The outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which results in the development of coronavirus disease 2019 (COVID-19), has been associated with significant morbidity and mortality. The risk of vertical transmission from infected pregnant women to their fetuses is controversial. Recent studies have revealed the possibility of vertical transmission,1 , 2 contrary to previous reports of no evidence of vertical transmission of SARS-CoV-2.3 Whether vertical transmission occurs and if so, at what frequency, remains unknown.4
We present a case of rapid clinical deterioration in a woman at 28 weeks’ gestation caused by severe COVID-19. Using electron microscopy to evaluate for potential viral transmission in the placenta, we visualized and identified coronavirus virions invading into the syncytiotrophoblasts in the placental villi. To our knowledge, this is the first report demonstrating direct evidence of SARS-CoV-2 invasion in placental tissue and placental infection associated with SARS-CoV-2.
Clinical Presentation
A 40-year-old Hispanic woman, G3P2002, at 28 weeks and 4 days of gestation, with no significant medical history, presented to the emergency department with worsening shortness of breath, cough, and hypoxia in the setting of a known COVID-19 infection, on day 2 of 5 of an azithromycin course. She was promptly admitted with the diagnosis of sepsis pneumonia secondary to COVID-19.
Ten hours after the initial presentation, her clinical condition deteriorated with progressively increasing oxygen requirements. She was intubated, sedated, and started on a norepinephrine infusion because of hypotension to maintain appropriate perfusion for the placenta. Antenatal corticosteroids for fetal lung maturity were administered in anticipation of a preterm delivery. Therapeutic anticoagulation with heparin was initiated because of the risk of venous thromboembolism in the setting of severe COVID-19 with elevated D-dimer. She received a one-time dose of 400 mg tocilizumab, an interleukin (IL)-6 receptor antagonist, while awaiting regulatory permission to start the use of the antiviral remdesivir. On hospital day 4, she developed metabolic acidosis (pH 7.19, pCO2 26 mm Hg, pO2 338 mm Hg, HCO3 9.9 mmol/L, base deficit 17 mmol/L) and, despite a bicarbonate infusion, her condition continued to deteriorate. The decision was made to proceed with delivery to optimize maternal treatment and decrease fetal morbidity. She received a 4 g bolus dose of magnesium sulfate for fetal neuroprotection. An uncomplicated repeat cesarean delivery was performed in a negative pressure operating room with all personnel in personal protective equipment of a female infant weighing 2 lb and 15 oz (1340 g). The cord blood arterial gas was pH 7.26, PCO2 46, PO2 38, HCO3 20.6, and base deficit 7. The Apgar scores were 3, 5, and 6, at 1, 5, and 10 minutes, respectively. Polymerase chain reaction (PCR) testing was not performed on the placenta or amniotic fluid.
Postoperatively, the patient received a 10-day course of remdesivir. She recovered well with progressively lower oxygen requirements and resolution of metabolic acidosis. The patient was discharged home on postoperative day 10 with therapeutic enoxaparin for 12 weeks. The infant had a negatve result for COVID-19 testing on day of life (DOL) 2 and 3.
Laboratory Methods and Analysis
Patients with suspected COVID-19, including infants, are tested for SARS-CoV-2 with PCR of a nasopharyngeal swab, using the Cepheid Xpert Xpress SARS-CoV-2 RT-PCR assay under emergency use authorization as per our institution’s policy. All placentas from COVID-19–positive mothers are submitted for gross and histologic evaluation in our institution. In this case, the placenta was submitted to the pathology laboratory without fixative; fresh tissue was taken, using appropriate personal protective gear, under the Fisher Scientific Safety Flow Lab Fume Hood. Two 1-mm fragments were taken, 1 from the chorionic villi deep within the placental parenchyma and 1 from the decidua on the maternal surface. The tissue was fixed in 4% glutaraldehyde for electron microscopic evaluation. The placenta was then fixed in 10% buffered formalin for 72 hours before sectioning. Ten representative, 3-mm-thick tissue sections were submitted from the placental parenchyma, membranes, and umbilical cord for histologic evaluation.
Given the severity of the patient’s clinical course, suspected viremia, and the presence of angiotensin-converting enzyme 2 (ACE2) receptors in the placenta,5 transmission electron microscopy (TEM) was used as an opportunity to learn more about the potential viral transmission in the placenta. To perform the TEM, placental tissue samples were fixed in 4% glutaraldehyde buffered in 0.1 M sodium cacodylate buffer, at a pH of 7.5, washed in sodium cacodylate buffer, postfixed in buffered 1% osmium tetroxide, en bloc stained with a saturated solution of uranyl acetate in 40% ethanol, dehydrated in a graded series of ethanol, infiltrated in propylene oxide with Epon epoxy resin (LADD LX112, Ladd industries, Burlington, VT), and embedded. The blocks were sectioned with a Reichert Ultracut microtome at 70 nm. The resulting grids were then poststained with a 1% aqueous uranyl acetate followed by 0.5% aqueous lead citrate and scoped on a Zeiss EM 900 transmission electron microscope retrofitted with an SIA L3C digital camera (SIA, Duluth, GA).
Findings
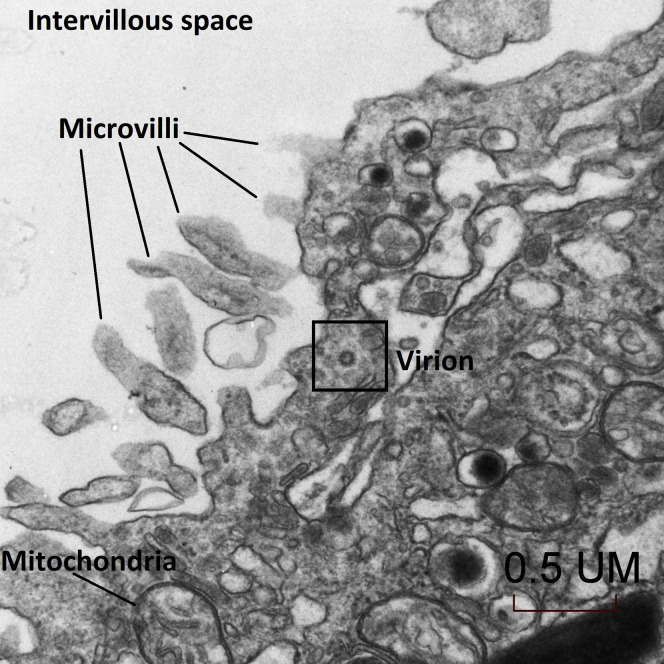
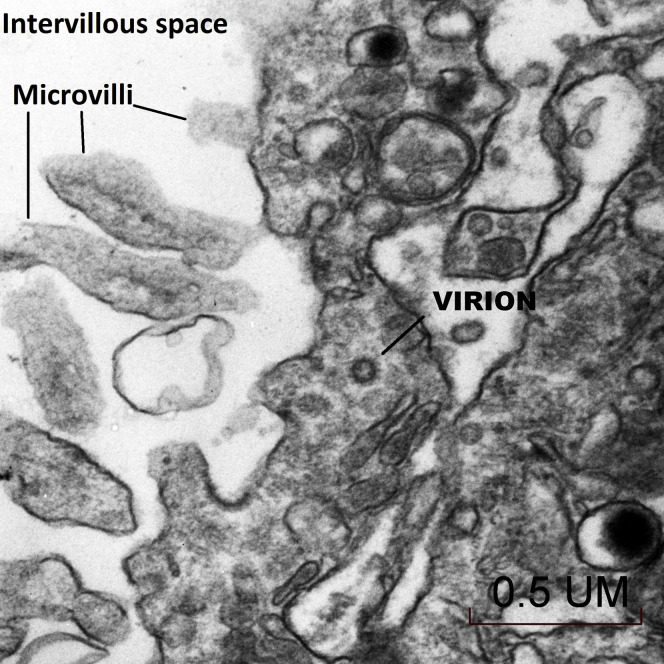
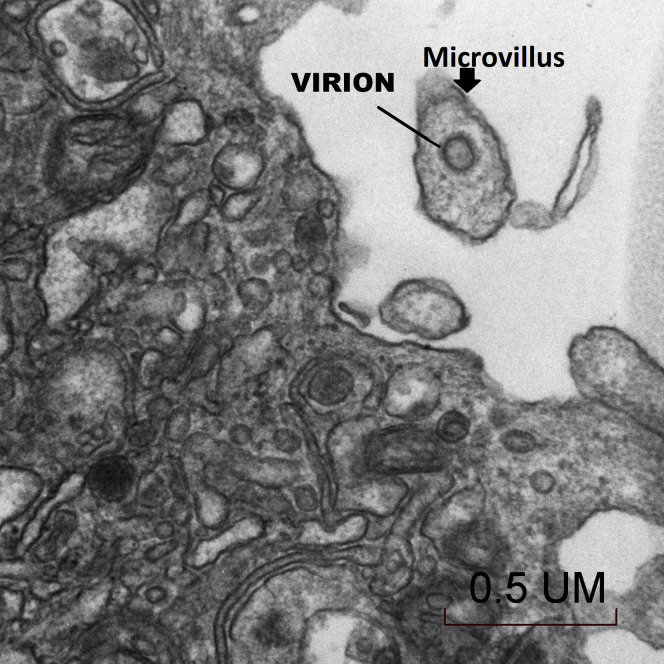
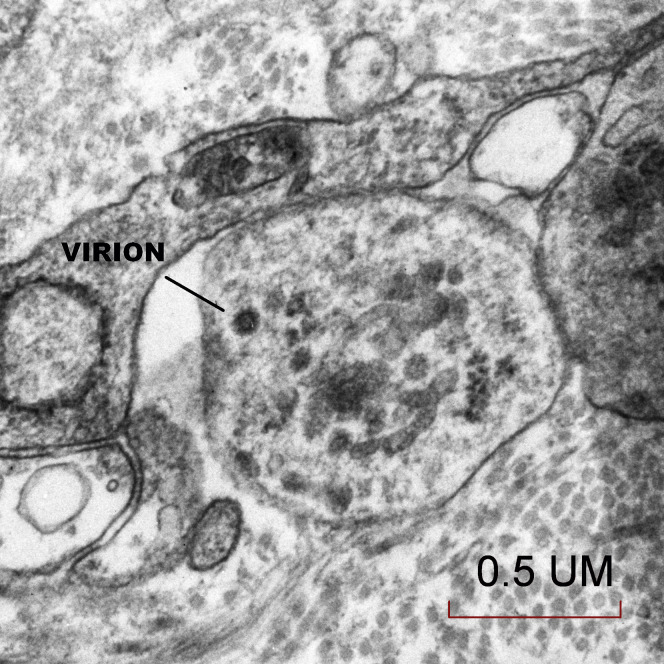
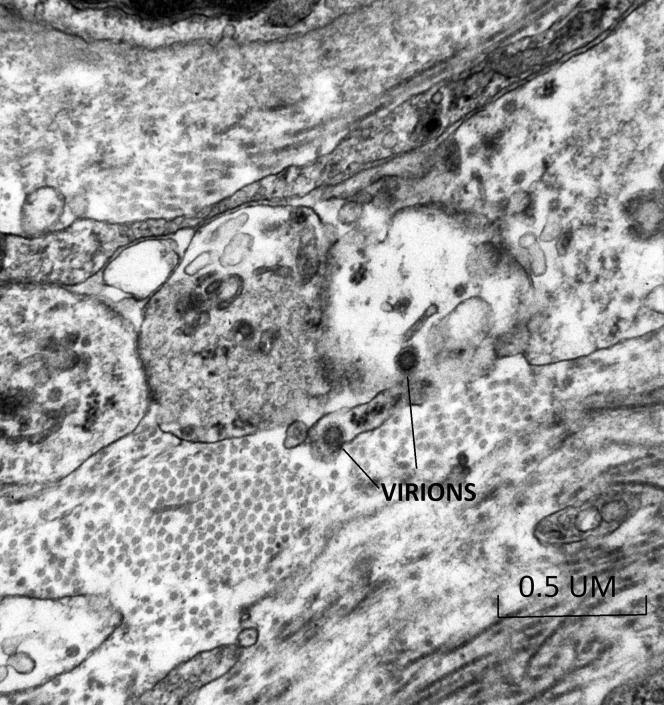
The placenta weighed 271 g (75th–90th percentile). Sections showed mature chorionic villi with focal villous edema and an area of decidual vasculopathy on the maternal surface. Survey from a placental thick section showed the terminal villi containing fetal blood vessels (Figure 1 ). This area was used for the TEM and contained syncytiotrophoblasts, fibroblasts, endothelial cells, and fetal red blood cells. A single virion was visible invading a syncytiotrophoblast (Figure 2 ). This virion was again visualized under a higher magnification (Figure 3 ). A single virion was also visualized in a microvillus (Figure 4 ). In addition, at the highest magnification of the mesenchymal core of the terminal villus, likely in the cell processes of fibroblasts, a single virion was visible in 1 field (Figure 5 ) and 2 virions in another (Figure 6 ).
Figure 1.
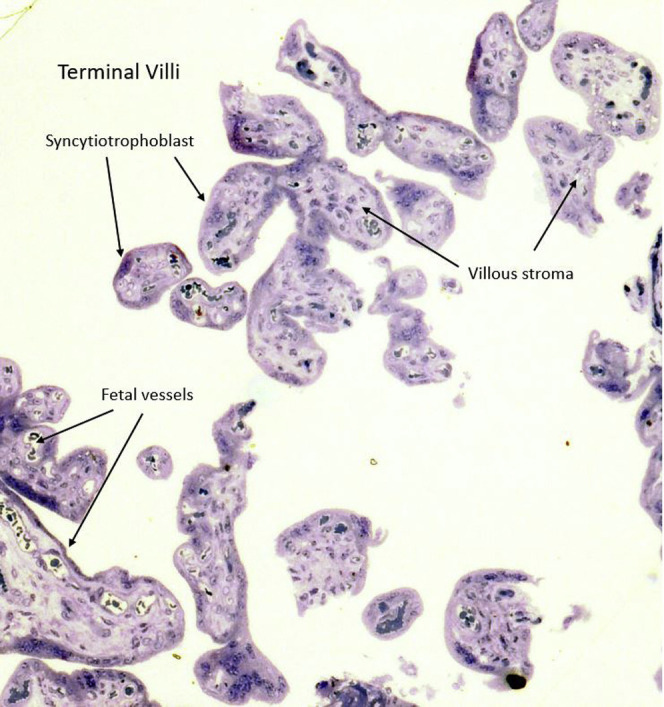
Placental thick section at 1 micron stained with toluidine blue showing the terminal villi containing fetal blood vessels (10×)
This area was used for transmission electron microscopy.
Algarroba. Visualization of SARS-CoV-2 invading the human placenta using electron microscopy. Am J Obstet Gynecol 2020.
Figure 2.
Transmission electron microscopy of a visible single virion invading a syncytiotrophoblast (30,000×)
Algarroba. Visualization of SARS-CoV-2 invading the human placenta using electron microscopy. Am J Obstet Gynecol 2020.
Figure 3.
Transmission electron microscopy of a visible single virion invading a syncytiotrophoblast at a higher magnification (50,000×)
Algarroba. Visualization of SARS-CoV-2 invading the human placenta using electron microscopy. Am J Obstet Gynecol 2020.
Figure 4.
Transmission electron microscopy of a single virion visualized in a syncytiotrophoblast microvillus (50,000×)
Algarroba. Visualization of SARS-CoV-2 invading the human placenta using electron microscopy. Am J Obstet Gynecol 2020.
Figure 5.
Transmission electron microscopy of the trophoblastic layer in the mesenchymal core of the terminal villus where a single virion was visible, likely in the cell processes of fibroblasts (50,000×)
Algarroba. Visualization of SARS-CoV-2 invading the human placenta using electron microscopy. Am J Obstet Gynecol 2020.
Figure 6.
Transmission electron microscopy of the trophoblastic layer in the mesenchymal core of the terminal villus where 2 virions were visible, likely in the cell processes of fibroblasts (50,000×)
Algarroba. Visualization of SARS-CoV-2 invading the human placenta using electron microscopy. Am J Obstet Gynecol 2020.
Discussion
This is the first visualization of SARS-CoV-2 in the human placenta. Using TEM, we were able to identify virions invading the syncytiotrophoblasts in the placental villi. In addition, we identified SARS-CoV-2 virions in the placental villi in the cell processes of fibroblasts. It seems that the cells are fibroblasts that may take the form of myofibroblasts as a result of response to injury or inflammation, in this case by the SARS-CoV-2.6 Our findings further contribute to the evidence of placental infection with SARS-CoV-2; however, there was no evidence of fetal infection.
The risk of intrauterine transmission is of particular interest as SARS-CoV-2 uses the ACE2 receptor for cell entry, and it is known that there is expression of the ACE2 receptor in the human placenta.5 Two recently published studies have provided evidence of the potential for vertical transmission. In a report by Zamaniyan et al,1 there was evidence of potential intrauterine infection in a woman with severe COVID-19 who delivered at 32 weeks’ gestation as shown by positive RT-PCR test results for COVID-19 in the amniotic fluid and repeat neonatal nasal and throat swabs; initial neonatal swabs, as well as vaginal secretions and umbilical cord blood were negative for COVID-19. In a study by Dong et al,2 a neonate born to a mother with COVID-19 of at least 20 days’ duration was shown to have positive immunoglobulin M (IgM) and immunoglobulin G antibodies and elevated IL-6 and IL-10 cytokines at 2 hours after birth, whereas the maternal vaginal secretions were negative for COVID-19. Although the infant was asymptomatic and had multiple negative nasopharyngeal swabs tested for SARS-CoV-2, in utero infection was suspected because IgM antibodies do not cross the placenta and the neonate mounted an innate immune response. There was additional evidence of vertical transmission supported by laboratory results that showed inflammation and liver injury. In contrast, other studies have reported no evidence of vertical transmission of COVID-19.3 Future studies are warranted for examining placental pathology and obstetrical and neonatal outcomes to assess the risk of vertical transmission of SARS-CoV-2.
Footnotes
The authors report no conflict of interest.
References
- 1.Zamaniyan M., Ebadi A., Aghajanpoor Mir S., Rahmani Z., Haghshenas M., Azizi S. Preterm delivery in pregnant woman with critical COVID-19 pneumonia and vertical transmission. Prenat Diagn. 2020 doi: 10.1002/pd.5713. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong L., Tian J., He S., et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323:1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H., Guo J., Wang C., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamouroux A., Attie-Bitach T., Martinovic J., Leruez-Ville M., Ville Y. Evidence for and against vertical transmission for SARS-CoV-2 (COVID-19) Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.039. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valdés G., Neves L.A., Anton L., et al. Distribution of angiotensin-(1–7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 2006;27:200–207. doi: 10.1016/j.placenta.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Baum J., Duffy H.S. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol. 2011;57:376–379. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]