Abstract
Suicide gene vectors are being developed in many laboratories as an attractive approach to cancer therapy. However, the development of these therapies is hampered by safety concerns and limitations of efficacy. The use of tumor-specific promoters, such as survivin promoter, can provide much needed specificity to target tumor cells. However, the expression levels from these promoters is often suboptimal and hence it is imperative to enhance the activity of the cytotoxic gene of interest. We tested apoptotic activity of several mutants of proapoptotic gene bax that constitutively translocate to the mitochondria and induce apoptosis. One of these mutants with deletion of serine at position S184 (S184del) was found to be most active and showed significant antitumor activity when expressed by the survivin promoter. In vitro testing shows that this vector (Sur-BaxS184del) induces cell killing in a variety of tumor cell lines of different origin with significantly higher efficacy than wild-type bax (Sur-BaxWT). The increase in cytotoxicity was a result of enhanced induction of apoptosis in tumor cells. In contrast to cytomegalovirus (CMV) promoter-driven bax (CMV-Bax), Sur-BaxS184del caused minimum toxicity in normal human dermal fibroblasts validating its specificity and safety. In a mouse tumor model (DA-3, murine breast cancer cells), we show that intratumoral injection of Sur-BaxS184del resulted in tumor growth retardation to the same level as CMV-Bax. This study highlights the effectiveness of using bax mutants in combination with survivin promoter for tumor-targeted suicide gene therapy in a nonviral vector.
Keywords: gene therapy, cancer, apoptosis, Bax, survivin promoter
Introduction
Gene therapy is emerging as a realistic treatment for various forms of cancer.1 In an attempt to develop safer and more effective gene therapy approaches, researchers are increasingly using tumor-specific promoters (TSP) to drive the expression of the gene of interest. Numerous TSPs have emerged as highly tumor specific and effective in selectively allowing expression of the gene of interest in cancer cells, including Htert,2 Her-23 and survivin4 promoters. Survivin is a member of the inhibitor of apoptosis family of proteins that has been known to regulate apoptosis in cancer cells.5 The overexpression of survivin6 and the upregulation of survivin promoter activity7 in a variety of tumors makes it an ideal candidate for tumor-targeted gene therapy. The specificity and utility of survivin promoter for tumor targeting has been tested and validated by numerous researchers.8–11
Although TSPs offer a tumor-specific activity, the expression level of the gene of interest from these promoters is often suboptimal. This is particularly important if the attempt is to drive the expression of suicide genes such as bax, bik, bid and other members of the bcl-2 proapoptotic gene family that are regulated by post-translational cellular signaling events to induce apoptosis.12 The expression of these proapoptotic genes from TSPs can be suboptimal for apoptosis induction, but in turn may sensitize cells to other apoptosis inducing agents and chemotherapeutics. One method to circumvent this problem is to increase the activity of the proapoptotic gene by making dominant active forms. This has been achieved for other members of the Bcl-2 proapoptotic family such as truncation of bid to tbid13 or mutations in the phosphorylation sites of bik.14
Bax, a member of the bcl2 family of genes, has been extensively studied as a candidate for suicide gene therapy.15–19 Although regulation of bax by hTERT promoter in an adenovirus vector has been shown to provide tumor-specific activity without hepatotoxicity,2,20 the use of bax as a suicide gene in nonviral plasmid-based vectors has only been successful with cytomegalovirus (CMV) promoter21,22 and not with TSPs.23 Recent evidence has shown that bax apoptotic activity is regulated by translocation of cytoplasmic bax to the mitochondria under apoptotic stimuli.12,24,25 This translocation is dependent on the conformational change in the C terminus of bax protein by dephosphorylation of serine at position 18426 and possibly threonine at position 182.24 The phosphorylation and dephosphorylation of bax have been suggested to be regulated by PKC zeta27 and PP2A,28 respectively.
The objective of this study was to generate a nonviral gene therapy vector with significant and selective activity in tumor cells. For this purpose, we developed and tested survivin promoter-driven bax constructs with several mutations in the C terminus of bax that make the protein constitutively active. Cytotoxicity assays showed a significant increase in tumor cell killing by survivin promoter-driven mutant bax with minimum effect on normal cells. The increase in activity was found to be due to increased apoptosis, both in vitro and in vivo, resulting in tumor growth retardation in a murine subcutaneous tumor models.
Materials and methods
Cell lines and reagent
Human cervical cancer cell line HeLa, breast cancer cell lines MCF-7, SKBR3, MDA-MB231, colon cancer cell line HT29 and murine breast cancer cell line DA-3 DMBA were cultured in Dulbecco’s modified Eagle’s Medium supplemented with 10% fetal bovine serum and penicillin streptomycin (5000 U ml-). Murine neuroblastoma cell line TBJ was cultured in RPMI media supplemented with 10% fetal bovine serum and penicillin streptomycin (5000 U ml−1). Caspase inhibitor Z-VADfmk was from Calbiochem (San Diego, CA) and used at 40 μM to inhibit apoptosis.
Construction of plasmids
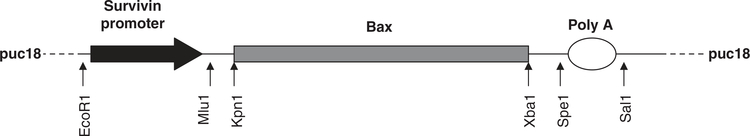
Genomic DNA was extracted from 293T cells using DNA extraction kit (Qiagen, Valencia, CA). The survivin (Sur) promoter region 1.1 kb was PCR amplified using the following primers: forward primer, 5′-CGAATTCGCGGCCGCCTGGCCATAGAACCAGAGAAGTGA-3′ and reverse primer, 5′-AAAGGTACCACGCGTCCACCTCTGCCAACGGGTCCCGCG-3′. PCR products were cloned into PCR3.1 vector using the TOPO cloning kit (Invitrogen, Carlsbad, CA) and sequenced. Subsequently, the Sur promoter region was excised with EcoRI and KpnI, and subcloned into puc18 vector. The Bax ORF from pCMV bax (Upstate-Millipore, Billerica, MA) was subcloned down streams of Sur promoter using KpnI and XbaI sites. Finally, the BGH polyadenylation signal was PCR amplified from pcDNA3.1 and cloned at the 3′ end of the bax gene to generate pSurBaxWT vector (Figure 1). Site-directed mutagenesis was used to induce mutations at positions T182A, S184A and deletion mutant S184del in bax gene using Quick Change site-directed mutagenesis kit (Stratagene, La Jolla, CA). All plasmids used for injection in mice were purified using the Endotoxin-free plasmid purification kit (Qiagen).
Figure 1.
Map of pSur-Bax wild type (WT). Arrows indicate the position of restriction sites used for cloning into puc18 backbone. Site-directed mutagenesis was used to generate T182A, S184A and S184del plasmids. pCMV-Bax was obtained commercially (Upstate-Millipore).
Cytotoxicity assay
Cell lines were seeded in 96-well plates at 10000 cells per well. Subsequent day the cells were transfected with 100 ng of luciferase reporter vector pGL3 control (Promega, Madison, WI) along with 200 ng per well of various bax vectors using ExGen 500 transfection reagent (Fermentas, Glen Burnie, MD). ExGen 500 is a 22 kDa linear polyethylenimine-based transfection reagent that binds and delivers DNA through the endocytic pathway. Cell survival was determined as luciferase activity 48 h after transfection and normalized to control vector (puc18)-transfected cells. For certain experiments, caspase inhibitor ZVAD fmk at 40 μM was added 30min before transfection. Cell viability after transfection with various plasmids was also determined using an MTS-based assay (Cell Titer one Aqueous solution, Promega) or by measuring adenosine triphosphate levels in cells using Cell Titer Glo Luminescent assay (Promega).
Apoptosis detection
HeLa cells were seeded in 24well plates at 105 cells per well. Subsequent day the cells were transfected with 1 μg per well of DNA using ExGen 500 transfection reagent. Apoptosis was determined 24 h later either as phosphatidyl serine exposure by annexin V FITC staining using the Apoalert Annexin V FITC staining kit (BD Biosceineces), or as mitochondrial depolarization by staining with 10 μM DiOC6, followed by flow cytometry on a BD FACScan Flow cytometer (BD Bioscience, San Jose, CA). At least 10000 events were collected and analyzed using cell quest software (BD Biosciences).
Western detection
HeLa cells transfected with various bax vectors were lysed in RIPA buffer 48 h after transfection. Lysates were run on 10% SDS-page gel transferred onto polyvinylidene difluoride membrane. Blots were blocked with 5% nonfat milk in tris-buffered saline for 2 h, followed by incubation with primary anti-bax antibody (Upstate) (1:1000) for 4 h. After three washes in tris-buffered saline, the blots were incubated with anti-rabbit horseradish-peroxidase conjugated antibody (1:10000) (Sigma, St Louis, MO) for 1 h. The blots were developed by enhanced chemiluminescence substrate using Western lightning reagent (Pierce, Rockford, IL). Quantification of gels was conducted using Quantity One software from Bio-Rad (Hercules, CA) and expressed as bax/tubulin ratio.
Animal studies
Six-week-old female Balb/c mice were injected with 106 DA-3 murine breast cancer cells in the flank. When tumors reached 5–6 mm in diameter, the mice were divided into different groups and treated with different vectors. Polyethyleneimine-based polymer jetPEI was used as a carrier for the DNA for in vivo studies. Polyethyleneimine is a cationic polymer that complexes with DNA to form nanoparticles that carry DNA into endosomes and then protect DNA from endocytic damage, allowing delivery of DNA to cells. Endotoxin-free DNA 50 μg was complexed with in vivo-jetPEI (polyplus transfection) and injected intratumorally every other day for a total of three injections. One mouse in each group was killed 48 h after the second injection, and the tumor was collected and fixed in neutral-buffered formalin for immunohistochemistry. Tumor volumes were measured in mice twice weekly and calculated based on the formula
where ‘a’ is the longest diameter and ‘b’ is the shortest diameter.
Results
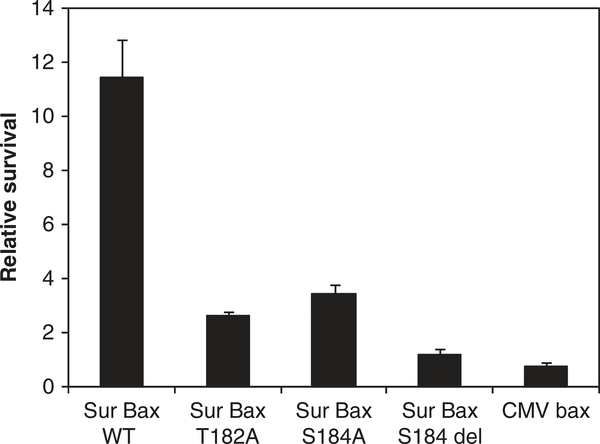
Mutations in the bax protein at positions T182 and S184 significantly increase tumoricidal activity
The suboptimal expression of suicide genes from TSPs, such as survivn promoter, warrants the need for more potent suicide genes. We approached this issue by making mutations in the C-terminal region of bax known to regulate mitochondrial translocation and apoptotic activity of bax.24,26 Mutations were made in the survivin promoter-driven bax construct (Sur-BaxWT) at positions T182A, S184A, and the deletion mutation S184del. Cytotoxicity assays were conducted in HeLa cells by co-transfection with a luciferase reporter plasmid and relative survival determined as luciferase activity compared with control. As seen in Figure 2, mutant forms of bax showed significantly higher cytotoxicity activity than Sur-BaxWT construct with the highest activity seen with S184del mutant. Although all the above bax mutations have been shown to constitutively translocate to the mitochondrial,24,26 a comparison of these mutants showed S184del to have the highest activity and hence was selected for use as the cytotoxic gene.
Figure 2.
Increase in cytotoxicity of bax mutants. HeLa cells were co-transfected with 200 ng of various bax constructs with 100 ng of pGL3 control luciferase reporter plasmid. Relative survival was determined as luciferase activity compared with puc18 control 48 h after transfection. Data are mean ± s.d. of triplicate observations. Experiments were repeated twice with similar results.
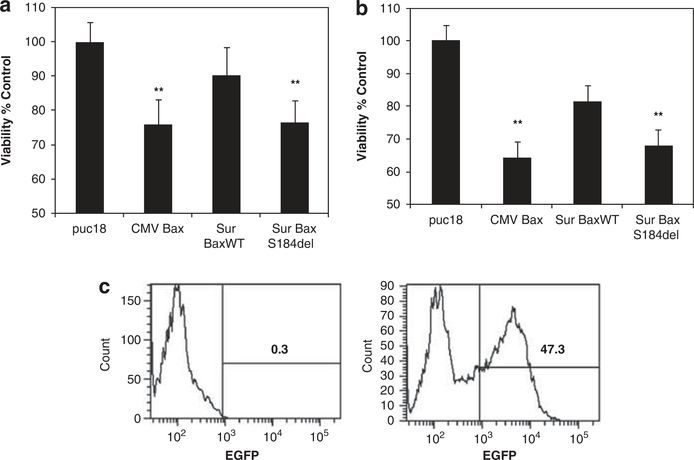
Further analysis of tumoricidal activity was conducted using cell viability assays after transfection. HeLa cells transfected with various bax constructs were assayed for cell viability 24 h later using either a MTS dye-based assay (Figure 3a) or measurement of cellular adenosine triphosphate levels (Figure 3b). Control cells transfected with a green fluorescent protein reporter and analyzed by flow cytometry showed around 47% transfection efficiency in these assays consistent with a similar loss of viability. Cell viability assays were consistent with the gene reporter assay. We saw a significant decline in cell viability with Sur-BaxS184del and CMV-Bax compared with vector-ransfected cells.
Figure 3.
Increased cytotoxicity of BaxS184del correlates with transfection efficiency: HeLa cells were transfected with indicated bax constructs or vector control. The cells were assayed 24 h after transfection for viability using either an (a) MTS assay or (b) adenosine triphosphate measurement as a correlate of cellular viability using a luminescence-based assay. Data are mean ± s.d. of triplicate observations (**P<0.01). (c) A typical transfection efficiency is shown using an EGFP plasmid transfected in HeLa cells under identical conditions as above.
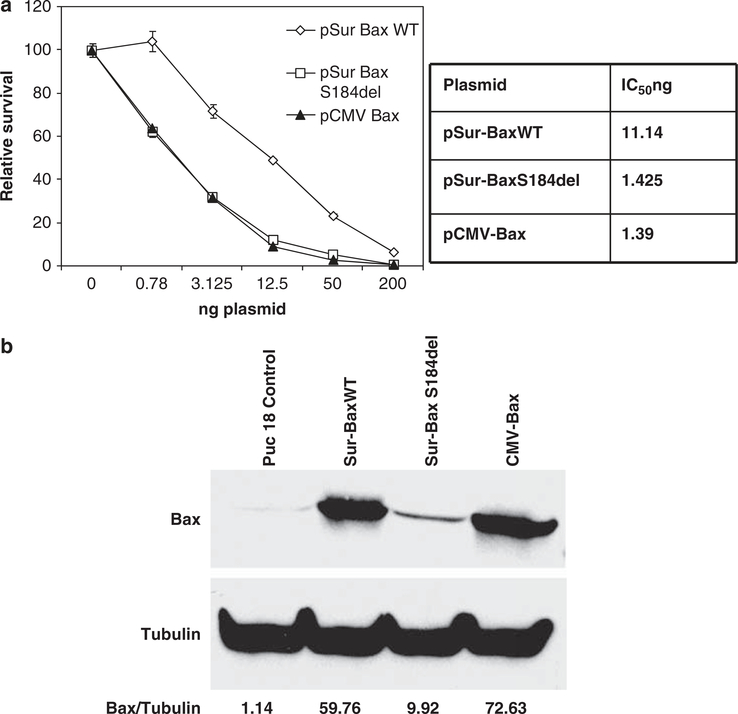
S184del mutation increases the activity of bax several fold with relatively small amounts of expression
We next wished to determine whether enhanced activity of Sur-BaxS184del would result in lower amounts of the vector needed for tumoricidal activity. A serial dilution of Sur-BaxWT, Sur-BaxS184del or CMV-Bax plasmids was used in a cytotoxicity assay in HeLa cells. As seen in Figure 4a, the S184del vector showed significantly higher activity than wild type (WT) at limiting amounts of DNA, which was comparable to CMV-Bax vector. After fitting the curves, the IC50 (expressed as ng of plasmid) for cytotoxicity with Sur-BaxS184del was found to almost 10-fold lower than Sur-BaxWT and similar to CMV-Bax. Further analysis of expression of bax protein with different constructs showed that although WT construct either under CMV or Sur promoter showed significant expression, the expression of mutant bax was relatively low (Figure 4b). Quantitative analysis shows that S184del mutant was expressed six- to seven fold lower than WT bax under Sur or CMV promoters, respectively. This is likely due to high cytotoxic activity of the mutant form, which requires only small amounts of protein accumulation before cell death. This is consistent with gene reporter-based cytotoxicity assay, in which luciferase expression is suppressed due to cytotoxicity. This suggests that the relatively low level of accumulation of the mutant form of bax is sufficient for cytotoxicity making it ideal for suicide gene therapy driven by the survivin promoter.
Figure 4.
Lower amounts of S184del vector and expression levels are needed for apoptosis induction. (a) HeLa cells were transfected with the indicated concentrations of bax plasmids along with 100 ng of luciferase reporter construct. Total amount of plasmid was 300 ng in each well and normalized in all the samples by adding the appropriate amount of puc18 control. Survival was determined as Luc activity 48 h after transfection. The curves were fit using Sigma plot software and IC50 calculated as ng of DNA required to induce 50% cytotoxicity. (b) Expression bax protein in HeLa cells transfected with various constructs detected 48 h after transfection using western blotting technique. Band intensities were quantified using Quantity One software from Bio-Rad and represented as bax/tubulin ratio. Lower levels of bax protein accumulation were detected in HeLa cells transfected with Sur-BaxS184del vector.
Increased cytotoxicity of S184del bax is due to enhanced apoptosis
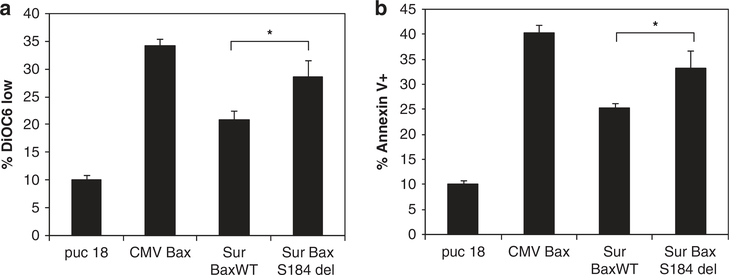
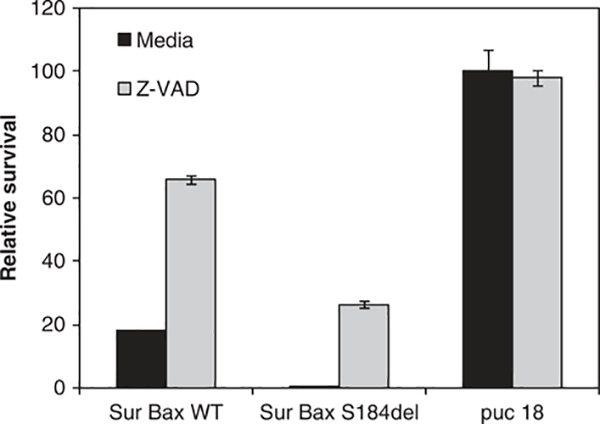
We next determined whether the increased activity of the Sur-BaxS184del vector was due to enhanced apoptosis induction. Two different markers of apoptosis, phosphatidyl serine exposure and mitochondrial membrane permeabilization, were determined in cells transfected with different suicide gene vectors. Sur-BaxS184del vector caused increased apoptosis compared with Sur-BaxWT (P<0.001), as determined by both phosphatidyl serine exposure (Figure 5b) and mitochondrial membrane permeabilization (Figure 5a). To determine whether the increased apoptosis was caspase dependent, we conducted a cytotoxicity assay in the presence or absence of pan caspase inhibitor Z-VADfmk. As seen in Figure 6, inhibition of caspase activity by Z-VAD significantly inhibited cytotoxicity in Sur-BaxWT-transfected cells, but only partially in Sur-BaxS184del-transfected cells. This suggests that the apoptosis mediated by mutant form of bax may also be partially caspase independent. These results suggest that Sur-BaxS184del causes enhanced apoptosis in cells by a partially caspase-independent mechanism.
Figure 5.
Increased cytotoxicity of BaxS184del is due to enhanced apoptosis. HeLa cells were transfected with indicated bax constructs or vector control. The cells were collected 24 h after transfection and analyzed for apoptosis. Apoptosis was determined either as (a) mitochondrial depolarization using mitochondrial potential sensitive dye DiOC6 or as (b) phosphatidyl serine exposure by Annexin V staining. Data are mean ± s.d. of triplicate observations (*P<0.01).
Figure 6.
HeLa cells were transfected with indicated bax constructs or vector control along with a luciferase reporter construct in the presence or absence of pan caspase inhibitor Z-VADfmk (40 μM). Survival was determined as Luc activity 48 h after transfection. Data are mean ± s.d. of triplicate observations. Experiment was repeated twice with similar results.
Survivin promoter-driven bax S184del shows increased tumoricidal activity in a variety of tumor cell lines
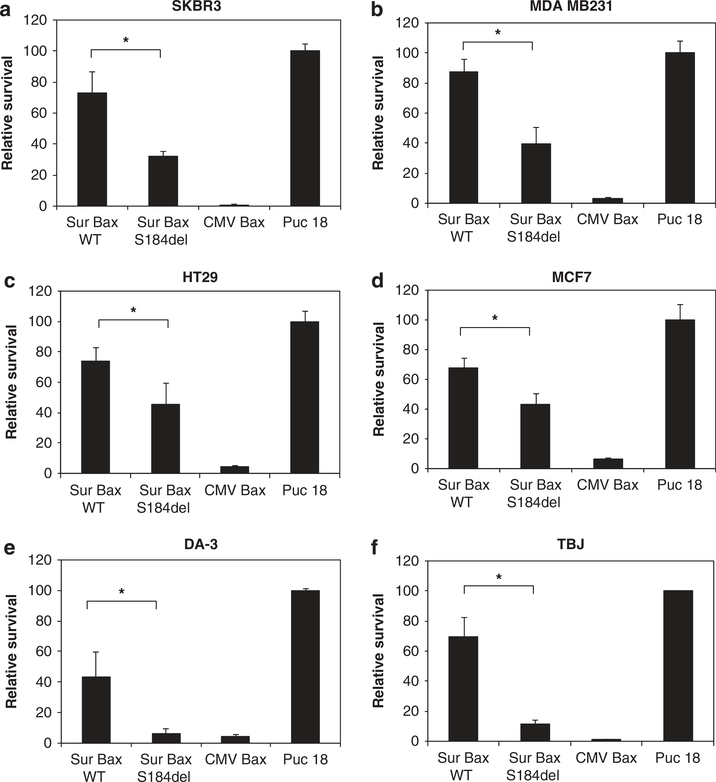
The activity of survivin promoter has been shown to be enhanced in a variety of tumor cells.7 To determine whether the enhanced activity of S184del mutant translates into increased tumoricidal activity in a variety of cells, we tested different cancer cell lines for cytotoxicity by the vectors. Both human and murine cell lines were used in cytotoxicity based on co-transfection of cells with luciferase reporter gene. As seen in Figure 7, transfection of cells with Sur-BaxS184del resulted in a significantly higher cytotoxicity than Sur-BaxWT vector in all of the cell lines tested. In most of the cell lines, although WT vector was almost nontoxic, S184del vector reduced survival to less than 50%. This suggests that mutation of bax (S184del) can significantly enhance its activity, especially in the context of a TSP, such as Sur promoter, making it reasonable to use for cancer gene therapy.
Figure 7.
Increased cytotoxicity of bax mutant in various tumor cell lines. Human breast cancer cell lines SKBR3 (a) MDA MB231 (b) and MCF7 (c) human colon cancer cell line HT29 (d) mouse breast cancer cell lines DA-3 (e) or mouse neuroblastoma cell line TBJ (f) were co-transfected with various bax constructs with a luciferase reporter plasmid as in previous experiments. Relative survival was determined 48 h later as percent luciferase activity of control-transfected cells. Data are mean ± s.d. of three independent observations (*P<0.001).
Survivin promoter-driven bax S184del shows relatively low cytotoxicity in normal human fibroblasts compared with CMV-driven bax
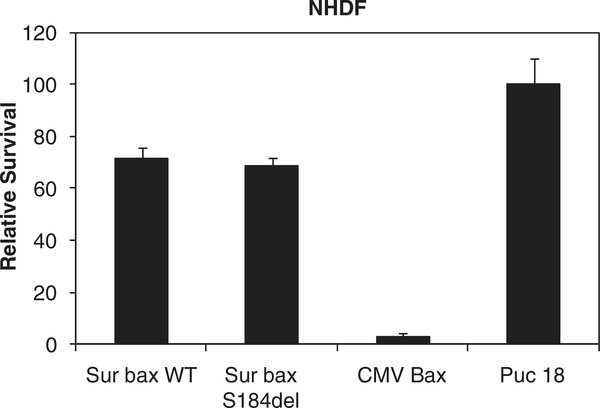
Mutational enhancement of apoptotic activity of bax raises the question whether this may result in higher toxicity in normal cells even when the expression is driven by a TSP. To address this issue, we used normal human dermal fibroblasts isolated from human skin to test the safety of the vectors. Interestingly, a gene reporter-based cytotoxicity assay in these noncancerous cells with Sur-BaxWT or Sur-BaxS184del resulted in minimal toxicity as determined by the gene reporter assay (Figure 8). On the other hand, CMV-bax was highly toxic similar to results with tumor cells, consistent with constitutive activity of CMV and lack of tumor specificity. This suggests that using mutant form of bax under the control of TSP provides significantly higher tumoricidal activity, although being nontoxic to normal tissue.
Figure 8.
Minimal toxicity of SurBaxS184del construct in normal human dermal fibroblasts. Normal human dermal fibroblasts (NHDF) were co-transfected with various bax constructs with a luciferase reporter plasmid. Relative survival was determined as percent luciferase activity of puc18 transfected.
Intratumoral injection of SurBaxS184del vector results in significant reduction in tumor growth with apoptosis induction
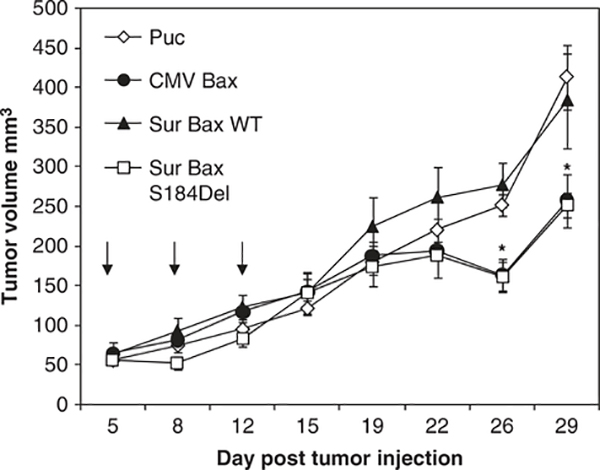
Finally, we asked whether the vectors generated would be effective in vivo using a subcutaneous murine tumor model. For this purpose, we chose the murine breast cancer cell line DA-3 shown to generate subcutaneous tumors in mice.29,30 Female Balb/c mice were injected subcutaneously with 106 DA-3 cells in the flank. When tumor size reached 5–6 mm in diameter, the mice were injected with plasmid DNA complexed with PEI cationic DNA delivery polymer. Mice received a total of three injections every other day. Tumor volumes were measured twice weekly. Forty-eight hours after the second injection, one mouse from each group was killed and the tumors were collected. Immunohistochemistry was performed on the tumors for detection of apoptosis using TdT-mediated dUTP nick end labeling assay. Mice treated with Sur-BaxS184del and CMV-Bax showed extensive apoptosis, determined by TdT-mediated dUTP nick end labeling staining (data not shown). Consistent with these results, we observed tumor growth retardation with both Sur-BaxS184del and CMV-Bax (P<0.05), although Sur-BaxWT exhibited tumor growth similar to vector control (Figure 9). These studies suggest that Sur-BaxS184del shows activity similar to CMV-bax and provides a good tumor-targeted gene therapy reagent.
Figure 9.
Balb/c mice were injected with 106 DA-3 murine breast cancer cell subcutaneously in the flank on day 0. When the tumors reached 5–6 mm in diameter, various plasmids complexed with jetPEI were injected intratumorally at 20 μg per mouse at days 5, 7 and 9. Tumor volumes were measured twice a week for 4 weeks. Data represents mean ± s.e.m. (n = 4–5 mice per group); *, where significant difference was seen with vector (puc18)-treated tumors (P<0.05). Arrows indicated days when tumors were injected with DNA PEI complex.
Discussion
Advances in cancer gene therapy have promoted the need for new and effective gene therapy vectors. Safety of gene therapy vectors can be achieved by use of promoters to drive the expression of gene of interest in specific cell types.1 In this regard, TSPs are being increasingly used for cancer gene therapy. Although the well-characterized TSPs such as Her-2,31 Htert2 and survivin7,32 promoters show highly regulated tumor-specific activity, the gene expression from these promoters can often be suboptimal. For the use of suicide genes under the control of TSPs, it is essential that the activity of the suicide gene be maximized for increased activity.
Bax is a proapoptotic member of the bcl2 family of genes that has been extensively used for suicide gene therapy.2,15,18,21,22,33–35 Bax apoptotic activity is regulated by the translocation of bax to the mitochondria, where it induces mitochondrial membrane permeabilization.24,25 It is believed that conformational changes in bax under apoptotic stimuli initiate bax translocation to the mitochondria.12,25,26 The C terminus of bax has been shown to regulate mitochondrial translocation with serine at position 18426 and Threonine at 18224 having a critical role in regulating this process. Recent evidence by Xin and Deng28 suggests that the Serine threonine phosphatase PP2A regulates bax phosphorylation under apoptotic stimuli. The authors show that dephosphorylation of bax at position S184 allows bax to translocate to the mitochondria and induce apoptosis. This information along with mutational studies of bax C-terminal domain suggest that a dominant active form of bax would be significantly more active that WT and beneficial for tumor-targeted suicide gene therapy.
Although WT bax under the control of CMV promoter has shown success in cancer gene therapy, the utilization of bax under a TSP has shown limited success, especially in adenoviral vector system.2 In nonviral system using WT bax with various TSP failed to show significant activity in vitro.23 We used several mutant forms of bax under the tumor-specific survivin promoter to test the efficacy of these nonviral vectors. Significantly increased activity of Sur-BaxWT could be achieved by the deletion mutation S184del in tumor cells. Although Sur-BaxS184del showed higher activity than WT both in vitro and in vivo, the vector was found to be nontoxic to normal human fibroblasts. This is in contrast to the CMV-Bax vector, which was equally toxic to both normal and tumor cells. The increased activity of S184del mutant was found to be due to enhanced apoptosis induction in a partially caspase-independent manner. We also show that relatively low amounts of mutant Bax were sufficient for apoptosis induction in agreement with the increased activity.
In vivo studies in DA-3 syngenic mouse tumor model showed that intratumoral injection of Sur-BaxS184del vector induces apoptosis similar to CMV-Bax vector, which was more than 50% apoptosis in tumor tissue with both the vectors. However, this apoptosis induction only translated to tumor growth retardation and not growth inhibition, most likely due to rapid growing nature of DA-3 cells in Balb/c mice. However, the activity of Sur-BaxS184del was similar to CMV-bax suggesting that the vector can significantly enhance the activity of bax under tumor-specific survivin promoter. Overall, this study provides a new improved system of using bax proapototic activity for gene therapy. Using constitutively active bax with surivivn promoter can provide a safe and effective gene therapy system that can be adapted for use with various tumors or delivery systems.
Acknowledgements
We thank Rei Ming Dai, Loretta Scheetz and Donna Butcher for technical help. This research was supported (in part) by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Lo HW, Day CP, Hung MC. Cancer-specific gene therapy. Adv Genet 2005; 54: 235–255. [DOI] [PubMed] [Google Scholar]
- 2.Gu J, Andreeff M, Roth JA, Fang B. hTERT promoter induces tumor-specific Bax gene expression and cell killing in syngenic mouse tumor model and prevents systemic toxicity. Gene Therapy 2002; 9: 30–37. [DOI] [PubMed] [Google Scholar]
- 3.Lanteri M, Ollier L, Giordanengo V, Lefebvre JC. Designing a HER2/neu promoter to drive alpha1,3galactosyltransferase expression for targeted anti-alphaGal antibody-mediated tumor cell killing. Breast Cancer Res 2005; 7: R487–R494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu ZB, Makhija SK, Lu B, Wang M, Kaliberova L, Liu B et al. Transcriptional targeting of tumors with a novel tumor-specific survivin promoter. Cancer Gene Ther 2004; 11: 256–262. [DOI] [PubMed] [Google Scholar]
- 5.Altieri DC. Survivin in apoptosis control and cell cycle regulation in cancer. Prog Cell Cycle Res 2003; 5: 447–452. [PubMed] [Google Scholar]
- 6.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene 2003; 22: 8581–8589. [DOI] [PubMed] [Google Scholar]
- 7.Bao R, Connolly DC, Murphy M, Green J, Weinstein JK, Pisarcik DA et al. Activation of cancer-specific gene expression by the survivin promoter. J Natl Cancer Inst 2002; 94: 522–528. [DOI] [PubMed] [Google Scholar]
- 8.Caldas H, Jaynes FO, Boyer MW, Hammond S, Altura RA. Survivin and Granzyme B-induced apoptosis, a novel anticancer therapy. Mol Cancer Ther 2006; 5: 693–703. [DOI] [PubMed] [Google Scholar]
- 9.Van Houdt WJ, Haviv YS, Lu B, Wang M, Rivera AA, Ulasov IV et al. The human survivin promoter: a novel transcriptional targeting strategy for treatment of glioma. J Neurosurg 2006; 104: 583–592. [DOI] [PubMed] [Google Scholar]
- 10.Zhu ZB, Chen Y, Makhija SK, Lu B, Wang M, Rivera AA et al. Survivin promoter-based conditionally replicative adenoviruses target cholangiocarcinoma. Int J Oncol 2006; 29: 1319–1329. [PubMed] [Google Scholar]
- 11.Zhu ZB, Makhija SK, Lu B, Wang M, Wang S, Takayama K et al. Targeting mesothelioma using an infectivity enhanced survivin-conditionally replicative adenoviruses. J Thorac Oncol 2006; 1: 701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol 1997; 139: 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madesh M, Antonsson B, Srinivasula SM, Alnemri ES, Hajnoczky G. Rapid kinetics of tBid-induced cytochrome c and Smac/DIABLO release and mitochondrial depolarization. J Biol Chem 2002; 277: 5651–5659. [DOI] [PubMed] [Google Scholar]
- 14.Li YM, Wen Y, Zhou BP, Kuo HP, Ding Q, Hung MC. Enhancement of Bik antitumor effect by Bik mutants. Cancer Res 2003; 63: 7630–7633. [PubMed] [Google Scholar]
- 15.Coll JL, Negoescu A, Louis N, Sachs L, Tenaud C, Girardot V et al. Antitumor activity of bax and p53 naked gene transfer in lung cancer: in vitro and in vivo analysis. Hum Gene Ther 1998; 9: 2063–2074. [DOI] [PubMed] [Google Scholar]
- 16.Haghighat P, Timiryasova TM, Chen B, Kajioka EH, Gridley DS, Fodor I. Antitumor effect of IL-2, p53, and bax gene transfer in C6 glioma cells. Anticancer Res 2000; 20: 1337–1342. [PubMed] [Google Scholar]
- 17.Norris JS, Hyer ML, Voelkel-Johnson C, Lowe SL, Rubinchik S, Dong JY. The use of Fas Ligand, TRAIL and Bax in gene therapy of prostate cancer. Curr Gene Ther 2001; 1: 123–136. [DOI] [PubMed] [Google Scholar]
- 18.Huh WK, Gomez-Navarro J, Arafat WO, Xiang J, Mahasreshti PJ, Alvarez RD et al. Bax-induced apoptosis as a novel gene therapy approach for carcinoma of the cervix. Gynecol Oncol 2001; 83: 370–377. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Marani M, Yu J, Nan B, Roth JA, Kagawa S et al. Adenovirus-mediated Bax overexpression for the induction of therapeutic apoptosis in prostate cancer. Cancer Res 2001; 61: 186–191. [PubMed] [Google Scholar]
- 20.Gu J, Kagawa S, Takakura M, Kyo S, Inoue M, Roth JA et al. Tumor-specific transgene expression from the human telomerase reverse transcriptase promoter enables targeting of the therapeutic effects of the Bax gene to cancers. Cancer Res 2000; 60: 5359–5364. [PubMed] [Google Scholar]
- 21.Okumura K, Nakase M, Nakamura S, Kamei T, Inui M, Tagawa T. Bax gene therapy for human osteosarcoma using cationic liposomes in vivo. Oncol Rep 2007; 17: 769–773. [PubMed] [Google Scholar]
- 22.Okumura K, Nakase M, Nakamura S, Inui M, Hiramoto K, Tagawa T. Antitumor activity of cationic liposome-mediated Bax gene transfer in osteosarcoma cells: induction of apoptosis and caspase-independent cell death. Int J Oncol 2005; 27: 433–438. [PubMed] [Google Scholar]
- 23.Kazhdan I, Long L, Montellano R, Cavazos DA, Marciniak RA. Targeted gene therapy for breast cancer with truncated Bid. Cancer Gene Ther 2006; 13: 141–149. [DOI] [PubMed] [Google Scholar]
- 24.Precht TA, Phelps RA, Linseman DA, Butts BD, Le SS, Laessig TA et al. The permeability transition pore triggers Bax translocation to mitochondria during neuronal apoptosis. Cell Death Differ 2005; 12: 255–265. [DOI] [PubMed] [Google Scholar]
- 25.Smaili SS, Hsu YT, Sanders KM, Russell JT, Youle RJ. Bax translocation to mitochondria subsequent to a rapid loss of mitochondrial membrane potential. Cell Death Differ 2001; 8: 909–920. [DOI] [PubMed] [Google Scholar]
- 26.Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J 1999; 18: 2330–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xin M, Gao F, May WS, Flagg T, Deng X. Protein kinase Czeta abrogates the proapoptotic function of Bax through phosphorylation. J Biol Chem 2007; 282: 21268–21277. [DOI] [PubMed] [Google Scholar]
- 28.Xin M, Deng X. Protein phosphatase 2A enhances the proapoptotic function of Bax through dephosphorylation. J Biol Chem 2006; 281: 18859–18867. [DOI] [PubMed] [Google Scholar]
- 29.Owen JL, Iragavarapu-Charyulu V, Gunja-Smith Z, Herbert LM, Grosso JF, Lopez DM. Up-regulation of matrix metalloproteinase-9 in T lymphocytes of mammary tumor bearers: role of vascular endothelial growth factor. J Immunol 2003; 171: 4340–4351. [DOI] [PubMed] [Google Scholar]
- 30.Grosso JF, Herbert LM, Owen JL, Lopez DM. MUC1/sec-expressing tumors are rejected in vivo by a T cell-dependent mechanism and secrete high levels of CCL2. J Immunol 2004; 173: 1721–1730. [DOI] [PubMed] [Google Scholar]
- 31.Yu L, Kamo S, Tagawa M. Identification of a minimal c-erbB-2 promoter region that mediates preferential expression of a linked foreign gene in human breast cancer cells. Int J Oncol 2002; 20: 607–610. [PubMed] [Google Scholar]
- 32.Chen JS, Liu JC, Shen L, Rau KM, Kuo HP, Li YM et al. Cancer-specific activation of the survivin promoter and its potential use in gene therapy. Cancer Gene Ther 2004; 11: 740–747. [DOI] [PubMed] [Google Scholar]
- 33.Tai YT, Strobel T, Kufe D, Cannistra SA. In vivo cytotoxicity of ovarian cancer cells through tumor-selective expression of the BAX gene. Cancer Res 1999; 59: 2121–2126. [PubMed] [Google Scholar]
- 34.Arafat WO, Gomez-Navarro J, Xiang J, Barnes MN, Mahasreshti P, Alvarez RD et al. An adenovirus encoding proapoptotic Bax induces apoptosis and enhances the radiation effect in human ovarian cancer. Mol Ther 2000; 1: 545–554. [DOI] [PubMed] [Google Scholar]
- 35.Groeger AM, Esposito V, Cassandro R, Baldi G, Rossiello L, De Luca L et al. A model of BAX gene delivery to human lung cancer. Anticancer Res 2001; 21: 3627–3630. [PubMed] [Google Scholar]