Abstract
Hot melt extrusion has been an exciting technology in the pharmaceutical field owing to its novel applicability. Twin-screw granulation presents a great potential and offers many advantages relative to conventional granulation processes. Different twin-screw granulation techniques, such as twin-screw dry granulation, twin-screw wet granulation, and twin-screw melt granulation, are currently being developed as robust and reproducible granulation processes. The competence of twin-screw granulation as a continuous manufacturing process has contributed to its suitability as an alternative granulation option within the pharmaceutical industry. In this article, different types of twin-screw granulation techniques were discussed. In addition, the screw elements, scale-up process, continuous twin-screw granulation which involves process analytical tools, and excipients were explored. This economical, industrially scalable process can be automated for continuous manufacturing to produce granules for the development of oral solid dosage forms. However, extensive research using process analytical tools is warranted to develop processes for the continuous manufacture of granules.
Keywords: Twin-screw dry granulation, Twin-screw wet granulation, Twin-screw melt granulation, Screw elements, Continuous manufacturing, Hot melt extruder
Graphical Abstract
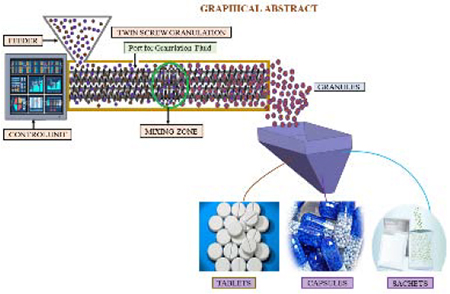
1. Introduction
Over the past 30 years, the hot melt extrusion (HME) technology has been widely utilized in the pharmaceutical industry. HME is the process of conveying a material by using rotating screws at an elevated temperature. This material is then pumped through a die to produce the desired product (Censi et al., 2018; Repka et al., 2018). Approximately 40% of new molecules have poor bioavailability owing to their low solubility. Thus, designing a dosage form for such molecules has proven to be a challenge for formulation scientists. However, the development of molecular dispersions for such poorly soluble molecules via HME has been well established to increase their solubility and bioavailability (Forster et al., 2001; Ndindayino et al., 2002; Takeichi et al., 2002). Besides enhancing solubility and bioavailability, the HME process also improves the stability, controls release rates, scale-up flexibility, and the accommodation of molecules of different sizes, and enables taste masking (Kallakunta et al., 2019; Tiwari et al., 2016). Several research groups have reported the suitability of the HME technology for developing different pharmaceutical dosage forms, such as pellets (Young et al., 2002), granules (Venkata Kallakunta et al., 2018; Liu et al., 2001; Robinson et al., 2003), immediate and modified release tablets (Crowley et al., 2002; Zhang and McGinity, 2000, 1999), oral fast dissolving systems (Sherry, 2008), transdermal (Aitken-Nichol et al., 1996; Repka and McGinity, 2001) and transmucosal delivery systems (Munjal et al., 2006b, 2006a; Prodduturi et al., 2005; Repka et al., 2005; Thumma et al., 2008), trans ungual delivery systems (Mididoddi and Repka, 2007; Mididoddi et al., 2006), implants (Bhardwaj and Blanchard, 1998; Sam, 1992), chronotherapeutic systems (Dumpa et al., 2018), semisolid dosage forms (Bhagurkar et al., 2016), abuse deterrent formulations (Wening et al., 2017), and co-crystals (Douroumis et al., 2017).
Recently, granulation by the HME technology has attracted the attention of academic scientists as well as workers in the pharmaceutical industry. As a result, the twin-screw granulation (TSG) was identified as an alternative approach for the transformation from batch processing to continuous manufacturing in the pharmaceutical industry. Continuous manufacturing has been mainly used in food and plastic industries. However, its use in the pharmaceutical industry was initially hindered because of the high regulatory standards for this industry (Sun, 2010). Most solid dosage forms are available as tablets, capsules, and powder formulations and they are preferably manufactured by different granulation techniques such as dry granulation, wet granulation, and melt granulation. The conventional wet granulation process is widely used in the pharmaceutical industry, and it enhances powder flowability, compactibility and uniformity (Wan et al., 1992), causes less segregation tendency (Keleb et al., 2004), and controls dissolution (Ochoa et al., 2010). High shear granulator and the fluid bed dryer are commonly used for conventional wet granulation (Leuenberger, 2001). Some active pharmaceutical ingredients (APIs) and excipients are sensitive to the granulating fluid, which causes serious stability and degradation issues (Lakshman et al., 2011). Twin screw melt granulation is an alternative approach where a molten binder enables the granulation process, ultimately avoiding the use of the granulating fluid (Hamdani et al., 2003).
TSG is the technique where material is conveyed to the mixing zone. The material is then kneaded to form agglomerates, with or without aid of specific binders in formulation, and then collected as granules at the discharge point. The kneading zone within the barrel can be adjusted in any location, as desired, to obtain suitable granules for post-granulation processing. The granules obtained by TSG can either be used directly for further processing or subjected to size reduction (milling) to obtain the desired size for the granules. The instrumentation consists of two co-rotating screws enclosed in a barrel and screw elements loaded onto a shaft rod. The process parameters include screw speed (rpm), feed rate (g/h or kg/h), L/S ratio (for wet granulation), screw configuration, barrel temperature, residence time, torque, and barrel fill volume. Different reports on TSG have demonstrated the significance of the process and formulation variables on the critical quality attributes for obtaining the desired quality granules (Dhenge et al., 2013; El Hagrasy and Litster, 2013; Hagrasy et al., 2013; Lee et al., 2012; Thompson and Sun, 2010; Tu et al., 2013). Further, TSG can be employed to manufacture amorphous granules (Majumder et al., 2018), which may aid in increasing the solubility and bioavailability of poorly water-soluble drugs.
1.1. Continuous twin screw granulation
The recent advancements in TSG and updates to regulatory guidelines (Sun, 2010), attracted pharmaceutical industries and regulatory authorities (Plumb, 2005) to get accustomed to this technology to improve the manufacturing output and product quality. Owing to the increased need for assurance of product quality and safety, the interest of health authorities has recently been fueled toward continuous manufacturing (Allison et al., 2015; Byrn et al., 2015; Nepveux et al., 2015; Srai et al., 2015). The FDA described the process analytical technology (PAT) and quality by design (QbD) concepts in guidance to industry document released in 2004, which is clearly emphasized in the ICH guidelines Q8, Q9, and Q10 (Guidance for industry Q10 pharmaceutical quality system, 2009, International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use ICH harmonised tripartite guideline quality risk management Q9, 2005, International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use pharmaceutical development Q8(R2), 2009). Developing formulation by the QbD approach aids in the achievement of a quality product.
Traditionally, pharmaceutical industries are manufacturing drug products in batches (the quantity of each is limited by the size of the equipment). The ability of TSG as a continuous process provides an option to overcome the limitations of batch manufacturing. Continuous manufacturing does not have any fixed batch size, but it can be used for any customized size requirement. Further, the scale-up process needed to meet commercial requirement is relatively easy by twin screw continuous manufacturing and requires minimum investment (Cartwright et al., 2013; Keleb et al., 2002; Vervaet and Remon, 2005). Conversely, the scale-up of the conventional batch process shows batch variability (Sochon et al., 2010), and requires high investments (Schaber et al., 2011). Continuous manufacturing does not rely on end product testing as it involves inline monitoring of drug product quality and critical process parameters. This continuous manufacturing requires implementation of PAT tools within the pharmaceutical processes (Rudd, 2004).
Continuous granulation reduces manpower, which in turn eliminates the probability of human errors (“Continuous Granulation | Thermo Fisher Scientific - US”). It also prevents any delay that may be encountered during material unloading from the previous process and reloading into another equipment for additional manufacturing processes. Continuous manufacturing does not require any space for storing in-process material as once the batch is initiated, the finished product will be the output of the process. Additionally, continuous manufacturing prevents the segregation of material, which occurs with different batch manufacturing processes during in-process material transfer from one process area to another process area (“Continuous Granulation | Thermo Fisher Scientific - US”). In continuous manufacturing lab scale equipment’s can be used for production scale (Vervaet and Remon, 2005). In conventional batch manufacturing, any discrepancies during the process may lead to rejection of the entire batch. However, because a specific amount of product is processed at each step in continuous manufacturing, any discrepancies may lead to the rejection of only limited product, thereby saving the unaffected material (Richter, 2019). The development of advanced inline monitoring techniques tracks the product at each individual stage of manufacturing and eventually saves additional analysis time, thereby enabling the direct release of the continuously manufactured batch into the market without delay (“Thermo Fisher Scientific US, Continuous Granulation,” n.d.). The Schematic representation of Continuous manufacturing by Twin screw granulation is depicted in Figure 1.
Fig. 1.
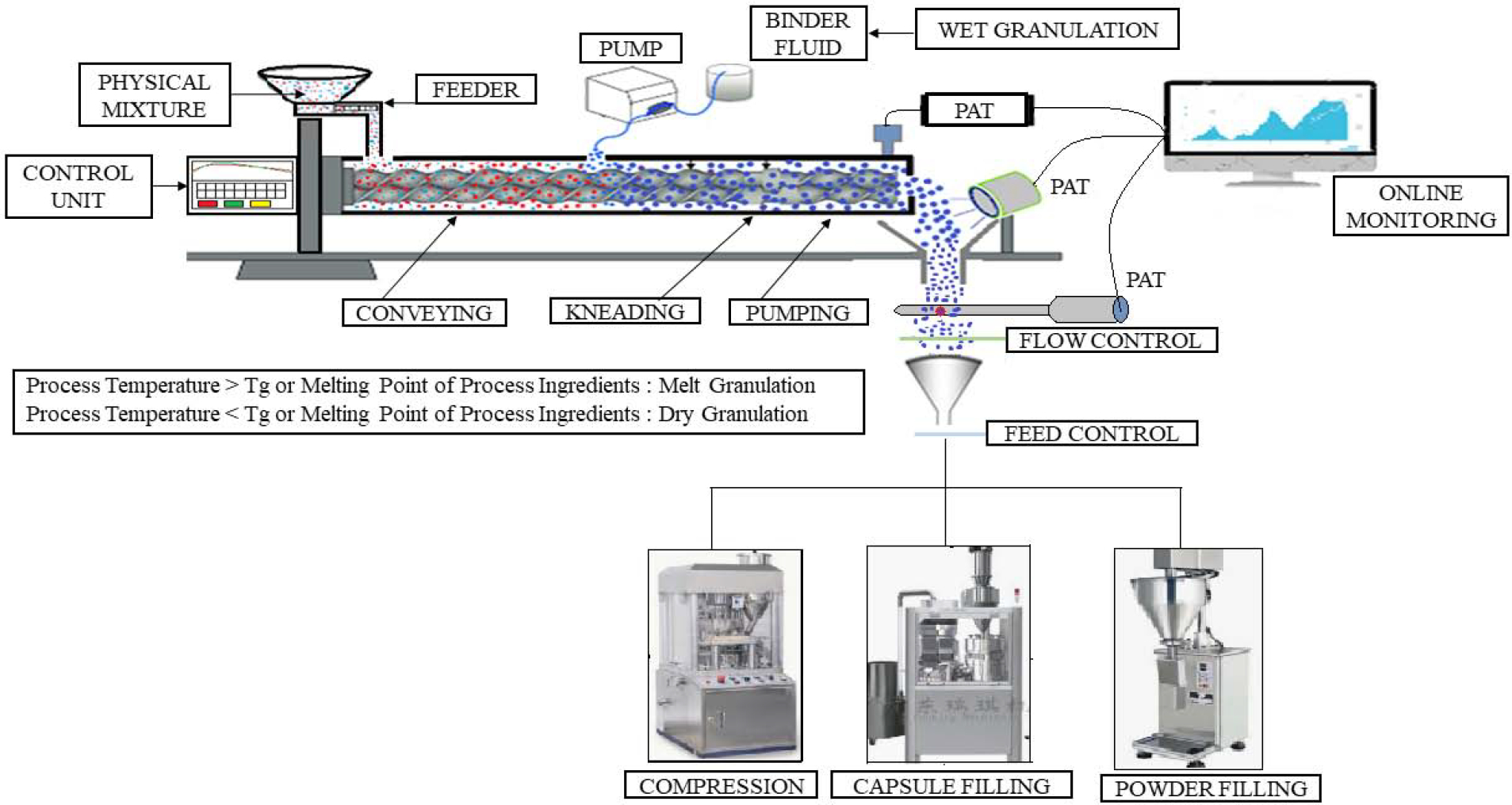
Schematic representation of Continuous manufacturing by Twin-screw granulation with monitoring and controls.
1.2. Single continuous manufacturing line for multiple products
As parts (product contact parts) of the twin-screw extruders can be replaced, this allows it to function as a flexible manufacturing process to produce multiple products in pipeline and prevent the cross contamination of products (“Continuous Granulation | Thermo Fisher Scientific - US”). The same equipment setup can be used for research and development, as materials can be analyzed inline at each step to understand the behavior of the product at each stage during early development. These extruders have been successfully investigated and proved to be an efficient equipment for both granulation and the HME processes, with desired modifications (Markarian, 2018). The applicability of extruders in granulation has been discussed elsewhere in this review article.
Mendez Torrecillas et al. (Mendez Torrecillas et al., 2017) evaluated various process variables affecting homogeneity of granules produced from continuous twin screw wet granulation using an 11 mm twin screw granulator using water as the granulating fluid at different L/S ratios. Two materials, microcrystalline cellulose (MCC) and alpha lactose monohydrate, were evaluated at screw speeds of 50–125 rpm and feed rate of 0.05–0.35 kg/h. It was reported that the homogeneity of the granules was influenced by feed rate, L/S ratio and torque velocities. These observations suggest the significance of feed rate and L/S ratio in producing uniform granules.
1.3. Process analytical technology
Process analytical technology (PAT) is used for inline monitoring of critical product parameters that are linked to product quality (Islam et al., 2014) and different PAT tools are available for inline monitoring of the granulation process. PAT tools aid in faster, reliable process development, and easy scale-up, thereby reducing the workload of process engineers. The main aim of any granulation technique is to obtain granules with the desired size, shape, and moisture content. The solid state of active components can be monitored by NIR and Raman spectroscopy and these techniques are suitable for identifying any small change in solid state (Fonteyne et al., 2015, 2012). Particle size distribution, and granule symmetry and surface can be monitored using an Eyecon high speed imaging camera/Parsum probe. This instrument can also be equipped to monitor granule characteristics during fluid bed coating and the milling process (El Hagrasy et al., 2013). The further implementation of PAT tools in continuous manufacturing enables the identification of critical process parameters (i.e., the process parameters that affect the quality of granules). Fonteyne et al. (Fonteyne et al., 2015, 2012) used photometric stereo imaging (Flashsizer FS3D) to evaluate the particle size distribution, roughness, and shape of wet granules. Additionally, these researchers employed NIR spectroscopy and Raman spectroscopy to respectively determine the moisture content of granules and the solid-state behavior of the theophylline active ingredient during the wet granulation process. A schematic illustration of continuous manufacturing line by twin screw granulation along with possible PAT tools is captured in Figure 1.
Sayin et al. (Sayin et al., 2015) investigated the effect of different screw elements, distributive feed screw (DFS) & kneading elements (KE’s) on particle size distribution (PSD) of granules produced using an 11 mm extruder (Thermo Fisher Scientific) at a screw speed of 482 rpm and feed rate of 1.11 kg/h by a twin screw wet granulation method. An Eyecon™ camera was used as an inline PAT tool for analyzing PSD. Two screw configurations, one with DFS and the other configuration with a kneading block containing 7 KE’s at 90˚ was investigated. A placebo blend containing α-lactose monohydrate (73.5%), Avicel PH101 (20%), HPMC (5%), Croscarmellose sodium (1.5%) was utilized for granulation with 0.1% w/w aqueous solution containing nigrosin dye as granulating fluid. Effects of multiple liquid to solid ratios (L/S; 0.15, 0.20, 0.25, 0.30) on PSD revealed increasing L/S ratios, increased particle size and decreased fines. Configuration with kneading elements produced granules between 125μm and 1 mm. Granules formed by kneading elements were less porous when compared to granules of DFS. Non-porous granules produced can be attributed to high shear or torque generated by KE’s during granulation. DFS generated relatively less shear (0.7–0.9 Nm) than KE’s (0.9–3.9 Nm). The DFS configuration resulted in similar granule size parameters (d10, d50, d90) and particle count at 0.15–0.25 L/S ratios.
2. Screw Elements
The availability of different screw elements allows it to serve as a versatile equipment for multiple applications. In this section of review, different types of screw elements, such as conveying, kneading, mixer, fractional lobed, distributive mixing, and cutter elements, that are used in granulation techniques are discussed. Screw shaft flexibility is a key success factor for co-rotating parallel twin screw granulation. As the screws are intermeshing and the flight of one screw cleans the surface of other screw in rotation, they are self-cleaning. Moreover, the screw configuration can be changed to meet various application requirements (“Thermo Scientific-16 mm Screw Elements Portfolio (PP-016),” 2013). Different screw elements used for Twin-screw granulation are presented in Figure 2.
Fig. 2.
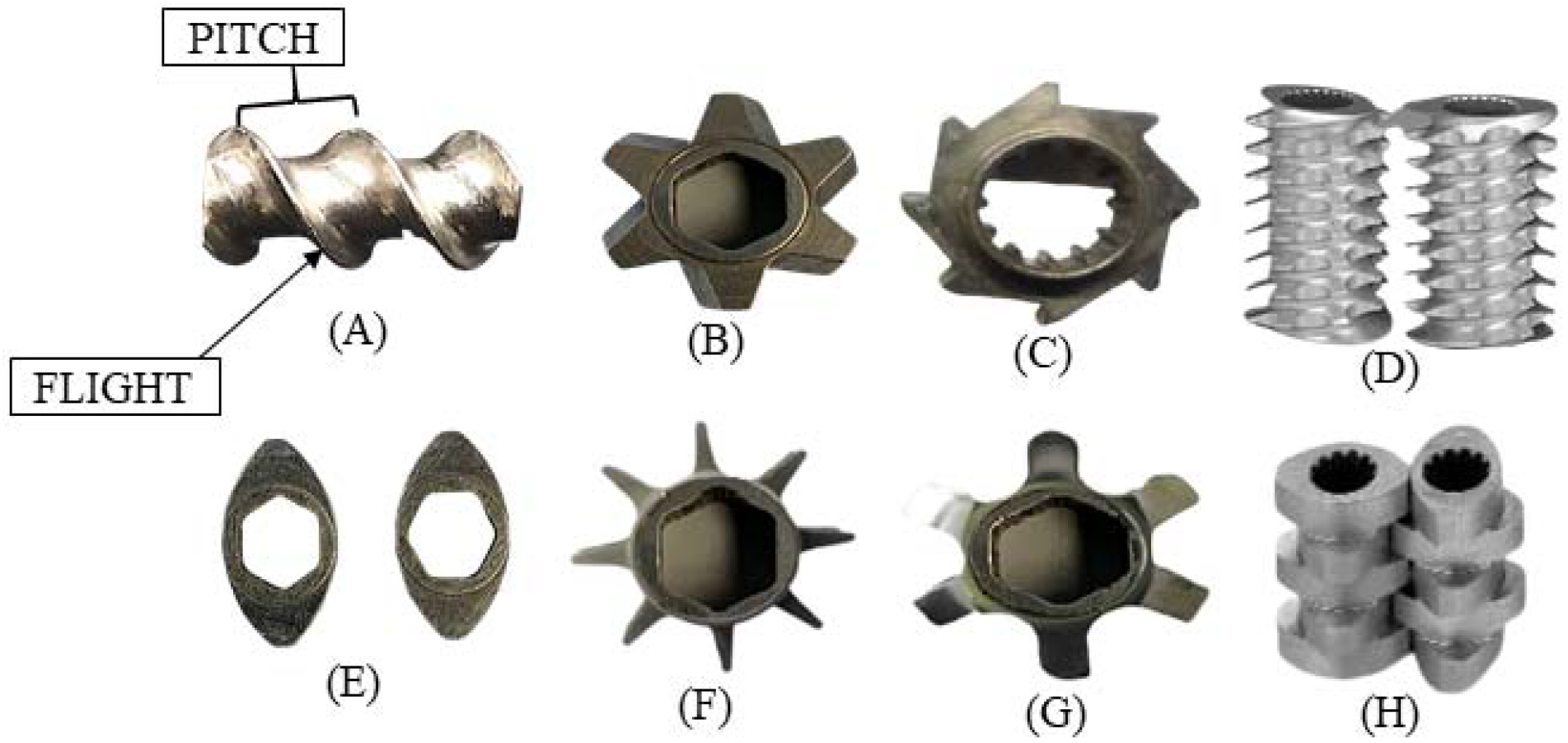
Different screw elements used for Twin-screw granulation. (A) Conveying element, (B) Wide toothed mixing element, (c) Cutter element, (D) Comb mixer element, (E) Kneading elements, (F) Narrow toothed mixing element, (G) Screw mixing element, (H) Kneading block.
2.1. Conveying elements
Conveying element (CEs) (Fig. 2A) or forwarding elements are used in conveying materials between mixing zones to carry material away from the feed zone and are mainly utilized in feeding zone. As their function is to convey materials, they impart low shear. Conveying property is improved with an increase in pitch, as more material will be conveyed in each revolution. Fill level is dependent on the feed rate, screw speed, and screw geometry. The reverse CEs can be placed at the end of the kneading or mixing zone to increase the residence time within the mixing section. Wide throat elements allow the feeding of larger sized particles, such as broken flakes, instead of pellets (“Thermo Scientific-16 mm Screw Elements Portfolio (PP-016),” 2013).
2.2. Mixing/Kneading elements
Mixing sections (Fig. 2E & 2H) are created by combining several single kneading elements (KEs). The offset between neighbored elements determine the conveying and mixing properties. The conveying properties decrease with increasing offset angles while the mixing properties increase. In extreme 90° offset sections have pure mixing and no conveying capabilities. By alternating elements with 0° and 90° on the hexagonal shaft orientation, 30° and 60° offsets can be achieved. The applied shear increases with an increase in offset angle (Sun, 2010) and the length of elements. For example, the Thermo fisher scientific 16 mm twin screw granulator has most commonly used mixing element of length 1/4 L/D; this is because a longer element (1/2 L/D) introduces high shear while shorter elements (1/8 L/D) introduces lower shear.
Mixing elements can be replaced by mixing blocks with fixed offset angles. The mixing block is a solid block of mixing elements because it is built from individual mixing elements, its geometry is the same. This solid block also has an increased overall strength (“Thermo Scientific-16 mm Screw Elements Portfolio (PP-016),” 2013).
2.3. Combing mixer element
Mixer elements (Fig. 2D) simultaneously perform both mixing and conveying functions and they are similar to CEs but have longitudinal slots for distributive mixing (Djuric and Kleinebudde, 2008). Comb mixing element is also called distributive mixing element (DME). By comparing its effect to that of the KE on granule properties, Li et al. (Li et al., 2018) have reported that the DME in the mixing or kneading region produce similar granules to those formed by KEs. DME in the forward or reverse configuration does not exhibit any effect on granule properties. Instead, it divides the agglomerates and recombines with the additional ungranulated fine material, leading to enhanced uniformity.
2.4. Cutter elements
Vercruysse et al. (Vercruysse et al., 2015) investigated the use of cutter element in twin-screw wet granulation (TSWG). These elements were placed in the last section of the screw to optimize the granule size to attain a size suitable for tableting. These elements were found to have neutral conveying activity, resulting in material retention and an increase in particle size. Nonetheless, incorporation of these elements resulted in increased torque values.
2.5. Miscellaneous Mixing Elements
Other types of mixing elements used in the TSG process area include narrow toothed mixing elements, wide toothed mixing elements, and screw mixing elements (SMEs).
Vercruysse et al. (Vercruysse et al., 2015) examined the impact of these mixing elements on granule properties. Narrow toothed mixing elements are found to have less conveying activity. Although incorporating narrow toothed mixing element resulted in reduced fines, a further increase in the number of elements did not have an impact on fines. Instead, a reduction in the oversized agglomerates was found. Conversely, wide toothed mixing elements are found to result in reduced fines and increased oversized agglomerates, owing to their non-conveying activity. An increase in the wide toothed mixing elements result in a subsequent increase in the shear and torque values. SMEs are found to result in the backflow of materials, resulting in increased residence time. Ishikawa et al. (Ishikawa et al., 2002) observed the distributive mixing process by incorporating the SMEs. Moreover, Fard et al. (Fard and Anderson, 2013) found low shear values with SMEs, a finding that was further confirmed in other reports by Vercruysse et al. (Vercruysse et al., 2015). The fines generated by SMEs was higher than those achieved with the KEs.
However, the advantages and applicability of these elements in granulation techniques are limited and extensive research is warranted to understand these elements in the manufacture of granules by TSG.
2.6. Screw elements with Fractional Lobed Geometry
Fractional lobed geometry elements are an alternative type of screw elements for conventional geometry elements (e.g., Unilobed, bilobed, trilobed). To understand the difference in geometry between novel fractional lobed and conventional elements, knowledge of tip angle (represented by ‘T’ in the image below Figure. 3) is of utmost importance. Figure 3A & 3B represents the end geometry of the conventional trilobed and the novel fractional trilobed elements, respectively. Tip angle remains constant (T1) in conventional elements but varies (T1 & T2) in fractional geometry elements. Fractional geometry elements maintain a constant gap between elements when rotating in the same direction. The number of lobes possible depend on the distance from the center to the barrel diameter ratio. A new geometry is thus formed by a transition between the two conventional elements. For example, Figure 3C is formed by the transition of the bi-lobed and four-lobed elements. Similarly, Figure 3D is formed by the transition of the uni-lobed and the bi-lobed element. The higher the number of lobes, the lower the tip angle. Increasing the tip angle will cause the element to display a shape that is nearly circular, thereby allowing the maintenance of a constant free volume between the element and barrel, which could not be achieved by conventional elements (Padmanabhan, 2004).
Fig. 3.
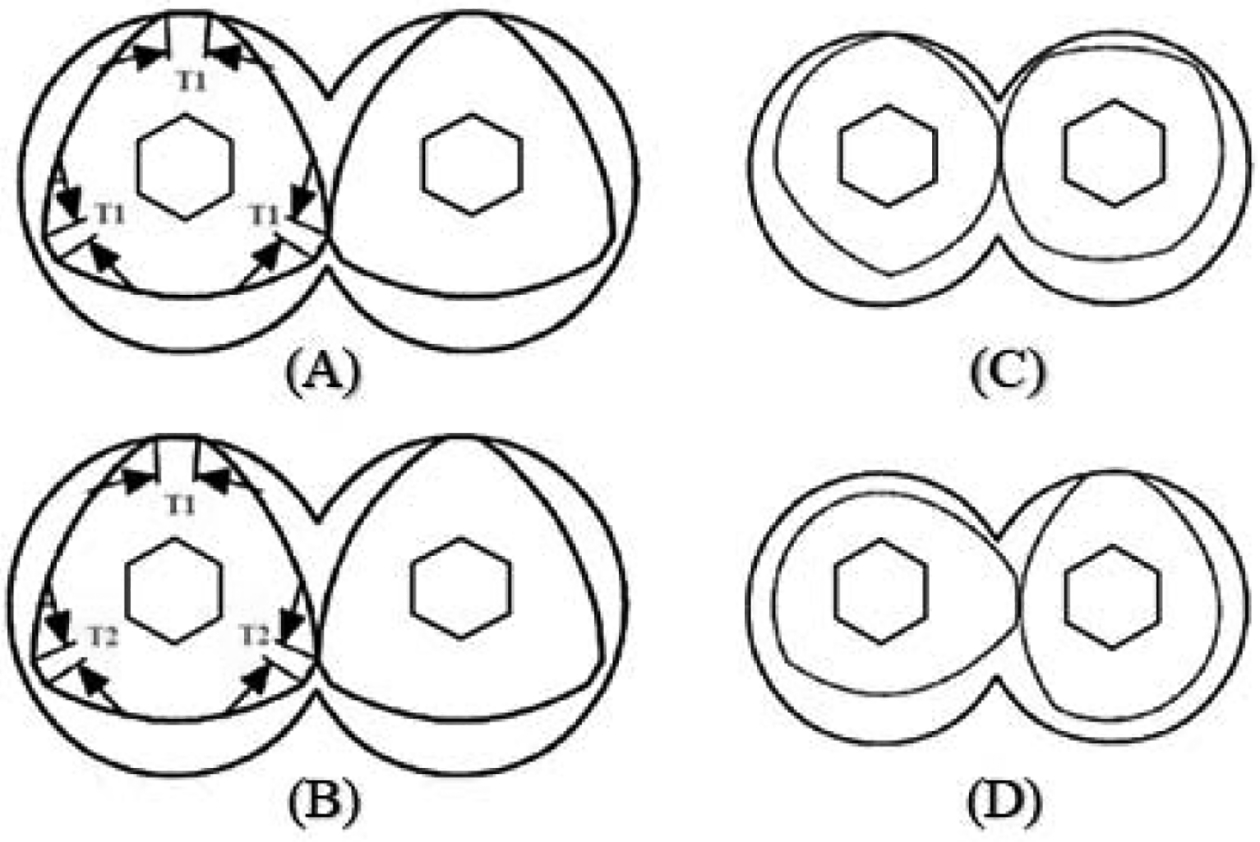
Illustration of elements geometry (A) Conventional Tri-lobed Geometry Elements (B) Novel Fractional Tri-lobed Geometry Elements (C) & (D) Fractional geometry of conventional Elements (Adapted from Padmanabhan, 2004).
Rao et al. (Rao et al., 2016) reported the advantages of incorporating screws with fractional lobed geometry relative to screws with conventional bilobed geometry. These novel fractional lobed geometry screws increased granular properties (tensile strength, friability, high mean particle diameter, low fines, symmetrical granules, better flow, high compressibility, low tablet weight variation) compared to screw elements with the conventional bilobed geometry. Moreover, the fractional lobed screws were demonstrated to be superior in high throughput, less torque, and uniform shear distribution. Only 58% torque (twin screw wet granulation) was recorded at the 20 kg/h feed rate and 900 rpm screw speed with the screws with fractional lobed geometry. Further research must be carried out to evaluate the suitability of these elements for other granulation techniques. The major advantage of using these elements is to eliminate the additional milling step as granules obtained are quite symmetrical and are suitable for coating or can be processed for downstream processing. A schematic personification of Fractional lobed geometry elements is captured in Figure 3.
3. Types of Twin-screw granulation
Granulation is the enlargement of particle size to enhance powder flowability, compressibility, and the homogeneity of the blend. Binders play a key role in the formation of granules. Based on binder’s utilization within the process TSG can be divided into three types: Twin-screw dry granulation (TSDG), Twin-screw wet granulation (TSWG), and Twin-screw melt granulation (TSMG).
3.1. Twin screw dry granulation
As indicated by its name, TSDG is a dry process where no solvent (cost-effective) is used to granulate the blend. TSDG is similar to the conventional roller compaction process, which was previously used for dry granulation (Mangal et al., 2016). The main limitation of this conventional process is the plastic deformation of the material, which reduces the granule strength (Malkowska et al., 1983). In the roller compacter, the material is compacted between two rolls to obtain ribbon-like clusters, which undergo further milling in the downstream process to retrieve granules of the desired size. Dry granulation mechanisms include the formation of strong bonds, such as solid bridges between the particles, and weaker intermolecular bonds such as van der Waals forces, hydrogen bonds, and electrostatic bonds in the pharmaceutical products (Adolfsson et al., 1997; Nystrom et al., 1993).
In the TSDG technique, the physical mixture containing the active ingredient, binder, and other excipients is fed into the extruder with the aid of a gravimetric hopper or force feeder. The feed material is then conveyed into the mixing or kneading zone with the aid of the CEs. The temperature of this process is maintained below the glass transition temperature (Tg) or melting point (MP) of the formulation components to ensure all materials in the granulation mixture remain in a dry state (non-molten state). The process temperature and shear generated in the kneading zone cause materials to soften and distribute uniformly in the extruder barrel, leading to granule formation. The formed granules are then conveyed and collected at the discharge point. This process is suitable and enhance stability of heat and solvent-sensitive drugs as it does not require any additional drying step in the granulation process (Vervaet and Remon, 2005). Moreover, this process uses a low binder concentration (10%) which enhances the drug loading capacity (Richter, 2017) of the formulations. Experimental setup utilized for twin screw dry granulation is represented in Figure 4A.
Fig. 4.
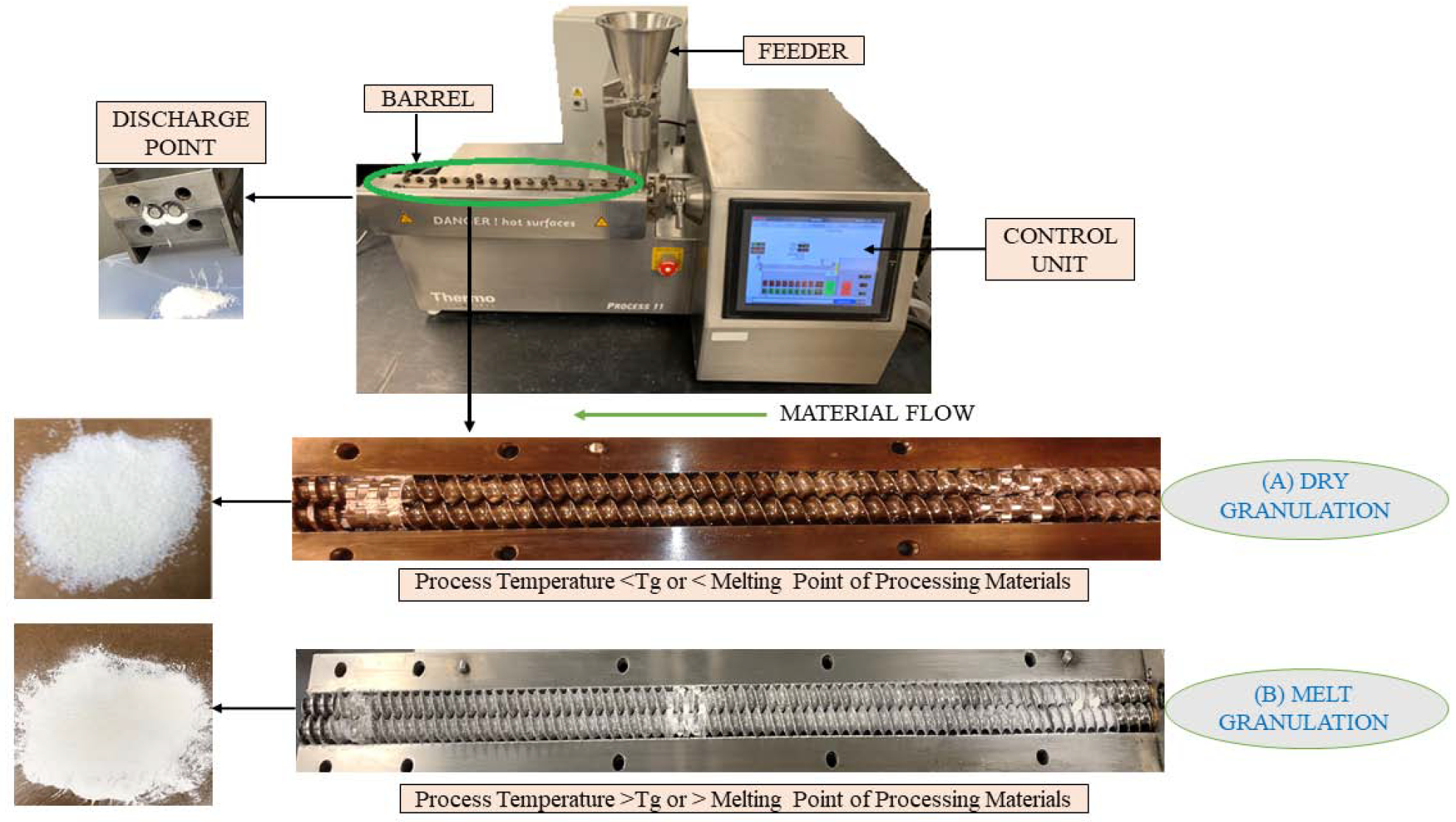
The experimental set-up for manufacture of granules A) Twin-screw dry granulation B) Twin-screw melt granulation.
Kallakunta et al. (VR Kallakunta et al., 2018) examined the effect of formulation ingredients (binders, sustained release agents), screw configuration, and feed rate on the development of sustained release formulations containing APIs with three different MPs (Theophylline: 272–274 °C, Acetaminophen: 169–171 °C, Lidocaine: 82 °C). The process was carried out below the MP or the Tg of all ingredients in the formulation to ensure all ingredients remained in the non-molten (i.e., dry) state. The main factors supporting the granulation process were heat energy and the shear applied in the mixing zones. The % drug load and the proportion of compressible material in the physical mixture were factors affecting granule formation. The granules showed good compression characteristics and were successfully compressed into sustained tablets for 24 h. DSC and XRD revealed the preservation of the crystallinity of the formulation components after dry granulation, indicating successful dry granulation. These results demonstrate the applicability of hot melt twin extruder in the manufacture of granules and the sustained release tablets containing low to high MP drugs.
Majumder et al. (Majumder et al., 2018) successfully produced amorphous granules of Benzoyl-methoxy-methylindol-acetic acid (BMA), a poorly soluble drug with amorphous magnesium aluminometasilicate (Neusilin® (US2)) as an inorganic carrier via twin screw granulation. Solution calorimetry (SolCal) was employed to identify the amorphous nature of the granules produced by the TSDG technique. The granules of all extrudates had a particle size < 115 μm and flow properties close to those of Neusilin®US2 (particle size, 130 μm). Both BMA and US2 were extruded in different drug-carrier ratios (1:4, 1:2.5, 1:1) at a temperature of 180 °C. The amorphous nature of the granules was further confirmed by DSC and XRD while SEM and optical microscopy studies revealed the entrapment of BMS within the porous cavity of Neusilin® US2. FTIR revealed molecular interactions between the carboxylic group of the drugs and the silanol groups of the carriers. Dynamic vapor sorption (DVS) showed the hygroscopic nature of extrudates exposed to higher humidity; thus, care must be exercised during storage. This confirms the capability of employing the TSG technique for the manufacture of amorphous granules with suitable excipients, thereby aiding poorly soluble drugs. Further, this study revealed that SolCal could be an alternative technique to DSC.
Upadhye et al. (Upadhye et al., 2017) successfully performed dry granulation for the physical mixture containing Sildenafil citrate with polymers, Klucel™ HF (Hydroxy Propyl Cellulose), Natrasol® (Hydroxy Ethyl Cellulose), and Aqualon™ N7 (Ethyl cellulose) in different ratios. The processing conditions examined were 100–200 rpm, 65 °C, and 3–5 g/min of feed rate with different screw configurations. The addition of magnesium stearate to the physical blend aided in overcoming material degradation and extruder noise during granulation. Similarly, the applicability of the dry granulation technique for thermolabile API (Ondansetron HCl dihydrate, OND) using the polymers, Klucel™ EF (HPC) and Ethocel standard 10 (Ethyl cellulose, EC), was investigated at a screw speed of 25–100 rpm and feed rate of 80 g/h. Extrusion was carried out at 70–90 °C using different screw configurations. The number of mixing elements in the screw configuration had an impact on the nature of the granules produced. An increase in the EC concentration resulted in fines whereas low feed rate and high screw rpm resulted in desirable granules. The granules obtained were compressed into tablet (140 kg/cm), which retarded drug release for 15.5 h. Altogether, this study emphasized granulation at low temperatures, which is suitable for heat-sensitive drugs.
Liu et al. (Liu et al., 2017) evaluated non-binder ingredients (micro crystalline cellulose (MCC) and lactose monohydrate) and polymers (Affinisol™ HPMC HME 100LV and Affinisol™ HPMC HME 4M) in the heat-assisted twin screw granulation technique. Melt granulation was carried out at 160, whereas dry granulation was carried at 160 in the mixing zone and 30 in the remaining zones. As reported previously by Upadhye et al., high screw speed and low feed rate resulted in good granules (Upadhye et al., 2017). The temperature of the granules at the discharge point was 40 °C for formulations containing 100LV and HPMC HME 4M, and 80 °C for those containing dehydrated lactose. The addition of a filler (MCC) resulted in a 50% decrease in particle size (506 μm to 227 μm). Formulations containing HPMC HME 4M and MCC had no agglomeration. The addition of caffeine resulted in a decrease in particle size compared to granules composed of the placebo blend, which accounted for the lower cohesion of friction of caffeine relative to that of other ingredients in the formulation. The polymers that were chemically identical and shared the same Tg (111 °C) had an impact as they differ in molecular weight and viscosity. Compared to the 4M polymer, the low viscosity polymer, 100LV, was the most suitable binder for dry granulation. Further phase transformation of the active ingredient (caffeine) and dehydration of the excipient (lactose monohydrate) were partial in twin screw granulation but complete in the melt granulation process. Such finding revealed the stability of the formulations prepared by TSDG over melt granulation.
Richter et al. (Richter, 2017) examined the effect of process temperature below and above the Tg of a polymeric binder using lactose (80–85%) as a substitute for API, 15% Kollidon VA64 (Tg: 105°C) or Soluplus® (Tg: 72°C) as binders, and 5% of MCC. Granulation using Kollidon VA64 as binder at 30–50 ℃, 1.5–2.0 kg/h feed rate, 150–300 rpm resulted in poor granulation as the process temperature was insufficient for granule formation. By using Soluplus® (15%) as a binder in the granulation process at 130–150 ℃, 0.5 kg/h, and 70–130 rpm, granule formation was achieved. A decrease in the processing temperature from 150 °C to 130 °C resulted in reduced fines and increased agglomerates. In TSDG, % torque is a very good indicator of the process as it is related to feed rate, processing temperature, and screw speed. At the lowest feed rate, the filling level in the extruder was found to be minimum and the material was simply conveyed forward rather than converted to granules. However, at a higher feed rate, a potential blockade is expected in the extruder, resulting in an unsuccessful process. Thus, the combination of these process parameters and the interaction between API and the excipients are critical aspects in the granulation process. TSDG enables the manufacture of granules and sustained release of tablets containing low to high MP drugs, amorphous granules of poorly soluble drugs, and granulation at low temperatures, which is suitable for heat-sensitive drugs and the stability of the formulations.
3.2. Twin-screw melt granulation
Melt granulation requires a binder with low MP, which acts as a binding liquid after melting (Perissutti et al., 2003). This process requires a low binder concentration (Vasanthavada et al., 2011). Similar to TSWG and TSDG, the physical mixture of the blends containing active ingredient, binder, and other excipients are conveyed at a process temperature near or above the MP of the binder to ensure that only the binder in the formulation is melted and uniformly distributed between the particles, leading to granule formation (Melkebeke et al., 2006). Binder selection plays a crucial role in TSMG. A binder that has a lower Tg or MP than all other materials in the formulation serve as the best candidates for use in TSMG. Schematic representation of twin screw melt granulation is captured in Figure 4B.
Verstraete et al. (Verstraete et al., 2016) examined the suitability of both hydrophilic (Tecophilic SP60D60, SP93A100, TG2000) and hydrophobic (Tecoflex EG72D) thermoplastic polyurethanes (TPU) for the sustained release of metformin HCl and sought to compare mini matrices manufactured by HME to tablets manufactured by injection molding (IM) and TSMG. Mini matrices and injection molding (0–70% of drug load) were carried out at 100–160 °C while TSMG (85–90% drug load) was carried out at 140 °C and 40 °C in zone next to the discharge point using a screw configuration with two kneading zones, 0.7 kg/h feed rate, and 200 rpm. A binder concentration <5% resulted in fines (<250 μm) while a concentration >15% resulted in coarse granules (>1000 μm). The separate use of hydrophilic and hydrophobic TPU binders did not successfully produce sustained release formulation, whereas the use of a TPU binders blend resulted in sustained drug release in the mini matrices and IM tablets, but not the TSMG tablets. This study reflects the role of binder concentration and the need for suitable binder concentrations to produce the desired granules for the manufacture of suitable dosage forms.
Keen et al. (Keen et al., 2015) performed TSMG using lipid binders (Compritol 888 ATO [C888], MP 80 °C) and Tramadol HCl as the model drug, with MCC (Avicel PH-102), magnesium stearate, lactose (Fast flo 316), and dicalcium phosphate as additional excipients. Extrusion was carried out at 80 °C with screw configuration containing two kneading blocks. An increase in the binder (C888) concentration resulted in reduced fines while a binder with a concentration above 40% resulted in paste-like mass. This was because the excess quantity of the molten lipid proportion promoted the lubricating action, thereby compromising its binding property. Granulation at low temperature (< 80 °C) helped to achieve easy, non-sticky, free-flowing granules with a smooth, spherical appearance. The formulation containing C888 lipid binders did not impact drug release in dose dumping studies (5%, 20%, & 40% V/V of EtOH in 0.1 N HCl). This study demonstrated the capability of developing successful sustained release tablets using the TSMG technique.
Monteyne et al. (T Monteyne et al., 2016) examined the effect of process and formulation parameters on granules and tablet properties using PEG4000 (Semi crystalline, Tm: 53 °C) and Soluplus® (Amorphous, Tg: 70 °C) as binders and metoprolol tartrate (MPT) and caffeine anhydrous (CAF) as the miscible and non-miscible model drugs, respectively. Four formulations (MPT/PEG, MPT/SLP, CAF/PEG, CAF/SLP) were extruded with a screw configuration containing one kneading zone and one mixing element (before the discharge point to break large lumps). When binder and API are non-miscible, high process temperatures above the Tg or Tm of the binder can be used because of the non-plasticizing effect of a drug on the binder. Granules obtained by Soluplus® are larger and harder than granules obtained from PEG, which are fragile for both drugs. Increasing the feed rate at low temperature and low binder concentration affected friability whereas high temperature and high binder concentration reduced friability (<0.5%). The formulation with PEG had a faster dissolution than that with Soluplus®, which indicates the hydrophilicity of PEG. A lower screw speed produced more spherical granules due to more residence time, thereby leading to more interaction between the binder and API. Higher feed rate and a higher screw speed with shorter residence time produced broader size distribution of the granules. The granule shape was affected by drug-binder miscibility and the nature of the binder. When the drug-binder miscibility was low, needle-shaped granules were obtained. Formulation with PEG as a binder resulted in the fragmenting effect rather than the elongation effect, which can be attributed to the low viscous and brittle nature of the PEG.
Kallakunta et al. (Venkata Kallakunta et al., 2018) reported lipid-based sustained release (SR) tablets using TSMG technique. Compritol 888 ATO, Geleol, and Precirol ATO 5 were selected as the SR agents and a screw configuration with two kneading zones was employed. The second mixing zone involved the conversion of the partially formed granules into complete granules with acceptable strength. The first mixing zone was maintained at a higher temperature (40–45 °C above the MP of the lipid). The formulation containing HPC SL and Avicel PH 102 produced poor granules while that with Klucel EF and Kollidon VA64 binders resulted in the formation of good granules. Klucel EF was selected as a suitable binder because of the less fines produced at a lower concentration (2.5–7.5%). As feed rate and screw speed influence the shear produced in the barrel, they affected granule formation and the % of fines. Lower feed rate (1–2 g/min) resulted in fines >40% while a higher feed rate (>7 g/min) resulted in incomplete granule formation; by using a feed rate of 5–6 g/min, the desired granules were produced. FTIR studies revealed no interactions between API and lipids while DSC and XRD studies revealed the recrystallization of lipids upon cooling to room temperature. The formulations from wet granulation displayed incomplete release while those from direct compression did not display stability after testing at 40/75% RH. The effect of intragranular and extragranular binder content on the dissolution profiles was examined. The formulations prepared by hot melt granulation displayed a sustained release pattern up to 24 h and were stable at the tested conditions. This investigation further confirmed the suitability of TSMG as an alternative technique for the production of stable sustained release tablets relative to the wet granulation and direct compression methods. Thus, TSMG is an emerging technique for the production of suitable granules and involves the selection of appropriate materials in the development of solid oral dosage forms, such as tablets, capsules, or sachets.
Patil et al. (Patil et al., 2015) studied the enhancement of pH-dependent solubility of OND (MP: 189 °C), using the TSMG technique by employing EC, a hydrophobic non-swellable polymer, and hydroxy propyl cellulose (HPC), a hydrophilic swellable polymer and stearic acid (SA), as binder. Fumaric acid was used to enhance the solubility of OND by altering the microenvironment in alkaline media. Extrusion was carried out using a screw configuration with two kneading zones at 110 °C (First kneading zone) and 50–70 °C (Remaining zones), 7.2 g/min feed rate, and 100 rpm. The formulation with fumaric acid showed increased drug release in the alkaline pH media due to the altered microenvironment in the tablet. Due to the hydrophobic nature of EC and SA, an increase in their concentration retarded drug release rate for 24 h; however, formulations containing HPC had a relatively fast drug release in 7–9 h which can be attributed to its hydrophilic nature.
Batra et al. (Batra et al., 2016) performed melt granulation using different polymeric binders (<10% w/w) to enhance the compressibility of two poorly compressible drugs, acetaminophen (MP: 169 °C) and metformin HCl (MP: 222 °C). Process parameters, such as screw speed, feed rate, screw configuration, and barrel temperature were optimized to ensure that granules were formed with all of the polymers investigated at a low concentration (10%). Screw configuration with two kneading zones and CEs (high pitched in feeding zone) were used. The first kneading zone was maintained constant (60°) whereas the second kneading zone was altered at different angles with increasing shear (30°, 30° & 60°, 60° & 90°). Low shear (30° in second kneading zone) resulted in unsuccessful granulation whereas high shear configuration (60° & 90°) resulted in either a sticky product on the barrel and walls or partial melting of the API, resulting larger granules. Thus, a moderate shear configuration (30° & 60°) was employed. In the granulation process with both APIs, polymers such as Klucel, Eudragit, POLYOX N10, and Soluplus, resulted in acceptable tablet tensile strength (>2 MPa). The low MP polyethylene glycols did not produce granules with sufficient strength due to their waxy nature. Altogether, this study highlighted the importance of selecting appropriate screw configuration for the production of the desired granules and achieving acceptable tensile strength for the prepared tablets. This study also confirms the successful application of TSMG in the manufacture of granules and the development of tablets.
Monteyne et al. (Tinne Monteyne et al., 2016) manufactured SR matrix granules using stearic acid (SA), with MP of 69 °C, and polyethylene oxide (PEO) to retard drug release of the highly water-soluble drug, metoprolol tartrate (MPT). A screw configuration with one kneading zone was used. The interaction between SA and PEO resulted in hydrogen bonding. However, the preferential interaction between MPT and SA reduced the chance of hydrogen bonding, thereby improving the sustained release properties of the formulation. Higher concentration of SA led to burst release while the addition of PEO retarded drug release.
Melkebeke et al. (Melkebeke et al., 2006) worked on enhancing dissolution of class II molecule using PEG-400 and PEG4000 as binders and maltodextrin as a filler. Granulation was carried out with a screw configuration that had two kneading zones. The granulation and strength of the granules formed was dependent on PEG 400, PEG 4000, and the processing temperature. At a processing temperature of 30 ℃, the granules formed were soft and sticky while at 50 ℃, the desired granules were produced as the MP of PEG 4000 was not attained. At 65 °C, PEG 4000 was completely molten, activating its binding property, which resulted in stronger granules with lower friability. The formulations with higher liquid (PEG 400) fractions produced granules with higher friability because of the compromised binding properties caused by the improper ratio of PEGs. At 10% drug load, the use of PEGs improved the rate of dissolution to approximately 70% in 10 min compared to the pure drug (20% drug release). Adding surfactants, such as SLS (3%), caused 100% drug release, but compromised the yield (53%) and quality of the granules. Replacing SLS with polysorbate 80, Lutrol F127, and Cremophor RH 40 resulted in a yield >90% with 100% drug release in 10 min. Formulations containing polysorbate 80 and Cremophor RH 40 had soft granules, but a higher dissolution rate. The formulation containing Lutrol F127 had similar dissolution to that containing SLS. This study demonstrated the suitability of TSMG for enhancing the solubility of poorly soluble drugs and the formation of granules for development into solid products.
3.3. Twin-screw wet granulation
In the TSWG technique, granules are formed with the aid of granulating fluid (water/solvent/binder solution). Granulating fluid is described in terms of L/S ratio (Liquid to solid ratio). In wet granulation, the materials are conveyed within the barrel of an extruder and the granulating fluid is pumped into the barrel zone using a peristaltic pump. The wet mass of the material formed in the extruder is thoroughly mixed and kneaded in the mixing or kneading zone, leading to the formation of granules. The granules are dried during transit in the extruder and collected. This technique avoids the additional drying step in the manufacturing process in most of the drug and polymer combinations.
Further, TSWG utilizes relatively low amounts of granulating fluid, thereby aiding in rapid drying of granules before discharge (Sun, 2010). A binder can be directly added to the physical mixture and its function is activated once the granulating solution is introduced onto the material. Granulating fluid is added at a predetermined rate to ensure the uniform distribution of the solution onto the material throughout the process. Different studies have reported that the barrel temperature is maintained at ambient temperature 20–25 ℃ (Dhenge et al., 2011; Kleinebudde et al., 2016), which indicates that granule formation in TSWG is carried even at low temperatures and is suitable for thermolabile materials. An adumbration of twin screw wet granulation setup is captured in Figure 5.
Fig. 5.
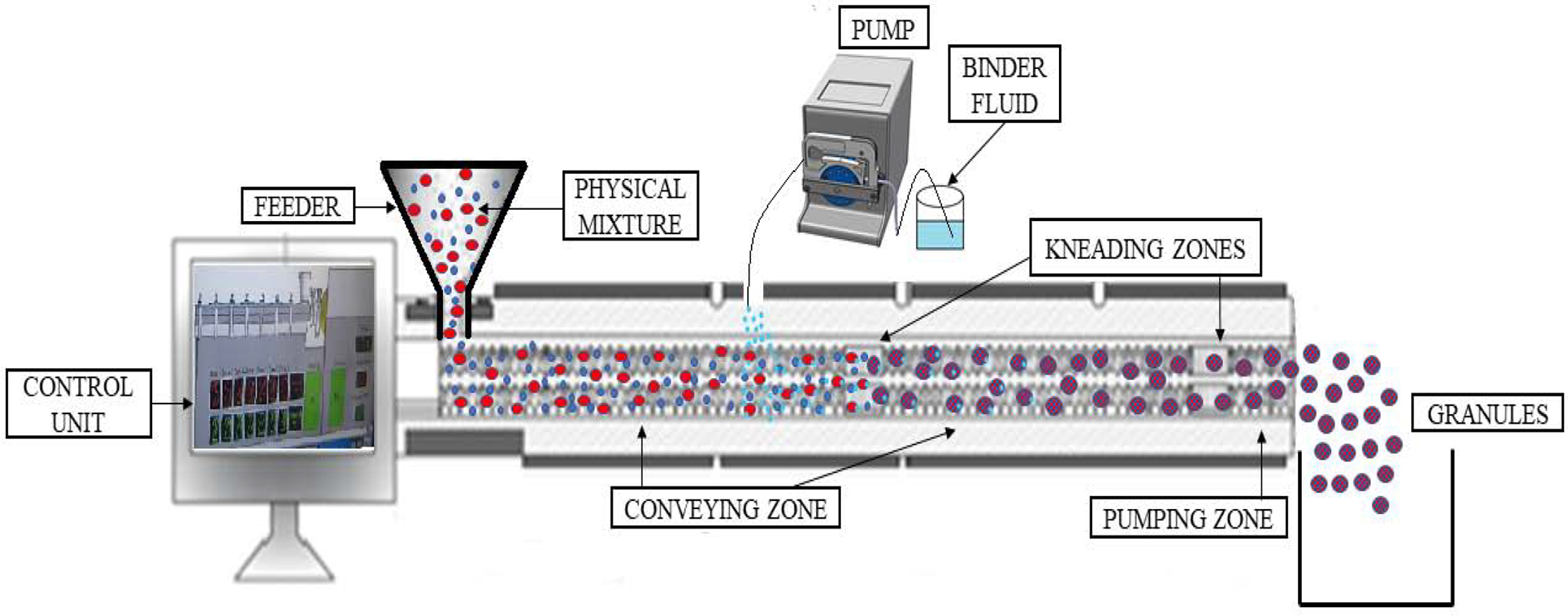
Schematic representation of Twin-screw wet granulation.
Dhenge et al. (Dhenge et al., 2011) conducted extensive research to elucidate the effect of different feed rates (2, 3.5, 5, 6 kg/h) on the nature of granules produced by TSWG. Physical mixture and binder solution were added into the barrel within the first zone, granulation was carried out at 25 °C, 400 screw rpm, and 0.3 L/S ratio, with two kneading zones in the screw configuration. Sodium chloride (0.5%) was used as the non-functional active ingredient to examine the release effect while HPC (5%) was employed as the binder. Other materials used in the granulation process were lactose monohydrate (73%), Avicel PH 101 (20%), and Crosscarmellose sodium (1.5%). Owing to low particle-particle interaction at a low feed rate, granules exhibit bimodal distribution. An increase in feed rate resulted in smoother granules with reduced porosity. This report elaborates the significance of feed rate in the manufacture of desirable and suitable granules.
Maniruzzaman et al. (Maniruzzaman et al., 2015) examined the enhancement of solubility of a poorly water-soluble drug, ibuprofen, by the TSWG technology with a polymer (Pharmacoat 603; HPMC), inorganic excipients (Magnesium Aluminometasillicate; MAS), binder (PEG 2000), and water (25–40% w/v) as the granulating fluid. Granulation was carried out for a physical mixture containing a 40% drug load by using a screw configuration with 3 kneading zones (increasing shear from one kneading zone to another zone), 1 kg/h feed rate, and 4.16–6.67 ml/min of granulating fluid. API was completely dispersed within the polymer matrix, which was confirmed by a scanning electron microscope; thus, the solubility of the active component was demonstrated to be enhanced. Moisture content of the granules is known to be affected by binder concentration and L/S ratio. A low moisture content was obtained at high binder concentration and low L/S ratio. The formulation containing MAS and HPMC in a 1:1 ratio showed higher drug release.
Kleinebudde et al. (Kleinebudde et al., 2016) performed extensive research on TSWG to determine the role of L/S ratio in the production of desired granules. The formulations were prepared using ibuprofen, MCC (Vivapur 102), Lactose monohydrate, polyvinyl pyrrolidine K30, and magnesium stearate. Granulation was carried at 20 °C and 0.5 kg/h feed rate using screw configuration with 2 kneading zones and distributive elements (before discharge point). Variation in the mass of granules was also examined to determine the wetting characteristics. Over wetting increased the mass of granules, leading to the formation of paste (wet mass). For the lactose-based formulation, L/S ratio (0.10–0.13) was very narrow for successful granulation and above this ratio, the product appeared in a paste form instead of granules due to over wetting. For the MCC-based formulation, a relatively broader (i.e., 0.4 to 1.1) L/S ratio was obtained. Combining lactose and MCC resulted in a wider L/S ratio. Hence, for materials, such as lactose, which have very narrow L/S ratio, the use of additional materials with a wide L/S ratio, such as MCC, is suggested to overcome the issues of over wetting. At higher L/S ratio, the material stuck to the metal surface of the twin-screw extruder and was more commonly identified near the extruder outlet.
Vercruysse et al. (Vercruysse et al., 2015) examined the effect of different screw elements on granule formation using lactose monohydrate and distilled water as the granulating fluid. Granulation using only CEs resulted in fines without granulation, even with an increase in the granulating fluid. Such finding indicated the conveying functionality of the elements. The addition of KEs resulted in a decrease in fines but enhanced the oversized granules, which were not resolved even by addition of another kneading zone or toothed mixing element or cutter element before exit. Cutter elements increased the oversized agglomerates as they do not possess the conveying property, while the addition of the narrow toothed mixing element in the last zone reduced the agglomerates but increased the fines. The addition of SME reduced the large oversized agglomerates and incorporating 2 or 4 SMEs in the last zone resulted in low torque values as their conveying property shifted the d50 from higher particle size to lower particle size. The geometry of the CEs and SMEs was similar; however, the use of SMEs alone resulted in the backflow of the material and increased the residence time, thereby causing reduced fines. Compared to KEs, the SMEs were found to maintain high % of fines. Granulation by the narrow toothed mixing element resulted in reduced fines and agglomerates, whereas wide toothed mixing element resulted in reduced fines and increased agglomerates. This study emphasized the selection of suitable screw elements in the production of granules with desired percent of fines and agglomerates. Similarly, Li et al. (Li et al., 2018) examined the effect of screw elements (i.e., conveying, kneading (60°), and DMEs) on granule properties using APAP (50% and 5% drug loads), Avicel PH 101 (45% and 90%), Hydroxy propyl methyl cellulose (5%), and granulating fluid containing 0.1% w/w nigrosine dye in deionized water to identify uniform distribution. The entire barrel zone was divided into 3 regions containing CEs in the upstream and downstream region, while the middle region was replaced with either CEs, KEs, or DMEs. The use of CEs in the middle region did not result in differences in the material collected from the upstream and downstream regions, thereby confirming that only the CEs could not form granules. When KEs and DMEs are used alone in the mixing region, the granules obtained were bimodal and unimodal in the upstream and downstream regions, respectively. As drug load increased from 5% to 90%, the binder solution required for granulation is decreased owing to the higher water absorption capacity of Avicel pH 101. KEs in the reverse configuration was found to produce granules with uniform binder distribution and less porosity compared to the screw configuration in the forward direction. DMEs did not have any influence on granule properties in either directions. This recent study demonstrates the granulation of drugs with low to high drug loads by TSWG through the selection of appropriate screw elements for the twin-screw extruder.
Lute et al. (Lute et al., 2018) examined the effect of barrel fill volume on mean residence time, granule properties, and tablet tensile strength using microcrystalline cellulose (Avicel pH 101) and alpha lactose monohydrate (Pharmatose 200M) by TSWG with distilled water as the granulating fluid. Extrusion was carried at 25 °C with different feed rates and the screw configuration contained KEs and CEs with short and large pitch. An increase in the barrel fill volume resulted in poor granulation of MCC (water insoluble) owing to the decreased interaction between MCC and the granulating fluid and the reduction in residence time. Lactose (water soluble) could maintain granule size at all fill levels. Thus, this report revealed the significance of material property, barrel fill level, and residence time in the formation of granules.
The above reports confirm the TSWG technique as an alternative to the traditional wet granulation process and its capability to reduce labor, time, and the space for equipment with respect to plant site. This technique is also suitable for thermolabile drugs as TSWG can be carried out at ambient temperature without the need for an additional drying step. Furthermore, coupling of the PAT tools to the twin-screw extruder creates an alternative continuous manufacturing process for the production of granules in the development of solid dosage forms.
Overall, the above reports support continued findings that the novel twin screw granulation techniques, such as TSDG, TSWG, and TSMG, are potential alternative granulation techniques to the conventional granulation process for use in the pharmaceutical industry. The different excipients examined for use in the TSG techniques and their properties are presented in Table 1.
Table 1:
Properties of different excipients examined for use in the twin-screw granulation process.
| S.No | Trade Name | Chemical Name | Category | Tg/MP (°C) | Molecular Weight (Dalton) | Reference |
|---|---|---|---|---|---|---|
| 1. | Klucel™ EF | Hydroxy propyl cellulose | Binder | 120/NA* | 80,000 | (VR Kallakunta et al., 2018) |
| 2. | Kollidon® VA64 | Vinylpyrrolidone-Vinyl acetate co-polymer. | Binder | 101/NA | 45,000–70,000 | |
| 3. | Aqualon™ T10 | Ethyl cellulose | Sustained release agent | 150–156/NA | 75,000 | |
| 4. | Neusilin® (US2) | Magnesium aluminometasilicate | Carrier | >300/ NA | 262.43 | (Majumder et al., 2018) |
| 5. | Affinisol™ HPMC HME 100LV | Hydroxy propyl methyl cellulose | Polymeric Binder | 111/NA | 180,000 | (Liu et al., 2017) |
| 6. | Affinisol™ HPMC HME 4M | Hydroxy propyl methyl cellulose | Polymeric Binder | 111/NA | 550,000 | |
| 7. | Klucel™ HF | Hydroxy propyl cellulose | Sustained release agent | NA/>170 | 1,150,000 | (Upadhye et al., 2017) |
| 8. | Natrasol™ 250 L | Hydroxy ethyl cellulose | Sustained release agent | NA/NA | 90,000 | |
| 9. | Ethocel standard 10 | Ethyl cellulose | Sustained release agent | 129–133/165–173 | NA | |
| 10. | PEG 2000 | Polyethylene glycol 2000 | Binder | NA/49–52 | 1800 | (Maniruzzaman et al., 2015) |
| 11. | Compritol 888 ATO | Glyceryl behenate | Lipid binder | NA/69–74 | 414.7 | (Keen et al., 2015) |
| 12. | PEG 4000 | Polyethylene glycol 4000 | Binder | NA/53 | 3600–4400 | (T Monteyne et al., 2016) |
| 13. | Soluplus | Polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol | Binder | 70/NA | 90,000–140,000 | |
| 14. | Hydroxy propyl cellulose SSL | Hydroxypropyl cellulose | Sustained release agent | NA/180–220 | 40,000 | (Venkata Kallakunta et al., 2018) |
| 15. | Stearic acid | Octadecanoic acid | Binder | NA/69.3 | 284.48 | (Patil et al., 2015) |
| 16. | Kollidon 12 PF | Polyvinylpyrrolidone | Polymeric Binder | 72/NA | 2000–3000 | (Batra et al., 2016) |
| 17. | Kollidon 30 | Polyvinylpyrrolidone | Polymeric Binder | 160/NA | 44,000–54,000 | |
| 18. | Klucel JXF | Hydroxypropyl cellulose | Polymeric Binder | ~19/~200 | ~140,000 | |
| 19. | Affinisol 6cP | Hydroxypropyl methylcellulose | Polymeric Binder | 95/NA | NA | |
| 20. | Aqoat® LG | Hydroxypropyl methylcellulose acetate succinate | Polymeric Binder | 122/NA | 17,000–2000 | |
| 21. | HPMC Phthalate HP 55 | Hydroxypropyl methylcellulose phthalate | Polymeric Binder | 145/NA | 45,600 | |
| 22. | Eudragit EPO | Poly (butyl methacrylate-codimeth laminoethyl methacrylate-comethyl methacrylate) (1:2:1) | Polymeric Binder | 45/NA | 47,000 | |
| 23. | Eudragit L100–55 | Poly (methacrylic acid-co-ethyl acrylate) (1:1) | Polymeric Binder | 110/NA | 32000 | |
| 24. | Lutrol micro F127 | Poloxamer | Polymeric Binder | NA/52 | 9840–14600 | |
| 25. | Kolliphor P188 | Poloxamer | Polymeric Binder | NA/52 | 7680–9510 | |
| 26. | Polyox N10 NF | Polyethylene oxide | Polymeric Binder | NA/68 | 100,000 | |
| 27. | SentryPolyox® WSR N12K | Polyethylene oxide | Sustained release agent | NA/68 | 1,000,000 | (Tinne Monteyne et al., 2016) |
NA: Not Available
4. Scale-up of Twin-screw granulation
Scale-up is a pre-requisite for commercial production that should not compromise the quality of the product. Different factors, such as process variables, equipment variables, formulation variables, and manufacturing plant conditions, which affect the quality of the granules, are presented in Figure 6. In this section, key aspects that should be considered during the scale-up of a manufacturing process are discussed.
Fig. 6.
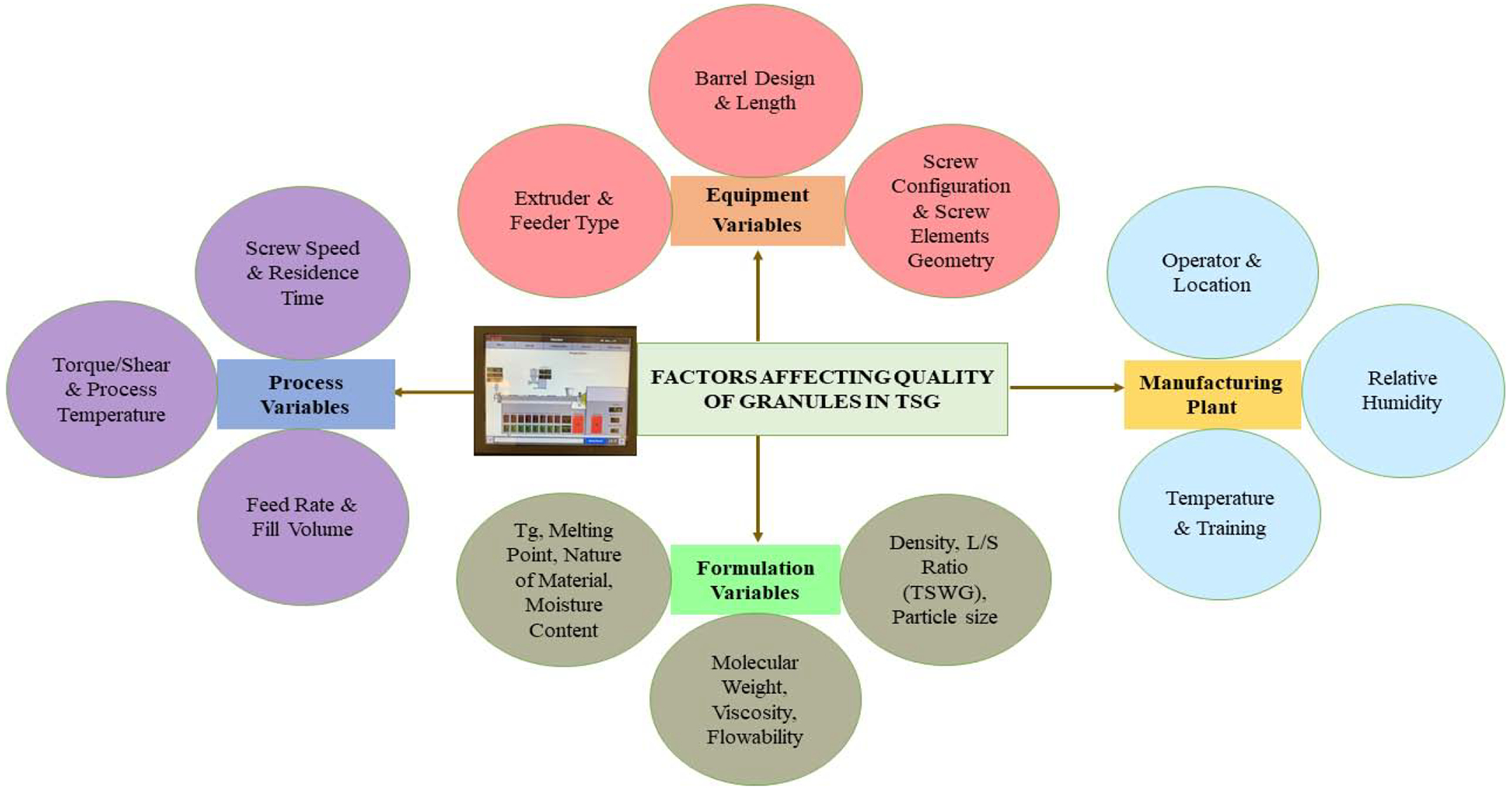
Factors affecting the quality of granules in Twin-screw granulation.
Agrawal et al. (Agrawal et al., 2016) reported their observations during scale-up of the HME process from 16 mm to 18 mm. For the change in equipment size, the impact of machine parameters, such as screw design, free volume, and process parameters, such as feed rate, screw speed, and barrel temperature, were examined. Product quality is affected by multiple machine parameters and process parameters; thus, understanding the effect of each parameter on product quality is essential. Changes in screw configuration affect the quality of the product. Shear, residence time, and the change in feed rate affects fill volume. Scale-up of a process from the lab scale (9 mm) to a large scale (16 or 18 mm) is critical as most equipment geometry will vary. Due to changes in the geometry of the extruders, the researchers calculated feed rate using a volumetric scale-up equation and modified the screw configuration to achieve a similar mechanical shear. Residence time remained the same, approximately 60 s (for 16 mm and 18 mm). Impurities within the 18-mm process was found to be less (0.14%) than that of the 16-mm process (0.25%). The less impurities is due to more flight depth clearance (3 mm) being available for the 18-mm process compared to the 16-mm process, which had a flight depth clearance of 1 mm. At higher clearance, the shear or torque experienced by the extruded material was less than that of the process with smaller clearance. This study highlights the various process and machine parameters that should be considered in scale-up of the equipment. It also confirms the feasibility of using the twin-screw extruder from the lab scale to the production scale, adding to its suitability as an equipment for commercial use in the pharmaceutical industry. Similarly, Jakob et al. (Bernd and Tom, 2018) reported the successful scale-up from 11 mm to 16 mm using the twin-screw process. As a result, they highly recommended that similar barrel geometry and screw configuration should at least be used. During the process of scale-up, the same temperature, residence time, and specific energy must be maintained. The starting feed rate is calculated according to Schuler rule and the specific energy is adjusted by changing the throughput value. Barrel surface availability, heating, and cooling rate depends on barrel diameter. The process in the 11-mm extruder at 1 kg/h throughput and 200 rpm resulted in 559 kJ/kg of specific energy and 55 s of residence time. During scale-up of the process to 16 mm at 3 kg/h of feed rate (as per Schuler rule), lower residence time and specific energy were achieved, and an adjustment of the feed rate to 2.5 kg/h resulted in similar residence time (55 s) and specific energy (566 kJ/kg). Instead of using a tracer and timer, a camera would serve as a better option to attain accurate measurements of retention time, which could also aid in achieving successful scale-up. Overall, this study by Jakob and colleagues provides insights into the scale-up parameters and aspects that should be monitored to obtain products of similar quality.
Maniruzzaman et al. (Maniruzzaman and Nokhodchi, 2017) described the approaches to be considered during the scale-up process of HME. Scale-up can be achieved via different strategies by increasing the run time of the lab scale equipment or increasing the feed rate, barrel diameter, and screw diameter. There are some challenges that affect product quality. For instance, the increase in feed rate affects residence time and an increase in barrel size without the optimization of feed rate will affect product quality. An increase in both feed rate and screw speed leads to an increase in shear and torque, eventually affecting process and product quality. To overcome these issues during scale-up, a complete understanding of the process parameters affecting product quality is essential and the fundamental geometry of a benchtop extruder must be matched with the scale-up extruder. The ratio of the outer and inner diameter of the screw is considered to be a key parameter. A screw profile should be kept constant between two extruders and an increase in the screw diameter leads to an increase in shear. As feeding the material in the upstream process is the initial step, it should also be considered as a critical process; any discrepancies in material feed will affect all downstream processes. There are several technical formulas that also play a vital role in the scale-up process:
- Shear rate determines the velocity gradient between two surfaces moving at different speeds; it is calculated by the following equation (Maniruzzaman and Nokhodchi, 2017):
- D= Screw diameter
- n= screw speed
- h= overflight clearance
- Stress experienced by conveying material inside the barrel is calculated as shear stress by using the following equation (Maniruzzaman and Nokhodchi, 2017):
- Shear stress= EC * shear rate
- EC = Viscosity (Barrel temperature affects viscosity; higher and lower viscosities of a material affect its uniformity. Higher viscosity of a material leads to dispersive mixing)
- Total amount of energy invested onto the material during high shear mixing is defined as specific mechanical energy consumption (SMEC) (Maniruzzaman and Nokhodchi, 2017):
- SMEC = T * n/w
- T= Torque
- n= screw speed
- ѡ= feed rate
- For extruders that are geometrically similar but have different diameters
- Feed rate is calculated using (Maniruzzaman and Nokhodchi, 2017):
- QT = QM* D3T /D3M * NT/NM
- QT & QM = Final and initial process feed rate
- DT & DM = Screw diameter after and before scaleup
- NT & NM = Screw speed after and before scaleup
- Throughput is calculated using (Maniruzzaman and Nokhodchi, 2017):
- QT = QM* D2T/D2M * NT/NM
- QT & QM = Final and initial process throughput
- DT & DM = Targeted and initial screw diameter
- NT & NM = Targeted and initial screw speeds
- Mechanical energy input is calculated using (Maniruzzaman and Nokhodchi, 2017):
- E = Emax * N/Nmax * T/Tmax * Gear box rating
- Emax, Nmax, Tmax = Predetermined
- The above studies provide attributes that are essential for the scaleup process in twin screw extruders.
Osorio et al. (Osorio et al., 2017) studied the effect of process variables (Froude number; Fr, L/S ratio; powder feed number; PFN) on granule size distribution (GSD), porosity and liquid distribution in 11 mm, 16 mm, and 24 mm twin screw granulators. A placebo blend containing α- lactose monohydrate, MCC PH101, hypromellose and croscarmellose sodium was granulated using 0.1% w/w aqueous solution containing nigrosine dye. Liquid to solid ratios varying from 0.15 to 0.30 were studied using a screw configuration containing distributive feed screw (DFS). The increasing L/S ratio resulted in decreased fines, decreased porosity and increased size of the granules. With increasing TSG (16 mm & 24 mm) scale employing DFS resulted in a bimodal GSD with no effect on porosity of the granules. Fr and PFN demonstrated a significant effect on d90 but not on d10 or d50. In TSG with increased granulator scale the size of granules increased. However, this can be optimized by reducing the L/S ratio. This study provides insights on significance of various parameters in scale up of TSG.
5. Conclusion
The TSG processes, such as TSMG, TSWG, and TSDG, are seething techniques in the pharmaceutical industry. The use of twin-screw extruders to produce granules for different types of drugs makes it a suitable alternative in the granulation process. In this review, the current state of the art of TSMG, TSWG, and TSDG was presented. Owing to increased interest in continuous manufacturing and the ability of TSG as a continuous process, makes it an effective alternative strategy for granulation. TSG can reduce batch loss and production time, and improves the safety, manufacturing output, and the quality of pharmaceutical products. TSWG is an alternative to the traditional wet granulation process; it reduces labor, time, and space, and is suitable for thermolabile drugs. This is because TSWG can be operated at ambient temperatures without additional drying steps. Similar to TSWG, TSDG enables the manufacture of granules and the sustained release of tablets containing low to high MP drugs, amorphous granules of poorly soluble drugs, and granulation at low temperatures that are suitable for heat-sensitive drugs and enhancing the stability of formulations. In summary, novel twin screw granulation techniques serve as potential alternative granulation techniques to conventional granulation for the pharmaceutical industry. However, extensive research using different screw elements and process analytical tools is imperative to transform novel twin screw granulation as a continuous manufacturing process.
HIGHLIGHTS.
Twin screw granulation is an economical, industrially-scalable, continuous process.
Different TSG techniques are potential alternatives of conventional granulation.
TSG can be adapted for various drugs with different physicochemical properties.
Screw elements with different geometry are available for successful granulation.
Extensive study of process parameters is warranted for scaleup and quality granules.
Acknowledgement
This project was also partially supported by Grant Number P30GM122733-01A1, funded by the National Institute of General Medical Sciences (NIGMS) a component of the National Institutes of Health (NIH) as one of its Centers of Biomedical Research Excellence (COBRE).
Abbreviations
- HME
hot melt extrusion
- TSG
twin-screw granulation
- API
active pharmaceutical ingredient
- PAT
process analytical technology
- CE
Conveying element
- KEs
Kneading elements
- SME
screw mixing element
- TSWG
twin-screw wet granulation
- TSDG
Twin-screw dry granulation
- TSMG
Twin-screw melt granulation
- Tg
glass transition temperature
- MP
melting point
- BMA
Benzoyl-methoxy-methylindol-acetic acid
- Neusilin® US2
magnesium aluminometasilicate
- SolCal
Solution calorimetry
- DVS
Dynamic vapor sorption
- OND
Ondansetron HCl dihydrate
- EC
Ethyl cellulose
- MCC
micro crystalline cellulose
- TPU
thermoplastic polyurethanes
- IM
injection molding
- CAF
caffeine anhydrous
- SR
sustained release
- SA
stearic acid
- QbD
quality by design
- PEO
polyethylene oxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolfsson A, Olsson H, Nystrom C, 1997. Effect of particle size and compaction load on interparticulate bonding structure for some pharmaceutical materials studied by compaction and strength. Eur. J. Pharm. Biopharm 44, 243–251. 10.1016/S0939-6411(97)00136-7 [DOI] [Google Scholar]
- Agrawal AM, Dudhedia MS, Zimny E, 2016. Hot Melt Extrusion: Development of an Amorphous Solid Dispersion for an Insoluble Drug from Mini-scale to Clinical Scale. AAPS PharmSciTech. 17, 133–147. 10.1208/s12249-015-0425-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken-Nichol C, Zhang F, McGinity J, 1996. Hot melt extrusion of acrylic films. Pharm. Res 13, 804–808. 10.1023/a:1016076306279 [DOI] [PubMed] [Google Scholar]
- Allison G, Cain YT, Cooney C, Garcia T, Bizjak TG, Holte O, Jagota N, Komas B, Korakianiti E, Kourti D, Madurawe R, Morefield E, Montgomery F, Nasr M, Randolph W, Robert JL, Rudd D, Zezza D, 2015. Regulatory and quality considerations for continuous manufacturing May 20–21, 2014 Continuous manufacturing symposium. J. Pharm. Sci 104, 803–812. 10.1002/jps.24324 [DOI] [PubMed] [Google Scholar]
- Batra A, Desai D, Serajuddin A, 2016. Investigating the use of polymeric binders in twin screw melt granulation process for improving compactibility of drugs. J. Pharm. Sci 496, 140–150. 10.1016/j.xphs.2016.07.014 [DOI] [PubMed] [Google Scholar]
- Bernd J, Tom G, 2018. Relevant Process Parameters for Twin Screw Compounding. URL https://assets.thermofisher.com/TFS-Assets/CAD/Application-Notes/LR70-e-Relevant-process-parameters-twin-screw-compounding.pdf (accessed 09 November 2019).
- Bhagurkar AM, Angamuthu M, Patil H, Tiwari RV, Maurya A, Hashemnejad SM, Kundu S, Murthy SN, Repka MA, 2016. Development of an Ointment Formulation Using Hot-Melt Extrusion Technology. AAPS PharmSciTech 17, 158–66. 10.1208/s12249-015-0453-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj R, Blanchard J, 1998. In vitro characterization and in vivo release profile of a poly (d, l-lactide-co-glycolide)-based implant delivery system for the α-MSH analog, melanotan-I. Int. J. Pharm 170, 109–117. 10.1016/S0378-5173(98)00149-5 [DOI] [Google Scholar]
- Byrn S, Futran M, Thomas H, Jayjock E, Maron N, Meyer RF, Myerson AS, Thien MP, Trout BL, 2015. Achieving continuous manufacturing for final dosage formation: Challenges and how to meet them May 20–21, 2014 Continuous manufacturing symposium. J. Pharm. Sci 104, 792–802. 10.1002/jps.24247 [DOI] [PubMed] [Google Scholar]
- Cartwright J, Robertson J, D’Haene D, Burke M, 2013. Twin screw wet granulation: Loss in weight feeding of a poorly flowing active pharmaceutical ingredient. Powder Technol. 238, 116–121. 10.1016/j.powtec.2012.04.034 [DOI] [Google Scholar]
- Censi R, Rosa M, Id G, Casadidio C, Martino P. Di, 2018. Hot Melt Extrusion: Highlighting Physicochemical Factors to Be Investigated While Designing and Optimizing a Hot Melt Extrusion Process. Pharmaceutics 10, 89 10.3390/pharmaceutics10030089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley M, Zhang F, Koleng J, McGinity J, 2002. Stability of polyethylene oxide in matrix tablets prepared by hot-melt extrusion. Biomaterials 23, 4241–4248. 10.1016/S0142-9612(02)00187-4 [DOI] [PubMed] [Google Scholar]
- Dhenge R, Cartwright J, Doughty D, 2011. Twin screw wet granulation: Effect of powder feed rate. Adv. Powder Technol 22, 162–166. 10.1016/j.apt.2010.09.004 [DOI] [Google Scholar]
- Dhenge R, Washino K, Cartwright J, Hounslow M, 2013. Twin screw granulation using conveying screws: Effects of viscosity of granulation liquids and flow of powders. Powder Technol. 238, 77–90. 10.1016/j.powtec.2012.05.045 [DOI] [Google Scholar]
- Djuric D, Kleinebudde P, 2008. Continuous granulation with a twin-screw extruder. J. Pharm. Sci 97, 4934–4942. 10.1002/jps.21339 [DOI] [PubMed] [Google Scholar]
- Douroumis D, Ross S, Nokhodchi A, Ross SA, 2017. Advanced methodologies for cocrystal synthesis. Adv. Drug Deliv. Rev 117, 178–195. 10.1016/j.addr.2017.07.008 [DOI] [PubMed] [Google Scholar]
- Dumpa NR, Sarabu S, Bandari S, Zhang F, Repka MA, 2018. Chronotherapeutic Drug Delivery of Ketoprofen and Ibuprofen for Improved Treatment of Early Morning Stiffness in Arthritis Using Hot-Melt Extrusion Technology. AAPS PharmSciTech. 19, 2700–2709. 10.1208/s12249-018-1095-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hagrasy AS, Cruise P, Jones I, Litster JD, 2013. In-line size monitoring of a twin screw granulation process using high-speed imaging. J. Pharm. Innov 8, 90–98. 10.1007/s12247-013-9149-y [DOI] [Google Scholar]
- El Hagrasy AS, Litster JD, 2013. Granulation rate processes in the kneading elements of a twin screw granulator. AIChE J. 59, 4100–4115. 10.1002/aic.14180 [DOI] [Google Scholar]
- Fard A, Anderson P, 2013. Simulation of distributive mixing inside mixing elements of co-rotating twin-screw extruders. Comput. Fluids 87, 79–91. 10.1016/j.compfluid.2013.01.030 [DOI] [Google Scholar]
- Fonteyne M, Soares S, Vercruysse J, Peeters Elisabeth Burggraeve, Anneleen Vervaet C, Remon J, Sandler N, De Beer T, 2012. Prediction of quality attributes of continuously produced granules using complementary pat tools. Eur. J. Pharm. Biopharm 82, 429–436. 10.1016/j.ejpb.2012.07.017 [DOI] [PubMed] [Google Scholar]
- Fonteyne M, Vercruysse J, De Leersnyder F, Snick B. Van, Vervaet C, Remon JP, De Beer T, 2015. Process analytical technology for continuous manufacturing of solid-dosage forms. TrAC Trends Anal. Chem 67, 159–166. 10.1016/j.trac.2015.01.011 [DOI] [Google Scholar]
- Forster A, Hempenstall J, Rades T, 2001. Characterization of glass solutions of poorly water-soluble drugs produced by melt extrusion with hydrophilic amorphous polymers. J. Pharm. Pharmacol 53, 303–315. 10.1211/0022357011775532 [DOI] [PubMed] [Google Scholar]
- Guidance for Industry Q10 Pharmaceutical Quality System, 2009. http://www.fda.gov/cder/guidance/index.htmhttp://www.fda.gov/cber/guidelines.htm. (accessed 09 November 2019).
- Hagrasy A. El, Hennenkamp J, Burke M, 2013. Twin screw wet granulation: influence of formulation parameters on granule properties and growth behavior. Powder Technol. 238, 108–115. 10.1016/j.powtec.2012.04.035 [DOI] [Google Scholar]
- Hamdani J, Moës A, Amighi K, 2003. Physical and thermal characterisation of Precirol® and Compritol® as lipophilic glycerides used for the preparation of controlled-release matrix pellets. Int. J. Pharm 260, 47–57. 10.1016/S0378-5173(03)00229-1 [DOI] [PubMed] [Google Scholar]
- ICH-Q9 Quality Risk Management, 2005. https://database.ich.org/sites/default/files/Q9_Guideline.pdf (accessed 09 November 2019).
- ICH-Q8(R2) Pharmaceutical Development, 2009. https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf (accessed 09 November 2019).
- Ishikawa T, Amano T, Kihara SI, Funatsu K, 2002. Flow patterns and mixing mechanisms in the screw mixing element of a co-rotating twin-screw extruder. Polym. Eng. Sci 42, 925–939. 10.1002/pen.11002 [DOI] [Google Scholar]
- Islam MT, Maniruzzaman M, Halsey SA, Chowdhry BZ, Douroumis D, 2014. Development of sustained-release formulations processed by hot-melt extrusion by using a quality-by-design approach. Drug Deliv. Transl. Res 4, 377–387. 10.1007/s13346-014-0197-8 [DOI] [PubMed] [Google Scholar]
- Kallakunta VR, Patil H, Tiwari R, Ye X, Upadhye S, Vladyka R, Sarabu S, Kim D, Bandari S, Repka M, 2018. Exploratory studies in heat-assisted continuous twin-screw dry granulation: A novel alternative technique to conventional dry granulation. Int. J. Pharm 555, 380–393. 10.1016/j.ijpharm.2018.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallakunta VR, Tiwari R, Sarabu S, Bandari S, Repka M, 2018. Effect of formulation and process variables on lipid based sustained release tablets via continuous twin screw granulation: A comparative study. Eur. J. Pharm. Biopharm 121, 126–138. 10.1016/j.ejps.2018.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallakunta VR, Sarabu S, Bandari S, Tiwari R, Patil H, Repka MA, 2019. An update on the contribution of hot-melt extrusion technology to novel drug delivery in the twenty-first century: part I. Expert Opin. Drug Deliv 16, 539–550. 10.1080/17425247.2019.1609448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen J, Foley C, Hughey J, Bennett R, 2015. Continuous twin screw melt granulation of glyceryl behenate: development of controlled release tramadol hydrochloride tablets for improved safety. Int. J. Pharm 487, 72–80. 10.1016/j.ijpharm.2015.03.058 [DOI] [PubMed] [Google Scholar]
- Keleb E, Vermeire A, Vervaet C, Remon J, 2004. Twin screw granulation as a simple and efficient tool for continuous wet granulation. Int. J. Pharm 273, 183–194. 10.1016/j.ijpharm.2004.01.001 [DOI] [PubMed] [Google Scholar]
- Keleb E, Vermeire A, Vervaet C, Remon J, 2002. Continuous twin screw extrusion for the wet granulation of lactose. Int. J. Pharm 239, 69–80. 10.1016/S0378-5173(02)00052-2 [DOI] [PubMed] [Google Scholar]
- Kleinebudde P, Schmidt A, De Waard H, Moll K-P, Krumme M, Kleinebudde Peter, 2016. Quantitative Assessment of Mass Flow Boundaries in Continuous Tw in-screw Granulation. Chim. Int. J. Chem 70, 604–609. 10.2533/chimia.2016.604 [DOI] [PubMed] [Google Scholar]
- Lakshman JP, Kowalski J, Vasanthavada M, Tong WQ, Joshi YM, Serajuddin AT., 2011. Application of melt granulation technology to enhance tabletting properties of poorly compactible high-dose drugs. J. Pharm. Sci 100, 1553–1565. 10.1002/jps.22369 [DOI] [PubMed] [Google Scholar]
- Lee K, Ingram A, Rowson N, 2012. Twin screw wet granulation: the study of a continuous twin screw granulator using Positron Emission Particle Tracking (PEPT) technique. Eur. J. Pharm. Biopharm 81, 666–673. 10.1016/j.ejpb.2012.04.011 [DOI] [PubMed] [Google Scholar]
- Leuenberger H, 2001. New trends in the production of pharmaceutical granules: batch versus continuous processing. Eur. J. Pharm. Biopharm 52, 289–296. 10.1016/s0939-6411(01)00199-0 [DOI] [PubMed] [Google Scholar]
- Li J, Pradhan S, Wassgren C, 2018. Granule transformation in a twin screw granulator: Effects of conveying, kneading, and distributive mixing elements. Powder Technol. 346, 363–372. 10.1016/j.powtec.2018.11.099 [DOI] [Google Scholar]
- Liu J, Zhang F, McGinity J, 2001. Properties of lipophilic matrix tablets containing phenylpropanolamine hydrochloride prepared by hot-melt extrusion. Eur. J. Pharm. Biopharm 52, 181–190. 10.1016/s0939-6411(01)00162-x [DOI] [PubMed] [Google Scholar]
- Liu Y, Thompson M, O’Donnell K, 2017. Impact of non-binder ingredients and molecular weight of polymer binders on heat assisted twin screw dry granulation. Int. J. Pharm 536, 336–344. 10.1016/j.ijpharm.2017.11.061 [DOI] [PubMed] [Google Scholar]
- Lute SV, Dhenge Id RM, Salman AD, 2018. Twin Screw Granulation: An Investigation of the Effect of Barrel Fill Level. Pharmaceutics 10, 67 10.3390/pharmaceutics10020067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder M, Rajabnezhad S, Nokhodchi A, Maniruzzaman M, 2018. Chemico-calorimetric analysis of amorphous granules manufactured via continuous granulation process. Drug Deliv. Transl. Res 8, 1658–1669. 10.1007/s13346-018-0519-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkowska S, Khan KA, Lentle R, Marchant J, Elger G, 1983. Effect of Re-Compression on the Properties of Tablets Prepared by Moist Granulation. Drug Dev. Ind. Pharm 9, 349–361. 10.3109/03639048309044679 [DOI] [Google Scholar]
- Mangal H, Kirsolak M, Kleinebudde P, 2016. Roll compaction/dry granulation: suitability of different binders. Int. J. Pharm 503, 213–219. 10.1016/j.ijpharm.2016.03.015 [DOI] [PubMed] [Google Scholar]
- Maniruzzaman M, Nair A, Renault M, Nandi U, Scoutaris N, Farnish R, Bradley M, Snowden MJ, Douroumis D, 2015. Continuous twin-screw granulation for enhancing the dissolution of poorly water soluble drug. Int. J. Pharm 496, 52–62. 10.1016/j.ijpharm.2015.09.025 [DOI] [PubMed] [Google Scholar]
- Maniruzzaman M, Nokhodchi A, 2017. Continuous manufacturing via hot-melt extrusion and scale up: regulatory matters. Drug Discov. Today 22, 340–351. 10.1016/j.drudis.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Markarian J, 2018. Considering Twin-Screw Granulation. Pharm. Technol 42, 40–42. http://www.pharmtech.com/considering-twin-screw-granulation [Google Scholar]
- Melkebeke B, Vermeulen B, Vervaet C, Remon J, 2006. Melt granulation using a twin-screw extruder: a case study. Int. J. Pharm 326, 89–93. 10.1016/j.ijpharm.2006.07.005 [DOI] [PubMed] [Google Scholar]
- Mendez Torrecillas C, Halbert GW, Lamprou DA, 2017. A novel methodology to study polymodal particle size distributions produced during continuous wet granulation. Int. J. Pharm 519, 230–239. 10.1016/j.ijpharm.2017.01.023 [DOI] [PubMed] [Google Scholar]
- Mididoddi P, Repka M, 2007. Characterization of hot-melt extruded drug delivery systems for onychomycosis. Eur. J. Pharm. Biopharm 66, 95–105. 10.1016/j.ejpb.2006.08.013 [DOI] [PubMed] [Google Scholar]
- Mididoddi PK, Prodduturi S, Repka MA, 2006. Influence of tartaric acid on the bioadhesion and mechanical properties of hot-melt extruded hydroxypropyl cellulose films for the human nail. Drug Dev. Ind. Pharm 32, 1059–1066. 10.1080/03639040600683410 [DOI] [PubMed] [Google Scholar]
- Monteyne Tinne, Adriaensens P, Brouckaert D, Remon J-P, Vervaet C, De Beer T, 2016. Stearic acid and high molecular weight PEO as matrix for the highly water soluble metoprolol tartrate in continuous twin-screw melt granulation. Int. J. Pharm 512, 158–167. 10.1016/j.ijpharm.2016.07.035 [DOI] [PubMed] [Google Scholar]
- Monteyne T, Vancoillie J, Remon J, Vervaet C, 2016. Continuous melt granulation: Influence of process and formulation parameters upon granule and tablet properties. Eur. J. Pharm. Biopharm 107, 249–262. 10.1016/j.ejpb.2016.07.021 [DOI] [PubMed] [Google Scholar]
- Munjal M, ElSohly M, Repka M, 2006a. Polymeric systems for amorphous Δ9-tetrahydrocannabinol produced by a hot-melt method. Part II: Effect of oxidation mechanisms and chemical interactions on stability. J. Pharm. Sci 95, 2473–2485. 10.1002/jps.20711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munjal M, Stodghill S, ElSohly M, Repka M, 2006b. Polymeric systems for amorphous Δ9-tetrahydrocannabinol produced by a hot-melt method. Part I: Chemical and thermal stability during processing. J. Pharm. Sci 95, 1841–1853. 10.1002/jps.20667 [DOI] [PubMed] [Google Scholar]
- Ndindayino F, Vervaet C, Mooter G. Van den, 2002. Direct compression and moulding properties of co-extruded isomalt/drug mixtures. Int. J. Pharm 235, 159–168. [DOI] [PubMed] [Google Scholar]
- Nepveux K, Sherlock JP, Futran M, Thien M, Krumme M, 2015. How development and manufacturing will need to be structured-heads of development/manufacturing May 20–21, 2014 Continuous manufacturing symposium. J. Pharm. Sci 104, 850–864. 10.1002/jps.24286 [DOI] [PubMed] [Google Scholar]
- Nystrom C, Alderborn G, Duberg M, Karehill PG, 1993. Bonding surface area and bonding mechanism-two important factors fir the understanding of powder comparability. Drug Dev. Ind. Pharm 19, 2143–2196. 10.3109/03639049309047189 [DOI] [Google Scholar]
- Ochoa L, Igartua M, Hernández R, Solinis M, 2010. In vivo evaluation of two new sustained release formulations elaborated by one-step melt granulation: level A in vitro–in vivo correlation. Eur. J. Pharm. Biopharm 75, 232–237. 10.1016/j.ejpb.2010.02.008 [DOI] [PubMed] [Google Scholar]
- Osorio JG, Sayin R, Kalbag AV, Litster JD, Martinez-Marcos L, Lamprou DA, Halbert GW, 2017. Scaling of continuous twin screw wet granulation. AIChE J. 63, 921–932. 10.1002/aic.15459 [DOI] [Google Scholar]
- Padmanabhan B, 2004. Fractional and higher lobed co-rotating twin-screw elements. Google Patents. US6783270B1. https://patents.google.com/patent/US6783270B1/en
- Patil H, Tiwari R, Upadhye S, Vladyka R, 2015. Formulation and development of pH-independent/dependent sustained release matrix tablets of ondansetron HCl by a continuous twin-screw melt granulation. Int. J. Pharm 496, 33–41. 10.1016/j.ijpharm.2015.04.009 [DOI] [PubMed] [Google Scholar]
- Perissutti B, Rubessa F, Moneghini M, 2003. Formulation design of carbamazepine fast-release tablets prepared by melt granulation technique. Int. J. Pharm 256, 53–63. 10.1016/S0378-5173(03)00062-0 [DOI] [PubMed] [Google Scholar]
- Plumb K, 2005. Continuous processing in the pharmaceutical industry: changing the mind set. Chem. Eng. Sci 83, 730–738. 10.1205/cherd.04359. [DOI] [Google Scholar]
- Prodduturi S, Manek R, Kolling W, Stodghill S, Repka M, 2005. Solid-state stability and characterization of hot-melt extruded poly (ethylene oxide) films. J. Pharm. Sci 94, 2232–2245. 10.1002/jps.20437 [DOI] [PubMed] [Google Scholar]
- Rao V, Kulkarni V, Padmanabhan B, Ghike R, Roden R, Ford E, 2016. Novel Continuous Wet Granulation Using Twin Screw Fractional Lobe Processor. https://www.pharmacompass.com/jAssets/pdf/party/content/STEERLife-India-Pvt-Ltd-party-content-1492686490.pdf (accessed 11.9.19).
- Repka M, Bandari S, Kallakunta VR., 2018. Melt extrusion with poorly soluble drugs–an integrated review. Int. J. Pharm 535, 68–85. 10.1016/j.ijpharm.2017.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka M, Gutta K, Prodduturi S, Munjal M, Stodghill S, 2005. Characterization of cellulosic hot-melt extruded films containing lidocaine. Eur. J. Pharm. Biopharm 59, 189–196. 10.1016/j.ejpb.2004.06.008 [DOI] [PubMed] [Google Scholar]
- Repka MA, McGinity JW, 2001. Influence of chlorpheniramine maleate on topical hydroxypropylcellulose films produced by hot-melt extrusion. Pharm. Dev. Technol 6, 297–304. 10.1081/PDT-100002610 [DOI] [PubMed] [Google Scholar]
- Richter M, 2019. Continuous twin-screw granulation – What to consider in process design, development and scale-up (No.WP03). https://assets.thermofisher.com/TFS-Assets/MSD/Application-Notes/WP03-continuous-twin-screw-granulation-design-development-scale-up.pdf (accessed 09 November 2019).
- Richter M, 2017. Dry granulation as a twin-screw process in pharmaceutical applications (No. LR-79). https://assets.thermofisher.com/TFS-Assets/CAD/Application-Notes/LR79-e-Drygranulation-as-a-twinscrew-process-in-pharmaceutical-applications.pdf (accessed 08 November 2019).
- Robinson J, McGinity J, Delmas P, 2003. Effervescent granules and methods for their preparation. 6,649,186 B1. [Google Scholar]
- Rudd D, 2004. Pharmacopoeial implications of continuous processes: new concepts compared to batch processes, Presented at Process Analytical Technologies May 3–4, Cannes, France. [Google Scholar]
- Sam A, 1992. Controlled release contraceptive devices: a status report. J. Control. release 22, 35–46. 10.1016/0168-3659(92)90114-7 [DOI] [Google Scholar]
- Sayin R, Martinez-Marcos L, Osorio JG, Cruise P, Jones I, Halbert GW, Lamprou DA, Litster JD, 2015. Investigation of an 11 mm diameter twin screw granulator: Screw element performance and in-line monitoring via image analysis. Int. J. Pharm 496, 24–32. 10.1016/j.ijpharm.2015.09.024 [DOI] [PubMed] [Google Scholar]
- Schaber S., Gerogiorgis DI Ramachandran R, Evans JM., Barton P., Trout BL, 2011. Economic Analysis of Integrated Continuous and Batch Pharmaceutical Manufacturing. Ind. Eng. Chem. Res 50, 10083–10092. 10.1021/ie2006752 [DOI] [Google Scholar]
- Sherry R, 2008. Granules comprising paracetamol, a NSAID and a sugar alcohol made by melt extrusion. Google Patents. CN101035514A. https://patents.google.com/patent/CN101035514A/en [Google Scholar]
- Sochon R, Zomer S, Cartwright J, 2010. The variability of pharmaceutical granulation. Chem. Eng. J 164, 285–291. 10.1016/j.cej.2010.08.031 [DOI] [Google Scholar]
- Srai JS, Badman C, Krumme M, Futran M, Johnston C, 2015. Future supply chains enabled by continuous processing-opportunities and challenges May 20–21, 2014 Continuous manufacturing symposium. J. Pharm. Sci 104, 840–849. 10.1002/jps.24343 [DOI] [PubMed] [Google Scholar]
- Sun J, 2008. Wet granulation in a twin screw extruder. Beijing University of Chemical Technology; http://hdl.handle.net/11375/23276 [Google Scholar]
- Takeichi Y, Shimooka T, Yamabe K, Nagayasu A, Baba K, Kinoshita M, Minakuchi K, Houchi H, Azuma M, 2002. Improvement of solubility and oral bioavailability of a poorly water-soluble drug, TAS-301, by its melt-adsorption on a porous calcium silicate. J. Pharm. Sci 91, 362–370. 10.1002/jps.10026 [DOI] [PubMed] [Google Scholar]
- Thermo Fisher Scientific US, Continuous Granulation. https://www.thermofisher.com/us/en/home/industrial/pharma-biopharma/drug-formulation-manufacturing/continuous-granulation.html (accessed 09 November 2019).
- Thermo Scientific-16 mm Screw Elements Portfolio (PP-016), 2013. www.thermoscientific.com/mc (accessed 09 November 2019).
- Thompson M, Sun J, 2010. Wet granulation in a twin-screw extruder: Implications of screw design. J. Pharm. Sci 99, 2090–2103. 10.1002/jps.21973 [DOI] [PubMed] [Google Scholar]
- Thumma S, Majumdar S, Elsohly M, Gul W, Repka M, 2008. Chemical stability and bioadhesive properties of an ester prodrug of Δ9-tetrahydrocannabinol in poly (ethylene oxide) matrices: Effect of formulation additives. Int. J. Pharm 362, 126–132. 10.1016/j.ijpharm.2008.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari RV, Patil H, Repka MA, 2016. Contribution of hot-melt extrusion technology to advance drug delivery in the 21st century. Expert Opin. Drug Deliv 13, 451–464. 10.1517/17425247.2016.1126246 [DOI] [PubMed] [Google Scholar]
- Tu W, Ingram A, Seville J, 2013. Regime map development for continuous twin screw granulation. Chem. Eng. Sci 87, 315–326. 10.1016/j.ces.2012.08.015 [DOI] [Google Scholar]
- Upadhye SB, Vladyka ph R.R.S., Repka MA, Park J, Tiwari RV, Patil HG, Morott JT, LU W, 2017. Twin screw dry granulation for producing solid formulations. Google Patent. WO 2017/185040 A1. https://patents.google.com/patent/WO2017185040A1/en
- Vasanthavada M, Wang Y, Haefele T, 2011. Application of melt granulation technology using twin-screw extruder in development of high-dose modified-release tablet formulation. J. Pharm. Sci 100, 1923–1934. 10.1002/jps.22411 [DOI] [PubMed] [Google Scholar]
- Vercruysse J, Burggraeve A, Fonteyne M, 2015. Impact of screw configuration on the particle size distribution of granules produced by twin screw granulation. Int. J. Pharm 479, 171–180. 10.1016/j.ijpharm.2014.12.071 [DOI] [PubMed] [Google Scholar]
- Verstraete G, Mertens P, Grymonpré W, Van Bockstal PJ, De Beer T, Boone MN, Hoorebeke V, Remon JP, Vervaet C, 2016. A comparative study between melt granulation/compression and hot melt extrusion/Injection molding for the manufacturing of oral sustained release thermoplastic polyurethane matrices. Int. J. Pharm 513, 602–611. 10.1016/j.ijpharm.2016.09.072 [DOI] [PubMed] [Google Scholar]
- Vervaet C, Remon J, 2005. Continuous granulation in the pharmaceutical industry. Chem. Eng. Sci 60, 3949–3957. 10.1016/j.ces.2005.02.028 [DOI] [Google Scholar]
- Wan L, Heng P, Muhuri G, 1992. Incorporation and distribution of a low dose drug in granules. Int. J. Pharm 88, 159–163. 10.1016/0378-5173(92)90312-P [DOI] [Google Scholar]
- Wening K, Schwier S, Hans-J Stahlberg M, 2017. Application of hot-melt extrusion technology in immediate-release abuse-deterrent formulations. J. Opioid Manag 13, 473–484. 10.5055/jom.2017.0422. [DOI] [PubMed] [Google Scholar]
- Young C, Koleng J, McGinity J, 2002. Production of spherical pellets by a hot-melt extrusion and spheronization process. Int. J. Pharm 242, 87–92. 10.1016/s0378-5173(02)00152-7 [DOI] [PubMed] [Google Scholar]
- Zhang F, McGinity JW, 2000. Properties of hot-melt extruded theophylline tablets containing poly(vinyl acetate). Drug Dev. Ind. Pharm 26, 931–942. 10.1081/DDC-100101320 [DOI] [PubMed] [Google Scholar]
- Zhang F, McGinity JW, 1999. Properties of sustained-release tablets prepared by hot-melt extrusion. Pharm. Dev. Technol 4, 241–250. 10.1081/PDT-100101358 [DOI] [PubMed] [Google Scholar]


