Abstract
Echocardiographic strain imaging allows new insight into a complex cardiac mechanics and enables more precise evaluation of cardiac function. Hence, it has been shown to have clinical utility in a variety of valvular heart diseases. In particular, global longitudinal strain has been shown to be more sensitive to detect systolic dysfunction than left ventricular ejection fraction. In patients with valvular heart diseases, it provides both diagnostic and prognostic information in addition to standard echocardiographic and clinical parameters. In this review, we summarize current clinical application of strain echocardiography in patients with valvular heart diseases and discuss pathophysiological mechanisms that lead to respective findings in specific diseases.
Keywords: speckle tracking, strain, deformation, GLS, valve disease
Introduction
With a rapidly aging population, there is a significant increase in the number of patients with valvular heart disease and in its morbidity and mortality (1). Optimal clinical risk assessment and timing of intervention are crucial for good clinical outcome. Current guidelines frequently refer to systolic function assessment based on ejection fraction (EF) for clinical decision-making (2). However, over the last decade, there is growing evidence on myocardial deformation imaging, specifically strain echocardiography, as an indispensable technique for measurement of cardiac function. Especially global longitudinal strain (GLS) appears to offer incremental diagnostic and prognostic information and has the potential to become an important parameter for the management of patients with various valvular heart diseases (3–5).
In this paper, we review current clinical application of strain echocardiography in patients with valvular heart diseases, and discuss pathophysiological mechanisms that lead to respective findings in specific diseases.
Echocardiographic Assessment of Myocardial Strain
Principles of strain
Strain is a dimensionless measure of tissue deformation. It is defined as the relative length change of an object within a certain direction (6, 7). Myocardial deformation is determined by the spatial orientation of myofibers that simultaneously contract in different directions and cause a complex three-dimensional deformation. In order to completely describe this deformation, three normal strain and six shear strain components are needed. As this is impractical for clinical use, myocardial deformation is only described by three orthogonal strain components oriented along the coordinate system of the ventricle, i.e., longitudinal, circumferential, and radial. Rotation, twist, and torsion resulting mainly from circumferential–longitudinal shear strains are hardly used in the clinic. The complex left ventricular (LV) architecture and mechanics enables that only 15% of fiber shortening results into a 60% change in LVEF.
The interest in separately assessed deformation of endocardial, mid-myocardial, and epicardial layers resulted in the concept of layer-specific strain. However, the clinical usefulness of this concept has to be questioned. Physiologically, there is mechanical coupling between layers, which limits differential deformation. In addition, lateral resolution of echocardiographic imaging is insufficient to clearly differentiate layers in a myocardial wall of normal thickness (8).
During systole the ventricle undergoes, longitudinal and circumferential shortening (represented by negative strain values) and radial thickening (represented by positive strain values). All three normal strain components can be utilized for describing regional (segmental strain) or global (global strain) function of a chamber. The latter is defined as the respective deformation of the entire myocardium within an image plane. The most commonly used deformation component is global longitudinal strain (GLS). It is defined as the average myocardial deformation of the LV as measured from all three apical views. It is currently the most robust and reproducible deformation parameter, superior to conventional echocardiographic measurement, such as EF (9, 10). LV GLS in a healthy individual is on average approximately –20% (11). Segmental peak strain measurements have a higher variability than GLS (12), so that the analysis of segmental strain curve patterns might be a more robust alternative.
Further information on the concept of myocardial deformation and practical guidance for strain measurement are beyond the scope of this review, but may be found elsewhere (6, 8, 13).
Echocardiographic techniques for strain assessment
Echocardiography is currently the method of choice for clinical strain assessment. Tissue Doppler imaging (TDI) or speckle-tracking echocardiography (STE) is used to measure myocardial deformation.
TDI was the first echocardiographic technique for obtaining strain measurements. It has been well validated and has the highest temporal resolution among all deformation imaging modalities (6). However, the necessity of additional image acquisitions and time-consuming post-processing for a comprehensive LV analysis are major limitations for routine clinical application.
The newer approach, based on tracking speckles from frame to frame in the regular grayscale image, enables a fast and user-friendly semi- or fully-automatic strain analysis, which paved the way into clinical practice. However, STE depends on good image quality and geometry, which limits its applicability in patients with suboptimal echogenicity. Furthermore, the limited temporal resolution of underlying gray-scale images prevents a reliable analysis of short-lived events during isovolumetric periods and in diastole as well as the measurement of velocity and strain rate. Post-processing analysis is strongly vendor specific. For example, vendors report by default either endocardial strain or mid- or full-wall strain, which is a relevant cause of intervendor differences in strain measurements next to differences in post-processing algorithms (7). However, algorithm-related intervendor differences have been substantially reduced following efforts of the European Association of Cardiovascular Imaging, American Society of Echocardiography and Industry Task Force on strain standardization (7, 14). Latest intervendor comparisons proved an excellent reproducibility of GLS and only a moderate, yet significant, bias among vendors (10, 15). The incoherent use of endocardial and full-wall strain remains, although currently available evidence is in favour of the full wall approach (16, 17).
The problems with image quality, frame rate, and intervendor differences are even more pronounced in 3D STE compared with 2D STE; hence 3D STE cannot be considered ready for clinical use.
Assessing Cardiac Function in Valve Disease
Ejection fraction versus strain
LVEF is incorporated into current guideline-recommended treatment strategies for valve diseases, in order to perform interventions in asymptomatic patients with severe valvular dysfunction before developing irreversible LV damage (2). Therefore, surgery is recommended in asymptomatic patients with severe mitral regurgitation with early signs of LV systolic dysfunction (LVEF ≤60% and/or LV end-systolic diameter ≥45 mm). Treatment is recommended at LVEF <50% in patients with severe aortic regurgitation or aortic stenosis. However, assessment of systolic dysfunction in valvular heart disease is challenging. LVEF represents only a relative volume change from end-diastole to end-systole and does not account for the complexity of myocardial mechanics. This volume-based parameter has an important limitation in reflecting systolic function in altered loading (pressure and volume) conditions, which are inherently present in patients with valve diseases.
LVEF is often in the “supernormal” range in regurgitant valvular lesions and therefore a poor representative of systolic myocardial function (18, 19). In mitral regurgitation, LVEF overestimates cardiac function as it reflects both the aortic forward stroke volume and regurgitant volume pumped into the low-pressure left atrium. Consequently, LVEF can remain in a normal range for a long time and may mask subtle early reductions in contractility (19). Stenotic lesions present with concentric remodeling with increased wall thickness and reduced cavity diameter; hence EF can remain preserved despite reduced shortening of the myofibers (20). Therefore, LVEF can only detect LV systolic dysfunction in a relatively advanced stage of disease when a relevant myocardial contractile dysfunction has already developed.
Myocardial deformation imaging, however, may be superior in detecting subtle dysfunction (18, 19, 21). Longitudinal LV strain is the most vulnerable component of LV mechanics. In an early stage of disease, impaired longitudinal deformation is often compensated by an augmented circumferential function, which keeps the LVEF within a normal range (20). It has been shown that circumferential strain contributes more than twice as much to LV as longitudinal strain. Therefore, detection of early cardiac dysfunction in valve disease is one of the most promising indications of strain imaging (3–5, 22, 23).
Factors Influence Strain Values in Valve Disease
The management of valve disease relies to a large extent on the assessment of cardiac function. LVEF is considered to be an essential measurement for this, however, it is both preload- and afterload-dependent. The same accounts for strain, as myocardial deformation depends on contractile properties of the myocardial fibres (“contractility”), but also on their loading conditions (pre- and after-load), chamber geometry, dyssynchrony, and segment interactions (8). Therefore, abnormally low strain values are not necessarily a sign of myocardial dysfunction, while normal values do not automatically exclude diseases.
In this review, we explain how deformation should be interpreted in the light of changed loading, ventricular geometry, and shape with a focus on valve disease. Information on other pathology may be found elsewhere (8).
Loading
Basic physiological experiments showed that in the setting of an unchanged contractile state, a change in loading would influence myocardial deformation (24–26). Results confirmed that myocardial strain can decrease with increasing afterload and can increase with increasing preload.
In animal experiments, an acute afterload increase of 20 mm Hg by aortic banding resulted in an absolute decrease in longitudinal strain of approximately 3% (25). Similarly, in the experimental model mimicking the effects of progressive aortic valve stenosis with chronic increase in afterload, decrease in all deformation components were observed (27). Longitudinal strain was the most altered, particularly in the basal septal segments, which were shown to be the most sensitive segments to changes in pressure overload. On the other hand, rapid decrease in afterload induced by percutaneous aortic valve replacement led to acute increase in longitudinal strain. A mean decrease of transaortic mean pressure gradient of 38 mm Hg resulted in an increase of longitudinal strain measured with TDI from −11±5% to −17±9% (28). Additionally, multivariable analysis demonstrated that end-systolic strain relates more to altered afterload than to any other determinant of overall cardiac performance (24). These results indicated that the effect of afterload on strain is so profound that it can be dominating over changes in contractility.
In clinical experiments, preload reduction by a tilt-table test resulted in an acute 5% absolute and 25% relative decrease in GLS in an upright versus a supine position (29). A similar trend was also observed in circumferential strain components. These results indicate a clinically important preload dependency of myocardial strain and confirm the Frank-Starling concept that pre-stretching of myofibers augments myocardial contraction. In chronic preload increase (e.g. in patients with valve regurgitation), a biphasic behaviour of myocardial strain is observed: In the early phase of mitral or aortic regurgitation, when myocardial contractility is still preserved, strain values are increased (18, 19, 30). This suggests that strain values in the early stage of valvular regurgitation reflect volume overload rather than changes in contractility. Over time, strain normalizes with the adaptive remodeling of the ventricle and eventually progressively decreases when LV myocardium begins to fail.
Chamber geometry
Abovementioned example shows that interpretation of clinical strain measurements becomes complex when the evolution from acute to chronic effects is observed, as chronic load alterations result in myocardial remodelling, which additionally affects myocardial deformation.
Chronic pressure overload (e.g., in patients with valve stenosis) results in increasing wall thickness and decreasing chamber size that helps to keep wall stress in an acceptable range. Both changes augment EF and can virtually maintain a normal EF for a long time despite reduction of both longitudinal and circumferential strain (Fig. 1a) (20). In an advanced stage of chronic pressure overload, the increased wall stress results in myocardial fibrosis and myocyte degeneration (27), which leads to contractile dysfunction and impaired performance. Experimental studies showed that in LVs with chronically increased afterload and normal EF, longitudinal strain reduction is more the consequence of hypertrophic remodeling rather than loading and fibrotic remodeling. The relative contribution of hypertrophy to alter GLS was 62%, while the contribution of fibrotic remodeling and afterload was 38%. Therefore, a reduced strain per se is not a marker of impaired myocardial function in diseases with chronically increased afterload and cardiac remodeling.
Figure 1.
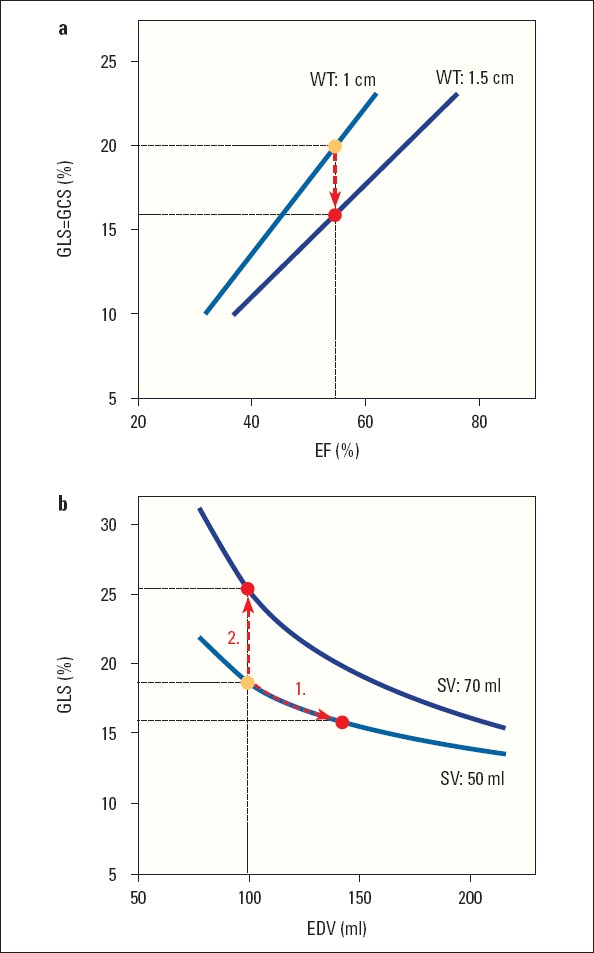
A theoretical model of relationship between chamber geometry and strain. (a) In the left ventricle (LV) with increased wall thickness, less global longitudinal (GLS) and circumferential (GCS) shortening is required to maintain the same ejection fraction (EF). Data from Stokke et al. (20) (b) An increase in LV end-diastolic volume (EDV) with no change in stroke volume (SV) results in a decrease in deformation (1.), while increased SV leads to increased deformation (2.). Data from Marciniak et al. (19)
A slow chronic increase in preload (e.g., in patients with valve regurgitation) imposes a volume overload on the chamber, which leads to an adaptive remodelling with increasing chamber size and increasing stroke volume. The complex dependency of deformation, chamber geometry, and stroke volume was very nicely explained in a mathematical simulation (19) which showed that increase in chamber size, without any change in stroke volume or contractility, leads to a decrease in deformation, while deformation increases with increasing stroke volume (Fig. 1b). Therefore, in the early phase of valve regurgitation, strain values increase or remain unchanged due to a balance of increased chamber dimensions and increased stroke volume (18, 19, 30). In advanced disease, when all compensatory mechanisms of remodeling are exhausted and wall stress becomes too high, irreversible changes occur in the myocardium and result in the development of contractile dysfunction and impaired myocardial deformation (18, 19). These studies therefore confirmed that strain is not directly related to the degree of mitral or aortic regurgitation itself, but determined by the interaction between chamber volume, stroke volume, and the contractility of the muscle.
In the clinic, loading effects and adaptive structural remodeling regularly influence each other, so that the contractile state of the myocardium is hard to establish from a single strain measurement alone. Different proposals have been made to overcome the dependency of strain on load and geometry: Some authors advised that myocardial strains should always be assessed together with end-systolic wall stress in order to separate the effect of myocardial dysfunction and afterload (31). Similarly, correcting myocardial strain for changes in geometry could be a sensitive way of detecting early LV dysfunction in valve disease with regurgitant lesions (18, 19, 21). Compared with the absolute strain values, normalized longitudinal strain values to end-diastolic volume demonstrated markedly less overlap between patients with aortic regurgitation and control group (18, 21). However, further larger outcome studies are needed to evaluate if these proposed approaches lead to parameters with better prognostic value.
Recent Clinical Applications
Recent studies suggested that strain echocardiography might be useful to improve the assessment of myocardial function in valve diseases, particularly in detecting subclinical dysfunction and defining the timing of interventions. However, a majority of studies focused on aortic and mitral valve disease, while data on right-sided valve diseases are limited. Table 1 summarizes the main echocardiographic studies that suggested a clinical utility of strain echocardiography in valvular heart diseases.
Table 1.
Clinical application of myocardial strain in valve diseases
| Author, year (ref.) | n | Clinical outcome | GLS cutoff (%) | Vendor |
|---|---|---|---|---|
| Aortic stenosis | ||||
| Ng et al., 2014 (32) | 688 | Predict all-cause mortality in a wide range of AS and EF | -14.0% | GE, EchoPac 108.1.5 |
| Kusunose et al., 2014 (33) | 395 | Predict all-cause mortality in moderate-severe and severe AS with preserved EF | N/A | Siemens Syngo VVI |
| Huded et al., 2018 (35) | 504 | Predict all-cause mortality | -17.0% | Siemens Syngo VVI |
| Salaun et al., 2018 (34) | 582 | Predict all-cause mortality in moderate and severe AS and preserved EF | -13.75%* | GE, EchoPac |
| Vollema et al., 2018 (22) | 220 | Predict symptoms development and need for aortic valve interventions in asymptomatic severe AS | -18.2% | Various (GE and TomTec) |
| Dahl et al., 2012 (3) | 125 | Predict outcome (cardiovascular mortality, cardiac hospitalization because of worsening of HF) in severe symptomatic AS | N/A | GE, EchoPac PC 08 |
| D’Andrea et al., 2019 (36) | 75 | Predict positive LV reverse remodeling after TAVR in LFLG AS | -12.0% | GE, EchoPac 202 |
| Aortic regurgitation | ||||
| Alashi et al., 2018 (4) | 1063 | Predict all-cause mortality in asymptomatic severe AR with preserved EF Predict outcome (all-cause mortality, perioperative complication, in-hospital stroke, atrial fibrillation, and readmission) after AVR |
-19.5% | Siemens Syngo VVI |
| Ewe et al., 2015 (23) | 129 | Predict progression during conservative management and need for AVR in asymptomatic moderately severe and severe AR and preserved EF | -17.4% | GE, EchoPac 110.0.0 |
| Alashi et al., 2020 (41) | 865 | Predict all-cause mortality after AVR in asymptomatic severe AR with preserved EF Persistent impaired GLS or worsening of GLS by 5% in absolute value from baseline was related with mortality after AVR |
-19.0% | Siemens Syngo VVI |
| Kusunose et al., 2014 (40) | 159 | Predict need for AVR in asymptomatic moderately severe to severe AR and preserved EF | N/A | Siemens Syngo VVI |
| Mitral regurgitation | ||||
| Mentias et al., 2016 (30) | 737 | Predict all-cause mortality in asymptomatic patients with significant primary MR and preserved LV EF | -21.0% | Siemens Syngo VVI |
| Kim et al., 2018 (5) | 506 | Predict outcome (HF, reoperation, and death) after surgery for severe primary MR Related with long-term LV dysfunction after valve procedure |
-18.1 % | TomTec, Image Arena version 4.6 |
| Witkowski et al., 2013 (51) | 233 | Predict postoperative LV dysfunction in moderate-severe primary MR | -19.9% | GE, EchoPAC 108.1.5 |
| Hiemstra et al., 2020 (42) | 593 | Predict outcome (all-cause mortality, HF, and cerebrovascular accident) after surgery for severe primary MR | -20.6% | GE, EchoPAC version 112 |
| Alashi et al., 2016 (43) | 448 | Predict postoperative LV dysfunction and mortality in asymptomatic severe primary MR | N/A | Siemens Syngo VVI |
| Namazi et al., 2020 (44) | 650 | Predict all-cause mortality in moderate and severe secondary MR | -7.0% | GE, EchoPAC 201.0.0 |
Apical four-chamber longitudinal strain
AR - aortic regurgitation; AS - aortic stenosis; AVR - aortic valve replacement; EF - ejection fraction; GLS - global longitudinal strain; HF - heart failure; LFLG - low-flow, low-gradient;
LV - left ventricle; MR - mitral regurgitation; N/A - not available; TAVR - transcatheter aortic valve replacement; n-number of patients
Aortic stenosis
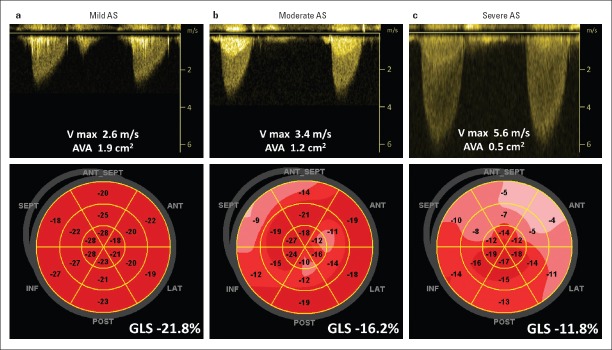
In patients with aortic stenosis, GLS progressively worsens with increasing disease severity (from −18.2±2.1% in mild to −13.3±3.7% in severe aortic stenosis) (Fig. 2) (32). Longitudinal strain has been shown to be a strong predictor of all-cause mortality independent of stenosis severity and EF or other known predictors (Fig. 3a) (32–35). Additionally, in symptomatic severe aortic stenosis, either high-gradient or low-flow, low-gradient, preoperative GLS was associated with long-term postoperative cardiac mortality and morbidity in all therapeutic approaches in either surgical or transcatheter aortic valve replacement (3, 36).
Figure 2.
Global longitudinal strain (GLS) in patients with various degrees of aortic stenosis (AS) and preserved ejection fraction. (a) Patient with mild AS, (b) patient with moderate AS, and (c) patient with severe AS. Upper panels: recordings demonstrated maximal velocity and calculated aortic valve area. Lower panels: demonstrated bull’s eyes of peak systolic strain and value of the GLS for corresponding patients
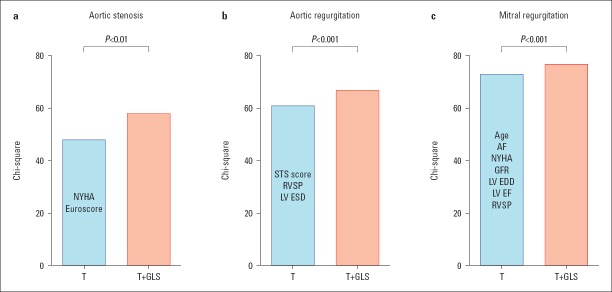
Figure 3.
Incremental prognostic value of left ventricular global longitudinal strain (GLS) to traditional risk factors (T) in predicting mortality in valve disease. (a) Aortic stenosis: traditional risk variables are New York Heart Association (NYHA) classification and additive EuroSCORE. Data from Kusonose et al. (33) (b) Aortic regurgitation: traditional risk variables are Society of Thoracic Surgeons score, right ventricular systolic pressure (RVSP), and indexed left ventricular end-systolic diameter (LV ESD). Data from Alashi et al. (4) (c) Mitral regurgitation: traditional risk variables are age, atrial fibrillation (AF), NYHA classification, estimated glomerular filtration rate, left ventricular end-diastolic diameter (LV EDD), left ventricular ejection fraction (LV EF), and right ventricular systolic pressure (RVSP). Data from Hiemstra et al. (42)
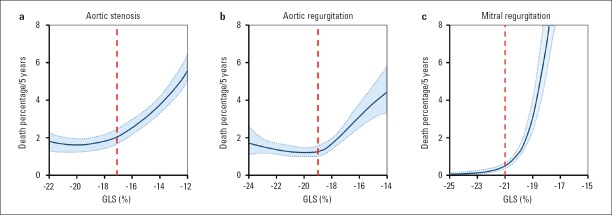
In asymptomatic severe aortic stenosis and normal EF, a GLS worse than −18.2% has been shown to be associated with disease progression, evidenced by symptoms onset and need for valve intervention (22). Similarly, others have shown that a GLS better than −17% is associated with excellent 5-year event-free survival (Fig. 4a) (35). In a recent meta-analysis including 1067 patients, a GLS worse than −14.7% predicted occurrence of death with 60% sensitivity and 70% specificity and was associated with a >2.5-fold increased risk of death (37). Current studies suggest that STE might identify asymptomatic patients with severe aortic stenosis who would benefit from earlier intervention than recommended in recent ESC/EACTS guidelines (2). Interestingly, the Heart Valve Clinic International Database (HAVEC) group recommended to incorporate GLS into therapeutic decision-making (38). Thus, patients with asymptomatic severe aortic stenosis with GLS worse than −16.0% and other high-risk factors (high-calcium score on cardiac computer tomography and myocardial fibrosis detected by magnetic resonance) may be considered for intervention. A similar algorithm was recently proposed by Dahl et al. (39).
Figure 4.
Normogram of estimated risk of death at 5 years for left ventricular global longitudinal strain (GLS) in valve diseases: (a) Aortic stenosis, (b) aortic regurgitation, and (c) mitral regurgitation. Solid blue line represents the 5-year parametric estimates of instantaneous risk of death, respectively, enclosed by 68% confidence interval (shaded area). The GLS value where the risk of death continuously increased is marked in every group by red dashed line. Data from Huded et al. (35), Alashi et al. (4), and Mentias et al. (30)
Aortic regurgitation
Patients with symptomatic moderate-to-severe and severe aortic regurgitation have more impaired GLS than those without symptoms (−14.9±3.0% vs. −16.8±2.5%) (23). In asymptomatic patients, the risk of death continuously increases as GLS worsens beyond −19% (Fig. 4b) (4). In the same study, GLS significantly improved the reclassification of mortality risk (Fig. 3b) and was independently associated with the need for aortic valve surgery in asymptomatic patients with moderately severe or severe aortic regurgitation and preserved EF (4, 40). In patients undergoing aortic valve surgery, preoperative GLS worse than −19% was associated with reduced long-term survival after surgical procedure (41).
Mitral regurgitation
In patients with severe primary mitral regurgitation undergoing interventions, preoperative GLS is an independent predictor of cardiovascular events and death and appears to have incremental predictive value over conventional clinical and echocardiographic risk factors (Fig. 3c) (5, 30, 42, 43). One of the largest studies revealed that mitral valve surgery in patients with asymptomatic severe mitral regurgitation was beneficial with respect to outcome (all-cause mortality), particularly in those with GLS worse than –21% (Fig. 4c) (30). In addition, preoperative GLS worse than -18.1% predicted postprocedural LV dysfunction in patients undergoing mitral valve surgery (5). Interestingly, the GLS reported in these studies was higher than what is considered the lower limit of normal, which indicates that strain values that are otherwise considered normal are already associated with impaired outcome in patients with primary mitral regurgitation.
Large studies evaluating the clinical utility of strain in secondary mitral regurgitation are limited. Recently, a retrospective analysis of 650 patients with moderate and severe secondary mitral regurgitation demonstrated that GLS worse than −7.0% was associated with increased risk for all-cause mortality, whereas LVEF was not (44). The lower GLS found in this study compared to those with primary regurgitation may be explained with specific high-risk patient population that was investigated. The study population mostly comprised of patients with advanced heart failure with a mean EF of 29±10%, half of which had an ischemic cardiomyopathy.
Mitral stenosis and right-sided valve diseases
Studies evaluating clinical application of strain echocardiography in mitral stenosis or right-sided valve diseases are small and limited.
Patients with severe mitral stenosis have reduced LV deformation which is related to the severity of mitral stenosis (45, 46). Improvement in strain values was detected within 72 hours after balloon mitral valvuloplasty.
The potential prognostic value of right ventricular (RV) longitudinal strain was found in patients with significant tricuspid regurgitation (47). Impaired RV free wall longitudinal strain (worse than −23%) was associated with worse outcome with a prognostic value beyond conventional echocardiographic parameters of RV systolic function.
In patients with pulmonary valve disease undergoing percutaneous pulmonary valve implantation, preinterventional RV longitudinal strain can predict improvement of exercise function after intervention (48).
Future Perspectives
An ever-growing evidence leaves no doubts about the future importance of strain imaging in valvular heart diseases. It has been convincingly shown that longitudinal strain has an independent and incremental prognostic value to standard echocardiographic and clinical parameters. However, some open issues need to be discussed before it is widely implemented in clinical practice.
First, the variability of proposed GLS cutoffs in predicting clinical outcome might be one of the main limitations for incorporating strain measurement into clinical guidelines and decision-making algorithms. Cutoff values vary significantly between different valvular heart diseases (Table 1), suggesting that underling pathology and disease`s specific alterations in chamber geometry and loading play a role. Therefore, disease-specific cutoffs need to be defined. As a rule of thumb, a strain value worse than normal is associated with worse outcome (Fig. 4). In addition, some variability may also be attributed to technical reasons, such as intervendor variability and strain definitions. Furthermore, different studies define clinical endpoints and follow-up periods differently.
Second, recent studies showed the usefulness of strain echocardiography for risk stratification of asymptomatic patients with severe valve disease, suggesting that GLS could be an important complementary parameter for patient management decisions toward earlier interventions. However, these results arise from retrospective or prospective observational cohort studies. Hence, large randomized trials are needed to analyze the effective benefit of GLS and early interventions in asymptomatic patients with valve diseases.
Finally, given the load dependence of strain measurements, a load-independent marker of systolic function is still needed. The new concept of myocardial work, which integrates deformation assessed by STE with afterload information estimated by LV pressure, potentially offers a solution (49). The recently proposed LV stress–strain loop areas as an index of myocardial work integrates in addition information on wall thickness and radius of curvature and uses so an estimate of wall stress rather than pressure (50). The concepts may be of particular interest in conditions where loading and geometry are altered, as it is often the case in valvular heart disease.
Conclusion
Strain echocardiography in valvular heart disease has demonstrated to be a useful complementary echocardiographic method that can identify patients at risk of developing symptoms or poor survival and might assist in therapeutic decision-making. However, a profound understanding of the complex interaction between loading conditions, chamber geometry, and contractility is necessary for the correct interpretation of myocardial deformation in order to draw appropriate conclusions in patients with valve disease.
Footnotes
Conflict of interest: Relationship with Industry and Financial Associations; M.C. was supported by a research grant from the European Association of Cardiovascular Imaging. J.U.V. holds a personal research mandate of the Flemish Research Council (FWO). He also received research support/consultancy fees from GE, Hitachi, Philips, and Siemens.
Peer-review: Internally peer-reviewed.
Authorship contributions: Concept – M.C., J.U.V.; Design – M.C., J.U.V.; Supervision – J.U.V.; Funding – N/A; Materials – N/A; Data collection and/or processing – N/A; Analysis and/or interpretation – M.C., J.U.V.; Literature search – M.C., J.U.V.; Writing – M.C., J.U.V.; Critical review – J.U.V.
References
- 1.Kodali SK, Velagapudi P, Hahn RT, Abbott D, Leon MB. Valvular Heart Disease in Patients ≥80 Years of Age. J Am Coll Cardiol. 2018;71:2058–72. doi: 10.1016/j.jacc.2018.03.459. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. ESC Scientific Document Group 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 3.Dahl JS, Videbæk L, Poulsen MK, Rudbæk TR, Pellikka PA, Møller JE. Global strain in severe aortic valve stenosis relation to clinical outcome after aortic valve replacement. Circ Cardiovasc Imaging. 2012;5:613–20. doi: 10.1161/CIRCIMAGING.112.973834. [DOI] [PubMed] [Google Scholar]
- 4.Alashi A, Mentias A, Abdallah A, Feng K, Gillinov AM, Rodriguez LL, et al. Incremental Prognostic Utility of Left Ventricular Global Longitudinal Strain in Asymptomatic Patients With Significant Chronic Aortic Regurgitation and Preserved Left Ventricular Ejection Fraction. JACC Cardiovasc Imaging. 2018;11:673–82. doi: 10.1016/j.jcmg.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Kim HM, Cho GY, Hwang IC, Choi HM, Park JB, Yoon YE, et al. Myocardial Strain in Prediction of Outcomes After Surgery for Severe Mitral Regurgitation. JACC Cardiovasc Imaging. 2018;11:1235–44. doi: 10.1016/j.jcmg.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics:ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr. 2011;12:167–205. doi: 10.1093/ejechocard/jer021. [DOI] [PubMed] [Google Scholar]
- 7.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, et al. Definitions for a common standard for 2D speckle tracking echocardiography:consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2015;16:1–11. doi: 10.1093/ehjci/jeu184. [DOI] [PubMed] [Google Scholar]
- 8.Voigt JU, Cvijic M. 2- and 3-Dimensional Myocardial Strain in Cardiac Health and Disease. JACC Cardiovasc Imaging. 2019;12:1849–63. doi: 10.1016/j.jcmg.2019.01.044. [DOI] [PubMed] [Google Scholar]
- 9.Yingchoncharoen T, Agarwal S, Popović ZB, Marwick TH. Normal ranges of left ventricular strain:A meta-analysis. J Am Soc Echocardiogr. 2013;26:185–91. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Farsalinos KE, Daraban AM, Ünlü S, Thomas JD, Badano LP, Voigt JU. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors:The EACVI/ASE Inter-Vendor Comparison Study. J Am Soc Echocardiogr. 2015;28:1171–81. doi: 10.1016/j.echo.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults:an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–70. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 12.Mirea O, Pagourelias ED, Duchenne J, Bogaert J, Thomas JD, Badano LP, et al. EACVI-ASE-Industry Standardization Task Force. Variability and Reproducibility of Segmental Longitudinal Strain Measurement:A Report From the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc Imaging. 2018;11:15–24. doi: 10.1016/j.jcmg.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Negishi K, Negishi T, Kurosawa K, Hristova K, Popescu BA, Vinereanu D, et al. Practical guidance in echocardiographic assessment of global longitudinal strain. JACC Cardiovasc Imaging. 2015;8:489–92. doi: 10.1016/j.jcmg.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Marwick TH, Fukuda N, Oe H, Saito M, Thomas JD, et al. Improvement in Strain Concordance between Two Major Vendors after the Strain Standardization Initiative. J Am Soc Echocardiogr. 2015;28:642–8. doi: 10.1016/j.echo.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Ünlü S, Mirea O, Duchenne J, Pagourelias ED, Bézy S, Thomas JD, et al. Comparison of Feasibility, Accuracy, and Reproducibility of Layer-Specific Global Longitudinal Strain Measurements Among Five Different Vendors:A Report from the EACVI-ASE Strain Standardization Task Force. J Am Soc Echocardiogr. 2018;31:374–80. doi: 10.1016/j.echo.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Dobrovie M, Bėzy S, Ünlü S, Chakraborty B, Petrescu A, Duchenne J, et al. How Does Regional Hypertrophy Affect Strain Measurements With Different Speckle-Tracking Methods? J Am Soc Echocardiogr. 2019;32:1444–50. doi: 10.1016/j.echo.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Ünlü S, Duchenne J, Mirea O, Pagourelias ED, Bézy S, Cvijic M, et al. Impact of apical foreshortening on deformation measurements:a report from the EACVI-ASE Strain Standardization Task Force. Eur Hear J Cardiovasc Imaging. 2020;21:337–43. doi: 10.1093/ehjci/jez189. [DOI] [PubMed] [Google Scholar]
- 18.Marciniak A, Sutherland GR, Marciniak M, Claus P, Bijnens B, Jahangiri M. Myocardial deformation abnormalities in patients with aortic regurgitation:a strain rate imaging study. Eur J Echocardiogr. 2009;10:112–9. doi: 10.1093/ejechocard/jen185. [DOI] [PubMed] [Google Scholar]
- 19.Marciniak A, Claus P, Sutherland GR, Marciniak M, Karu T, Baltabaeva A, et al. Changes in systolic left ventricular function in isolated mitral regurgitation A strain rate imaging study. Eur Heart J. 2007;28:2627–36. doi: 10.1093/eurheartj/ehm072. [DOI] [PubMed] [Google Scholar]
- 20.Stokke TM, Hasselberg NE, Smedsrud MK, Sarvari SI, Haugaa KH, Smiseth OA, et al. Geometry as a Confounder When Assessing Ventricular Systolic Function:Comparison Between Ejection Fraction and Strain. J Am Coll Cardiol. 2017;70:942–54. doi: 10.1016/j.jacc.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 21.Smedsrud MK, Pettersen E, Gjesdal O, Svennevig JL, Andersen K, Ihlen H, et al. Detection of left ventricular dysfunction by global longitudinal systolic strain in patients with chronic aortic regurgitation. J Am Soc Echocardiogr. 2011;24:1253–9. doi: 10.1016/j.echo.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Vollema EM, Sugimoto T, Shen M, Tastet L, Ng AC, Abou R, et al. Association of Left Ventricular Global Longitudinal Strain With Asymptomatic Severe Aortic Stenosis:Natural Course and Prognostic Value. JAMA Cardiol. 2018;3:839–47. doi: 10.1001/jamacardio.2018.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ewe SH, Haeck ML, Ng AC, Witkowski TG, Auger D, Leong DP, et al. Detection of subtle left ventricular systolic dysfunction in patients with significant aortic regurgitation and preserved left ventricular ejection fraction:speckle tracking echocardiographic analysis. Eur Heart J Cardiovasc Imaging. 2015;16:992–9. doi: 10.1093/ehjci/jev019. [DOI] [PubMed] [Google Scholar]
- 24.Ferferieva V, Van den Bergh A, Claus P, Jasaityte R, Veulemans P, Pellens M, et al. The relative value of strain and strain rate for defining intrinsic myocardial function. Am J Physiol Heart Circ Physiol. 2012;302:H188–95. doi: 10.1152/ajpheart.00429.2011. [DOI] [PubMed] [Google Scholar]
- 25.Donal E, Bergerot C, Thibault H, Ernande L, Loufoua J, Augeul L, et al. Influence of afterload on left ventricular radial and longitudinal systolic functions:a two-dimensional strain imaging study. Eur J Echocardiogr. 2009;10:914–21. doi: 10.1093/ejechocard/jep095. [DOI] [PubMed] [Google Scholar]
- 26.Dahle GO, Stangeland L, Moen CA, Salminen PR, Haaverstad R, Matre K, et al. The influence of acute unloading on left ventricular strain and strain rate by speckle tracking echocardiography in a porcine model. Am J Physiol Heart Circ Physiol. 2016;310:H1330–9. doi: 10.1152/ajpheart.00947.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reant P, Metras A, Detaille D, Reynaud A, Diolez P, Jaspard-Vinassa B, et al. Impact of Afterload Increase on Left Ventricular Myocardial Deformation Indices. J Am Soc Echocardiogr. 2016;29:1217–28. doi: 10.1016/j.echo.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Bauer F, Eltchaninoff H, Tron C, Lesault PF, Agatiello C, Nercolini D, et al. Acute improvement in global and regional left ventricular systolic function after percutaneous heart valve implantation in patients with symptomatic aortic stenosis. Circulation. 2004;110:1473–6. doi: 10.1161/01.CIR.0000134961.36773.D6. [DOI] [PubMed] [Google Scholar]
- 29.Negishi K, Borowski AG, Popović ZB, Greenberg NL, Martin DS, Bungo MW, et al. Effect of Gravitational Gradients on Cardiac Filling and Performance. J Am Soc Echocardiogr. 2017;30:1180–8. doi: 10.1016/j.echo.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Mentias A, Naji P, Gillinov AM, Rodriguez LL, Reed G, Mihaljevic T, et al. Strain Echocardiography and Functional Capacity in Asymptomatic Primary Mitral Regurgitation With Preserved Ejection Fraction. J Am Coll Cardiol. 2016;68:1974–86. doi: 10.1016/j.jacc.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Slimani A, Melchior J, de Meester C, Pierard S, Roy C, Amzulescu M, et al. Relative Contribution of Afterload and Interstitial Fibrosis to Myocardial Function in Severe Aortic Stenosis. JACC Cardiovasc Imaging. 2020;13:589–600. doi: 10.1016/j.jcmg.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 32.Ng ACT, Prihadi EA, Antoni ML, Bertini M, Ewe SH, Ajmone Marsan N, et al. Left ventricular global longitudinal strain is predictive of all-cause mortality independent of aortic stenosis severity and ejection fraction. Eur Heart J Cardiovasc Imaging. 2018;19:859–67. doi: 10.1093/ehjci/jex189. [DOI] [PubMed] [Google Scholar]
- 33.Kusunose K, Goodman A, Parikh R, Barr T, Agarwal S, Popovic ZB, et al. Incremental prognostic value of left ventricular global longitudinal strain in patients with aortic stenosis and preserved ejection fraction. Circ Cardiovasc Imaging. 2014;7:938–45. doi: 10.1161/CIRCIMAGING.114.002041. [DOI] [PubMed] [Google Scholar]
- 34.Salaun E, Casalta AC, Donal E, Bohbot Y, Galli E, Tribouilloy C, et al. Apical four-chamber longitudinal left ventricular strain in patients with aortic stenosis and preserved left ventricular ejection fraction:Analysis related with flow/gradient pattern and association with outcome. Eur Heart J Cardiovasc Imaging. 2018;19:868–78. doi: 10.1093/ehjci/jex203. [DOI] [PubMed] [Google Scholar]
- 35.Huded CP, Masri A, Kusunose K, Goodman AL, Grimm RA, Gillinov AM, et al. Outcomes in Asymptomatic Severe Aortic Stenosis With Preserved Ejection Fraction Undergoing Rest and Treadmill Stress Echocardiography. J Am Heart Assoc. 2018;7:pii, e007880. doi: 10.1161/JAHA.117.007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D'Andrea A, Carbone A, Agricola E, Riegler L, Sperlongano S, Tocci G, et al. Predictive Value of Left Ventricular Myocardial Deformation for Left Ventricular Remodeling in Patients With Classical Low-Flow, Low-Gradient Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement. J Am Soc Echocardiogr. 2019;32:730–6. doi: 10.1016/j.echo.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Magne J, Cosyns B, Popescu BA, Carstensen HG, Dahl J, Desai MY, et al. Distribution and Prognostic Significance of Left Ventricular Global Longitudinal Strain in Asymptomatic Significant Aortic Stenosis:An Individual Participant Data Meta-Analysis. JACC Cardiovasc Imaging. 2019;12:84–92. doi: 10.1016/j.jcmg.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Dulgheru R, Pibarot P, Sengupta PP, Piérard LA, Rosenhek R, Magne J, et al. Multimodality Imaging Strategies for the Assessment of Aortic Stenosis:Viewpoint of the Heart Valve Clinic International Database (HAVEC) Group. Circ Cardiovasc Imaging. 2016;9:e004352. doi: 10.1161/CIRCIMAGING.115.004352. [DOI] [PubMed] [Google Scholar]
- 39.Dahl JS, Magne J, Pellikka PA, Donal E, Marwick TH. Assessment of Subclinical Left Ventricular Dysfunction in Aortic Stenosis. JACC Cardiovasc Imaging. 2019;12:163–71. doi: 10.1016/j.jcmg.2018.08.040. [DOI] [PubMed] [Google Scholar]
- 40.Kusunose K, Agarwal S, Marwick TH, Griffin BP, Popović ZB. Decision making in asymptomatic aortic regurgitation in the era of guidelines incremental values of resting and exercise cardiac dysfunction. Circ Cardiovasc Imaging. 2014;7:352–62. doi: 10.1161/CIRCIMAGING.113.001177. [DOI] [PubMed] [Google Scholar]
- 41.Alashi A, Khullar T, Mentias A, Gillinov AM, Roselli EE, Svensson LG, et al. Long-Term Outcomes After Aortic Valve Surgery in Patients With Asymptomatic Chronic Aortic Regurgitation and Preserved LVEF:Impact of Baseline and Follow-Up Global Longitudinal Strain. JACC Cardiovasc Imaging. 2020;13:12–21. doi: 10.1016/j.jcmg.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 42.Hiemstra YL, Tomsic A, van Wijngaarden SE, Palmen M, Klautz RJM, Bax JJ, et al. Prognostic Value of Global Longitudinal Strain and Etiology After Surgery for Primary Mitral Regurgitation. JACC Cardiovasc Imaging. 2020;13:577–85. doi: 10.1016/j.jcmg.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 43.Alashi A, Mentias A, Patel K, Gillinov AM, Sabik JF, Popović ZB, et al. Synergistic Utility of Brain Natriuretic Peptide and Left Ventricular Global Longitudinal Strain in Asymptomatic Patients With Significant Primary Mitral Regurgitation and Preserved Systolic Function Undergoing Mitral Valve Surgery. Circ Cardiovasc Imaging. 2016;9:pii:e004451. doi: 10.1161/CIRCIMAGING.115.004451. [DOI] [PubMed] [Google Scholar]
- 44.Namazi F, van der Bijl P, Hirasawa K, Kamperidis V, van Wijngaarden SE, Mertens B, et al. Prognostic Value of Left Ventricular Global Longitudinal Strain in Patients With Secondary Mitral Regurgitation. J Am Coll Cardiol. 2020;75:750–8. doi: 10.1016/j.jacc.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 45.Sengupta SP, Amaki M, Bansal M, Fulwani M, Washimkar S, Hofstra L, et al. Effects of percutaneous balloon mitral valvuloplasty on left ventricular deformation in patients with isolated severe mitral stenosis:a speckle-tracking strain echocardiographic study. J Am Soc Echocardiogr. 2014;27:639–47. doi: 10.1016/j.echo.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 46.Roushdy AM, Raafat SS, Shams KA, El-Sayed MH. Immediate and short-term effect of balloon mitral valvuloplasty on global and regional biventricular function :a two-dimensional strain echocardiographic study. Eur Heart J Cardiovasc Imaging. 2016;17:316–25. doi: 10.1093/ehjci/jev157. [DOI] [PubMed] [Google Scholar]
- 47.Prihadi EA, van der Bijl P, Dietz M, Abou R, Vollema EM, Marsan NA, et al. Prognostic Implications of Right Ventricular Free Wall Longitudinal Strain in Patients With Significant Functional Tricuspid Regurgitation. Circ Cardiovasc Imaging. 2019;12:e00⇚. doi: 10.1161/CIRCIMAGING.118.008666. [DOI] [PubMed] [Google Scholar]
- 48.Chowdhury SM, Hijazi ZM, Fahey JT, Rhodes JF, Kar S, Makkar R, et al. Speckle-Tracking Echocardiographic Measures of Right Ventricular Function Correlate With Improvement in Exercise Function After Percutaneous Pulmonary Valve Implantation. J Am Soc Echocardiogr. 2015;28:1036–44. doi: 10.1016/j.echo.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russell K, Eriksen M, Aaberge L, Wilhelmsen N, Skulstad H, Remme EW, et al. A novel clinical method for quantification of regional left ventricular pressurestrain loop area:A non-invasive index of myocardial work. Eur Heart J. 2012;33:724–33. doi: 10.1093/eurheartj/ehs016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cvijic M, Duchenne J, Ünlü S, Michalski B, Aarones M, Winter S, et al. Timing of myocardial shortening determines left ventricular regional myocardial work and regional remodelling in hearts with conduction delays. Eur Heart J Cardiovasc Imaging. 2018;19:941–9. doi: 10.1093/ehjci/jex325. [DOI] [PubMed] [Google Scholar]
- 51.Witkowski TG, Thomas JD, Debonnaire PJ, Delgado V, Hoke U, Ewe SH, et al. Global longitudinal strain predicts left ventricular dysfunction after mitral valve repair. Eur Heart J Cardiovasc Imaging. 2013;14:69–76. doi: 10.1093/ehjci/jes155. [DOI] [PubMed] [Google Scholar]