curve, which showed strong discriminative power to accurately fit predictive events with actual events, with a mean absolute error of 0.039. Furthermore, we performed an internal validation of the nomogram, and the C-index was 0.791, indicating a moderate predictive ability. Tumor stage, age, tumor location, Borrmann classification, TNM stage and tumor infiltrating growth pattern were reported as independent risk factors for GCPD. In addition, clinicopathological parameters such as the depth of invasion, lymph node status, and differentiation status were closely related to peritoneal dissemination ( 29-31), which is consistent with the findings of our study.
In general, SRCC in advanced GC indicates more invasion and is associated with poor prognosis. It was reported that SRCC tumors were more prone to tumor recurrence and peritoneal dissemination (32). Our study showed that SRCC was one of the main risk factors influencing peritoneal metastasis with GC, with T4 and N3b stage also being in the top three risk factors; thus, we consider it a tool for decision-making in clinical treatment.
CT scans are limited in accurately judging the staging of a tumor due to their sensitivity. Indeed, endoscopic ultrasound can improve sensitivity in T staging. However, in our study, the patients in the development group were included between 2001 and 2010, when endoscopic ultrasound was not yet widely used in SYSUCC. However, some of the patients included in the validation group had available endoscopic ultrasound data. Therefore, the preoperative T stage may be more precise. As a result, we obtained good predictive capability in our external validation, with an AUC of 0.779, which indicated that our model could still be used for other institutions under current technical circumstances. In addition, the T stage mentioned in the patient demographics table represented the preoperative clinical stage. The accuracy of the T stage was 55% for T1, 52% for T2, 83% for T3 and 82% for T4 in our study, which was similar to the findings of another study (33), and we believe that the correlation of the T stage obtained from CT scan with the pathologic stage may be acceptable.
Borrmann classification is commonly used in Eastern countries and has been accepted by an increasing number of clinical doctors worldwide in recent years. Borrmann IV tumors are described as diffuse and infiltrative, without diffuse ulceration or raised margins; the gastric wall is thickened and indurated, and the margin is unclear (34,35). In our study, Borrmann IV was found to be an independent risk factor in our risk model, with a risk score of approximately 83 points. Some researches even supposed that Borrmann IV GC should be classified as T4b disease due to the poor overall survival, which was consistent with the findings of our study (36). Furthermore, we found that tumor size (>5 cm) also plays a role in risk estimation. We believe that an increased extent of tumor invasion will increase the possibility of tumor recurrence and metastasis.
It is well known that GC patients have less time for radical surgical treatment once they develop peritoneal dissemination. Thus, accurately predicting peritoneal dissemination is vital to tailoring individual treatment. CT evaluates cancer carcinomatosis with a sensitivity ranging from 8% to 67%, depending on the size of metastatic nodules, the location, and the chemotherapy that has been administered (11). Furthermore, it is difficult to use CT to distinguish between peritoneal reactive hyperplasia and metastasis (13). A quantified risk prediction nomogram allows surgeons to more objectively estimate the progression of GC, especially peritoneal dissemination. In our study, we developed an ROC curve for the nomogram, identifying the cut-off value for predicting peritoneal dissemination as 0.155. At this specific point, the sensitivity of our model for predicting peritoneal dissemination was 90.8%, which was much higher than conventional CT examination. That is, when the risk value determined by each clinicopathological variable exceeds 0.155, we tend to judge that more than 90% of such patients may have peritoneal metastasis or micrometastasis. In these cases, we should fully explain the patients’ tumor burden, especially the possibility of peritoneal metastasis. For treatment management, instead of blindly performing aggressive surgery, we first provided patients with a comprehensive chemotherapy-based treatment plan or used other treatment protocols that proved to be beneficial. As a result, it helped reduce the pain caused by invasive diagnosis; moreover, better treatment management also offered the best survival benefit for patients. In addition, the external validation of the nomogram obtained a satisfactory result, with an AUC of 0.779 and a 95% CI of 0.724−0.833, which indicated that using our nomogram in other institutions was a possibility. We look forward to our risk model providing strength to clinical decision makers for the early diagnosis of peritoneal metastasis and further tailoring individual treatments for GC patients.
Several nomograms that were mainly focused on predicting the prognosis of GC were constructed in previous studies (37-40). However, we built a nomogram specifically for the prediction of GCPD, and we believe that it could help clinicians more successfully tailor individual treatments in the future.
However, we acknowledge that there are several limitations in this study. First, traditional imaging diagnostic techniques may inevitably cause some measurement bias for the analysis. Moreover, given that neoadjuvant chemotherapy has been a hot topic in recent years and several publications suggest that neoadjuvant chemotherapy could lower tumor stage and prevent tumor recurrence, patients with advanced GC are therefore recommended for neoadjuvant chemotherapy before surgery in some guidelines (41). However, we did not include patients with neoadjuvant chemotherapy in this study, which may limit the application of this model. The reasons we did not include this population in the analysis were as followed: 1) Patients with and without neoadjuvant chemotherapy represented two groups of populations respectively with different characteristics, for instance, the peritoneal metastasis in patients with neoadjuvant chemotherapy was usually more elusive compared with that in patients without neoadjuvant chemotherapy; 2) Even among the patients with neoadjuvant chemotherapy, the efficacy varied in patients with different stages and location of GC. In addition, other variables, such as tumor markers and nutrition status, were also reported to be risk factors of GC (42,43). We did not include these variables due to the fact that some data in this part were missing. Ideally, more multicenter databases should be used for external validation to verify the predictive accuracy and generalization capacity of our nomogram. Despite these limitations, we are convinced that this nomogram model could still help provide a very strong reference for clinicians in tailoring personalized treatment plans for patients with GC.
Conclusions
We constructed a robust nomogram using clinicopathological variables associated with GCPD that was confirmed both internally and externally as having good predictive ability for peritoneal dissemination in GC patients. We hope it may strengthen the early diagnosis of GCPD and further assist clinicians in tailoring optimal individualized therapy. However, more multicenter databases must be used for external validation to verify the predictive accuracy and generalization capacity of our nomogram.
Acknowledgements
This work was supported by Young Teacher Foundation of Sun Yat-sen University (No. 17ykpy65), The Sun Yat-sen University (No. 53000-71020005) and National Key Clinical Discipline.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Yingbo Chen, Email: chenyb@sysucc.org.cn.
Junsheng Peng, Email: pengjunsheng@tom.com.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Sun K, Zheng R, et al Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30:1–12. doi: 10.21147/j.issn.1000-9604.2018.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller KD, Siegel RL, Lin CC, et al Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 4.Strong VE, Wu AW, Selby LV, et al Differences in gastric cancer survival between the U.S. and China. J Surg Oncol. 2015;112:31–7. doi: 10.1002/jso.23940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomassen I, van Gestel YR, van Ramshorst B, et al Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134:622–8. doi: 10.1002/ijc.28373. [DOI] [PubMed] [Google Scholar]
- 6.Kono K, Yong WP, Okayama H, et al Intraperitoneal chemotherapy for gastric cancer with peritoneal disease: experience from Singapore and Japan. Gastric Cancer. 2017;20:122–7. doi: 10.1007/s10120-016-0660-y. [DOI] [PubMed] [Google Scholar]
- 7.Coccolini F, Gheza F, Lotti M, et al Peritoneal carcinomatosis. World J Gastroenterol. 2013;19:6979–94. doi: 10.3748/wjg.v19.i41.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yonemura Y, Prabhu A, Sako S, et al Long term survival after cytoreductive surgery combined with perioperative chemotherapy in gastric cancer patients with peritoneal metastasis. Cancers (Basel) 2020:12. doi: 10.3390/cancers12010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo HL, He L, Zhu YC, et al Comparison between multi-slice spiral CT and magnetic resonance imaging in the diagnosis of peritoneal metastasis in primary ovarian carcinoma. Onco Targets Ther. 2018;11:1087–94. doi: 10.2147/OTT.S147700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diop AD, Fontarensky M, Montoriol PF, et al CT imaging of peritoneal carcinomatosis and its mimics. Diagn Interv Imaging. 2014;95:861–72. doi: 10.1016/j.diii.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Koh JL, Yan TD, Glenn D, et al Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2009;16:327–33. doi: 10.1245/s10434-008-0234-2. [DOI] [PubMed] [Google Scholar]
- 12.de Bree E, Koops W, Kröger R, et al Preoperative computed tomography and selection of patients with colorectal peritoneal carcinomatosis for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2006;32:65–71. doi: 10.1016/j.ejso.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Jacquet P, Jelinek JS, Steves MA, et al Evaluation of computed tomography in patients with peritoneal carcinomatosis. Cancer. 1993;72:1631–6. doi: 10.1002/1097-0142(19930901)72:5<1631::aid-cncr2820720523>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Low RN, Barone RM, Lucero J Comparison of MRI and CT for predicting the Peritoneal Cancer Index (PCI) preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol. 2015;22:1708–15. doi: 10.1245/s10434-014-4041-7. [DOI] [PubMed] [Google Scholar]
- 15.Lim JS, Yun MJ, Kim MJ, et al CT and PET in stomach cancer: preoperative staging and monitoring of response to therapy. Radiographics. 2006;26:143–56. doi: 10.1148/rg.261055078. [DOI] [PubMed] [Google Scholar]
- 16.Dromain C, Leboulleux S, Auperin A, et al Staging of peritoneal carcinomatosis: enhanced CT vs. PET/CT. Abdom Imaging. 2008;33:87–93. doi: 10.1007/s00261-007-9211-7. [DOI] [PubMed] [Google Scholar]
- 17.Kawanaka Y, Kitajima K, Fukushima K, et al Added value of pretreatment 18F-FDG PET/CT for staging of advanced gastric cancer: Comparison with contrast-enhanced MDCT. Eur J Radiol. 2016;85:989–95. doi: 10.1016/j.ejrad.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Fukagawa T Role of staging laparoscopy for gastric cancer patients. Ann Gastroenterol Surg. 2019;3:496–505. doi: 10.1002/ags3.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altman DG, Vergouwe Y, Royston P, et al Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338:b605. doi: 10.1136/bmj.b605. [DOI] [PubMed] [Google Scholar]
- 20.Gamboa AC, Winer JH Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for gastric cancer. Cancers (Basel) 2019:11. doi: 10.3390/cancers11111662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rau B, Brandl A, Piso P, et al Peritoneal metastasis in gastric cancer: results from the German database. Gastric Cancer. 2020;23:11–22. doi: 10.1007/s10120-019-00978-0. [DOI] [PubMed] [Google Scholar]
- 22.Bonnot PE, Piessen G, Kepenekian V, et al Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): A propensity score analysis. J Clin Oncol. 2019;37:2028–40. doi: 10.1200/JCO.18.01688. [DOI] [PubMed] [Google Scholar]
- 23.Yasufuku I, Nunobe S, Ida S, et al Conversion therapy for peritoneal lavage cytology-positive type 4 and large type 3 gastric cancer patients selected as candidates for R0 resection by diagnostic staging laparoscopy. Gastric Cancer. 2020;23:319–27. doi: 10.1007/s10120-019-00994-0. [DOI] [PubMed] [Google Scholar]
- 24.Li K, Cannon JGD, Jiang SY, et al Diagnostic staging laparoscopy in gastric cancer treatment: A cost-effectiveness analysis. J Surg Oncol. 2018;117:1288–96. doi: 10.1002/jso.24942. [DOI] [PubMed] [Google Scholar]
- 25.Irino T, Sano T, Hiki N, et al Diagnostic staging laparoscopy in gastric cancer: a prospective cohort at a cancer institute in Japan. Surg Endosc. 2018;32:268–75. doi: 10.1007/s00464-017-5673-z. [DOI] [PubMed] [Google Scholar]
- 26.Lee KF, Tsai MM, Tsai CY, et al DEK is a potential biomarker associated with malignant phenotype in gastric cancer tissues and plasma. Int J Mol Sci. 2019:20. doi: 10.3390/ijms20225689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemtsova MV, Kalinkin AI, Kuznetsova EB, et al Clinical relevance of somatic mutations in main driver genes detected in gastric cancer patients by next-generation DNA sequencing. Sci Rep. 2020;10:504. doi: 10.1038/s41598-020-57544-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shuai Y, Ma Z, Liu W, et al TEAD4 modulated LncRNA MNX1-AS1 contributes to gastric cancer progression partly through suppressing BTG2 and activating BCL2. Mol Cancer. 2020;19:6. doi: 10.1186/s12943-019-1104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeldan E, Oguz S, Usta U, et al Risk factors for peritoneal dissemination of gastric cancer. Minerva Chir. 2015;70:91–6. [PubMed] [Google Scholar]
- 30.Huang B, Sun Z, Wang Z, et al Factors associated with peritoneal metastasis in non-serosa-invasive gastric cancer: a retrospective study of a prospectively-collected database. BMC Cancer. 2013;13:57. doi: 10.1186/1471-2407-13-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casariego Vales E, Pita Fernández S, Rabuñal Rey R, et al Metastasis of gastric cancer: influence of the localization of the primary tumor. Rev Esp Enferm Dig (in Spanish) 1994;85:249–83. [PubMed] [Google Scholar]
- 32.Lu M, Yang Z, Feng Q, et al The characteristics and prognostic value of signet ring cell histology in gastric cancer: A retrospective cohort study of 2199 consecutive patients. Medicine (Baltimore) 2016;95:e4052. doi: 10.1097/MD.0000000000004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan C, Zhu ZG, Yan M, et al Value of multidetector-row computed tomography in the preoperative T and N staging of gastric carcinoma: a large-scale Chinese study. J Surg Oncol. 2009;100:205–14. doi: 10.1002/jso.21316. [DOI] [PubMed] [Google Scholar]
- 34.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 35.Agnes A, Estrella JS, Badgwell B The significance of a nineteenth century definition in the era of genomics: linitis plastica. World J Surg Oncol. 2017;15:123. doi: 10.1186/s12957-017-1187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang JY, Wang ZN, Lu CY, et al Borrmann type IV gastric cancer should be classified as pT4b disease. J Surg Res. 2016;203:258–67. doi: 10.1016/j.jss.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 37.Chen S, Chen X, Nie R, et al A nomogram to predict prognosis for gastric cancer with peritoneal dissemination. Chin J Cancer Res. 2018;30:449–59. doi: 10.21147/j.issn.1000-9604.2018.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang Y, Jin C, Yu H, et al. Development and validation of a deep learning ct signature to predict survival and chemotherapy benefit in gastric cancer: A multicenter, retrospective study. Ann Surg 2020. [Epub ahead of print].
- 39.Zhang W, Fang M, Dong D, et al Development and validation of a CT-based radiomic nomogram for preoperative prediction of early recurrence in advanced gastric cancer. Radiother Oncol. 2019;145:13–20. doi: 10.1016/j.radonc.2019.11.023. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Wu Z, Lin E, et al Systemic prognostic score and nomogram based on inflammatory, nutritional and tumor markers predict cancer-specific survival in stage II-III gastric cancer patients with adjuvant chemotherapy. Clin Nutr. 2019;38:1853–60. doi: 10.1016/j.clnu.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 41.Waddell T, Verheij M, Allum W, et al Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi57–63. doi: 10.1093/annonc/mdt344. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Li B, Liu Z, et al HER2 copy number of circulating tumour DNA functions as a biomarker to predict and monitor trastuzumab efficacy in advanced gastric cancer. Eur J Cancer. 2018;88:92–100. doi: 10.1016/j.ejca.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 43.Caccialanza R, Cereda E, Klersy C, et al Early intravenous administration of nutritional support (IVANS) in metastatic gastric cancer patients at nutritional risk, undergoing first-line chemotherapy: study protocol of a pragmatic, randomized, multicenter, clinical trial. Ther Adv Med Oncol. 2020;12:1758835919890281. doi: 10.1177/1758835919890281. [DOI] [PMC free article] [PubMed] [Google Scholar]