To the Editor,
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is affecting different regions of the world since the end of 2019, causing infection named COVID-19 by World Health Organization (WHO).
Up to the date (i.e. April 28, 2020) 3,063,814 cases have been confirmed by the Center for System Science and Engineering (CSSE) at Johns Hopkins University (JHU) (https://coronavirus.jhu.edu/map.html).
The infection may produce, after a period of cough, fever and thoracic pain, a severe pulmonary failure requiring in more than 15% of the patients assisted ventilation (Zhou et al., 2020). Due to the lack of specific therapy and vaccine, the consequences of hospitals overload have produced a dangerous growth of the number of deaths.
Sars-CoV-2 and Sars-CoV, responsible of the outbreak in 2003, share a high homology in the structure of spike protein, which bind host cells receptor (Zhang et al., 2020).
Angiotensin-converting enzyme 2 (ACE2) is the host functional receptor recognized by viral protein (spike) and allows to the Sars-CoV-2 to go into the cell (Hoffmann et al., 2020).
The affinity to bind ACE2 receptor is documented as more efficiently for Sars-CoV-2 than his predecessor, explaining the higher rate of transmission.
The expression of ACE2 is ubiquitous, although lung represents the target and more vulnerable organ due to the high presence of ACE2 on the type II alveolar epithelial cell allowing adhesion, translocation and facilitating replication.
However, different tissues such as gastrointestinal tract and heart among others, representing a further possible entry site, may be hit by Sars-CoV-2, characterizing the clinical picture for extra-pulmonary manifestations (Zhang et al., 2020; Zheng et al., 2020; Gu et al., 2020).
Moreover, the different molecular expression of ACE2, limiting the ingress of Sars-CoV-2 into the cells, seems to be crucial to track the incidence of COVID-19 in different populations and to explain their dissimilar susceptibility.
Regarding the potential racial heterogeneous molecular expression of ACE2, first Zhao et al. (Zhao et al., 2020) have analyzed lung cells through single-cell RNA sequencing (scRNA-Seq) and have interestingly found higher ACE2 pulmonary levels in Asian than white and African American donors. Additional studies in literature, analyzing big datasets such as the cancer genome atlas (TCGA), have not confirmed this evidence, indicating a similar molecular expression of ACE2 in the lung cells, without difference of race (Chen et al., 2020; Cai, 2020).
Evidences result controversial, although recently has been documented the highest expression of ACE2 in the upper respiratory tract, particularly in the nasal epithelial cells, explaining a possible lack in the previous studies limited only to analysis of lung tissue (Sungnak et al., 2020).
However, focusing on black population, a reduced molecular expression of ACE2 is documented and represents the key to explain the higher predisposition to develop essential arterial hypertension and an early end organ damage than in others races (Cohall et al., 2020).
Physiologically, ACE2 enzymatic activity is involved in the pathway counter to Angiotensin Converting Enzyme (ACE) leading to the formation of Angiotensin II (Ang II); in fact, ACE2 represents a modulator of the Renin Angiotensin Aldosterone System (RAAS) able to mitigate the largely known deleterious effects.
The peptides produced by ACE2, Angiotensin 1–7 (Ang 1–7) and Angiotensin 1–9 (Ang 1–9), have demonstrated to exert a protective role for cardiovascular system among others, reducing the availability of substrate for ACE action, being Ang II associated to cardiac remodeling, hypertrophy and fibrosis leading to heart failure (HF). ACE-inhibitors (ACEi) and Angiotensin II Receptor Blockers (ARBs) use this mechanism to treat Heart Failure and prevent cardiac remodeling, reducing circulating Ang II (Patel et al., 2016).
Pathogenesis of hypertension is strongly associated to RAAS system dysfunction and recent evidences suggest that an unbalanced symmetry of ACE/ACE2 pathways play a crucial role leading high level of blood pressure, vascular disease and organ damage (Soro-Paavonen et al., 2012; Patel et al., 2014).
In this context, as discussed by Patel et al. (Patel et al., 2014), patients with risk factors or known evidence of cardiovascular disease (CVD) have documented elevation of plasma ACE2 activity; conversely, a deficiency of ACE2 may lead to primary hypertension.
Paradoxically, African descendent populations such as African Americans and particularly subjects affected by pre-hypertensive status, diabetes and renal disease have shown a reduced plasma ACE2 activity (Soro-Paavonen et al., 2012; Patel et al., 2014). The molecular response to CVD risk factors is completely specular in these subjects, depressing ACE2 pathway, than in the normal population, in which as previous described it has been documented high levels of ACE2 to balance activation of RAAS.ss
This biological variability is probably correlated to environmental genetic selection for renal sodium-retainers affecting RAAS (Williams et al., 2014).
Moreover, hypertension profile in black race is characterized by low circulating plasma renin resulting in the documented resistance to RAAS inhibitors, explained by deficit of ACE2 pathway. In fact, lacking the biological counterbalance represented by ACE2 axis, normal or low level of RAAS components exposing constantly to Ang II, leading to long-standing vasoconstriction, hypertrophy and fibrosis (Cohall et al., 2020; Williams et al., 2014).
Therefore, taking these evidences our hypothesis consists in the potential COVID-19 less incidence in the black race, if only biological factors are considered.
Available online data and statistics to support this hypothesis are not manifest due to mostly missing or unspecified race and ethnicity data on reports in Europe and United States (U.S.).
Data about Latin America and Africa are defective due to deficiencies in screening, highlighted by the low number of oro/nasopharyngeal swabs performed (https://www.worldometers.info/coronavirus/#countries).
Center for disease controls (CDC) began reporting available data about African American community in U.S., which represents approximately the 14% of total population (https://www.census.gov/prod/cen2010/cph-2-1.pdf).
Opposite to our hypothesis, up to date April 152,020, data showed a unequal rate of incidence and deaths of black people, with higher number of confirmed cases in 20 of 31 and 19 of 24 analyzed states, respectively (https://www.kff.org/coronavirus-policy-watch/growing-data-underscore-communities-color-harder-hit-covid-19/).
However, what emerges by reports is a higher distribution of confirmed COVID-19 cases in the racial and ethnicity minority groups, being more affected than white also Hispanic and Asian individuals.
Predisposing factors explaining the disparity may be associated to living conditions, particularly in high populated areas, work circumstances and lower access to care, as accurately reported by CDC, potentially answer also to the exceptional higher mortality rate.
Nevertheless, despite a socioeconomic status and life styles choices, the previous underlined biological factors may play a role for the major incidence in black people of severe infection and mortality.
Although deficiency of ACE2 may be protective against Sars-CoV-2 ability to entry human cells, once acquired infection, the deficit of molecular pattern may result unfavorable for the host.
Sars-CoV-2 ability to downregulate ACE2 contributes to move balance towards over-activation of RAAS system; molecular pathway results unbalanced leading to the formation of Ang II with consequent worsening of clinical picture; increasing severity of the disease characterized by progression of inflammatory and thrombotic process (Verdecchia et al., 2020; Guo et al., 2020).
The high prevalence of risk factors for CVD in black population may give a determinant contribution, offering to viral replication a pathogenetic substrate characterized by chronic inflammation of endothelium, to develop more frequently the severe disease leading potentially to death; as also confirmed analyzing retrospectively predisposing risk factors in affected patients (Zhou et al., 2020; Vinciguerra et al., 2020; Varga et al., 2020).
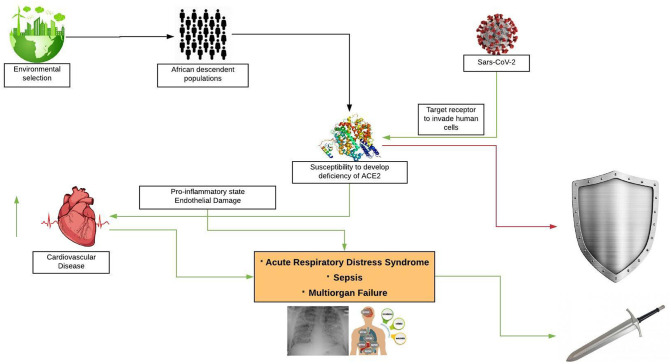
In conclusion, as summarized in the Fig. 1 we think that black race may be less subject to COVID-19 but, once infection is acquired, due to the same reason of potential ‘immunity’, clinical manifestation may be worse leading definitely to adverse outcome.
Fig. 1.
Proposed pathogenetic mechanism of COVID-19 in black populations: deficiency of ACE2 may act as shield not allow Sars-CoV-2 entry human cells; vice versa once infection is acquired deficiency of ACE2 may be responsible of severe clinical manifestations, potentially leading to death and acting as blade.
References
- Cai G. Bulk and single-cell transcriptomics identify tobacco-use disparity in lung gene expression of ACE2, the receptor of 2019-nCov. MedRxiv. 2020 pre-print publication available on medrxiv.org. [Google Scholar]
- Chen Y., Shan K., Qian W. 2020. Asians and Other Races Express Similar Levels of and Share the Same Genetic Polymorphisms of the SARS-CoV-2 Cell-Entry Receptor. [Google Scholar]
- Cohall D., Ojeh N., Ferrario C.M., Adams O.P., Nunez-Smith M. Is hypertension in African-descent populations contributed to by an imbalance in the activities of the ACE2/Ang-(1-7)/Mas and the ACE/Ang II/AT1 axes? J. Renin-Angiotensin-Aldosterone Syst. 2020;21(1) doi: 10.1177/1470320320908186. 1470320320908186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020;158(6):1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Huang Z., Lin L., Lv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J. Am. Heart Assoc. 2020;9(7) doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S.…Müller M.A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.K., Velkoska E., Freeman M., Wai B., Lancefield T.F., Burrell L.M. From gene to protein—experimental and clinical studies of ACE2 in blood pressure control and arterial hypertension. Front. Physiol. 2014;5:227. doi: 10.3389/fphys.2014.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V.B., Zhong J.C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1–7 axis of the renin–angiotensin system in heart failure. Circ. Res. 2016;118(8):1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soro-Paavonen A, Gordin D., Forsblom C., Rosengard-Barlund M., Waden J., Thorn L.…FinnDiane Study Group Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. Journal of hypertension. 2012;30(2):375–383. doi: 10.1097/HJH.0b013e32834f04b6. [DOI] [PubMed] [Google Scholar]
- Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M.…Worlock K. B. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020:1–7. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S.…Moch H. Endothelial cell infection and endotheliitis in COVID-19. The Lancet (London, England) 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Int. Med. 2020;S0953-6205(20):30151–30155. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinciguerra M., Romiti S., Greco E. Atherosclerosis as Pathogenetic Substrate for Sars-Cov2 “Cytokine Storm”. Preprints. 2020 doi: 10.20944/preprints202004.0430.v1. 2020040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S.F., Nicholas S.B., Vaziri N.D., Norris K.C. African Americans, hypertension and the renin angiotensin system. World J. Cardiol. 2014;6(9):878. doi: 10.4330/wjc.v6.i9.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020:1–5. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. BioRxiv. 2020 doi: 10.1164/rccm.202001-0179LE. pre-print publication available on biorxiv.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z.…Guan L. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet (London, England) 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]