Abstract
Objective:
Transcatheter aortic valve implantation (TAVI) is an established alternative to surgical aortic valve replacement. Our study aimed to evaluate the implementation of TAVI at our large-volume center, having an all-comer patient population with short and long-term follow-ups.
Methods:
This retrospective, single-center analysis included 556 consecutive patients with symptomatic severe aortic stenosis (AS) who underwent TAVI between July 2011 and December 2019.
Results:
The mean age of the entire population was 77.6±7.9 years, and 54.9% were women. The Society of Thoracic Surgeons (STS) mean score of the cohort was 6.0%±3.5%. The balloon-expandable valve (Sapien XT, Sapien 3; Edwards Lifesciences, Irvine, California) was the most frequently used valves in this cohort (94.6%). Transfemoral access was used in 96.3% of patients. Implantation success was achieved in 96.6% of cases. During the TAVI procedure, 7.2% of patients required permanent pacemaker implantation, with 37.5% in the Lotus valve group needing the most permanent pacemakers. The mean length of hospital stay for the entire cohort was 4.5±2.3 days. Overall, 22 (3.9%) in-hospital deaths occurred before hospital discharge. The mean follow-up period was 15.1±14.9 months for all patients, and a significant improvement was noted in all echocardiographic parameters and functional capacity. Paravalvular leak (PVL) was documented in 18.9% patients, mild in 17.9%, and moderate in 1% at discharge. No cases with severe PVL, necessitating additional procedures. The multiple logistic regression analysis revealed that sex, STS score, baseline SYNTAX score, bicuspid valve morphology, common femoral artery diameter, and post-TAVI PVL were independent predictors of overall mortality.
Conclusion:
To our knowledge, this study, which is the largest single-center real-world experience of TAVI in Turkey, demonstrated low complication rates with favorable short- and mid-term THV performance in patients undergoing TAVI.
Keywords: TAVI, aortic stenosis, single-center, clinical outcomes
Introduction
Transcatheter aortic valve implantation (TAVI) is an established alternative to surgical aortic valve replacement (sAVR). It has become the new standard to treat symptomatic aortic stenosis (AS) in high- and intermediate risk patients (1). Since it was first performed in 2002, TAVI has gradually expanded with the development of transcatheter heart valve (THV), the transition to a minimalist approach, and the completion of the learning curve (2). Notably, the success of TAVI depends on the complete coordination of the heart team. Several studies have revealed that the experience of the center influences the outcomes, as well as operator training (3).
Several national registries [e.g., German registry (GARY), US Transcatheter Valve Therapy Registry (TVT) registry, UK TAVI registry, FRANCE TAVI registry (France-II) and Society of Thoracic Surgeons and the American College of Cardiology Transcatheter Valve Therapy Registry (STS/ACC TVT)] have documented the “real-world” use of TAVI and allowed us to obtain valuable data in terms of its applicability, availability, complications, and durability of the THV in this frail patient population (4-7). The most critical limitation of the registries is the patient selection from various centers; therefore, single-center studies have garnered significance. Because with the increase in the number of centers and operators, it becomes challenging to obtain homogenous data owing to non-uniformity.
Therefore, this study aimed to evaluate the implementation of TAVI at our large-volume center, having an all-comer patient population with short and mid-term follow-ups.
Methods
Patient population and preprocedural planning
This retrospective, single-center analysis included 556 consecutive patients with severe symptomatic AS [aortic valve area (AVA) <1.0 cm2 or mean gradient ≥40 mm Hg or maximum jet velocity ≥4.0 m/s, AVA index <0.6 cm2/m2] who underwent TAVI between July 2011 and December 2019. Treatment options were decided by a dedicated heart team consisting of experienced clinical and interventional cardiologists, cardiac imaging specialists, cardiovascular surgeons, and anesthesiologists. The baseline characteristics, laboratory and echocardiographic data, procedural data, and outcome data were retrospectively collected. Aortic root anatomy and peripheral access site of all patients were evaluated using a multimodality approach with multi-slice computed tomography (MSCT) and echocardiography. The aortic root anatomy was assessed in detail, including aortic annulus measurements (diameter, perimeter, and area), the valve morphology (bicuspid or tricuspid), calcification distribution and density, maximal sinus Valsalva diameter, sino-tubular junction diameter, and coronary take-offs from the hinge points. All patients underwent invasive coronary angiography to detect obstructive coronary artery disease (CAD) before the procedure. The presence of CAD was defined as the presence of one or more lesions >70% in the epicardial coronary arteries, as well as vessels with a diameter >1.5 mm (>50% for left main) (8). The surgical risk was calculated based on the standard STS and updated EuroSCORE II risk estimation tools (9, 10). The STS scores of 4%, 4–8%, and >8%, were stratified as low, intermediate, and high risk, respectively. “Low flow state” across the aortic valve was defined as an indexed stroke volume <35 ml/m2. Until 2017, classical low flow low gradient (LFLG) AS was described as a mean transvalvular gradient <40 mm Hg, effective orifice area (EOA) <1.0 cm2, and left ventricular ejection fraction (LVEF) <40%, as per the ESC valvular heart disease guidelines (1). After that, the new definition was used according to the current guidelines. Dobutamine stress echocardiography was used to assess stenosis severity in patients with LFLG-AS. Post-procedural follow-up was performed after 30 days and 12 months. The final data on survival was obtained in January 2020. Written informed consent was obtained from all patients before the procedure, and the present study was approved by the Ethics Committee of our hospital.
Procedural details
Patients were selected for the transfemoral (TF) approach or alternative methods according to the iliofemoral arterial size, calcification, and tortuosity. The type of THV was selected based on the primary operator’s preference. The following four types of aortic valves were available: Edwards Sapien XT, Sapien 3 valve (Edwards Lifesciences, Irvine, California), Lotus™ valve system (Boston Scientific, MA, USA), and ACURATE neo™ (Boston Scientific). Depending on the valve size, the adapted sized sheath was introduced into the access route. TAVI was performed in the first 74 patients under general anesthesia and transesophageal echocardiography (TEE) guidance. On the other hand, in the subsequent patients, TAVI was performed using a conventional minimalist approach under conscious sedation without TEE. Hemostasis was achieved using the percutaneous closure devices Prostar XL (Abbott Vascular, Santa Clara, CA, USA) or Perclose ProGlide 6Fr suture devices (Abbott Vascular). Once the sheath was removed, peripheral angiography was performed to evaluate the access site patency. Surgical cut-down was performed in transfemoral procedures that were not amenable to percutaneous closure (e.g., in the presence of anterior calcification). In some technically difficult cases, the safety wire in the iliac or femoral artery was replaced, or micropuncture needles were used to identify the optimal site. After a successful TAVI procedure, patients were placed on dual antiplatelet therapy consisting of clopidogrel 75 mg and aspirin 100 mg for 3 to 6 months. Individualized antiplatelet treatment was provided considering the risk of bleeding in patients with atrial fibrillation.
In-hospital death, 30-day and 1-year all-cause mortality, stroke, vascular complications, bleeding complications, acute kidney injury, device success, and adverse events were defined based on the consensus document of the Valve Academic Research Consortium-2 (VARC-2) (11). Death was verified through hospital records and family contacts. Details regarding paravalvular leakage (PVL) after TAVI, pacemaker implantation, and new-onset atrial fibrillation (AF) were also collected retrospectively during the index hospitalization.
Statistical analysis
Data were analyzed using SPSS version 23.0 (SPSS Inc., Chicago, Illinois, United States). All data were presented as the mean±standard deviation (SD) for parametric variables, as the median (interquartile range) for nonparametric variables, and as percentages for categorical variables. Continuous variables were checked for normal distribution assumptions by using the Kolmogorov-Smirnov test. Categorical variables were presented as frequencies and percentages and compared using Pearson’s, Fisher’s exact, and the chi-square tests. One-way repeated-measures ANOVA was used for normally distributed continuous variables, and the Friedman test was used for non-normally distributed variables. Stepwise multiple logistic regression analysis was performed to investigate the relationship between mortality and sex, STS, baseline SYNTAX score (bSS), bicuspid, common femoral artery (CFA) diameter, and post-TAVI PVL in the study population. A two-tailed p value of <0.05 was considered statistically significant.
Results
Between July 2011 and December 2019, 556 patients underwent TAVI in our tertiary care center. The number of TAVI procedures progressively increased over time, as seen in Figure 1. The mean age of the entire population was 77.6±7.9 years, 305 (54.9%) were women, and 164 (29.7%) were diabetic. All patients were symptomatic, with 396 (71.7%) in class III-IV, and 148 (26.1%) in class II per the New York Heart Association (NYHA) functional class. Among the study patients, 458 (83%) were hypertensive, 381 (44.5%) had a known history of CAD, 43 (7.8%) had a known history of peripheral artery disease, and 24 (4.4%) had a history of valve surgery. The mean STS score of the cohort was 6.0%±3.5%, with most patients in the co classified as intermediate risk (STS score <4%, n=180; 4%–8%, n=237; >8%, n=139). Before the TAVI procedure, 133 (24%) patients had a diagnosis of permanent AF, 33 (6%) patients had a previous stroke, and 13 (2.4%) patients were undergoing renal replacement therapy. Baseline echocardiography parameters were logical with severe aortic valve stenosis with a mean AVA of 0.67±0.16 cm2, mean aortic valve gradient of 50.4±15 mm Hg, and 69 (12.7%) had moderate-to-severe mitral regurgitation (MR). Moreover, 4.4% of patients had both AS and moderate-to-severe aortic regurgitation. In 71 (13.2%) patients, the aortic valve had a bicuspid morphology. Furthermore, 33 (6.6%) patients were diagnosed with LFLG-AS according to the criteria stated earlier, and 7 (1.3%) patients with paradoxical LFLG-AS. Among the patients undergoing MSCT, the mean diameter of the aortic annulus was 24.6±2.4 mm, the mean aortic annulus perimeter was 77.4±7.5 mm, and the mean aortic annulus area was 481.9±95.9 mm2. The mean CFA size was 7.5±1.1 mm. Table 1 demonstrates the baseline clinical characteristics of patients.
Figure 1.
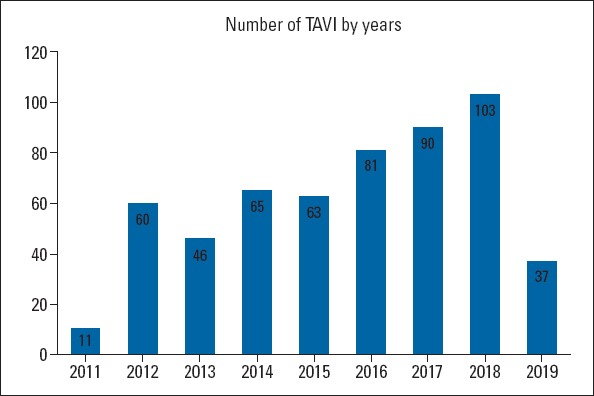
Number of TAVI procedures
Table 1.
Baseline clinical characteristics of patients
| Parameters | All Patients (n=556) |
|---|---|
| Age (years) | 77.6±7.9 |
| Female n (%) | 305 (54.9) |
| BMI (kg/m2) | 27.7±6.1 |
| NYHA n (%) | |
| 2 | 148 (26.1) |
| 3 | 313 (56.7) |
| 4 | 83 (15.0) |
| Pulmonary edema | 12 (2.2) |
| DM n (%) | 164 (29.7) |
| HT n (%) | 458 (83.0) |
| HL n (%) | 277 (50.2) |
| Previous PCI n (%) | 115 (20.9) |
| Previous CABG n (%) | 130 (23.6) |
| Previous valve replacement n (%) | |
| AVR | 7 (1.3) |
| MVR | 14 (2.5) |
| Mitral Ring | 2 (0.4) |
| Mitral Commissurotomy | 1 (0.2) |
| Previous MI n (%) | 66 (12.0) |
| Peripheral artery disease n (%) | 43 (7.8) |
| Moderate-to-severe COPD n (%) | 235 (42.4) |
| AF n (%) | 133 (24.0) |
| Stroke n (%) | 33 (6.0) |
| STS score % | 6.0±3.5 |
| EuroSCORE I % | 13.7±4.9 |
| EuroSCORE II % | 9.0±5.7 |
| Logistic EuroSCORE % | 22.6±14.7 |
| CAD n (%) | |
| Obstructive | 134 (24.4) |
| Non-obstructive | 247 (43.8) |
| Normal | 175 (31.8) |
| CAD n (%) | |
| 1 vessel disease | 74 (56.1) |
| 2 vessel disease | 49 (37.1) |
| 3 vessel disease | 9 (6.8) |
| Chronic kidney disease n (%) | |
| Stage 1 | 66 (11.7) |
| Stage 2 | 266 (47.9) |
| Stage 3a | 115 (20.6) |
| Stage 3b | 87 (15.8) |
| Stage 4 | 32 (4.1) |
| Renal replacement therapy n (%) | 13 (2.4) |
| Serum glucose | 127.4±54.3 |
| Creatinine mg/dL | 1.06±0.5 |
| GFR ml/min/1.73 m2 | 65.1±20.5 |
| Hemoglobin mg/dL | 11.6±1.9 |
| Troponin | 83.6±111.6 |
| CK-MB | 4.4±10.7 |
| Bicuspid n (%) | 71 (13.2) |
| Aortic stenosis classification n (%) | |
| HG-AS | 392 (70.6) |
| LFLG-AS | 33 (6.4) |
| Paradoxical LFLG-AS | 7 (1.3) |
| VS-AS | 124 (21.8) |
| LVEF (%) | 51.7±14.0 |
| LVEDD (cm) | 4.7±0.6 |
| LVESD (cm) | 3.1±0.8 |
| LA (cm) | 4.6±0.6 |
| LVH (%) | 84.3 |
| Aortic velocity | 4.4±0.6 |
| Aortic max gradient (mm Hg) | 81.9±23.0 |
| Aortic mean gradient (mm Hg) | 50.4±15.1 |
| AVA (cm2) | 0.67±0.16 |
| AVA index (cm2/BSA) | 0.37±0.09 |
| sPAP (mm Hg) | 44.1±16.9 |
| Aortic regurgitation-moderate-to-severe (%) | 4.4 |
| Mitral regurgitation-moderate-to-severe (%) | 12.7 |
| TTE or TEE mean aortic annulus (cm) | 2.15±0.20 |
| MSCT mean aortic annulus (mm) | 24.6±2.4 |
| MSCT mean aortic annulus area (mm2) | 481.9±95.9 |
| MSCT mean aortic annulus perimeter (mm) | 77.4±7.5 |
| MSCT mean aortic sinus valsalva (mm) | 31.6±3.7 |
| MSCT mean ascending aorta (mm) | 36.4±4.6 |
| Aortic annulus-LMCA distance (cm) | 13.5±2.2 |
| Aortic annulus-RCA distance (cm) | 14.2±2.2 |
| Mean CFA size (mm) | 7.5±1.1 |
BMI - body mass index; NYHA - New York Heart Association; DM - diabetes mellitus; HT - hypertension; PCI - percutaneous coronary intervention; CABG - coronary artery bypass grafting; AVR - aortic valve replacement; MVR - mitral valve replacement;
MI - myocardial infarction; COPD - chronic obstructive pulmonary disease;
AF - atrial fibrillation; STS - Society of Thoracic Surgeons; CAD - coronary artery disease; GFR - glomerular filtration rate; HG-AS - high gradient-aortic stenosis;
LFLG - low flow low gradient; VS - very severe; LVEF - left ventricular ejection fraction; LVEDD- left ventricular end diastolic diameter; LVESD - left ventricular end systolic diameter; LA- left atrium; LVH - left ventricular hypertrophy; AVA - aortic valve area; sPAP - systolic pulmonary artery pressure; TTE - transthoracic echocardiography;
TEE - transesophageal echocardiography; MSCT - multi-slice computed tomography;
LMCA - left main coronary artery; RCA - right coronary artery; CFA - common femoral artery
Procedural outcomes
Of the 556 patients, 526 (96.3%) underwent TF-TAVI, 20 (3.7%) underwent transaxillary TAVI, and one patient underwent transaortic TAVI. The balloon-expandable THVs (Sapien XT, Sapien 3) (Edwards Lifesciences, Irvine, California) was the most frequently used valves in this cohort (94.6%). The other THVs used were mechanically-expandable Lotus and self-expandable ACURATE neo valves (4.3% vs. 1.1%, respectively). In most patients (63.5%) who underwent percutaneous closure with ProGlide, 23 mm and 26 mm THVs were frequently used (41.9% vs. 41.0%, respectively). Most patients underwent TAVI with an indication of native senile aortic valve degeneration, and only 7 patients (1.2%) had valve-in-valve (ViV) TAVI. Per the VARC-2, device implantation success was achieved in 96.6% of cases. Notably, the success rate of Sapien 3 and ACURATE neo valve implantation was 100%, and the lowest device success rate was noted in patients who had Lotus valve implantation (Table 2).
Table 2.
Procedural details
| Access site n (%) | |
| Transaxillary | 20 (3.7) |
| Transfemoral | 536 (96.3) |
| Cut-down | 31 (5.7) |
| Prostar | 183 (33.0) |
| ProGlide | 322 (61.3) |
| Valve size mm n (%) | |
| 20 | 2 (0.4) |
| 23 | 233 (41.9) |
| 25 | 14 (2.5) |
| 26 | 228 (41.0) |
| 27 | 6 (1.1) |
| 29 | 73 (13.2) |
| SAPIEN XT n (%) | 480 (86.3) |
| Edwards SAPIEN 3 n (%) | 46 (8.3) |
| LOTUS n (%) | 24 (4.3) |
| ACURATE neo n (%) | 6 (1.1) |
| Valve-in-valve n (%) | 7 (1.2) |
| Pre-dilatation n (%) | 396 (71.3) |
| Post-dilatation n (%) | 19 (3.5) |
| Device success n (%) | 537 (96.6) |
| SAPIEN XT | 459 (96.8) |
| Edwards SAPIEN 3 | 46 (100.0) |
| LOTUS | 21 (87.5) |
| ACURATE neo | 6 (100.0) |
Procedural complications and outcomes are described in Table 3. During the TAVI procedure, overall, 40 patients (7.2%) required permanent pacemaker (PPM) implantation, with 9 (37.5%) of patients in the Lotus valve group needing PPM the most. Three patients (0.7%) developed a disabling stroke within 24 hours of the procedure. However, no incidences of a transient ischemic attack were noted. Emergent pericardiocentesis was required in five patients (0.9%), in four of them for treatment of perforation related to the temporary pacemaker lead in the right ventricle and one of them for perforation related to wire manipulations in the left ventricle. The most common in-hospital arrhythmia was AF observed in 20 patients (3.6%), followed by left bundle branch block in 14 (2.5%) and ventricular tachycardia in 3 patients (0.5%). According to VARC 2 criteria, 2 patients (0.4%) developed acute renal failure, 33 (6%) developed major vascular complications, and 4 (0.7%) developed major bleeding. Two patients developed coronary obstruction because of the displacement of the calcification from the native valve, thereby obstructing the coronary ostium. One of them was treated with the percutaneous coronary intervention (PCI), whereas the other patient died. Annular rupture occurred in one patient during valve implantation with 23 mm Sapien XT THV. When this patient was examined in detail, the most logical explanation was that she had severe AS with an aortic velocity of 7 cm/sn and a mean gradient of 147 mm Hg. Overall, three patients had device embolizations during the TAVI procedure. Of these three patients, two had TAVI devices embolized in the left ventricle, and both underwent emergency surgery; however, one of them died. The third patient had TAVI embolized in the aorta and consequently underwent deployment of another THV in the appropriate location. The mean length of hospital stay for the entire cohort was 4.5±2.3 days. Overall, 22 (3.9%) in-hospital deaths were observed before hospital discharge, with 3 of these occurring in the catheter laboratory because of annular rupture and cardiac tamponade.
Table 3.
Procedure related complications and outcomes
| Pacemaker n (%) | 40 (7.2) |
| SAPIEN XT | 30 (6.3) |
| Edwards SAPIEN 3 | 1 (2.3) |
| LOTUS | 9 (37.5) |
| ACURATE neo | - |
| Stroke n (%) | 4 (0.7) |
| Cardiac tamponade n (%) | 5 (0.9) |
| In-hospital arrhythmia n (%) | |
| New-onset AF | 20 (3.6) |
| LBBB | 14 (2.5) |
| VT | 3 (0.5) |
| Acute renal failure n (%) | 2 (0.4) |
| Major bleeding n (%) | 4 (0.7) |
| Major vascular complication n (%) | 33 (6.0) |
| Per closure device failure n (%) | 11 (2.0) |
| Coronary obstruction n (%) | 2 (0.4) |
| Device embolization n (%) | 3 (0.5) |
| Left ventricle n | 2 |
| Aorta n | 1 |
| Annular rupture n | 1 |
| Discharge time (day) | 4.5±2.3 |
| In-hospital mortality n (%) | 21 (3.9) |
| 30-day mortality n (%) | 11 (2.2) |
| 1st year mortality n (%) | 51 (12.3) |
| Total mortality n (%) | 158 (28.7) |
| 30-day NYHA n (%) | |
| 1 | 139 (41.6) |
| 2 | 171 (51.2) |
| 3 | 24 (7.2) |
| 1st year NYHA n (%) | |
| 1 | 67 (79.8) |
| 2 | 16 (19.0) |
| 3 | 1 (1.2) |
| Mean follow-up time (month) | 15.1±14.9 (0-77.0) |
| Post-TAVI creatinine mg/dL | 0.98±0.4 |
| Post-TAVI GFR ml/min/ | 68.5±20.4 |
| Post-TAVI hemoglobin mg/dL | 10.5±2.0 |
| Post-TAVI troponin | 247.7±551.0 |
| Post-TAVI CK-MB | 12.7±84.5 |
AF - atrial fibrillation; LBBB - left bundle branch block; VT - ventricular tachycardia; NYHA - New York Heart Association; GFR - glomerular filtration rate
Changes in patient outcome over time
The mean follow-up period was 15.1±14.9 months for all patients. When the effect of the TAVI procedure on patient outcomes was evaluated, an improvement was observed in the NYHA functional class over time (Fig. 2). Continued improvement was observed in AVA, LVEF, and systolic pulmonary artery pressure (sPAP) at 30 days and 1-year follow-ups (Table 4). After TAVI, all echocardiographic parameters significantly improved, the mean AVA increased to 1.83±0.55 cm2, and the mean gradient decreased to 10.5±3.9 mm Hg (Fig. 3). In addition, a statistically significant increase in LVEF and a substantial decrease in estimated sPAP was observed over time (p<0.001). The most striking improvement was in MR, with a moderate-to-severe MR of 69 (12.7%) at baseline, and 14 (2.7%) at discharge. Paravalvular leak (PVL) was documented in 18.9% patients, mild in 94 (17.9%), and moderate in 5 (1%) at discharge. No severe PVL occurred, necessitating additional procedures. Figure 4 illustrates the change in PVL from discharge to 1-year post-TAVI. Table 5 presents the comparison of PVL and short-term mortality according to the THVs. Post-TAVI PVL was significantly lower in the Sapien 3 group with no moderate PVL (p<0.001), but no difference was noted between short-term mortality rates. Multiple logistic regression analyses were used to determine the independent predictors of total mortality after TAVI procedure. The multiple logistic regression analysis revealed that sex, STS score, bSS, bicuspid valve morphology, CFA diameter, and post-TAVI PVL were independent predictors of total mortality (Table 6). The most common antiplatelet±anticoagulant combinations are described in Table 7. Aspirin+P2Y12, the most frequently used combination, was prescribed for 67.6% of patients. Anticoagulation was prescribed in 29.2% of patients (22.1% warfarin, 7.1% direct oral anticoagulant).
Figure 2.
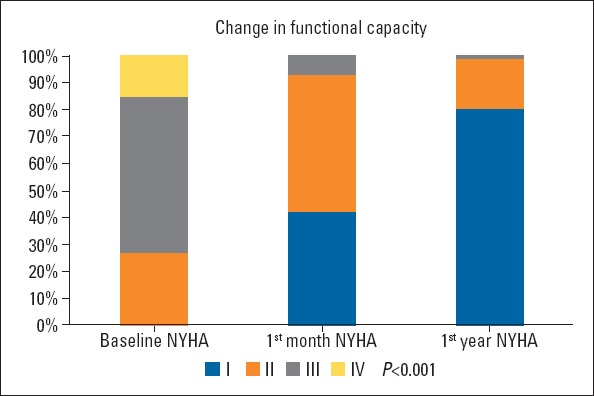
Improvement in NYHA functional class
Table 4.
Comparison of baseline and post-TAVI echocardiographic parameters
| Parameters | Baseline (1) | Discharge (2) | 30-Day (3) | 1st Year (4) | P value | Post hoc test | |
|---|---|---|---|---|---|---|---|
| LVEF (%) | Mean±SD median (IQR) | 51.7±14.0 55.0 (9.0-70.0) | 54.1±12.7 60.0 (12.0-70.0) | 55.2±11.4 60.0 (20.0-70.0) | 58.4±8.7 60.0 (30.0-65.0) | <0.001 | 1-2; 1-3; 1-4 |
| Aortic mean gradient (mm Hg) | Mean±SD median (IQR) | 50.4±15.1 49.0 (20.0-114.0) | 10.5±3.9 10.0 (4.0-24.0) | 11.0±4.4 11.0(5.0-36.0) | 12.2±4.5 12.0 (4.0-28.0) | <0.001 | 1-2; 1-3; 1-4; 2-4; 3-4 |
| AVA or EOA (cm2) | Mean±SD | 0.67±0.16 | 1.83±0.55 | 1.86±0.62 | 1.89±0.63 | <0.001 | 1-2; 1-3; 1-4 |
| sPAP (mm Hg) | Mean±SD median (IQR) | 44.1±16.9 40.0 (15.0-90.0) | 36.9±13.8 35.0 (15.0-75.0) | 36.6±13.8 35.0 (15.0-75.0) | 36.0±14.5 35.0 (15.0-90.0) | <0.001 | 1-2; 1-3; 1-4; |
| PVL | |||||||
| Mild | n (%) | NA | 94 (17.9) | 52 (17.2) | 23 (23.7) | 0.117 | |
| Moderate | 5 (1.0) | 6 (2.0) | - | ||||
| Moderate-to-severe MR | n (%) | 69 (12.7) | 14 (2.7) | 5 (1.7) | 2 (2.1) | <0.001 |
LVEF - left ventricular ejection fraction; AVA - aortic valve area; EOA - effective orifice area; sPAP - systolic pulmonary artery pressure; MR - mitral regurgitation; PVL - paravalvular leakage
Figure 3.
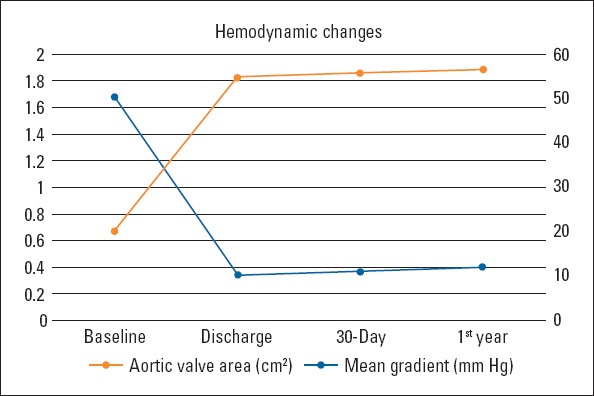
Change in mean gradient and AVA from baseline to 1-year post-TAVI
Figure 4.
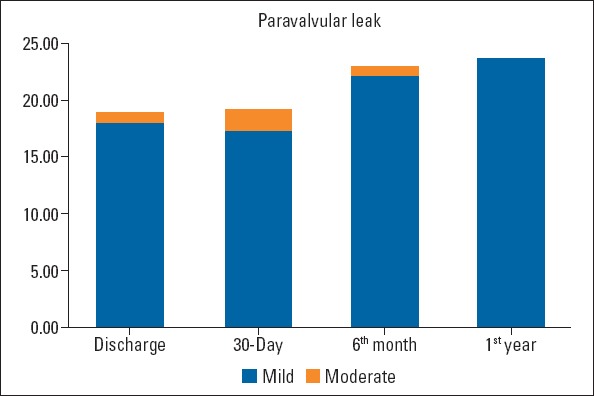
Change in PVL from baseline to 1-year post-TAVI
Table 5.
Comparison of THVs according to endpoints
| Parameters | SAPIEN XT n=480 | Edwards SAPIEN 3 n=46 | LOTUS n=24 | ACURATE neo n=6 | P value |
|---|---|---|---|---|---|
| In-hospital mortality n (%) | 19 (4.1) | - | 2 (8.3) | - | 0.487 |
| 30-day mortality n (%) | 11 (2.6) | - | - | - | 0.762 |
| Post-TAVI PVL n (%) | |||||
| Mild | 89 (19.6) | 5 (11.4) | 4 (17.7) | 1 (16.7) | <0.001 |
| Moderate | 4 (0.9) | - | 1 (4.5) | 1 (16.7) | |
| 30-day PVL n (%) | |||||
| Mild | 48 (18.0) | 4 (21.1) | 4 (25.4) | NA | 0.354 |
| Moderate | 5 (1.9) | - | 1 (6.7) |
PVL - paravalvular leakage
Table 6.
The predictors of overall mortality per multiple logistic regression analysis
| Odds ratio | 95% CI | P value | |
|---|---|---|---|
| Sex | 1.57 | 1.07–2.29 | 0.019 |
| STS | 1.11 | 1.03-1.18 | 0.003 |
| Baseline SYNTAX score | 1.08 | 1.01-1.15 | 0.021 |
| Bicuspid | 1.98 | 1.05-3.74 | 0.033 |
| CFA diameter | 3.46 | 1.48-8.05 | 0.004 |
| Post-TAVI PVL | 1.77 | 1.09-2.89 | 0.021 |
STS - Society of Thoracic Surgeons; CFA - common femoral artery; PVL - paravalvular leakage
Table 7.
Antiplatelet/anticoagulant regimen at discharge
| Post-Antiplatelet/Anticoagulation n (%) | |
| ASA or P2Y12 alone | 17 (3.2) |
| ASA+P2Y12 | 364 (67.6) |
| Warfarin alone | 36 (6.8) |
| ASA+Warfarin | 24 (4.5) |
| ASA+Warfarin+Clopidogrel | 27 (5.1) |
| Warfarin+Clopidogrel | 30 (5.7) |
| DOAC alone | 30 (5.7) |
| DOAC+Clopidogrel | 5 (1.0) |
| DOAC+ASA+Clopidogrel | 2 (0.4) |
ASA - acetyl salicylic acid; DOAC - direct oral anticoagulant
Discussion
To our knowledge, this study reports the largest single-center real-world experience of TAVI in Turkey. Based on our data, the most remarkable findings are as follows: (1) Our total device success was 96.6%, higher for Sapien 3 and ACURATE neo THVs. (2) In line with the trend in the world, our patient population tended to be intermediate risk over time based on the STS score. (3) Because of the operator’s experience and completion of the learning curve, our complication rates were lower compared with large registries. (4) Sex, bicuspid valve morphology, CFA diameter, and post-TAVI PVL were strong independent predictors of total mortality. (5) Continued stable improvement was observed in functional capacity, EOA, and aortic mean gradient during follow-ups.
Our center started performing the TAVI procedure earlier than other centers. The early start has provided an advantage of fewer complication rates because of the completion of the learning curve, besides providing the opportunity to follow-up patients in the short- and mid-term. The average STS score of the cohort was 6.0%±3.5%. By looking at the STS score, it may be thought that these patients are not at high risk, but all patients were evaluated by the heart team and were considered high risk not only based on their STS scores but also their comorbidities. Some of these comorbidities were as follows: 23.6% with CABG (redo surgery is considered high risk by surgeons), 42.4% with moderate-to-severe COPD, 22.7% with heart failure (LVEF <40%), 6% with stroke, and 6.8% with active cancer or cancer survivors. Although our patient population was intermediate risk based on the STS score, it should be noted that the STS score is not a TAVI-specific scoring system. Because the heart team firmly discussed each of our patients, it was decided to perform TAVI considering patients as high or prohibitive risk for open-heart surgery despite the STS score indicating intermediate risk.
As expected, TAVI procedures gained momentum, and the number of procedures increased, except in 2019. The low volume of TAVI procedures in 2019 reflects the result of reimbursement restrictions for TAVI in Turkey. Nevertheless, the high number of TAVI procedures enabled operators to complete their learning curve quickly; therefore, our outcomes were favorable compared with the other real-world data. With the accelerated increase in TAVIs, it shifted from being a “surgical-like” procedure to a “PCI-like” procedure. Notably, there is a trend worldwide for a minimalist approach in the procedural steps (12). In our clinical practice, general anesthesia was used only in exceptional circumstances after the learning curve was completed, the “TEE-guided procedure” was terminated, and, if anatomically appropriate, the transfemoral access was used as the first option.
The transfemoral access was preferred for 96.3% of our patients, but we did not perform a statistical comparison because of the insufficient number of non-TF access cases. Similarly, according to the data of Kumar et al. (13), TF-TAVI was associated with significantly lower in-hospital mortality [odds ratio (OR) 0.61, 95% confidence interval (CI) 0.42 to 0.88, p=0.01], and postoperative stroke (OR 1.19, 95% CI 0.67 to 2.10, p=0.56) compared with transapical (TA)-TAVI. Our priority has always been to prefer TF access. Even in patients with severe femoral or iliac artery stenosis, we first performed percutaneous intervention for stenosis and then performed TAVI via the TF route. However, the peripheral arteries should be carefully examined before TF access because, per our findings, the CFA dimension was one of the crucial predictors of total mortality. Therefore, our priority has always been to evaluate both peripheral arteries and aortic annulus by using MSCT. MSCT has always been evaluated by the same cardiologist experienced in this field. In fact, in the case of renal impairment, we decided to evaluate both coronary arteries, peripheral arteries, and aortic annulus simultaneously before TAVI by using MSCT instead of invasive coronary angiography.
Previous studies have demonstrated that PVL is associated with poor prognosis after TAVI (14, 15). In our study, none of the patients had severe PVL, and the S3 group did not even have moderate PVL. Although the polyethylene terephthalate fabric skirt structure of the S3 THV reduces PVL, our PVL ratios were lower than other THVs compared with other studies (16). The reason for the low PVL ratio was that we paid attention to careful evaluation of the annulus eccentricity index, landing zone, and extent of calcification. Moreover, the selection of the appropriate size of the prosthesis based on the aortic annulus size is one of the critical elements of PVL prevention.
In our cohort, the need for PPM implantation was low and significantly different among THVs. This finding is somewhat inconsistent with earlier studies that reported PPM implantation rates of 7%–17% for the S3 and 4%–13% for the Sapien XT. Nonetheless, we determined lower rates for both, especially the S3 and ACURATE neo THVs (17, 18). Our PPM indications were AV complete block, second degree AV block, and trifascicular block. We decided to implant PPM within 48 hours at the latest, and implanted it immediately after TAVI in some patients to prevent the development of asystole during follow-up. PPM implantation was needed in 37.5% of patients in the Lotus valve group. This rate was similar to the PPM implantation rate of 32% (37% among pacemaker-naive patients) described in the RESPOND (Repositionable Lotus Valve System-Post-Market Evaluation of Real World Clinical Outcomes)-an extensive, international, multicenter, prospective registry (19).
The disabling stroke rate (0.7%) was relatively low in our cohort compared with earlier TAVI registries (5, 7). Early post-procedure stroke was primarily because of the displacement of atherosclerotic debris from the aorta and aortic valve or periprocedural AF, whereas the subsequent events were associated with patient-specific factors. Compared with previous registries and single-center data, our in-hospital stroke rates were lower, and this may be attributable to the higher use of the balloon-expandable valve in our cohort (20). One of the limitations of our study is that every patient was not routinely evaluated using magnetic resonance imaging, but each patient underwent a neurological examination by the cardiologists after TAVI.
Comparing mortality rates among registries and our study is challenging because of the small number of our research alongside these large registries (4-7). However, the in-hospital mortality rate of our center was lower than that stated in national registries. Although the 30-day mortality rates were not determined in certain registries, our results were still favorable compared with those reported. Like our study, Tanner et al. (20), who published Ireland TAVI data, reported in-hospital mortality as 3.1% and 30-day mortality as 3%. In a single-center study published by Alatawi et al. (21), wherein the self-expandable valve was used predominantly (69.2%), periprocedural mortality was reported as 3.8% and 10% during the follow-up. In the PARTNER 2 study, wherein intermediate risk patients were evaluated, 30-day mortality owing to cardiac causes was 3.3%, and all-cause mortality was 3.9% (22).
Compared with other TAVI studies, our echocardiographic data revealed improvement in AVA, LVEF, mean gradient, and MR during both immediate and mid-term follow-ups. However, bioprosthetic valve degeneration progresses with time, and follow-up duration of 15.1±14.9 months might not indicate the actual durability of THVs.
According to the 5-year results of the recently published PARTNER2 study, it was concluded that the mean gradient of the balloon-expandable valve was identical to sAVR at the end of 5 years. Nevertheless, the re-intervention rate was higher because of PVL (23).
Conclusion
In conclusion, our study provides an up-to-date analysis of outcomes achieved with TAVI among patients with severe, symptomatic AS at a tertiary center in Turkey. Our results revealed excellent mortality and procedural outcomes in patients who underwent TAVI with either the balloon-expandable or the mechanically-expandable THVs.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – B.D.K., T.K.; Design – B.D.K., E.B.; Supervision – H.A., E.B.; Funding – B.D.K., H.A., E.B.; Materials – B.D.K., H.A., T.K.; Data collection and/or processing – B.D.K., H.A., E.B.; Analysis and/or interpretation – B.D.K., H.A.; Literature search – B.D.K., H.A., T.K.; Writing – B.D.K., H.A., E.B.; Critical review – T.K., E.B.
References
- 1.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, III, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease:a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–95. doi: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 2.Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis:first human case description. Circulation. 2002;106:3006–8. doi: 10.1161/01.cir.0000047200.36165.b8. [DOI] [PubMed] [Google Scholar]
- 3.Gurvitch R, Tay EL, Wijesinghe N, Ye J, Nietlispach F, Wood DA, et al. Transcatheter aortic valve implantation:lessons from the learning curve of the first 270 high-risk patients. Catheter Cardiovasc Interv. 2011;78:977–84. doi: 10.1002/ccd.22961. [DOI] [PubMed] [Google Scholar]
- 4.Werner N, Zahn R, Backmann A, Bauer T, Bleiziffer S, Hamm CW, et al. Patients at intermediate risk undergoing isolated interventional or surgical aortic valve replacement for severe symptomatic aortic valve stenosis One year results from the German Aortic Valve Registry (GARY) Circulation. 2018;138:2611–23. doi: 10.1161/CIRCULATIONAHA.117.033048. [DOI] [PubMed] [Google Scholar]
- 5.Grover FL, Vemulapalli S, Carroll JD, Edwards FH, Mack MJ, Thourani VH, et al. 2016 Annual Report of The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J Am Coll Cardiol. 2017;69:1215–30. doi: 10.1016/j.jacc.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Ludman PF, Moat N, de Belder MA, Blackman DJ, Duncan A, Banya W, et al. UK TAVI Steering Committee and the National Institute for Cardiovascular Outcomes Research Transcatheter aortic valve implantation in the United Kingdom:temporal trends, predictors of outcome, and 6-year follow-up:a report from the UK Transcatheter Aortic Valve Implantation (TAVI) Registry 2007 to 2012. Circulation. 2015;131:1181–90. doi: 10.1161/CIRCULATIONAHA.114.013947. [DOI] [PubMed] [Google Scholar]
- 7.Holmes DR, Jr, Nishimura RA, Grover FL, Brindis RG, Carroll JD, Edwards FH, et al. Annual Outcomes With Transcatheter Valve Therapy:From the STS/ACC TVT Registry. J Am Coll Cardiol. 2015;66:2813–23. doi: 10.1016/j.jacc.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The SYNTAX Score:an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–27. [PubMed] [Google Scholar]
- 9.Ad N, Holmes SD, Patel J, Pritchard G, Shuman DJ, Halpin L. Comparison of EuroSCORE II, Original EuroSCORE, and The Society of Thoracic Surgeons Risk Score in Cardiac Surgery Patients. Ann Thorac Surg. 2016;102:573–9. doi: 10.1016/j.athoracsur.2016.01.105. [DOI] [PubMed] [Google Scholar]
- 10.Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41:734–44. doi: 10.1093/ejcts/ezs043. [DOI] [PubMed] [Google Scholar]
- 11.Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Update standardized endpoint definitions fro transcatheter aortic valve implantation:the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33:2403–18. doi: 10.1093/eurheartj/ehs255. [DOI] [PubMed] [Google Scholar]
- 12.Akodad M, Lefèvre T. TAVI:Simplification Is the Ultimate Sophistication. Front Cardiovasc Med. 2018;5:96. doi: 10.3389/fcvm.2018.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar N, Khera R, Fonarow GC, Bhatt DL. Comparison of Outcomes of Transfemoral Versus Transapical Approach for Transcatheter Aortic Valve Implantation. Am J Cardiol. 2018;122:1520–6. doi: 10.1016/j.amjcard.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Jilaihawi H. Paravalvular Regurgitation After Transcatheter Aortic Valve Replacement:Striving to Perfect its Prognostic Evaluation With Hemodynamic Data. JACC Cardiovasc Interv. 2016;9:712–4. doi: 10.1016/j.jcin.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1):a randomised controlled trial. Lancet. 2015;385:2477–84. doi: 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 16.Vendrik J, van Kesteren F, van Mourik MS, Piek JJ, Tijssen JG, Henriques JPS, et al. Procedural Outcome and Midterm Survival of Lower Risk Transfemoral Transcatheter Aortic Valve Implantation Patients Treated With the SAPIEN XT or SAPIEN 3 Device. Am J Cardiol. 2018;121:856–61. doi: 10.1016/j.amjcard.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Sawaya FJ, Spaziano M, Lefevre T, Roy A, Garot P, Hovasse T, et al. Comparison between the SAPIEN S3 and the SAPIEN XT transcatheter heart valves:A single-center experience. World J Cardiol. 2016;8:735–45. doi: 10.4330/wjc.v8.i12.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Husser O, Hengstenberg C. Conduction Abnormalities and Pacemaker Implantations After SAPIEN 3 Vs SAPIEN XT:Depending on Who Is Implanted and How You Implant Response. Rev Esp Cardiol (Engl Ed) 2016;69:456. doi: 10.1016/j.rec.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Van Mieghem NM, Wöhrle J, Hildick-Smith D, Bleiziffer S, Blackman DJ, Abdel-Wahab M, et al. Use of a Repositionable and Fully Retrievable Aortic Valve in Routine Clinical Practice:The RESPOND Study and RESPOND Extension Cohort. JACC Cardiovasc Interv. 2019;12:38–49. doi: 10.1016/j.jcin.2018.10.052. [DOI] [PubMed] [Google Scholar]
- 20.Tanner R, Moran B, Margey R, Blake G, McGorrian C, Geraghty J, et al. Clinical experience with trans-catheter aortic valve implantation at a tertiary hospital in the Republic of Ireland. Ir J Med Sci. 2020;189:139–48. doi: 10.1007/s11845-019-02030-7. [DOI] [PubMed] [Google Scholar]
- 21.Alatawi FO, Abuelatta RA, AlAhmedi AB, Alharbi IH, Alghamdi SS, Sakrana AA, et al. Clinical outcomes with transcatheter aortic valve implantation at a single cardiac center in Saudi Arabia. Ann Saudi Med. 2018;38:167–73. doi: 10.5144/0256-4947.2018.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. PARTNER 2 Investigators Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609–20. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 23.Makkar RR, Thourani VH, Mack MJ, Kodali SK, Kapadia S, Webb JG, et al. PARTNER 2 Investigators Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N Engl J Med. 2020;382:799–809. doi: 10.1056/NEJMoa1910555. [DOI] [PubMed] [Google Scholar]


