Abstract
Patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection mainly present with upper and lower respiratory tract symptoms, with complications related to cytokine storm syndrome and acute respiratory distress syndrome. It has also been described to predispose to venous and arterial thromboembolism; however, limited published data is available regarding thrombosis in coronavirus disease 2019 (COVID-19). Here we are presenting a case of arterial thrombosis in a patient with COVID-19 and a systematic review on coagulopathy associated with COVID-19.
Keywords: Acute limb ischemia, Arterial thrombosis, COVID-19, Severe acute respiratory syndrome coronavirus 2
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is an enveloped RNA beta-coronavirus that belongs to the same family as SARS-CoV, is a novel coronavirus responsible for the current global pandemic resulting in an escalating number of cases and fatalities worldwide [1], [2]. Since the first case reported in Wuhan, China, almost 3 million cases have been reported in 205 countries and territories as of April 2020, and the count keeps rising each day. Most infections are not severe. According to the Chinese Center for Disease Control and Prevention, 81% patients have mild-to-moderate disease (asymptomatic or mild pneumonia), 14% have severe disease (dyspnea, hypoxia, or >50% lung involvement on imaging within 24–48 h), and only 5% have critical disease with multiple organ failure (respiratory failure, shock, or multiorgan dysfunction) [3].
The association of COVID-19 with coagulopathy has gained increasing interest recently. As per American Society of Hematology, some patients with severe coronavirus disease 2019 (COVID-19) have fulminant activation of coagulation and consumption of coagulation factors, which meets the criteria for disseminated intravascular coagulation as per International Society on Thrombosis and Haemostasis. A meta-analysis was conducted by Xiong et al. [4] which indicated that prothrombin time and D-dimer levels were significantly higher in patients with severe COVID-19 than in those with the mild disease. Here we report an unusual presentation of a 71-year-old diabetic male patient who presented with COVID-19 viral pneumonia and also had acute limb ischemia in the right upper extremity which required open thromboembolectomy with endarterectomy.
Case presentation
A 71-year-old Hispanic male with a past medical history of diabetes mellitus presented to the emergency department with complaints of subjective fever, myalgia, nonproductive cough, and exertional dyspnea over 10 days. Vital signs on presentation revealed blood pressure of 143/74 mmHg, heart rate of 97 beats/minute, respiratory rate of 20 breaths/minute, temperature of 37.7 °C, and oxygen saturation of 88% on room air. On examination, there were coarse crackles over the bilateral lung bases. On initial laboratory evaluation, the following values were noted: hemoglobin 15.2 g/dL (12–15.5 g/dL), total leucocyte count 8.6 × 103/µL (4.5–11 × 103/µL), absolute neutrophil count 7.1 × 103/µL (1.7–7 × 103/µL), absolute lymphocyte count 0.9 × 103/µL (0.9–2.9 × 103/µL), platelets 331 × 103/µL (140–440 × 103/µL), blood urea nitrogen 30 mg/dL (8.6–10.3 mg/dL), serum creatinine 1.45 mg/dL (0.60–1.30 mg/dL), troponin 0.046 ng/mL (<0.03 ng/mL), prothrombin time 12.1 s (9.9–13 s), international normalized ratio 0.9 (0.9–1.1), partial thromboplastin time 29.8 s (25.2–37.4 s), D-dimer 1.85 mcg/mL (≤0.50 mcg/mL), lactate dehydrogenase 2010 U/L (140–271 U/L), C-reactive protein 111.9 mg/L (≤9.9 mg/L), ferritin level 1137 ng/mL (16.4–294 ng/mL), creatinine kinase >100,000 U/L (30–223 U/L), and lactic acid was 1.7 mmol/L (0.5–2.2 mmol/L). Blood gas analysis was significant for hypoxemia and respiratory alkalosis. Chest X-ray showed multifocal infiltrates. Electrocardiogram showed sinus tachycardia of 103 beats/minute. Nasopharyngeal swab was positive for COVID 19. The patient was started on azithromycin, hydroxychloroquine, and ceftriaxone.
On Day 5 of hospitalization, the patient reported sudden-onset severe pain in the right arm. A Doppler ultrasound showed echogenic material (intraluminal thrombus) within the right brachial and radial artery. Computed tomography angiogram showed a nonocclusive thrombus of the right brachiocephalic trunk, occlusive thrombus with the axillary artery with reconstituted flow in the diminutive brachial artery extending to the radial, proximal ulnar and interosseous arteries, and incomplete visualization of the distal ulnar artery (Fig. 1 ). Transthoracic echocardiogram was negative for intracardiac thrombus and ejection fraction was 55–60%. The patient was started on therapeutic anticoagulation with unfractionated heparin and underwent open thromboembolectomy of the right brachiocephalic, subclavian, axillary, brachial, radial, ulnar arteries and endarterectomy of the right brachial artery. Post embolectomy, patient had palpable right radial and ulnar pulses with normal sensation and motor function. The patient was maintained on therapeutic anticoagulation with low molecular weight heparin (LMWH) post procedure. Unfortunately, the patient had a cardiopulmonary arrest due to persistent hypoxemia during hospitalization and passed away.
Fig. 1.
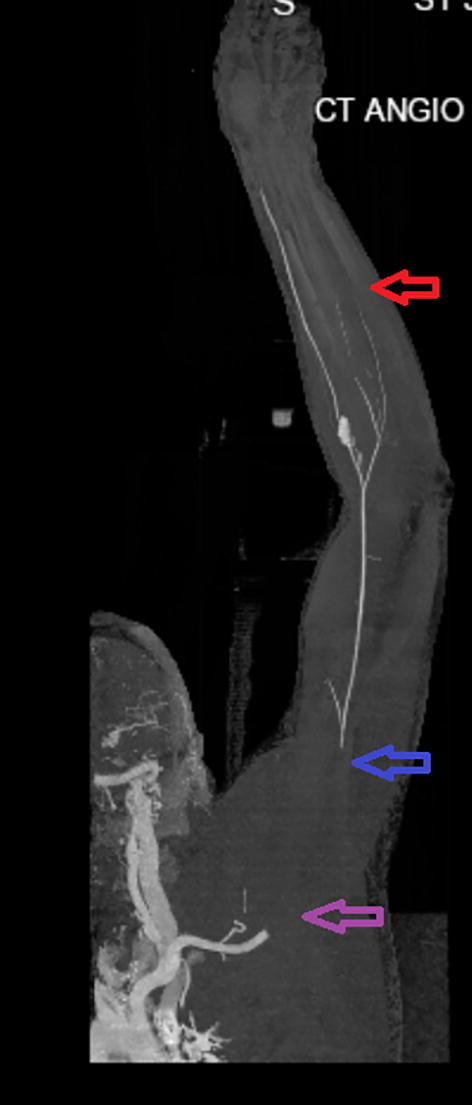
Computed tomography (CT) with angiogram showing filling defects; Purple arrow indicates absent flow in the right axillary artery; blue arrow indicates reconstituted flow in the diminutive brachial artery; and red arrow indicates incomplete visualization of the distal ulnar artery.
Discussion
COVID-19 can present with a spectrum of clinical manifestations including fever, myalgia, cough, dyspnea, and less frequently headache, diarrhea, nausea, and vomiting. Although respiratory symptoms predominate, thrombosis may also occur with COVID-19. Multiple studies demonstrate evidence of coagulation dysfunction in patients with COVID-19 and its association with poor prognosis. The non-survivors had significantly higher D-dimer and fibrin degradation product levels as well as longer prothrombin time and activated partial thromboplastin time than survivors on admission [5].
The prevalence of thrombosis among patients with COVID-19 is not fully established as most of the literature focus on hospitalized patients who are more likely to have comorbid conditions than individuals with asymptomatic or mild disease burden. Cui et al. [6] in a retrospective study analyzed 81 patients with severe COVID-19 in the intensive care unit (ICU). The incidence of venous thromboembolism (VTE) in these patients was 25% (20/81) and may be related to poor prognosis. Furthermore, Klok et al. [7] analyzed 184 patients with COVID-19 in ICU; the incidence of thrombosis was 31%; 27% were VTE and 3.7% were arterial thrombotic events (three cases of ischemic strokes). Our case adds to the literature that life-threatening acute limb ischemia can occur in COVID-19 patients.
Excessive complement activation can lead to diffuse thrombotic microangiopathy (TMA) and end-organ dysfunction. Complement inhibition was associated with favorable outcomes in SARS-CoV and Middle East respiratory syndrome-related coronavirus (MERS-CoV) murine models and reversed cardiac dysfunction in atypical hemolytic uremic syndrome-TMA, which mimics the pathological findings seen in COVID-19 [8], [9]. Thus, complement inhibition may be a reasonable treatment for COVID-19-related systemic thrombosis by reducing the innate immune-mediated consequences of severe coronavirus infection [10].
The ASH recommends that all hospitalized patients with COVID-19 should receive pharmacologic thromboprophylaxis with LMWH or fondaparinux, unless bleeding risk exists, and full therapeutic-intensity anticoagulation in the appropriate clinical scenario. Anticoagulant therapy mainly with LMWH appears to be associated with better prognosis in patients with severe COVID-19 meeting sepsis-induced coagulopathy criteria or with markedly elevated D-dimer levels [5].
Conclusion
In conclusion, we report a COVID-19 patient who developed arterial thrombosis leading to acute ischemia in the right upper extremity. Our case and review of literature reveals that health care providers should be aware of life-threatening thromboembolic events associated with COVID-19 so that prompt and appropriate intervention can be undertaken.
Funding
We confirm that there was no financial support or funding for our case.
Authors’ contributions
Study design: P.K., F.Q., M.S., Y.S., M.M.
Literature search: P.K., F.Q., A.R., Y.S., M.S.
Manuscript preparation: P.K., A.R., Y.S., M.S.
Manuscript review: P.K., F.Q., A.R., M.S., F.S., M.M., M.S.
All authors have approved the final article.
References
- 1.World Health Organization (WHO). WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. Geneva, Switzerland: WHO; 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 (accessed 6 May 2020).
- 2.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., Mcgoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Xiong M., Liang X., Wei Y.D. Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis. Br J Haematol. 2020 doi: 10.1111/bjh.16725. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020 doi: 10.1111/jth.14830. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.04.041. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gralinski L.E., Sheahan T.P., Morrison T.E., Menachery V.D., Jensen K., Leist S.R., et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. Mbio. 2018;9:e01753–18. doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Y., Zhao G., Song N., Li P., Chen Y., Guo Y., et al. Blockade of the C5a–C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg Microbes Infect. 2018;7:77. doi: 10.1038/s41426-018-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell C.M., Kahwash R. Will complement inhibition be the new target in treating COVID-19 related systemic thrombosis? Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047419. in press. [DOI] [PubMed] [Google Scholar]


