Abstract
Background
Individuals with a family history (FH+) of alcohol use disorder (AUD) have a higher risk for developing an AUD than those with no family history (FH-) of AUD. In addition, FH+ individuals tend to perform worse on neuropsychological measures and show heightened impulsivity, which may be due to underlying differences in brain structure such as cortical thickness. The primary aim of this study was to investigate differences in cortical thickness in FH+ compared to FH- adolescents. Secondary aims were to: a) investigate differences in executive functioning and impulsivity, and b) examine associations between brain structure and behavior.
Method
Brain scans of 95 FH- and 93 FH+ subjects aged 13–18 were obtained using magnetic resonance imaging (MRI). FH+ subjects were required to have at least 1 biological parent with a history of an AUD. FH+ and FH- individuals had limited or no past alcohol use, thereby minimizing potential effects of alcohol. Subjects were evaluated on impulsivity and executive functioning tasks. Thicknesses of cortical lobes and subregions were analyzed using FreeSurfer. Regions showing group differences were examined for group by age interactions and correlations with neuropsychological and personality measures.
Results
FH+ adolescents had thinner cortices in frontal and parietal lobes, notably in the medial orbitofrontal, lateral orbitofrontal, and superior parietal cortices. The difference in cortical thickness between family history groups was strongest among the youngest subjects. FH+ subjects were also more impulsive and had poorer performance on a spatial memory task.
Conclusions
These findings demonstrate frontal and parietal structural differences in FH+ adolescents that might underlie cognitive and behavioral characteristics associated with AUD risk.
Keywords: alcohol use disorder, family history, MRI, cortical thickness, adolescence
Introduction
Alcohol use disorder (AUD, defined as alcohol abuse or alcohol dependence) is a widespread problem affecting more than 17 million adults in the United States (NIAAA, 2012). Roughly 1 in 4 children in the United States grow up with AUD in their immediate families (Grant, 2000), and individuals who have a family history of AUD (FH+) are approximately 4 times more likely to develop an AUD in their lifetimes compared to individuals with no such family history (FH-) (Cotton, 1979, Goodwin et al., 1973). In addition, the likelihood of developing alcohol dependence is correlated with the number of affected first- and second-degree relatives (Dawson et al., 1992). One reason for this increased risk is AUD’s genetic heritability, which has been demonstrated through twin and adoption studies (Enoch and Goldman, 1999),
There are a number of traits that are thought to contribute to the elevated risk of AUD in FH+ individuals. Among these, problems with behavioral and cognitive control, as manifest in poor executive functioning and heightened impulsivity, are considered to be particularly important (Sher, 1997, Sher et al., 1991). Furthermore, there is evidence that FH+ adolescents may show differences in brain structure compared to age-matched FH- peers. For instance, lower amygdala volume has been reported in FH+ adolescents and emerging adults prior to onset of AUD (Hill et al., 2001, Hill et al., 2013b, Dager et al., 2015). Focusing on the striatum, Cservenka and colleagues found evidence for higher nucleus accumbens volume in FH+ subjects (2015). Hill and colleagues found no differences in caudate volume between FH+ and FH- subjects, but externalizing symptoms were correlated with caudate volume. (Hill et al., 2013a). Furthermore, cerebellar volume has been shown to be higher in FH+ individuals (Hill et al., 2007).
Relatively few studies, however, have focused on cortical thickness differences in FH+ subjects. Previously, Hill and colleagues conducted a region of interest (ROI) analysis specifically on the orbitofrontal cortex in a sample of adolescents and emerging adults (mean age = 17.6). While there were no differences between groups on orbitofrontal cortex volume, the ratio of right to left orbitofrontal cortex volume was lower in FH+ compared to FH- subjects (Hill et al., 2009). Recently, it was demonstrated using voxel-based morphometry that FH+ adults show lower grey matter volume in several cortical regions, including lingual gyrus, fusiform gyrus, and insula (Sharma and Hill, 2017).
The relative lack of investigation into cortical thickness differences in FH+ adolescents is surprising given that a family history of AUD is associated with higher impulsivity and poor executive functioning, which are both thought to rely on an assemblage of cortical brain regions (Sher et al., 1991, Kamarajan et al., 2015, Dougherty et al., 2015, Nigg et al., 2004, Adkison et al., 2013). Imaging studies have demonstrated altered patterns of cortical processing underlying behavioral control, decision making, and spatial working memory in FH+ adolescents (Spadoni et al., 2008, Cservenka, 2016), and studies using diffusion weighted imaging to measure white matter integrity also point to differences between FH+ and FH- subjects (Acheson et al., 2014, Herting et al., 2010). In addition, neuropsychological investigations have shown that adolescent binge drinkers have impaired spatial working memory (Squeglia et al., 2011), a difference that may in fact predate heavy drinking and serve as a risk factor for AUD. Thus, while previous studies of adolescents point to altered neural activity in FH+ individuals, potential underlying structural differences in cortical morphology—which have been extensively investigated in adults with a current or past AUD (De Bellis et al., 2005, Pfefferbaum et al., 1998, Medina et al., 2008, Chanraud et al., 2007)—have not been well studied in FH+ individuals prior to heavy drinking.
Neuroimaging studies have shown that cortical maturation follows an inverted U-shape pattern over the course of development (Giedd et al., 1999). Cortical thickness increases during childhood and then, at the onset of puberty, pruning of synaptic connections and reductions in dendritic spines leads to steady declines in cortical thickness throughout adolescence (Blakemore, 2008). Higher order association areas such as portions of the prefrontal cortex (PFC) and parietal cortex are among the latest regions to mature and continue to show significant thinning beyond the adolescent years (Gogtay et al., 2004). Although not focusing on structural imaging measures, Hardee and colleagues found an age x family history effect in the strength of neural activation in the prefrontal cortex on an fMRI task designed to assess behavioral inhibition (Hardee et al., 2014). As they got older, FH- subjects showed lower activation in the middle frontal gyrus—a component of the PFC—while performing the task, whereas FH+ subjects had greater activation in this same region (Hardee et al., 2014).
Building on these findings, the primary aim of this project was to investigate differences in cortical thickness between FH+ and FH- adolescents. In order to minimize confounding effects of alcohol exposure as opposed to pre-existing neurobiological differences, we stipulated that all subjects must have no more than minimal past alcohol use, as measured by the Alcohol Use Disorders Identification Test (AUDIT), and minimal prenatal alcohol exposure. In addition, given the significant age-related differences that occur within adolescence (Roper et al., 2014, Defoe et al., 2015), and because differences between family history groups may interact with age, we designed the study to incorporate an equal number of 13–14, 15–16, and 17–18 year-old adolescents in both the FH+ and FH- groups.
In conjunction with the cortical thickness findings, we also examined differences between the two family history groups on neuropsychological tasks and a behavioral measure of impulsivity. We hypothesized that FH+ adolescents would show poorer performance in executive function and greater impulsivity than FH-. Also, given that impulsivity and addictive behaviors have each been associated with lower cortical thickness in adolescents, especially in the frontal lobes (Schilling et al., 2013, Almeida et al., 2010, Yuan et al., 2013, Hong et al., 2013) and individual differences in frontal lobe measures have been shown to be correlated with impulsivity related measures (Hill et al., 2009), we hypothesized that the FH+ group would show lower cortical thickness than the FH- group in the frontal cortex. In addition to comparing FH+ and FH- subjects on cortical thickness, neuropsychological functioning, and impulsivity, a third aim was to identify relationships between these cognitive/behavioral constructs and cortical thickness in FH+ adolescents. No specific regional or direction-of-association hypotheses were made about these associations given the limited literature on this topic.
Materials and Methods
Participants
Neuroanatomical, neuropsychological, and behavioral data were collected for 95 FH+ (47 male, 48 female) and 96 FH- adolescents (48 male, 48 female) 13–18 years of age. Two subjects were excluded from data analysis due to excessive motion on MRI scans, and 1 was excluded due to abnormally large total intracranial volume (ICV). Subjects were required to be right handed as measured by the Annett Handedness Questionnaire (Annett, 1970) and have an IQ of 80 or higher as measured by the brief form of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). Participants were excluded if they had ever undergone neurological surgery; had a concussion in the last 6 months; experienced severe head trauma in the last 3 years; were diagnosed by a physician with a major mental health disorder, other than externalizing disorders (EDs), as reported by a parent; had a serious, unstable medical condition; had major hearing or vision issues; were pregnant; or had metal in the body that could interfere with MRI acquisition. FH- adolescents were excluded for diagnosed EDs including attention deficit hyperactivity disorder (ADHD), conduct disorder (CD), and oppositional defiant disorder (ODD) based on parent and child report. FH+ subjects were not excluded for these conditions because EDs are known to be more prevalent in FH+ adolescents and may be inextricable from relevant alcohol risk (Kuperman et al., 2001). Past or current use of substances other than alcohol, marijuana, and tobacco was exclusionary for FH+ and FH- groups. Problem level use (scores > 7 on the AUDIT or ASSIST) of alcohol, marijuana, and tobacco was also exclusionary.
FH+ subjects had to have at least 1 biological parent screen positive (≥ 3 DSM-IV abuse or dependence items) for a history of AUD on the Semi-Structured Assessment for the Genetics of Alcoholism IV (SSAGA) interview (Bucholz et al., 1994). If the affected parent was unavailable for interview, the Family History Assessment Module (FHAM) was used with the other biological parent or the adolescent subject (if he/she was 18 years of age) as the informant. In contrast, subjects in the FH- group were required to have both biological parents screen negative for AUD using the FHAM. Participants were excluded if their mothers reported having more than 3 drinks of alcohol per week at any point during pregnancy. The highest education level of the subject’s two parents was used as a proxy for socioeconomic status (see Supplementary Information for details). Participants were interviewed using the SSAGA (Child SSAGA for participants under the age of 18) to measure history of alcohol, tobacco, and marijuana use as well as mental health symptomology.
All subjects were analyzed for number of ED symptoms, regardless of ED diagnosis, using the criterion items in the SSAGA from the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) (First, 1994). Total ED symptoms were calculated by summing number of inattention, hyperactivity/impulsivity, ODD, and conduct disorder symptoms. Note that due to technical errors, data for externalizing and depression symptom counts were properly saved only for 88 of 95 FH- and 85 of 93 FH+ subjects).
This study was reviewed and approved by the Institutional Review Board at the University of Iowa. Parents provided consent for themselves and offspring below 18; adolescents 13 and older also provided consent. Subjects were consented after initial screening but prior to any other testing. Parents were consented prior to parent screening and all other testing due to the sensitive nature of the screening.
Brain image acquisition and processing
MRI scans were obtained on a Siemens Trio 3T MRI scanner equipped with a 12-channel phased array head coil at the University of Iowa Magnetic Resonance Research Facility. High-resolution T1-weighted MP-RAGE images (TR = 2300 ms, TE = 2.82 ms, flip angle = 10 degrees, voxel size = 1.1 × 1.1 × 1.1 mm, series = interleaved), and T2-weighted images (TR = 4130 ms, TE = 11 ms, flip angle = 120 degrees, voxel size = 1.3 × 0.9 × 2.5 mm, series = interleaved) were collected for all subjects.
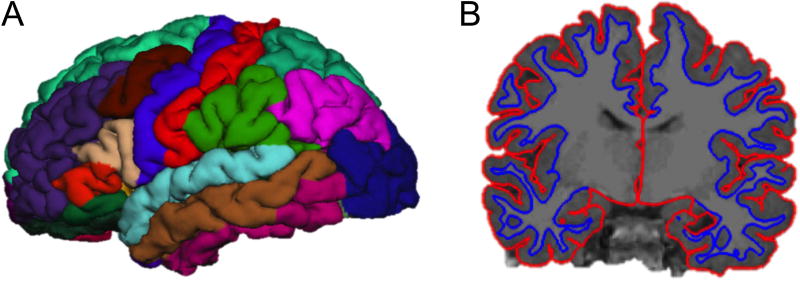
T1 MP-RAGE scans were segmented into anatomical regions using FreeSurfer (version 5.3). Image analysis in FreeSurfer has been previously described in detail (Fischl and Dale, 2000). Briefly, after basic preprocessing (including intensity correction, skull stripping, and motion correction), the white matter and pial surfaces were identified by creating a mesh around the white matter and pial voxels. Surface-based maps of each individual scan were constructed by segmenting images into distinct cortical and sub-cortical regions based on probability estimates obtained from another, manually labeled training dataset (Desikan et al., 2006). Total ICV was calculated for each subject using BRAINS AutoWorkup (Pierson et al., 2011) by adding all gray matter, white matter, and cerebrospinal fluid (CSF) volumes. The anatomical accuracy of FreeSurfer’s automated parcellations were visually inspected, and manual edits were made using standard intervention procedures (documented on the FreeSurfer website) to correct any unacceptable parcellations deemed necessary by two independent reviewers. Finally, cortical thickness measurements for each cortical region were obtained through automated calculation of the distances between the cortical surface and white matter border (See Fig. 1.B) (Fischl and Dale, 2000).
Figure 1.
(A) FreeSurfer subregion parcellations of a representative subject brain (see Table 1 for regions included in each lobe). (B) FreeSurfer white and grey matter surface labels used to assess cortical thickness in a coronal slice of a representative subject brain.
Cortical lobes (frontal, parietal, temporal, and occipital) were delineated according to Desikan et al. (Desikan et al., 2006) by combining together the respective subregions encompassed in each lobe (Table 1, Fig. 1.A). The cingulate (including the rostral anterior, caudal anterior, posterior, and isthmus divisions) and insular cortices were analyzed separately from the four lobes.
Table 1.
FreeSurfer Lobe Parcellation.
| Lobe | Subregions |
|---|---|
| Frontal | frontal pole, superior frontal gyrus, rostral middle frontal gyrus, caudal middle frontal gyrus, pars opercularis, pars triangularis, pars orbitalis, lateral orbitofrontal cortex, medial orbitofrontal cortex, precentral gyrus, paracentral lobule |
| Parietal | postcentral gyrus, supramarginal gyrus, superior parietal cortex, inferior parietal cortex, and precuneus |
| Temporal | entorhinal cortex, parahippocampal gyrus, temporal pole, fusiform gyrus, superior temporal gyrus, middle temporal gyrus, inferior temporal gyrus, transverse temporal cortex, banks of the superior temporal sulcus |
| Occipital | lingual gyrus, pericalcarine cortex, cuneus cortex, lateral occipital cortex |
Subregions involved in each of the four major lobes according to FreeSurfer parcellation (Desikan et al., 2006).
Neuropsychological test battery
Neuropsychological tests assessing spatial working memory and executive functioning were administered using the Computerized Multiphasic Interactive Neuropsychological DualDisplay System (CMINDS) (http://www.neurocomp.com/Solutions/Cminds). The following computerized tests were administered: Trails A and B (Trail Making Test Parts A and B), Digit Span (Forward and Backward), Letter Fluency, Category Fluency, and Visuospatial Sequencing Test (Forward and Reverse) (O’Halloran et al., 2008). The Visuospatial Sequencing Test was added after data collection had already begun, so these data are available for only 89 FH- and 81 FH+ subjects.
Impulsivity
We examined group differences on a behavioral measure of impulsivity, the Delay Discounting Task (DDT) (Mitchell, 1999). The DDT assesses impulsivity and reward valuation by requiring subjects to choose between a smaller hypothetical monetary reward available immediately or a larger hypothetical monetary reward available at a later time. This task measures a subject’s rate of discounting (k) where larger gains at a later time become valued as preferable to smaller gains with no delay (future value discounting). A logarithmic (base 10) transformation was applied to the k values of the DDT to normalize the distributions for analysis (Reynolds et al., 2004). Note that 1 FH+ subject scored a k value of 0.0000, so that value was adjusted to 0.0001 in order to apply a logarithmic transform.
Statistical analyses
A similar number of 13–14, 15–16, and 17–18 year-old subjects were deliberately recruited, with an equal numbers of males and females in each family history group. Measurements of cortical thickness were imported from FreeSurfer into PASW Statistics (SPSS) version 23. Full factorial univariate analysis of variance (UNIANOVA) was conducted for cortical thickness in regions of interest using SPSS with age bin and FH group as fixed factors of primary interest and gender and total ICV as covariates. Given that FH+ and FH- groups differed significantly on parental education, analyses were re-run with highest parental education level as an additional covariate, and patterns of results remained highly similar (Table S1). Differences in cortical thickness between the left and right hemispheres of the frontal and parietal lobes within each FH group were investigated using paired T-tests. Group differences in hemispheric asymmetry were analyzed using an independent samples T-test comparing the groups on differences in thickness between the left and right hemispheres of each lobe.
Regional analyses first targeted the individual cortical lobes (frontal, parietal, occipital, and temporal), cingulate cortex, and insula. Lobes showing group effects of p<0.05 were then broken down into smaller FreeSurfer subregions for further analysis. Main effects of group on neuropsychological and impulsivity test scores were analyzed using UNIANOVA with group and age bin included as fixed factors and gender as a covariate. Two-tailed partial correlations controlling for age, total ICV and gender were calculated between DDT and neuropsychological performance for regions showing significant group differences.
Results
Participants
FH groups did not differ significantly in age or gender (by design). Parents of FH+ subjects had significantly lower levels of education than parents of FH- (p=0.001) (Table 2). Relatively few FH+ had been diagnosed with an ED (ADHD: N=3, ODD: N=1, CD: N=0; EDs were exclusionary for FH-). Both groups reported relatively few externalizing symptoms, though the FH+ group did score higher on this externalizing composite measure (p<0.001). FH+ subjects also had more symptoms of depression than FH- as obtained from the SSAGA (p=0.008). FH+ and FH- adolescents did not significantly differ on lifetime use of alcohol, tobacco or marijuana. Among those adolescents that had used substances, AUDIT and ASSIST scores showed that the severity of alcohol use did not differ between groups, though it differed marginally for tobacco (p=0.061) and significantly for marijuana use (p=0.024), with FH+ subjects exhibiting greater severity overall. Of the FH+ subjects in this study, 63 had a father affected with AUD, 22 had a mother affected with AUD, and 8 had both parents affected with AUD.
Table 2.
Participant Demographics.
| FH- | FH+ | Statistic | p | |
|---|---|---|---|---|
| N | 95 | 93 | ||
| Age (years) | 15.45 (1.74) | 15.51 (1.70) | t = −0.21 | 0.834 |
| Female | 48 | 48 | χ2 = 0.02 | 0.882 |
| IQ | 115.31 (9.00) | 108.85 (10.05) | t = 4.64 | <0.001 |
| Highest parent education level | 5.75 (1.45) | 4.91 (1.96) | t = 3.33 | 0.001 |
| Number of externalizing symptoms | 1.16 (2.67) | 3.50 (4.87) | t = −3.89 | <0.001 |
| Number of depressive symptoms | 0.33 (1.42) | 1.19 (2.58) | t = −2.70 | 0.008 |
| Alcohol Use | ||||
| Subjects reporting any alcohol use | N=11 | N=20 | χ2 = 3.36 | 0.067 |
| AUDIT total score | 2.73 (2.20) | 3.50 (1.54) | t = −1.15 | 0.260 |
| Tobacco Use | ||||
| Subjects reporting any tobacco use | N=7 | N=10 | χ2 = 0.65 | 0.419 |
| ASSIST tobacco total score | 1.86 (0.38) | 4.60 (4.03) | t = −2.14 | 0.061 |
| Marijuana Use | ||||
| Subjects reporting any marijuana use | N=7 | N=10 | χ2 = 0.65 | 0.419 |
| ASSIST marijuana total score | 1.86 (2.27) | 6.60 (4.60) | t = −2.51 | 0.024 |
Demographic, mental health, and substance use breakdown of the 189 subjects analyzed for this study. Group values are mean(SD). Parental education levels were coded as follows: 0=less than high school, 1=high school GED, 2=high school diploma, 3=some college, 4=trade technical training certification, 5=associates (2 year), 6=bachelors (4 year), 7=masters, 8=doctorate. This scale was treated as continuous for purposes of data analysis. Independent samples t-tests for each ordinal variable of interest were run by family history (FH) group to obtain t statistic. Pearson chi-square tests were run for each categorical variable of interest by FH group to obtain the χ2 statistic. Two-tailed p-values for all findings reported. Equal variances were assumed whenever Levene’s Test did not reject the assumption of Equality of Variances (p>0.05). Where equal variances could not be assumed, the degrees of freedom were adjusted using the Welch-Satterthwaite method in SPSS. Equal variances could not be assumed for Highest parent education level, Number of externalizing symptoms, Number of depressive symptoms, and ASSIST tobacco total score.
Group differences in cortical thickness
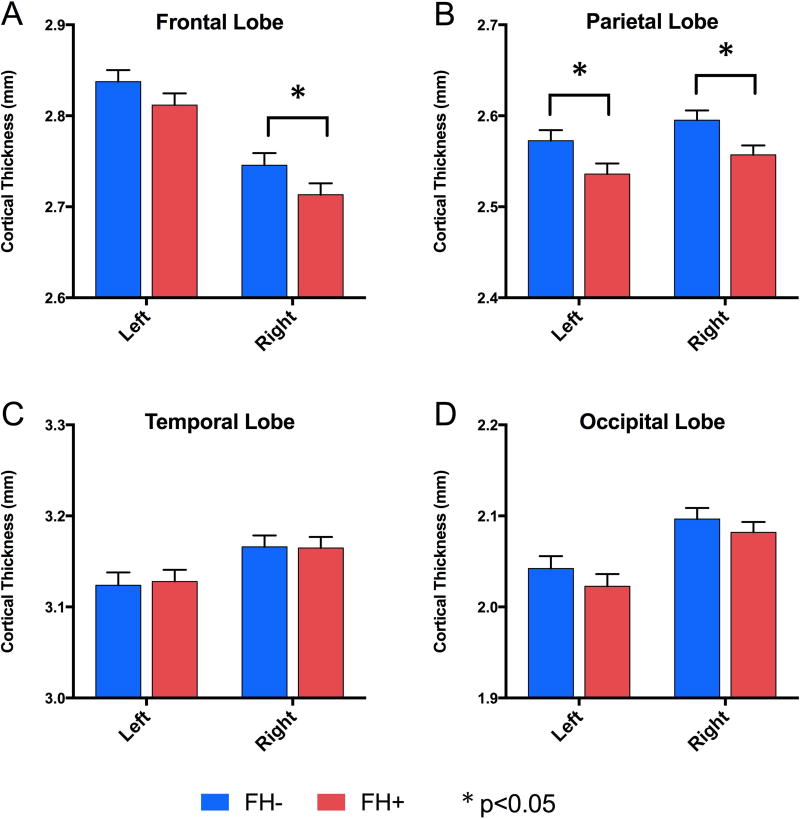
Analyses were conducted with FH status and age group as fixed factors in the same model and with gender as a covariate. Here, we first report the main effects of FH group. Lobe level analyses revealed that FH+ subjects had significantly thinner cortices in the right frontal (p=0.038), left parietal (p=0.050), and right parietal (p=0.047) lobes (Fig. 2.A,B). No differences were found for the temporal or occipital lobes (ps>0.10, Fig. 2.C,D). Each group did show significant hemispheric asymmetry in the frontal and parietal lobes (see supplementary Table S1 for values), but these asymmetries did not differ significantly between the groups. (ps>0.10). Since there were no group differences in hemispheric asymmetry, all further analyses were simply conducted on regions in the right or left hemispheres individually. Analysis of the cingulate cortex and insula revealed no differences between groups (ps>0.10). Cortical thickness values for each FH group are provided in supplementary Table S1. Since groups differed on measures of alcohol, tobacco, and marijuana use (Table 2), analyses of cortical thickness at the lobe level were re-run excluding all subjects who reported any use of alcohol, tobacco, or marijuana. Patterns of group differences were highly similar when focusing on alcohol and drug naïve subjects.
Figure 2.
Main effects of cortical lobe gray matter thickness (mm) across groups were found using UNIANOVA with family history group and age bin as fixed factors and gender and total intracranial volume as covariates. Left and right hemisphere measures are shown for (A) frontal lobe, (B) parietal lobe, (C) temporal lobe, and (D) occipital lobe. Mean values with SEM bars displayed. * p<0.05.
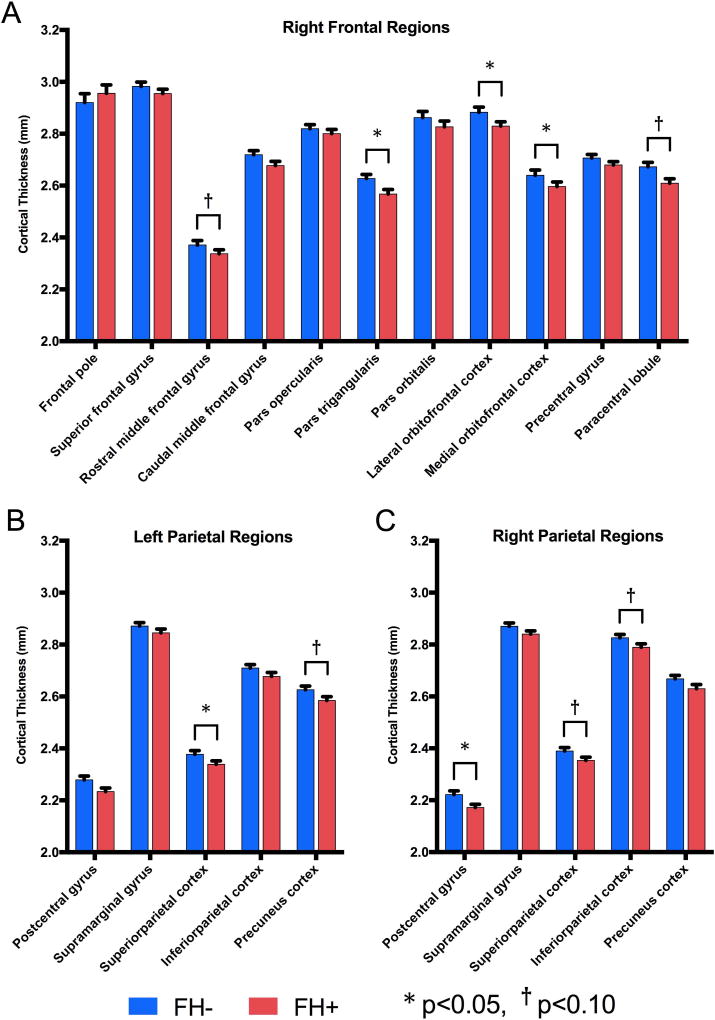
Because the FH+ group was found to have lower cortical thickness in the right frontal lobe and bilateral parietal lobes than the FH- group, sub-regions within those lobes were investigated to determine which specific regions accounted for these differences. Within the right frontal lobe, significantly thinner cortices were found in FH+ adolescents in the pars triangularis (p=0.010), lateral orbitofrontal cortex (p=0.016), and medial orbitofrontal cortex (p=0.014); and trending effects in the same direction were found in the rostral middle frontal gyrus (p=0.086) and paracentral lobule (p=0.070, Fig. 3.A). Within the left parietal lobe, FH+ subjects showed significantly thinner superior parietal cortices (p=0.047), and evidence of thinner precuneus cortices at a trend level (p=0.082) in FH+ (Fig. 3.B). In the right parietal lobe, the postcentral gyrus was significantly thinner (p=0.036) in FH+ adolescents, and the superior parietal cortex and inferior parietal cortex were marginally thinner (p=0.083 and p=0.077, respectively) in FH+ adolescents (Fig. 3.C). Note that some of these regions showed age by FH group interactions as described in the next section.
Figure 3.
Main effects of regional cortical thickness (mm) in (A) right frontal, and (B) left parietal, and (C) right parietal regions across groups were found using UNIANOVA with family history group and age bin as fixed factors and gender and total intracranial volume as covariates. Mean values with SEM bars shown. * p<0.05, † p<0.10.
Age effects on cortical thickness
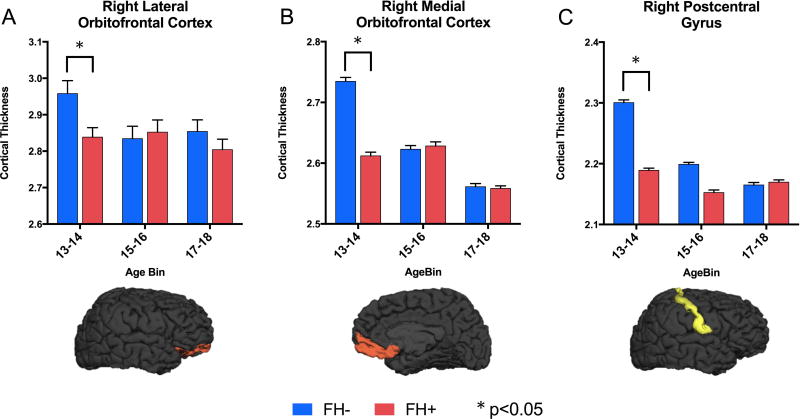
Beyond main effects of FH group on cortical thickness, 3 cortical regions showed group by age interaction effects (Fig. 4). The right postcentral gyrus, right lateral orbitofrontal cortex, and right medial orbitofrontal cortex showed significant group by age interactions (p=0.010, p=0.012, and p=0.008, respectively). Breaking groups down by age bin demonstrated that the effect in these regions is largely driven by the 13–14 year old subjects (values are provided in Supplemental Table S2). There were, however, no age by FH group interaction effects at the lobe level. See supplemental Figure S1 for main effects of age group.
Figure 4.
Breakdown of cortical thickness (mm) by age bin in regions showing family history (FH) group by age interaction effects. (A) Right lateral orbitofrontal cortex in the frontal lobe, (B) right medial orbitofrontal cortex in the frontal lobe, (C) right postcentral gyrus in the parietal lobe. Effects are evident primarily in the youngest age bin (13–14 years) where FH+ adolescents show lower cortical thickness than FH- controls. * p<0.05. See Supplemental Table S2 for group by age interaction values.
Group differences in neuropsychological and impulsivity tasks
Analyses of group differences on neuropsychological tasks (Table 3) indicate that FH+ adolescents took significantly longer (p=0.042) to complete the Trails B task (but did not differ on Trails A), and performed worse than their FH- peers on both VST Forward (p=0.017) and Reverse (p=0.027). FH+ adolescents also demonstrated significantly higher scores on the DDT (p=0.033), indicating greater impulsivity. There were no significant group by age interactions on any of these tasks (ps>0.10).
Table 3.
Neuropsychological and Delay Discounting Tasks.
| FH- | FH+ | Statistic | p | |
|---|---|---|---|---|
| Neuropsychological Tests | ||||
| Trails A (seconds) | 25.88 (7.49) | 27.62 (8.11) | F = 2.60 | 0.108 |
| Trails B (seconds) | 53.14 (20.45) | 60.58 (32.18) | F = 4.20 | 0.042 |
| Digit Span Forward (sequence length) | 6.57 (0.99) | 6.33 (1.13) | F = 2.48 | 0.117 |
| Digit Span Backward (sequence length) | 4.74 (1.19) | 4.63 (1.23) | F = 0.26 | 0.611 |
| Category Fluency (total correct) | 53.65 (12.50) | 52.31 (14.09) | F = 0.60 | 0.444 |
| Letter Fluency (total correct) | 41.17 (11.30) | 39.25 (12.52) | F = 1.69 | 0.195 |
| VST Forward (sequence span) | 6.35 (0.91) | 5.81 (1.02) | F = 5.79 | 0.017 |
| VST Reverse (sequence span) | 6.02 (0.85) | 5.46 (1.01) | F = 5.00 | 0.027 |
| Impulsivity | ||||
| Delay Discounting Task (Log k) | −2.32 (0.83) | −2.06 (0.82) | F = 4.60 | 0.033 |
Main effects of group on neuropsychological (Trails A and B, Digit Span (Forward and Backward), Category Fluency, Letter Fluency, and Visuospatial Sequencing Test (VST, Forward and Reverse)) and impulsivity (Delay Discounting Task) test scores were analyzed using UNIANOVA with group and age bin included as fixed factors and gender as a covariate. Group values are mean(SD).
Correlations of cortical thickness with impulsivity and executive functioning
The 3 lobes (right frontal, left parietal, right parietal) showing group main effects on cortical thickness (Fig. 2) were analyzed for correlations with DDT score to identify associations between regional structural differences and impulsivity. Among FH- subjects, a marginally significant negative correlation was found between impulsivity and left parietal lobe thickness (r=−0.18, p=0.082), but no significant correlations were found for FH+ subjects (see Supplemental Table S3). Correlations were also calculated between cortical thicknesses in these 3 lobes and neuropsychological tests that showed main effects of group (Table 2): Trails B, VST Forward, and VST Reverse. Because VST Forward and Reverse were significantly correlated (r=0.32, p=0.001), the two measures were summed to create one “VST” variable. In the FH- group, right frontal lobe thickness showed a significant positive correlation with time to complete Trails B (worse performance) (r=0.24, p=0.022). The left parietal lobe showed a significant negative correlation with VST score (r=−0.28 p=0.009) in the FH- group, and the right parietal lobe showed a weak negative correlation with VST score (r=−0.19, p=0.075) in the FH- group. No significant correlations were found for FH+ subjects (ps>0.10). When the entire sample was analyzed as a whole, no significant correlations were found between cortical thickness and neuropsychological performance or impulsivity. The left frontal and parietal lobes of all subjects grouped together did show trend-level correlations with VST performance (r=−0.14, p=0.064; and r=−0.136, p=0.080, respectively).
Discussion
The purpose of this study was to identify differences in cortical thickness, executive functioning, and impulsivity between FH+ and FH- adolescents. FH+ subjects showed lower cortical thickness relative to their FH- peers in the right frontal and bilateral parietal lobes. We controlled for total ICV in our sample to eliminate confounds of differences in overall head size. These findings remained even when subjects reporting alcohol, tobacco, and/or marijuana use were excluded from the analysis, indicating that the effect is likely not driven by the subject’s prior substance use.
Cortical thickness was chosen as the primary measure of interest since previous work has shown cortical thickness to be more sensitive to group differences than grey matter volume, a composite of cortical thickness and surface area, in imaging studies (Winkler et al., 2010). Future work may be useful to disentangle the contributions of area, thickness, and volume to meaningful differences in brain structure, but that is beyond the scope of this study and would be particularly well suited for a longitudinal design.
Several regions within the frontal and parietal lobes showed differences in cortical thickness between groups: the right medial orbitofrontal cortex, right lateral orbitofrontal cortex, right pars triangularis, left superior parietal cortex, and right postcentral gyrus were significantly thinner in FH+ adolescents (p<0.05). The right rostral middle frontal gyrus, right paracentral lobule, left precuneus cortex, right superior parietal cortex, and right inferior parietal cortex also showed some evidence of thinner cortices in FH+ adolescents (.05<p<0.10). These findings demonstrate hemispheric specificity of group differences and are supported by the within-group differences in cortical thickness found between the left and right hemispheres of the frontal and parietal lobes. Because there were no between-group differences in hemispheric asymmetry, it is unlikely that overall differences in lateralization between FH+ and FH- groups are responsible for these results.
The orbitofrontal cortex has been previously implicated in impulsivity, decision-making, and reward monitoring (Elliott et al., 2000, Berlin et al., 2004), and the parietal cortex has been associated with executive functions such as spatial working memory and attention allocation (Koenigs et al., 2009, Corbetta et al., 1995, Fein et al., 2009). A recent report found thickness in part of the left parietal lobe to be an important neural predictor of adolescent onset alcohol use (Squeglia et al., 2016). That the right, but not left, frontal lobe showed group differences is in line with previous research that has implicated reduced volume in regions of the right frontal lobe corresponding to both impulse control (Boes et al., 2009) and risk for addiction (Benegal et al., 2007). The present findings further support previous work showing alterations in the orbitofrontal cortex of individuals at high familial risk for alcohol dependence (Hill et al., 2009) and major depression (Peterson and Weissman, 2011).
FH+ adolescents did perform more poorly than FH- on neuropsychological tasks of executive functions including visual attention and spatial working memory and demonstrated greater behavioral impulsivity on the DDT. Based on our findings that there were significant differences in the cortical thickness of the frontal and parietal lobes between groups, we conducted additional analyses to determine if those structural differences were associated with the variability in impulsivity and executive functioning. Although there were a small number of significant correlations in the FH- group, there were no significant correlations between cortical thickness and executive functioning or impulsivity (as measured by DDT) in the FH+ group.
The negative correlations found between frontal and parietal cortical thickness and spatial working memory performance scores in FH- adolescents align with previous work in healthy adolescents demonstrating an association between regional cortical thinning and neuropsychological performance (Tamnes et al., 2010, Squeglia et al., 2013). Absence of this finding in FH+ adolescents suggests that they may have an altered structure-function association relative to FH-.
Interestingly, we did not find the patterns of correlation expected between structural and neuropsychological or impulsivity measures. There are many possible explanations for this apparent inconsistency in brain-behavior correlations. For instance, it is possible that the FH+ group was not representative of subjects at highest risk for AUD and thus could not be distinguished from FH- subjects as completely as possible. Additionally, we may not have used the potential measures with the greatest sensitivity to detect the behavioral alterations associated with differences in cortical thickness in the frontal and parietal lobes. For example, selective attention and interference from distraction, such as that measured by Stroop (Stroop, 1935), was not explicitly measured for this study but may be more highly correlated with cortical thickness differences between groups. Future research will be necessary to resolve these open questions.
Thus, while our results, in addition to earlier studies, point to altered brain morphology (Hill et al., 2001, Hill et al., 2013b, Dager et al., 2015), greater impulsivity (Sher, 1997, Sher et al., 1991, Kamarajan et al., 2015, Dougherty et al., 2015), and poorer executive functioning (Nigg et al., 2004, Adkison et al., 2013, Spadoni et al., 2008), reduced cortical thickness in the frontal and parietal lobes does not appear to strongly relate to impulsivity and neuropsychological performance in FH+ adolescents. It will be important for future studies to investigate both factors that contribute to altered cortical thickness in FH+ subjects and the impact that reduced cortical thickness has on cognitive and behavioral functioning.
Three regions of the frontal and parietal lobes - the right postcentral gyrus, right lateral orbitofrontal cortex, and left lateral orbitofrontal cortex - showed group by age interactions. As shown in Fig. 4, the largest differences between FH+ and FH- was in the 13–14 year old age group. There are several possible explanations for this finding. One intriguing possibility is that gray matter cortical maturation may be disrupted prior to adolescence in FH+ individuals, leading to lower cortical thickness in our youngest age groups. Cortical thinning may then occur at different rates across groups, ultimately leading to similar levels of cortical thickness in both groups by mid- to late-adolescence. This possibility for developmental delay in FH+ subjects is supported by previous longitudinal research using event-related potentials to measure developmental changes in children ages 8–18 at high risk for developing alcoholism (Hill et al., 1999). Another possibility for the major differences occurring in 13–14 year olds is that our study captured those 13–14 year olds at highest risk for AUD who will begin using alcohol in the next few years but excluded older subjects who already started using alcohol. Thus, FH+ subjects may not be homogeneous across age groups.
The results presented here should be considered in light of some possible limitations. First, it is known that early age of onset of alcohol use is associated with AUD (Grant, 1998), so it is possible, as noted above, that older adolescents who had already begun drinking and were thus excluded might have otherwise contributed to group differences in this study. This explanation could be best examined by a longitudinal design with initial assessments occurring prior to adolescence. In general, the age by group interactions do suggest a developmental trajectory as a meaningful factor in cortical maturation of FH+ subjects. Additionally, it is possible that since the FH- subjects in our sample came from homes with higher parental education levels, structural differences between groups were driven by above average cortical thickness in the FH- group, as opposed to below average cortical thickness in the FH+ group. However, if this were the case, we would have expected parental education levels, as a proxy indicator for potential genetic and environmental factors, to impact the effect sizes of the structural differences much more than it did (see Supplemental Table S1). Family history of AUD is also known to be associated with externalizing disorders (EDs) (Kuperman et al., 2001). Since FH+ subjects with EDs were not excluded, though few had formal diagnoses, it is difficult to disentangle FH status and EDs as causal for our findings. It may, then, be valuable to include FH- subjects with EDs in order to better isolate differences between family history groups that are a result of externalizing symptomatology specifically versus familial risk more generally. An additional limitation of this study is that age of puberty was not obtained for subjects. Because the trajectory of brain development changes around the time of puberty (Blakemore, 2008), and the age of puberty can vary widely across individuals, further research should be done to disentangle the effects of age and puberty on FH+ brains.
On the whole, these findings lead to a broader understanding of addiction, adolescent brain development, and neurobiological correlates of behavior. Differences in cortical thickness of regions underlying impulsivity, working memory, and attention may mediate personality traits and neuropsychological functions associated with addiction. Future research is necessary to continue parsing neurobiological and neuropsychological differences either predating or resulting from substance use disorders, as well as to continue identifying differences that predate substance use disorders. From a clinical perspective, this study may benefit future diagnostic and preventative measures for AUD by elucidating those parts of the brain systematically affected by a family history of AUD.
Supplementary Material
Acknowledgments
The authors would like to thank members of the Collaborative Studies on the Genetics of Alcoholism (COGA) project, the University of Iowa Interdisciplinary Summer Undergraduate Research Program, and especially Carinda Linkenmeyer, Alexander Wallace, Lindsey Fuhrmeister, Rose Nguyen, and Eric Axelson for their invaluable assistance in data collection and analysis. We would also like to thank the anonymous reviewers who provided helpful feedback to greatly improve the quality of this manuscript. This work was supported by the National Institute on Alcohol Abuse and Alcoholism grant 5R01AA018405 (D.S.O.).
Dr. O’Leary reports support from NIH grant R01AA021165-01A1: 3R01AA021165-02S1 for his work on The Relationship of Adolescent Binge Drinking to Measures of Brain and Behavior. Dr. Langbehn reports that he is a paid consultant for clinical trial design in Huntington’s Disease for Roche pharmaceutical, Voyager pharmaceutical, and Teva pharmaceutical.
Footnotes
CONFLICTS OF INTEREST
Dr. Vaidya, Dr. Kramer, Dr. Kuperman, and Ms. Henderson report no biomedical financial interests or potential conflicts of interest.
MRI data in this study were previously presented in a poster (K.E.H., presented under the name Kate E. Rasmussen) at the 2016 Annual Meeting of the Society for Neuroscience in San Diego, CA and in an oral presentation (J.G.V.) at the 2016 annual scientific meeting of the Research Society on Alcoholism in New Orleans, LA.
References
- Acheson A, Wijtenburg SA, Rowland LM, Winkler AM, Gaston F, Mathias CW, Fox PT, Lovallo WR, Wright SN, Hong LE, Dougherty DM, Kochunov P. Assessment of whole brain white matter integrity in youths and young adults with a family history of substance-use disorders. Hum Brain Mapp. 2014;35:5401–13. doi: 10.1002/hbm.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkison SE, Grohman K, Colder CR, Leonard K, Orrange-Torchia T, Peterson E, Eiden RD. Impact of fathers’ alcohol problems on the development of effortful control in early adolescence. J Stud Alcohol Drugs. 2013;74:674–83. doi: 10.15288/jsad.2013.74.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida LG, Ricardo-Garcell J, Prado H, Barajas L, Fernández-Bouzas A, Ávila D, Martínez RB. Reduced right frontal cortical thickness in children, adolescents and adults with ADHD and its correlation to clinical variables: a cross-sectional study. Journal of psychiatric research. 2010;44:1214–1223. doi: 10.1016/j.jpsychires.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. British journal of psychology. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. 1984 doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addict Biol. 2007;12:122–32. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127:1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Boes AD, Bechara A, Tranel D, Anderson SW, Richman L, Nopoulos P. Right ventromedial prefrontal cortex: A neuroanatomical correlate of impulse control in boys. Social Cognitive and Affective Neuroscience. 2009;4:1–9. doi: 10.1093/scan/nsn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the ssaga. J Stud Alcohol. 1994;55:149–58. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–38. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, Miezin FM, Petersen SE. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science. 1995;270:802. doi: 10.1126/science.270.5237.802. [DOI] [PubMed] [Google Scholar]
- Cotton NS. The familial incidence of alcoholism: A review. Journal of studies on alcohol. 1979;40:89–116. doi: 10.15288/jsa.1979.40.89. [DOI] [PubMed] [Google Scholar]
- Cservenka A. Neurobiological phenotypes associated with a family history of alcoholism. Drug Alcohol Depend. 2016;158:8–21. doi: 10.1016/j.drugalcdep.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Gillespie AJ, Michael PG, Nagel BJ. Family History Density of Alcoholism Relates to Left Nucleus Accumbens Volume in Adolescent Girls. Journal of Studies on Alcohol and Drugs. 2015;76:47–56. [PMC free article] [PubMed] [Google Scholar]
- Dager AD, Mckay DR, Kent JW, Curran JE, Knowles E, Sprooten E, Göring HHH, Dyer TD, Pearlson GD, Olvera RL. Shared genetic factors influence amygdala volumes and risk for alcoholism. Neuropsychopharmacology. 2015;40:412–420. doi: 10.1038/npp.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Harford TC, Grant BF. Family history as a predictor of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1992;16:572–575. doi: 10.1111/j.1530-0277.1992.tb01419.x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent–onset alcohol use disorders and comorbid mental disorders. Alcoholism: Clinical and Experimental Research. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Defoe IN, Dubas JS, Figner B, Van Aken MAG. A meta-analysis on age differences in risky decision making: Adolescents versus children and adults. American Psychological Association. 2015 doi: 10.1037/a0038088. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on mri scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Lake SL, Mathias CW, Ryan SR, Bray BC, Charles NE, Acheson A. Behavioral impulsivity and risk-taking trajectories across early adolescence in youths with and without family histories of alcohol and other drug use disorders. Alcohol Clin Exp Res. 2015;39:1501–9. doi: 10.1111/acer.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable Functions in the Medial and Lateral Orbitofrontal Cortex: Evidence from Human Neuroimaging Studies. Cerebral Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Enoch M-A, Goldman D. Genetics of alcoholism and substance abuse. Psychiatric Clinics of North America. 1999;22:289–299. doi: 10.1016/s0193-953x(05)70077-0. [DOI] [PubMed] [Google Scholar]
- Fein G, Shimotsu R, Chu R, Barakos J. Parietal Gray Matter Volume Loss is Related to Spatial Processing Deficits in Long-Term Abstinent Alcoholic Men. Alcoholism, clinical and experimental research. 2009;33:1806–1814. doi: 10.1111/j.1530-0277.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB. Diagnostic and statistical manual of mental disorders. DSM IV-4th edition. APA. 1994 1994. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: A longitudinal mri study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin DW, Schulsinger F, Hermansen L, Guze SB, Winokur G. Alcohol problems in adoptees raised apart from alcoholic biological parents. Archives of General Psychiatry. 1973;28:238–243. doi: 10.1001/archpsyc.1973.01750320068011. [DOI] [PubMed] [Google Scholar]
- Grant BF. The impact of a family history of alcoholism on the relationship between age at onset of alcohol use and dsm-iv alcohol dependence: Results from the national longitudinal alcohol epidemiologic survey. Alcohol Research and Health. 1998;22:144. [PMC free article] [PubMed] [Google Scholar]
- Grant BF. Estimates of us children exposed to alcohol abuse and dependence in the family. American Journal of Public Health. 2000;90:112–115. doi: 10.2105/ajph.90.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee JE, Weiland BJ, Nichols TE, Welsh RC, Soules ME, Steinberg DB, Zubieta JK, Zucker RA, Heitzeg MM. Development of impulse control circuitry in children of alcoholics. Biol Psychiatry. 2014;76:708–16. doi: 10.1016/j.biopsych.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Schwartz D, Mitchell SH, Nagel BJ. Delay discounting behavior and white matter microstructure abnormalities in youth with a family history of alcoholism. Alcohol Clin Exp Res. 2010;34:1590–602. doi: 10.1111/j.1530-0277.2010.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biological Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Lichenstein S, Wang S, Carter H, Mcdermott M. Caudate volume in offspring at ultra high risk for alcohol dependence: Comt val158met, drd2, externalizing disorders, and working memory. Adv J Mol Imaging. 2013a;3:43–54. doi: 10.4236/ami.2013.34007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, Mcdermott M, Keshavan M. Cerebellar volume in offspring from multiplex alcohol dependence families. Biol Psychiatry. 2007;61:41–7. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Steinhauer SR, Konicky C, Lowers L, Connolly J. Developmental delay in p300 production in children at high risk for developing alcohol-related disorders. Biological Psychiatry. 1999;46:970–981. doi: 10.1016/s0006-3223(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Hill SY, Wang S, Carter H, Mcdermott MD, Zezza N, Stiffler S. Amygdala volume in offspring from multiplex for alcohol dependence families: The moderating influence of childhood environment and 5-httlpr variation. J Alcohol Drug Depend. 2013b;(Suppl 1) doi: 10.4172/2329-6488.S1-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Kostelnik B, Carter H, Holmes B, Mcdermott M, Zezza N, Stiffler S, Keshavan MS. Disruption of orbitofrontal cortex laterality in offspring from multiplex alcohol dependence families. Biol Psychiatry. 2009;65:129–36. doi: 10.1016/j.biopsych.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S-B, Kim J-W, Choi E-J, Kim H-H, Suh J-E, Kim C-D, Klauser P, Whittle S, Yücel M, Pantelis C. Reduced orbitofrontal cortical thickness in male adolescents with internet addiction. Behavioral and Brain Functions. 2013;9:11. doi: 10.1186/1744-9081-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Pandey AK, Chorlian DB, Manz N, Stimus AT, Anokhin AP, Bauer LO, Kuperman S, Kramer J, Bucholz KK, Schuckit MA, Hesselbrock VM, Porjesz B. Deficient event-related theta oscillations in individuals at risk for alcoholism: A study of reward processing and impulsivity features. PLoS One. 2015;10:e0142659. doi: 10.1371/journal.pone.0142659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Barbey AK, Postle BR, Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci. 2009;29:14980–6. doi: 10.1523/JNEUROSCI.3706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman S, Schlosser SS, Kramer JR, Bucholz K, Hesselbrock V, Reich T, Reich W. Developmental sequence from disruptive behavior diagnosis to adolescent alcohol dependence. Am J Psychiatry. 2001;158:2022–6. doi: 10.1176/appi.ajp.158.12.2022. [DOI] [PubMed] [Google Scholar]
- Medina KL, Mcqueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: Unique gender effects. Alcoholism: Clinical and Experimental Research. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146:455–64. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- NIAAA. Alcohol use disorder [Online] National Institute on Alcohol Abuse and Alcoholism; 2012. Available: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-use-disorders [Accessed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, Puttler LI, Adams KM, Zucker RA. Neuropsychological executive functioning in children at elevated risk for alcoholism: Findings in early adolescence. J Abnorm Psychol. 2004;113:302–14. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- O’Halloran JP, Kemp AS, Gooch KN, Harvey PD, Palmer BW, Reist C, Schneider LS. Psychometric comparison of computerized and standard administration of the neurocognitive assessment instruments selected by the catie and matrics consortia among patients with schizophrenia. Schizophrenia Research. 2008;106:33–41. doi: 10.1016/j.schres.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Weissman MM. A brain-based endophenotype for major depressive disorder. Annual review of medicine. 2011;62:461–474. doi: 10.1146/annurev-med-010510-095632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55:905–12. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Pierson R, Johnson H, Harris G, Keefe H, Paulsen JS, Andreasen NC, Magnotta VA. Fully automated analysis using brains: Autoworkup. NeuroImage. 2011;54:328–336. doi: 10.1016/j.neuroimage.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behavioural Processes. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Roper ZJ, Vecera SP, Vaidya JG. Value-driven attentional capture in adolescence. Psychol Sci. 2014;25:1987–93. doi: 10.1177/0956797614545654. [DOI] [PubMed] [Google Scholar]
- Schilling C, Kuhn S, Paus T, Romanowski A, Banaschewski T, Barbot A, Barker GJ, Bruhl R, Buchel C, Conrod PJ, Dalley JW, Flor H, Ittermann B, Ivanov N, Mann K, Martinot JL, Nees F, Rietschel M, Robbins TW, Smolka MN, Strohle A, Kathmann N, Garavan H, Heinz A, Schumann G, Gallinat J. Cortical thickness of superior frontal cortex predicts impulsiveness and perceptual reasoning in adolescence. Mol Psychiatry. 2013;18:624–630. doi: 10.1038/mp.2012.56. [DOI] [PubMed] [Google Scholar]
- Sharma VK, Hill SY. Differentiating the Effects of Familial Risk for Alcohol Dependence and Prenatal Exposure to Alcohol on Offspring Brain Morphology. Alcoholism: Clinical and Experimental Research. 2017;41:312–322. doi: 10.1111/acer.13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ. Psychological characteristics of children of alcoholics. Alcohol health and research world. 1997;21:247–254. [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Walitzer KS, Wood PK, Brent EE. Characteristics of children of alcoholics: Putative risk factors, substance use and abuse, and psychopathology. J Abnorm Psychol. 1991;100:427–48. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory BOLD response in adolescents. Alcohol Clin Exp Res. 2008;32:1135–45. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Ball TM, Jacobus J, Brumback T, Mckenna BS, Nguyen-Louie TT, Sorg SF, Paulus MP, Tapert SF. Neural predictors of initiating alcohol use during adolescence. American journal of psychiatry. 2016 doi: 10.1176/appi.ajp.2016.15121587. appi-ajp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Sorg SF, Jernigan TL, Tapert SF. Early adolescent cortical thinning is related to better neuropsychological performance. Journal of the International Neuropsychological Society. 2013;19:962–970. doi: 10.1017/S1355617713000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcoholism: Clinical and Experimental Research. 2011;35:1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of experimental psychology. 1935;18:643. [Google Scholar]
- Tamnes CK, Østby Y, Walhovd KB, Westlye LT, Due-Tønnessen P, Fjell AM. Neuroanatomical correlates of executive functions in children and adolescents: A magnetic resonance imaging (MRI) study of cortical thickness. Neuropsychologia. 2010;48:2496–2508. doi: 10.1016/j.neuropsychologia.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. Psychological Corporation; 1999. [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, Cheng P, Dong T, Bi Y, Xing L, Yu D, Zhao L, Dong M, Von Deneen KM, Liu Y. Cortical thickness abnormalities in late adolescence with online gaming addiction. PloS one. 2013;8:e53055. doi: 10.1371/journal.pone.0053055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.