Abstract
Aim
An increased risk of venous thromboembolism (VTE) in patients with COVID-19 pneumonia admitted to intensive care unit (ICU) has been reported. Whether COVID-19 increases the risk of VTE in non-ICU wards remains unknown. We aimed to evaluate the burden of asymptomatic deep vein thrombosis (DVT) in COVID-19 patients with elevated D-dimer levels.
Method
In this prospective study consecutive patients hospitalized in non-intensive care units with diagnosis of COVID-19 pneumonia and D-dimer > 1000 ng/ml were screened for asymptomatic DVT with complete compression doppler ultrasound (CCUS). The study was approved by the Institutional Ethics Committee.
Results
The study comprised 156 patients (65.4% male). All but three patients received standard doses of thromboprophylaxis. Median days of hospitalization until CCUS was 9 (IQR 5–17). CCUS was positive for DVT in 23 patients (14.7%), of whom only one was proximal DVT. Seven patients (4.5%) had bilateral distal DVT. Patients with DVT had higher median D-dimer levels: 4527 (IQR 1925-9144) ng/ml vs 2050 (IQR 1428-3235) ng/ml; p < 0.001. D-dimer levels > 1570 ng/ml were associated with asymptomatic DVT (OR 9.1; CI 95% 1.1–70.1). D-dimer showed an acceptable discriminative capacity (area under the ROC curve 0.72, 95% CI 0.61–0.84).
Conclusion
In patients admitted with COVID-19 pneumonia and elevated D-dimer levels, the incidence of asymptomatic DVT is similar to that described in other series. Higher cut-off levels for D-dimer might be necessary for the diagnosis of DVT in COVID-19 patients.
Keywords: COVID-19, SARS-CoV-2 infection, Venous thromboembolism, Deep vein thrombosis, D-dimer, Doppler ultrasound
Highlights
-
•
An increased risk of VTE in patients with COVID-19 pneumonia admitted to intensive care unit has been reported.
-
•
The most consistent hemostatic abnormalities with COVID-19 include mild thrombocytopenia and increased D-dimer levels.
-
•
In COVID-19 patients with high D-dimer levels, the incidence of asymptomatic DVT is similar to that described in other series.
-
•
Higher cut-off levels for D-dimer might be necessary for the diagnosis of DVT in COVID-19 patients.
1. Introduction
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are the main manifestations of venous thromboembolism (VTE). DVT and PE share common risk factors and, in most cases, PE is a consequence of DVT [1]. D-dimer is a product of degradation of fibrin acting as a surrogate marker for fibrinolysis and is usually elevated in thrombotic events [2].
Since December 2019, when coronavirus disease 2019 (COVID-19) emerged in Wuhan province and spread rapidly through China and the rest of the world, biological and clinical-epidemiological characteristics of the infection have been published [3]. Thromboprophylaxis seems to be associated with lower mortality and is recommended for patients admitted with COVID-19 unless contraindicated [4]. An increased risk of VTE has recently been suggested in intensive care unit (ICU) patients with COVID-19 infection despite adequate thromboprophylaxis [5]. Whether incidence of VTE is higher in patients with COVID-19 is still an issue of debate, and some authors are suggesting increased doses of thromboprophylaxis in these patients.
The aim of our study was to evaluate the burden of asymptomatic lower limbs DVT in in hospitalized patients in non-intensive care units with COVID-19 pneumonia and elevated D-dimer. As a secondary objective we evaluated the optimal D-dimer levels for the diagnosis of DVT in patients with COVID-19 pneumonia.
2. Method
This was a prospective observational study performed at a third-level hospital in Madrid. In the first half of April 2020, all consecutive patients hospitalized in non-intensive care units with diagnosis of COVID-19 pneumonia were screened for asymptomatic DVT. Patients were included in the study if they were older than 18 years, D-dimer levels were higher than 1000 ng/ml and they had been hospitalized for at least 48 h. COVID-19 diagnosis was defined by positive PCR in nasopharyngeal swab or by the presence of radiological and analytical findings highly suggestive of the disease.
Patients were excluded if they were receiving therapeutic doses of anticoagulation, had a history of deep vein thrombosis of lower limbs or had clinical signs or symptoms of DVT at the moment of the inclusion in the study. Oral informed consent was obtained in all patients prior to their participation in the study. Local protocol for thromboprophylaxis consisted in enoxaparin 40 mg per day or bemiparin 3500 UI per day. In case of diagnosis of DVT, decision on therapy was taken by the treating physician in each case. Local protocol in our center recommended 3 months of anticoagulant therapy for patients with distal DVT unless bleeding risk is very high according to RIETE score.
Patients included in the study underwent complete compression doppler ultrasound (CCUS) of both legs, which included the proximal territory (common femoral vein, shapeno-femoral junction, femoral vein, popliteal vein) and distal territory (calf veins). CCUS was performed by two clinicians with at least 50 h of accredited training in CCUS, >400 CCUS performed and frequent use of the technique in the setting of VTE clinic. In case of discrepancy, CCUS images were reviewed by two additional investigators.
2.1. Ethics and risks
This study was carried out following the international ethical recommendations for conducting research in humans in the latest revision of the Declaration of Helsinki, as well as those established in the Good Clinical Practice Guidelines and in the current legislation. The Institutional Ethics Committee approved the study. Participants were assured confidentiality and privacy of all collected information.
2.2. Statistical analysis
Qualitative variables are presented by the frequency distribution and percentages. Quantitative variables are presented by the mean and the standard deviation (SD) in case they have a normal distribution, or median and the interquartile range (IQR) if they have a non-normal distribution. The association between qualitative variables was studied with Chi-squared Test and Fisher's Exact. For numerical variables, the t-test or the Mann-Whitney U test was used, depending on the normality of the variable. Receiver operating characteristic (ROC) curve was generated to determine the accuracy and optimal threshold of D-dimer for DVT diagnosis. Several cutoff points of the D-dimer were chosen arbitrarily by the researchers from different coordinates of the ROC curve to know the sensitivity, specificity, positive predictive value and negative predictive value of these cut points. In this sense, a cutoff point with higher sensitivity, another with more specificity and another intermediate were obtained. Univariable and multivariable logistic regression analysis were used for predicting the risk of DVT based on the D-dimer levels (estimation of the odds ratio [OR]) and to determine the independence from other factors includings age, presence of active cancer, sex and body mass index. A logarithmic transformation of the variables that did not present a normal distribution was made prior to their inclusion in the logistic regression model. A comparison of the predicted and observed rate of DVT was performed using the Hosmer-Lemeshow test. A two-tailed p-value < 0.05 was considered to be statistically significant for all analyses. IBM SPSS Statistics for Mac, Version 26. Armonk, NY: IBM Corp. was used for all calculations.
3. Results
One hundred and ninety-eight patients were initially selected for the study; of those, 37 patients were excluded because they were receiving therapeutic doses of anticoagulation (31 due to atrial fibrillation, 3 due to VTE history and 3 due to mechanical heart valves), 4 patients had previous history of DVT of lower limbs and one patient had symptoms of DVT. This patient was diagnosed with proximal DVT and thus excluded of the study. Thus, the study comprised 156 patients. All but three patients received standard doses of thromboprophylaxis. These three patients had high bleeding risk, and they received intermittent pneumatic compression. Mean age of the sample was 68.1 (SD 14.5) and 65.4% were male. Median days of hospitalization until CCUS was 9 (IQR 5–17). The baseline characteristics of the sample are summarized in Table 1 . CCUS was positive for DVT in 23 patients (14.7%, 95% CI 9.6–21.3), of whom only one was proximal and 22 were distal DVT. Seven patients (4.5%) had bilateral distal DVT. Of the 133 patients with negative CCUS, none was diagnosed with PE or DVT at the time of study closure.
Table 1.
Basal characteristics, laboratorty tests and CCUS results of hospitalized patients with COVID-19 pneumonia.
| Variable | N = 156 |
|---|---|
| Male, n (%) | 102 (65.4) |
Ethnicity, n (%):
|
122 (78.2) 30 (19.2) 4 (2.6) |
| Past history of VTE, n (%) | 2 (1.3) |
| Known thrombophilia, n (%)⁎ | 1 (0.6) |
| Active cancer, n (%) | 16 (10.3) |
| Body mass index, kg/m2 (SD) | 26.9 (4.2) |
| Clinical characteristics | |
| SARS-COV2 confirmed by PCR, n (%) | 133 (85.3) |
| Days of hospitalization until CCUS, median (IQR) | 9 (5–17) |
| Transferred from ICU, n (%) | 16 (10.3) |
| Oxygen saturation, % (SD) | 94.9 (2.8) |
Oxygen therapy, n (%)
|
32 (20.5) 83 (53.2) 25 (16.0) 10 (6.4) 6 (3.8) |
| Laboratory tests | |
| Lymphocyte (×109/l), median (IQR) | 0.9 (0.6–1.3) |
| Platelets (×109/l), mean (SD) | 264.5 (132.4) |
| D-dimer, ng/ml (IQR) | 2148 (1532–4002) |
| Interleukin-6, pg/ml (IQR)$ | 46.1 (11.0–122.1) |
| Ferritin, ug/l (IQR)& | 881.5 (409–1595) |
| LDH, U/l (IQR)% | 336 (251–418) |
| C-reactive protein in mg/dL, median (IQR) | 5.1 (1.2–16.1) |
| CCUS results | |
Deep vein thrombosis, n (%):
|
23 (14.7) 1 (0.6) 22 (14.1) 7 (4.5) 16 (10.3) |
* Included genetic thrombophilia (Factor V Leiden, Prothrombin G20210A, Protein C deficiency, Protein S deficiency and Antithrombin deficiency) and acquired thrombophilia (antiphospholipid syndrome).
When the data were not available in all patients, it is indicated below:
&data available in 124 patients; $data available in 101 patients; %data available in 152 patients.
CCUS: complete compression doppler ultrasound; ICU: intensive care unit; VTE: Venous thromboembolism.
Patients with DVT had higher D-dimer levels: 4527 (IQR 1925–9144) ng/ml vs 2050 (IQR 1428–3235) ng/ml; p < 0.001. These differences persisted after multivariable logistic regression analysis adjusted for age, sex, body mass index and presence of active cancer (adjusted OR 9.8; CI 95% 2.9–33.7; p < 0.001). There were no significant differences in the rest of the variables between both groups (Table 2 ).
Table 2.
Differential characteristics of COVID-19 pneumonia patients with and without DVT.
| Variable | DVT (N = 23) | Non-DVT (N = 133) | p-value |
|---|---|---|---|
| Age, years (SD) | 66.7 (15.2) | 68.4 (14.4) | 0.603 |
| Male, n (%) | 14 (60.9) | 88 (66.2) | 0.622 |
Ethnicity, n (%):
|
19 (82.6) 3 (13.0) 1 (4.3) |
103 (77.4) 27 (20.3) 3 (2.3) |
0.403 |
| Active cancer, n (%) | 2 (8.7) | 14 (10.5) | 1.000 |
| Body mass index, kg/m2 (SD) | 27.7 (5.5) | 26.8 (3.9) | 0.425 |
| Days of hospitalization, median (IQR) | 12 (5–19) | 9 (4.5–15.5) | 0.136 |
| Required ICU admission, n (%) | 3 (13.0) | 13 (9.8) | 0.708 |
| Oxygen saturation, % (SD) | 94.5 (3.2) | 95 (2.7) | 0.453 |
| Lymphocyte (×109/l), median (IQR) | 1.0 (0.6–1.3) | 0.9 (0.6–1.3) | 0.483 |
| Platelets (×109/l), mean (SD) | 292.2 (147.4) | 259.7 (129.7) | 0.280 |
| D-dimer, ng/ml (IQR) | 4527 (1925–9144) | 2050 (1428–3535) | <0.001 |
| Interleukin-6, pg/ml (IQR) | 66.9 (13.0–184.7) | 43.0 (10.2–119.5) | 0.403 |
| Ferritin, ug/l (IQR) | 742 (323.5–1082.5) | 885 (455.0–1610.0) | 0.311 |
| LDH, U/l (IQR) | 360 (289.0–570.0) | 325 (247.0–410.5) | 0.053 |
| C-reactive protein, mg/dl (IQR) | 2.5 (0.7–9.6) | 5.2 (1.5–16.5) | 0.174 |
DVT: deep venous thrombosis; SD: standard deviation; IQR: interquartile range.
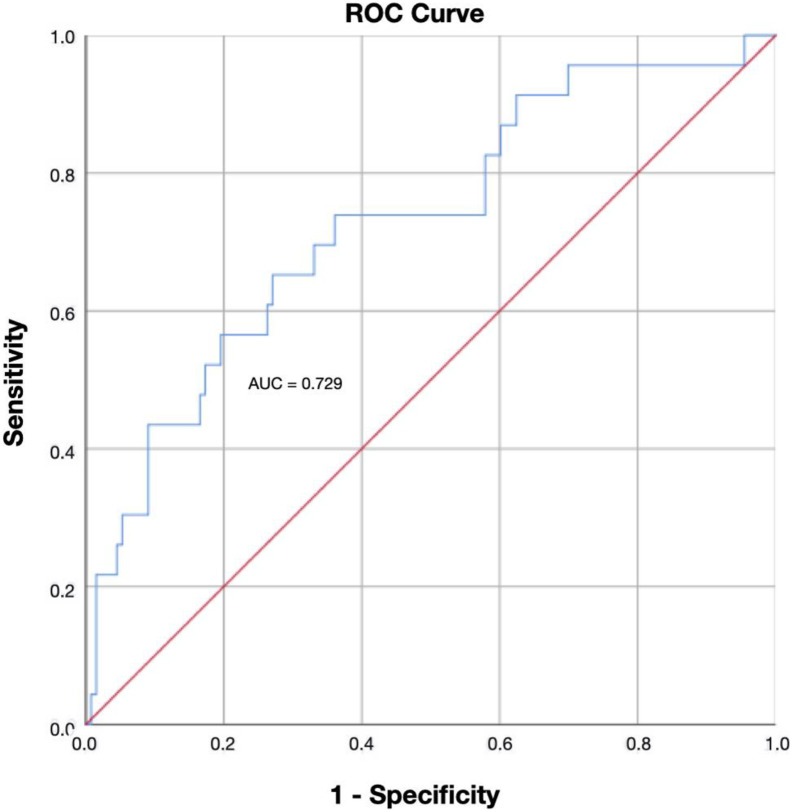
A D-dimer cutoff point of 1570 ng/ml showed a sensitivity of 95.7%, specificity of 29.3%, positive predictive value of 19% and negative predictive value of 97.5%, and was chosen as the optimal cutoff point for ruling out asymptomatic DVT. D-dimer levels over 1570 ng/ml were associated with higher incidence of asymptomatic DVT (crude OR: 9.1; CI 95% 1.1–70.1). D-dimer levels were categorized into three ranges: 1000–1800, 1801–2650 and >2650 ng/ml. When we compared the 3 groups, we found differences in the prevalence of DVT: 3.8%, 10.5% and 25.8% respectively (p = 0.003). D-dimer showed an acceptable discriminative capacity (area under the ROC curve 0.72, 95% CI 0.61–0.84) (Fig. 1 ). The predicted and the observed DVT rate showed no significant differences (p = 0.807) (Table 3 ).
Fig. 1.
Area under ROC curve for D-dimer in DVT diagnosis. (AUC: Area under ROC curve; ROC: receiver operating characteristic; DVT: deep vein thrombosis).
Table 3.
Comparison between predicted and observed DVT rate. p = 0.807.
| Predicted |
||||
|---|---|---|---|---|
| DVT | Non-DVT | Percentage correct | ||
| Observed | DVT | 4 | 19 | 17.4 |
| Non-DVT | 2 | 131 | 98.5 | |
| Overall percentage | 86.5 | |||
DVT: deep venous thrombosis.
4. Discussion
COVID-19, a viral respiratory illness caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), may predispose patients to thrombotic disease, both in the venous and arterial circulation, due to excessive inflammation, platelet activation, endothelial dysfunction, and stasis [6].
Several recent studies have reported a higher incidence of arterial and venous thrombosis in COVID-19 patients [[7], [8], [9]]. However, scarce data has been published about incidental VTE in patients with COVID-19. A recent study from China reported that 40% of hospitalized patients with COVID-19 were at high risk of VTE using the Padua model. The study did not provide data about the use of thromboprophylaxis or the VTE events [10]. In a retrospective study, the incidence of VTE was 25% among 81 patients with severe COVID-19 admitted to ICU. Of note, none of the patients had received thromboprophylaxis [11]. A multicenter study of 184 patients with severe COVID-19 from the Netherlands reported 28 (15.2%) episodes of VTE, estimating a VTE cumulative incidence of 27% (95% CI 17–37%). All patients received thromboprophylaxis [5]. Another study reported a high incidence of symptomatic VTE in ICU patients (28%), while it was lower in ward patients (3.3%) [9]. All these results suggested that VTE remains underdiagnosed in patients with severe COVID-19.
Our study showed a high incidence (14.7%) of asymptomatic DVT in a cohort of patients admitted in non-intensive care units with COVID-19 pneumonia. However, this incidence is similar to that reported in other recent study about asymptomatic DVT in internal medicine setting (16.2%) [12] and even lower than that reported in orthopedic surgery setting [13,14]. Of note, patients in our study had an expectedly high thrombotic risk, since median days of admission was 9 and all patients had COVID-19 pneumonia and a cut-off D-dimer level of 1000 ng/ml.
The most consistent hemostatic abnormalities with COVID-19 include mild thrombocytopenia [15] and increased D-dimer levels [16]. These hemostatic changes, together with the higher rate of mortality associated with them, indicate some form of coagulopathy that may predispose to thrombotic events, although the cause is uncertain [6]. D-dimer can be elevated in various conditions, such as pregnancy, postoperative state, malignancy, and sepsis, which lowers its specificity [17]. In combination with a low pretest probability for VTE, a normal D-dimer has been shown to rule out VTE due to its high sensitivity (80%–100%) and NPV up to 100% [18].
Elevated D-dimer levels are a common finding in patients with COVID-19 [15] and diagnosis of VTE might be challenging. Thus, the index of suspicion for VTE should be high in the case typical DVT symptoms, hypoxemia disproportionate to known respiratory pathologies, or acute unexplained right ventricular dysfunction [6]. Validated diagnostic algorithms in patients with suspected PE, like the YEARS clinical decision rule, incorporate different D-dimer cutoff values at presentation to increase the specificity when compared to the fixed threshold of 500 ng/ml [19]. In this respect, a recent prospective study showed that a combination of a low clinical pretest probability and a d-dimer level < 1000 ng/ml in outpatients allows to rule out PE [20]. Only one previous study has retrospectively assessed the role of D-dimer for the diagnosis of VTE in patients with COVID-19 pneumonia. A single center study from China included 81 critically ill patients and suggested that D-dimer levels > 1500 ng/ml had a sensitivity of 85.0%, a specificity of 88.5% and a NPV of 94.7% for detecting VTE events. However, the study was limited by small sample size and lack of validation [8]. Therefore, we prospectively searched for the optimal D-dimer cutoff point to exclude DVT, resulting in 1570 ng/ml with a NPV of 97.5%, similar results to the previously reported.
This study has several limitations: First, the study was limited to screening for asymptomatic DVT and therefore the incidence of pulmonary embolism and the role of D-dimer in the diagnosis of PE in this population remains unknown. Second, the restricted sample size could have limited the significance of our findings.
5. Conclusion
In patients admitted with COVID-19 pneumonia and elevated D-dimer levels, the incidence of asymptomatic DVT is similar to that described in other series. Higher cut-off levels for D-dimer might be necessary for the diagnosis of DVT in COVID-19 patients.
Footnotes
The study did not receive any funding.
References
- 1.Bruni-Fitzgerald K.R. Venous thromboembolism: an overview. J Vasc Nurs. 2015;33(3):95–99. doi: 10.1016/j.jvn.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Ordieres-Ortega L., Demelo-Rodríguez P., Galeano-Valle F., Kremers B.M.M., Ten Cate-Hoek A.J., Ten Cate H. Predictive value of D-dimer testing for the diagnosis of venous thrombosis in unusual locations: a systematic review. Throm Res. 2020;189:5–12. doi: 10.1016/j.thromres.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C., He J.X. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Throm Res. 2020 doi: 10.1016/j.thromres.2020.04.013. pii: S0049-3848(20)30120-1 [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., Der Nigoghossian C. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.031. pii: S0735-1097(20)35008-7 [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann H. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020 doi: 10.7326/M20-2003. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14888. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang T., Chen R., Liu C. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7(5):e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020 doi: 10.1111/jth.14830. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciuti G., Grifoni E., Pavellini A., Righi D., Livi R., Perfetto F., Abbate R., Prisco D., Pignone A.M. Incidence and characteristics of asymptomatic distal deep vein thrombosis unexpectedly found at admission in an Internal Medicine setting. Thromb Res. 2012;130(4):591–595. doi: 10.1016/j.thromres.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Clayton R.A.E., Gaston P., Watts A.C., Howie C.R. Thromboembolic disease after total knee replacement: experience of 5100 cases. Knee. 2009;16:18–21. doi: 10.1016/j.knee.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Kassai B., Boissel J., Cucherat M., Sonie S., Shah N.R., Leizorovicz A. A systematic review of the accuracy of ultrasound in the diagnosis of deep venous thrombosis in asymptomatic patients. Thromb Haemost. 2004;91(4):655–656. doi: 10.1267/THRO04040655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lippi G., Plebani M., Michael Henry B. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lippi G., Favaloro E.J. D-dimer is associated with severity of coronavirus disease 2019 (COVID-19): a pooled analysis. Thromb Haemost. 2020;120(5):876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Righini M., Van Es J., Den Exter P.L. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311(11):1117–1124. doi: 10.1001/jama.2014.2135. [DOI] [PubMed] [Google Scholar]
- 18.Wells P.S., Anderson D.R., Rodger M., Stiell I., Dreyer J.F., Barnes D., Forgie M., Kovacs G., Ward J., Kovacs M.J. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98–107. doi: 10.7326/0003-4819-135-2-200107170-00010. [DOI] [PubMed] [Google Scholar]
- 19.van der Hulle T., Cheung W.Y., Kooij S., Beenen L.F.M., van Bemmel T., van Es J., Faber L.M., Hazelaar G.M. Years Study Group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289–297. doi: 10.1016/S0140-6736(17)30885-1. [DOI] [PubMed] [Google Scholar]
- 20.Kearon C., de Wit K., Parpia S., Schulman S., Afilalo M., Hirsch A. Study Investigators. Diagnosis of pulmonary embolism with d-Dimer adjusted to clinical probability. N Engl J Med. 2019;381(22):2125–2134. doi: 10.1056/NEJMoa1909159. [DOI] [PubMed] [Google Scholar]