Abstract
Summary
Brown tumors (BTs) are expansile osteolytic lesions complicating severe primary hyperparathyroidism (PHPT). Clinical, radiological and histological features of BTs share many similarities with other giant cell-containing lesions of the bone, which can make their diagnosis challenging. We report the case of a 32-year-old man in whom an aggressive osteolytic lesion of the iliac crest was initially diagnosed as a giant cell tumor by biopsy. The patient was scheduled for surgical curettage, with a course of neoadjuvant denosumab. Routine biochemical workup prior to denosumab administration incidentally revealed high serum calcium levels. The patient was diagnosed with PHPT and a parathyroid adenoma was identified. In light of these findings, histological slices of the iliac lesion were reviewed and diagnosis of a BT was confirmed. Follow-up CT-scans performed 2 and 7 months after parathyroidectomy showed regression and re-ossification of the bone lesion. The aim of this case report is to underline the importance of distinguishing BTs from other giant cell-containing lesions of the bone and to highlight the relevance of measuring serum calcium as part of the initial evaluation of osteolytic bone lesions. This can have a major impact on patients’ management and can prevent unnecessary invasive surgical interventions.
Learning points:
Although rare, brown tumors should always be considered in the differential diagnosis of osteolytic giant cell-containing bone lesions.
Among giant cell-containing lesions of the bone, the main differential diagnoses of brown tumors are giant cell tumors and aneurysmal bone cysts.
Clinical, radiological and histological characteristics can be non-discriminating between brown tumors and giant cell tumors. One of the best ways to distinguish these two diagnoses appears to be through biochemical workup.
Differentiating brown tumors from giant cell tumors and aneurysmal bone cysts is crucial in order to ensure better patient care and prevent unnecessary morbid surgical interventions.
Patient Demographics: Adult, Male, Other, Canada
Clinical Overview: Bone, Parathyroid, Bone, PTH, Brown tumour, Hyperparathyroidism (primary), Parathyroid adenoma
Diagnosis and Treatment: Bone lesions, Hypercalcaemia, Bone biopsy, Calcium (serum), Histopathology, CT scan, PTH, 25-hydroxyvitamin-D3, MRI, Phosphate (serum), X-ray, Sestamibi scan, Parathyroidectomy, Denosumab, Cholecalciferol, Calcium carbonate, Calcitriol
Related Disciplines: Pathology, Surgery, Radiology/Rheumatology
Publication Details: Error in diagnosis/pitfalls and caveats, April, 2020
Background
Primary hyperparathyroidism (PHPT) can cause several skeletal abnormalities, collectively known as osteitis fibrosa cystica (OFC). OFC is characterized clinically by bone pain and radiologically by the presence of subperiosteal bone resorption, salt-and-pepper skull demineralization, osteolysis of the distal clavicles as well as brown tumors (BTs) (1, 2). Although historically considered a classical finding, OFC is nowadays a very rare presentation of PHPT, especially in developed countries where measurement of serum calcium is performed routinely (2).
BTs represent a terminal stage of the PHPT-related bone disease. They are expansile osteolytic lesions caused by an excessive activity of osteoclasts. In these sites where bone resorption is especially rapid, the normal marrow content is replaced by hemorrhage, proliferating fibrous tissue and reparative granulation tissue, forming BTs. Their diagnosis is based on clinical manifestations, radiological imaging and histological findings. However, as these can be non-specific, a high index of suspicion is required, especially in a patient without known hyperparathyroidism (2).
The differential diagnosis of BTs includes various giant cell-containing bone lesions (Table 1), among which are giant cell tumors (GCTs) (3, 4). The distinction between these two conditions can be challenging, sometimes leading to diagnostic errors and unnecessary surgical procedures.
Table 1.
Differential diagnosis of giant cell lesions of the bone (4).
| Reactive | Benign | Malignant |
|---|---|---|
| Brown tumor | Giant cell reparative granuloma | Giant cell rich osteosarcoma |
| Haemophiliac pseudotumor | Nonossifying fibroma | Clear cell chondro-sarcoma |
| Intraosseous hemorrhage | GCT | Metastatic carcinoma |
| Aneurysmal bone cyst | Undifferenciated pleomorphic sarcoma | |
| Chrondroblastoma | Malignant GCT (1–2%) |
GCT, giant cell tumor.
Case presentation
A 32-year-old man initially presented to his general practitioner with a 6-month history of constant back pain radiating to his left buttock. No accompanying symptom was notable other than slight nycturia. His past medical history and his family’s medical history were also unremarkable. In the course of his evaluation, he underwent a lumbar MRI which revealed a heterogenous lesion of the left iliac crest. A subsequent pelvic CT showed a 7.3 × 2.9 × 8.7 cm well-defined expansile osteolytic lesion of the left posterior iliac crest, as well as other smaller osteolytic foci in both iliac bones (Fig. 1).
Figure 1.
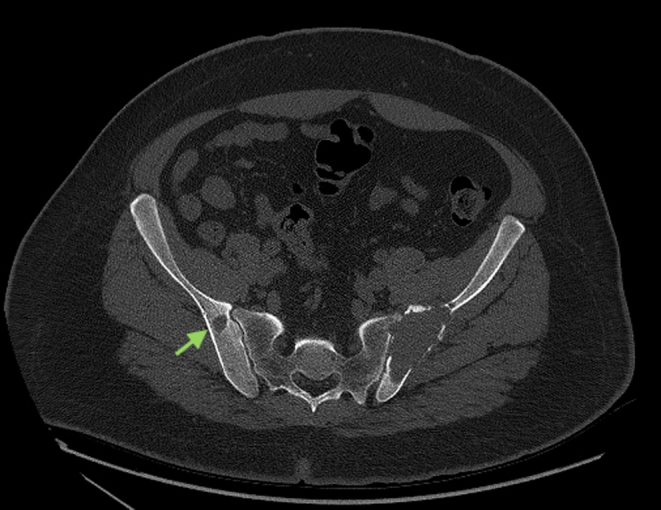
Image of the patient’s pelvic CT showing a large osteolytic lesion of the left iliac crest and another osteolytic focus in the right iliac bone (green arrow).
A biopsy of the main left iliac lesion showed numerous osteoclast-like multinucleated giant cells scattered evenly in a sparse stroma, intermixed with round and spindle-shaped mononuclear cells without cytological atypias, as well as abundant extravasated red blood cells (Fig. 2). This was consistent with the histological characteristics of a GCT.
Figure 2.
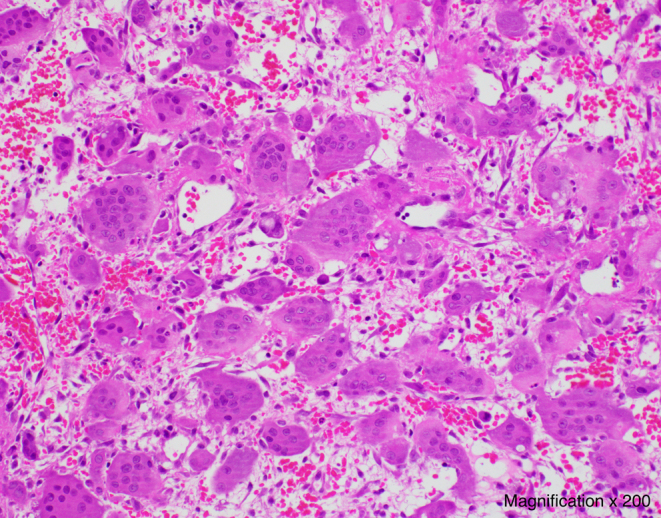
Histological slice of the biopsy of the left iliac lesion showing multiple multinucleated giant cells scattered evenly in a sparse stroma, intermixed with round and spindle-shaped mononuclear cells, as well as extravasated red blood cells (Magnification× 200).
After evaluation, the patient was diagnosed with a benign GCT of bone. He was scheduled by the orthopedics team for a surgical curettage after a course of neoadjuvant denosumab. On routine biochemical workup prior to denosumab administration, a high serum calcium level (3.33 mmol/L (reference range (RR): 2.20–2.60 mmol/L)) was discovered. Consequently, an endocrinology consultation was requested.
Investigation
Upon investigation of the hypercalcemia, the patient was found to have an elevated parathyroid hormone (PTH) level (63.2 pmol/L (RR: 1.6–6.9 pmol/L)), low serum phosphorus (0.49 mmol/L (RR: 0.7–1.4 mmol/L)) and a normal kidney function. 25-hydroxyvitamin D (25(OH)D) levels were also measured as low (18.2 nmol/L (RR: 75–150 nmol/L)).
The diagnosis of PHPT was confirmed, and a Technetium-99m sestamibi of the parathyroid glands later revealed a right inferior parathyroid adenoma.
Newly diagnosed PHPT prompted reassessment of the osteolytic bone lesion’s diagnosis. Skeletal radiographs were ordered to look for stigmata of OFC. Hand radiographs (with a mammographic technique) showed multiple sites of resorption of the phalangeal tufts, subperiosteal resorption of the middle phalanges bilaterally and a lytic lesion of the fifth left distal phalanx suggestive of a BT (Fig. 3).
Figure 3.

Hand x-ray showing a lytic lesion of the fifth distal phalanx suggestive of a BT (green arrow), subperiosteal bone resorption of the middle phalanges (red arrows) and resorption of the phalangeal tufts (blue arrows).
In light of these findings, histological slices of the iliac lesion were reviewed. Given the clinical context, the observed features were confirmed to be consistent with those of a BT.
Treatment
After two weekly doses of 120 mg of denosumab, the serum calcium levels gradually normalized. Vitamin D deficiency was also aggressively corrected preoperatively with cholecalciferol 20,000 IU weekly (25(OH)D level on the day of surgery: 64.0 nmol/L). The patient underwent parathyroidectomy of a 1100 mg adenoma, with no postoperative complications.
Outcome and follow-up
Postoperatively, the patient presented transient hypocalcemia that was corrected with oral calcium carbonate, calcitriol and cholecalciferol supplementation. Supplement doses were repeatedly evaluated in the first postoperative month and adjusted according to serum calcium levels (Table 2). The patient was kept on calcium carbonate 1000 mg three times a day, calcitriol 0.25 µg twice daily and cholecalciferol 10,000 IU daily until 3 months postoperatively, after which a tapering was initiated. All supplements other than cholecalciferol were completely stopped at 5 months after surgery. On last follow-up (7 months postoperatively), serum calcium and 25(OH)D levels were normal but PTH levels remained slightly elevated.
Table 2.
Postoperative variations of serum calcium and PTH levels as well as required vitamin D supplementation.
| Normal range | Before surgerya | Post-operative | ||||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 2 | Day 8 | 2 months | 5 months | 7 months | |||
| Total Ca2+ (mmol/L) | 2.20–2.60 | 2.63 | 2.09 | 2.19 | 2.42 | 2.37 | 2.68 | 2.53 |
| PTH (pmol/L) | 1.6–6.9 | 96.5 | – | – | – | 16.2 | 6.8 | 12.3c |
| Oral supplementation | ||||||||
| Calcitriolb | None | 0.25 µg/day | 0.25 µg twice/day | 0.25 µg twice/day | 0.25 µg twice/day | 0.25 µg/day | None | |
| Calcium carbonate | none | 500 mg twice/day | 500 mg thrice/day | 1000 mg thrice/day | 1000 mg thrice/day | 1000 mg twice/day | None | |
aLaboratory results from 3–6 days preoperatively; bPatient was simultaneously taking cholecalciferol 10,000 IU/week; c25(OH)D level was 76.1 nmol/L.
Follow-up CT-scans performed 2 and 7 months after parathyroidectomy showed regression and progressive re-ossification of the left iliac lesion, as well as complete re-ossification of the other small osteolytic foci (Fig. 4).
Figure 4.
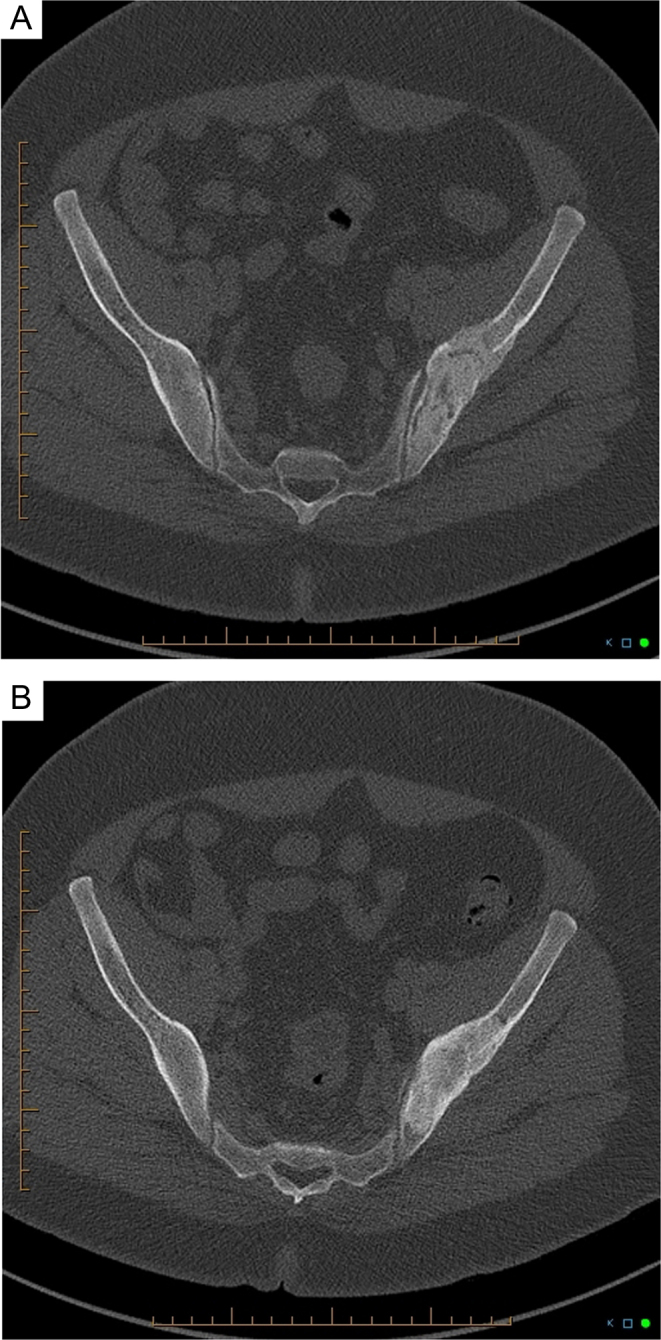
Follow-up pelvic CT scans 2 (A) and 7 months (B) after parathyroidectomy showing progressive re-ossification of the left iliac crest lesion.
Based on the patient’s age, it was decided to request a MEN workup and to refer him for genetic testing in order to eliminate a familial form of PHPT (5, 6). Serum levels of calcitonin, prolactin, metanephrins and normetanephrins were normal. As for genetic testing, no mutation was identified in any of the following tested genes known to be associated with hereditary PHPT: CASR, CDC73, CDKN1B, GCM2, MEN1 and RET.
Discussion
BTs are a rare manifestation of prolonged severe PHPT. Clinically, they cause swelling, bone pain and pathological fractures. On imaging, they appear as lytic lesions with well-defined margins and sometimes a sclerotic rim (2, 3, 4). Biopsy is the gold standard for definitive diagnosis of BTs, showing lobules separated by fibrous septa and composed of fibroblasts, extravasated red blood cells, hemosiderin-laden macrophages and scattered multinucleated giant cells that cluster around areas of hemorrhage (4).
Among giant cell-containing lesions of the bone, one of the main differential diagnoses of BTs is GCTs (3, 4). These are mainly benign bone tumors that also manifest with bone pain and swelling. They present radiologically as a lytic mass with non-sclerotic margins. Histologically, they are made up of a syncytium of round and oval mononuclear stromal cells, admixed with numerous large multinucleated giant cells scattered evenly throughout the tumor (4).
It is notable that clinical, radiological and histological features of BTs share many similarities with those of GCTs. However, some elements can be helpful in distinguishing these two entities (summarized in Table 3). Clinically, although they cause the same symptoms, they affect different parts of the skeleton. BTs can develop in various bones, while GCTs mostly arise in the epiphysis and metaphysis of long bones in patients with closed growth plates (2, 3, 4), making the iliac crest a very unusual site for a GCT. Moreover, GCTs present generally as solitary bone lesions, whereas BTs are frequently multiple. On histological evaluation, they are both composed of a fibrotic stroma with multinucleated giant cells (3, 4). However, the mononuclear cells are round in GCTs but spindled in BTs, and giant cells tend to be distributed uniformly in GCTs, while, in BTs, they are arranged in clusters around areas of hemorrhage (3, 4).
Table 3.
| Brown tumor | Giant cell tumor | |
|---|---|---|
| Etiology | Prolonged hyperparathyroidism | Mainly benign primary bone neoplasm |
| Epidemiology | Peak incidence fifth–sixth decade | Peak incidence third–fifth decade |
| Typically affected bones | Jaw, clavicles, ribs, pelvis and extremities | Distal femur, proximal tibia and distal radius |
| Localization within bones | Variable | Epiphysis/metaphysis |
| Biochemistry | Elevated Ca2+ | Ca2+ usually normal |
| Elevated PTH | ||
| Imaging | Expansile lytic lesion | Expansile lytic lesion |
| Well defined margins | Non-sclerotic margins | |
| Sclerotic rim | Neocortex | |
| Can be multiple or solitary | Most often solitary | |
| Histology | Lobular structure | Round/oval stromal cells |
| Multinucleated giant cells in clusters | Multinucleated giant cells distributed evenly | |
| Spindled stromal cells | ||
| Extravasated red blood cells |
One of the best ways to distinguish BTs from other giant cell-containing bone lesions is through biochemical workup: hypercalcemia with elevated PTH levels in the presence of a giant cell-rich osteolytic lesion is highly suggestive of the diagnosis of BT. However, it is to keep in mind that there are a few cases reporting the concomitant presence of a GCT and PHPT (3, 7).
Management of BTs consists mainly of treating the hyperparathyroidism, often leading to spontaneous regression of the bone lesions, as observed in our patient. On the contrary, GCTs are most commonly treated with aggressive surgical curettage or en-bloc resection of the tumor (3, 7, 8, 9). This supports the importance of rapidly distinguishing BTs from GCTs in order to offer the right treatment to patients. Indeed, there have been cases reported in the literature of patients who have undergone unnecessary surgical interventions because of a diagnostic mix-up between these two pathologies (7, 8, 9). A biochemical workup including serum calcium and phosphorus levels is therefore essential in the evaluation of a patient presenting with a lytic bone lesion.
Most cases of PHPT are sporadic and are due to a solitary parathyroid adenoma. Only 5 to 10% of cases of PHPT are associated with a hereditary syndrome, which include multiple endocrine neoplasia (MEN) type1 syndrome, MEN 2A syndrome, MEN 4 syndrome, hyperparathyroid-jaw tumor syndrome and familial isolated primary hyperparathyroidism (5).
Despite his young age and aggressive disease, no genetic mutation associated with a familial form of PHPT was found in our patient. It can be noted that our patient’s serum 25(OH)D level was low, which could have contributed to the severity of the bone disease. In fact, in asymptomatic PHPT, low 25(OH)D levels are associated with increased disease activity and parathyroid gland weight, as well as a lower total hip and forearm bone mineral density (1, 10).
In conclusion, this case report emphasizes the importance of considering BTs in the differential diagnosis of osteolytic giant cell-containing bone lesions. Since clinical, histological and radiological features can be non-discriminating between the various etiologies of such lesions, routine measurement of serum calcium and phosphorus in their initial evaluation can help identify the right diagnosis. This would ensure better patient care and prevent unnecessary and morbid surgical interventions.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. Approval from the local Research Ethics Board has been obtained prior to writing the case.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent has been obtained from the patient.
Author contributions and acknowledgements
L B, M J Bég and S M were the physicians who contributed to the patient’s care. S H drafted the manuscript and all authors contributed to the reviewing and editing process. M J Ber provided expert opinion on all radiological investigations undertaken. J D was the pathologist who interpreted the tumor pathology and provided the microscopic images for publication. All others gave their approval to submit the manuscript for publication.
References
- 1.Khan AA, Hanley DA, Rizzoli R, Bollerslev J, Young JE, Rejnmark L, Thakker R, D’Amour P, Paul T, Van Uum S, et al Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management. A Canadian and International Consensus. Osteoporosis International 2017. 28 1–19. ( 10.1007/s00198-016-3716-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Misiorowski W, Czajka-Oraniec I, Kochman M, Zgliczynski W, Bilezikian JP. Osteitis fibrosa cystica – a forgotten radiological feature of primary hyperparathyroidism. Endocrine 2017. 58 380–385. ( 10.1007/s12020-017-1414-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossi B, Ferraresi V, Appetecchia ML, Novello M, Zoccali C. Giant cell tumor of bone in a patient with diagnosis of primary hyperparathyroidism: a challenge in differential diagnosis with brown tumor. Skeletal Radiology 2014. 43 693–697. ( 10.1007/s00256-013-1770-9) [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg AE, Nielsen GP. Giant cell containing lesions of bone and their differential diagnosis. Current Diagnostic Pathology 2001. 7 235–246. ( 10.1054/cdip.2001.0080) [DOI] [Google Scholar]
- 5.Walker MD, Silverberg SJ. Primary hyperparathyroidism. Nature Reviews: Endocrinology 2018. 14 115–125. ( 10.1038/nrendo.2017.104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcocci C, Cetani F. Clinical practice. Primary hyperparathyroidism. New England Journal of Medicine 2011. 365 2389–2397. ( 10.1056/NEJMcp1106636) [DOI] [PubMed] [Google Scholar]
- 7.Panagopoulos A, Tatani I, Kourea HP, Kokkalis ZT, Panagopoulos K, Megas P. Osteolytic lesions (brown tumors) of primary hyperparathyroidism misdiagnosed as multifocal giant cell tumor of the distal ulna and radius: a case report. Journal of Medical Case Reports 2018. 12 176 ( 10.1186/s13256-018-1723-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aghaghazvini L, Sharifian H, Rasuli B. Primary hyperparathyroidism misdiagnosed as giant cell bone tumor of maxillary sinus: a case report. Iranian Journal of Radiology 2016. 13 e13260 ( 10.5812/iranjradiol.13260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vera L, Dolcino M, Mora M, Oddo S, Gualco M, Minuto F, Giusti M. Primary hyperparathyroidism diagnosed after surgical ablation of a costal mass mistaken for giant-cell bone tumor: a case report. Journal of Medical Case Reports 2011. 5 596 ( 10.1186/1752-1947-5-596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bilezikian JP, Brandi ML, Rubin M, Silverberg SJ. Primary hyperparathyroidism: new concepts in clinical, densitometric and biochemical features. Journal of Internal Medicine 2005. 257 6–17. ( 10.1111/j.1365-2796.2004.01422.x) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a