Abstract
Objective
To analyze the risk factors for pulmonary embolism (PE) in patients infected with COVID-19.
Methods
We conducted an observational, retrospective study. Patients with severe infection with COVID-19 and suspected PE were included.
Results
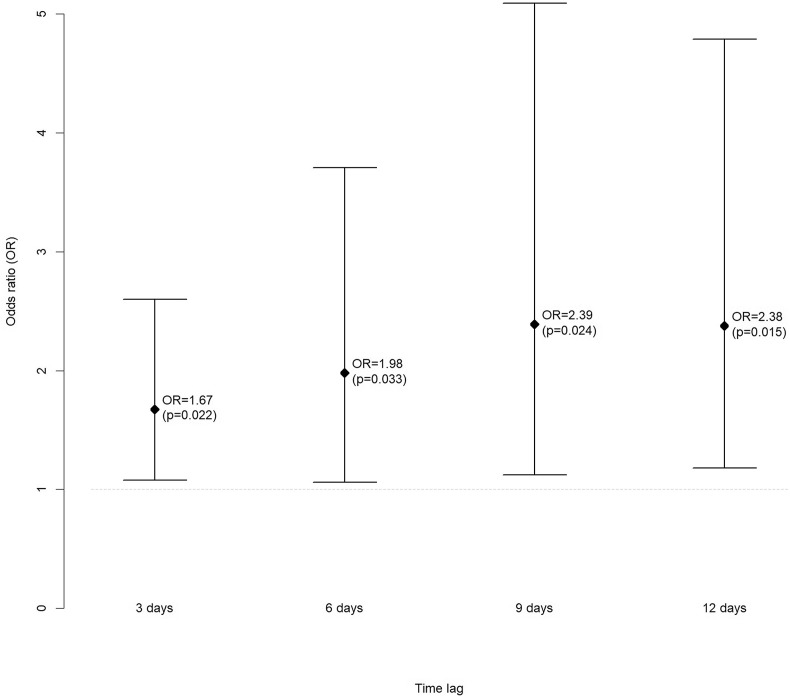
Patients with higher levels of D-dimer and those requiring intubation were at a higher risk of developing PE. Higher D-dimer levels were associated with a greater probability of PE 3, 6, 9 and 12 days after determining D-dimer levels with an OR of 1.7, 2.0, 2.4 and 2.4, respectively.
Conclusion
In conclusion, patients infected with COVID-19 requiring OTI with higher levels of D-dimer have an increased risk of developing PE.
Keywords: COVID-19, D-dimer, Pulmonary embolism
1. Introduction
Since the beginning of the COVID-19 pandemic, some authors have suggested that the infection may directly impact cardiovascular disease, either by increasing the risk of severe disease and death in patients with preexisting cardiovascular disease (CVD) or by the association of COVID-19 infection with cardiovascular complications including acute myocardial injury, arrhythmias and venous thromboembolism (VTE) [1].
Our objective was to describe our experience with patients infected with COVID-19 in whom pulmonary embolism (PE) has been suspected and to analyze the associated factors.
2. Methods
2.1. Location
This was an observational, retrospective study, conducted in a referral hospital in Badalona (Barcelona, Spain), with 600 beds, covering a population of 700,000 inhabitants.
2.2. Study subjects
Patients hospitalized for severe infection with COVID-19 and suspected PE during a two-week period were included. Suspicion was mainly based on a worsening of respiratory symptoms and increases in D-dimer values. Patients with suspected PE underwent diagnostic procedures in order to confirm the diagnosis.
2.3. Statistical analysis
Categorical variables are expressed as frequencies, while quantitative variables are expressed as the mean and standard deviation (SD). Qualitative variables were compared with Fisher's exact test, while quantitative variables were compared using the t-student test. These analyses were conducted with version 15 of STATA.
We were also interested in assessing whether D-dimer levels might predict the development of PE in the following days. To this end, we first recorded, for each determination of D-dimer levels, whether the patient had developed PE 3, 6, 9 or 12 days after that determination. Then, separately for each of these time lags (e.g., 3 days), we fitted a mixed-effects logistic regression, with the presence of PE as the dependent binary variable (i.e., the future outcome that we wished to predict), D-dimer levels as the independent variable (i.e., the variable that we wished to use for predicting), and the subject identification as a random factor (to adjust for repeated measures). Initially we also aimed to conduct this analysis using shorter time lags (1 or 2 days), but we could not correctly fit the corresponding mixed-effects logistic regressions due to singularity. This analysis was conducted with the “lme4″ package for R [2].
2.4. Ethical considerations
The research protocol was approved by the regional ethics committee (reference PI-20-131).
3. Results
Twenty-one patients were included in the analysis. Briefly, 48% of them were female, with a mean age of 59.9 years (SD 10.9). Days between admission and suspicion of PE was 9.7, and mean dimer-D levels on admission and on suspicion of PE were 1.6 μg/mL and 5.1 μg/mL respectively, with normal D-dimer values in our laboratory being those under 0.5 μg/mL.
3.1. Potential risk factors
Neither age, gender nor days of hospitalization were not associated with an increased risk of developing PE. Patients with confirmed PE showed higher levels of D-dimer (p = 0.023). Patients who had been intubated showed a higher risk of PE (p = 0.008).
3.2. D-dimer
Higher D-dimer levels were associated with a higher probability of PE three days after the determination of the D-dimer levels (OR = 1.7, p = 0.022), 6 days after the determination (OR = 2.0, p = 0.033), 9 days after the determination (OR = 2.4, p = 0.024) and 12 days after the determination (OR = 2.4, p = 0.015) (Fig. 1 ).
Fig. 1.
Association between D-dimer levels and probability of PE 3, 6, 9 and 12 days after the determination.
4. Discussion
Our data show that in severe COVID-19 pneumonia the risk of developing PE is associated with higher progressive incremental levels of D-dimer than those observed in the group without PE. The risk of PE increases at day 3 (OR 1.67), day 6 (OR 1.98) and day 9 (OR 2.39) after the first D-dimer determination. The risk does not increase significatively after day 9 (OR 2.38).
Potential association between COVID-19 and venous thromboembolism is not new. Several authors have described hematological parameters alterations in patients infected with COVID-19 [[3], [4], [5], [6]]. Among them, some have described an association between the severity of the disease and levels of D-dimer [[4], [5], [6]]. Zhou and cols, in a retrospective, multicentric cohort study conducted in China described increasing odds of in-hospital death associated with d-dimer greater than 1 μg/mL on admission [4]. Tang et al. [5], in a series that included 183 patients with confirmed COVID-19 infection, found that patients with abnormal coagulation results, especially markedly elevated D-dimer and fibrin degradation product levels, had a higher risk of death. Furthermore, Gao and cols found that D-dimer levels were closely related to the occurrence of severe COVID-19 disease in adult patients [6]. In line with these findings, anticoagulant treatment has been found to be associated with decreased mortality in patients with severe COVID-19 disease meeting the criteria for sepsis-induced coagulopathy or with markedly elevated D-dimer levels [7].
Recently, several authors have suggested an association between COVID-19 pneumonia and VTE [8]. Some authors have described the prevalence of VTE in severely ill patients [9,10]. In the study by Cui et al. [9] the prevalence of VTE was 25%, with a sensitivity, specificity and negative predictive value of D-dimer cut-off value of 1.5 μg/mL of 85, 88.5 and 94.7%, respectively. Klok and cols [10] reported an incidence of 31% of thrombotic complications in ICU patients with COVID-19 infection. In this study, the authors did not analyze the association between levels of D-dimer and the occurrence of thrombotic events.
Although this is a short analysis it is important to note that it is one of the few studies that has investigated the risk of PE in patients infected with COVID-19, and analysed the association between D-dimer levels and PE over time. Our data would suggest that despite the high D-dimer value at admission, its rapid increment is associated with a significant risk of PE. Although the ultimate aim of our study would be to use D-dimer levels to predict the development of PE and thus prevent it, we would like to highlight that our results should be taken with caution until they are replicated with other data.
To conclude, patients with severe COVID-19 disease requiring orotracheal intubation and higher levels of D-dimer have an increased risk of developing PE. Prospective studies with a larger sample size are required in order to obtain D-dimer cut-off values to create protocols both for anticoagulant treatment and diagnostic evaluation when PE is suspected.
Author contribution
Study design: IGO, JAC, AR.
Data collecting: IGO, HSP.
Statistical analysis: IGO, JR.
Wrote the manuscript: IGO, HSP.
Read critically the manuscript: IGO, HSP, JR, JAC, AR.
Declaration of competing interest
The authors do not have any financial or personal relationships with people or organizations that may have inappropriately influenced their work in the present article.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J. Am. Coll. Cardiol. 2020 Mar 18 doi: 10.1016/j.jacc.2020.03.031. pii: S0735-1097(20)34637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates D., Maechler M., Bolker B. Fitting linear mixed-effects models using lme4. J. Stat. Software. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 3.Fan B.E., Chong V.C.L., Chan S.S.W. Hematologic parameters in patients with COVID-19 infection. Am Hematol. 2020 Mar 4 doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N., Li D., Wang X. Abnormal Coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020. Apr;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao Y., Li T., Han M. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020 Mar 17 doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang N., Bai H., Chen X. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemostasis. 2020 Mar 27 doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danzi G.B., Loffi M., Galeazzi G. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui S., Chen S., Li X., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020 doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020 doi: 10.1016/j.thromres.2020.04.13. [DOI] [PMC free article] [PubMed] [Google Scholar]