Abstract
Objective
This study describes early-phase development of a behavioral intervention to reduce weight regain following bariatric surgery. We utilized the Obesity-Related Behavioral Intervention Trials (ORBIT) model to guide intervention development and evaluation. We sought to establish recruitment, retention, and fidelity monitoring procedures; evaluate feasibility of utilizing weight from the electronic medical record (EMR) as an outcome; observe improvement in behavioral risk factors; and evaluate treatment acceptability.
Methods
The intervention comprised four weekly telephone calls addressing behavior change strategies for diet, physical activity, and nutrition supplement adherence and five biweekly calls addressing weight loss maintenance constructs. Veterans (N=33) who received bariatric surgery 9–15 months prior consented to a 16-week, pre-post study. Self-reported outcomes were obtained by telephone at baseline and 16 weeks. Clinic weights were obtained from the EMR six months pre- and post-consent. Qualitative interviews were conducted at 16 weeks to evaluate treatment acceptability. We aimed to achieve a recruitment rate of ≥ 25%, retention rate of ≥ 80%, and have ≥50% of participants regain <3% of their baseline weight.
Results
Results supported the feasibility of recruiting (48%) and retaining participants (93% provided survey data; 100% had EMR weight). Pre-post changes in weight (73% with <3% weight regain) and physical activity (Cohen’s ds 0.38 to 0.52) supported the potential for the intervention to yield clinically significant results. Intervention adherence (mean 7.8 calls of 9 received) and positive feedback from interviews supported treatment acceptability.
Conclusions
The intervention should be evaluated in an adequately powered randomized controlled trial.
Registration
Clinicaltrials.gov identifier NCT03246672
Keywords: severe obesity, bariatric surgery, behavioral intervention, pilot study
Introduction
Severe obesity, defined as body mass index (BMI) ≥35 kg/m2 (class II or III), is associated with several chronic diseases (Kleinman et al., 2009), decreased quality of life (Kolotkin & Andersen, 2017), poor mental well-being (Efferdinger, Konig, Klaus, & Jagsch, 2017), and decreased lifespan (Global et al., 2016). Currently, 15% of Americans have severe obesity (Flegal, Kruszon-Moran, Carroll, Fryar, & Ogden, 2016). In people with severe obesity, bariatric surgery in combination with post-operative lifestyle modification is the most effective treatment for reducing weight, improving comorbid conditions, and enhancing qualify of life (Gloy et al., 2013; Golzarand, Toolabi, & Farid, 2017).
Although most people experience clinically significant weight loss from surgery, many regain some of the lost weight (Cooper, Simmons, Webb, Burns, & Kushner, 2015; Courcoulas et al., 2013; Funk et al., 2017; Sjostrom, 2013). On average, bariatric surgery patients achieve their nadir approximately one year following surgery and begin to regain weight thereafter (Voils, Adler, Liu, & Funk, 2017).
Significant weight regain is concerning because it can lead to recurrence of obesity-related comorbidities (e.g., diabetes, hypertension), revisional bariatric procedures, increased healthcare costs, and reduced quality of life. In the Scandinavian Obesity Surgery Registry, comprised of over 26,000 patients who received Roux-en-Y gastric bypass (RYGB), patients regained an average of 4.8 kg between two and five years following surgery; greater regain was associated with recurrence of type 2 diabetes (Sundbom et al., 2017).
Nonadherence to recommended lifestyle changes plays a large role in weight regain, suggesting the need for behavioral intervention. In a cross-sectional study of patients who had undergone RYGB, weight regain was associated with poor diet quality, physical inactivity, and lack of nutritional counseling during follow-up (Freire, Borges, Alvarez-Leite, & Toulson Davisson Correia, 2012). In a cross-sectional telephone survey of patients who had undergone RYGB two to sixteen years prior, sedentary behavior and lack of moderate-to-vigorous physical activity were independently associated with weight regain and maintenance of maximum weight loss (Herman, Carver, Christou, & Andersen, 2014). Focus group and individual interviews with bariatric surgery patients have revealed that lack of social support, eating for emotional regulation, and return to previous eating habits contribute to weight regain (Benson-Davies, Davies, & Kattelmann, 2013; Wood & Ogden, 2015). Behavioral interventions are needed to help people adhere to post-operative lifestyle changes, thus reducing rates of weight regain and associated negative outcomes following surgery.
Most studies of behavioral interventions to reduce weight regain have been conducted in non-surgical populations. In fact, history or planning of bariatric surgery is often an exclusion criterion in behavioral weight management intervention trials due to the unique dietary needs of this population (Sherwood et al., 2013; Voils, Olsen, et al., 2017; Yancy Jr et al., 2010). The literature on behavioral interventions to reduce weight regain among bariatric surgery patients is relatively sparse, comprising primarily small, underpowered efficacy trials. Two systematic reviews evaluated a combined 12 randomized and non-randomized studies of behavioral interventions to reduce post-operative weight regain (Rudolph & Hilbert, 2013; Stewart & Avenell, 2016). Most studies had significant limitations in design and trial implementation. For example, most interventions focused on a single health behavior, such as limiting caloric intake or increasing physical activity, rather than the multiple lifestyle behaviors needed to maintain weight loss and health after surgery. Several randomized controlled trials (RCTs) had sample sizes <85, and only three provided a pre-specified effect size or power analysis; thus, many were likely underpowered to detect clinically significant differences. The tested interventions varied in dose, interventionist training, and timing in relation to surgery. Although one intervention involved acceptance-based psychological treatment, others were not grounded in theory or a conceptual model.
Previously we developed a conceptual model that distinguishes the psychological constructs and behavioral skills involved in weight loss initiation versus maintenance (Voils, Gierisch, et al., 2014). One distinction is between favorable expectations about outcomes, associated with initiation, versus satisfaction with outcomes, associated with maintenance (Rothman, 2000). Another is that self-efficacy to initiate dietary change is different than self-efficacy to recover from a relapse or to maintain behavior (Schwarzer & Renner, 2000). The behavioral focus also differs, with goal setting and implementation intentions used to propel behavior change, and relapse prevention planning used once new habits have been established. Finally, social support may be derived primarily from the intervention setting during initiation (e.g., from similar others in group-based interventions), whereas during maintenance, the primary source of support is people in the existing social network outside the intervention setting.
We previously used this conceptual model to develop a weight loss maintenance intervention for behavioral weight loss in a non-surgical population. In an RCT, outpatients with obesity underwent a 16-week weight loss intervention focused on calorie and fat restriction (Voils, Olsen, et al., 2017). Participants who lost at least 4 kg were eligible to be randomized to our conceptually driven maintenance intervention (N=110) or usual care (N=112). The intervention was delivered via three group sessions and eight individualized telephone calls that addressed the aforementioned weight loss maintenance constructs. Over 56 weeks, participants in the intervention group regained significantly less weight (0.75 kg) than those in the usual care group (2.36 kg).
Although behavioral interventions have been effective in reducing weight regain in non-surgical weight loss populations (Kiernan et al., 2012; Sherwood et al., 2013; Svetkey et al., 2008), it is unclear to what extent they must be modified to address the unique needs of bariatric surgery patients. Due to the debilitating nature of severe obesity, bariatric surgery patients typically have lower levels of function prior to surgery and may be unable to engage in the same duration, intensity, and types of exercise as the general population of adults with obesity. Furthermore, patients with severe obesity are more likely to experience pain when they try to exercise, which can lead them to stop if not adequately managed. These considerations suggest that patients who undergo bariatric surgery will benefit most from a graduated physical activity program that addresses their unique physical challenges.
Bariatric surgery patients also have unique dietary behaviors. Surgical patients have reduced stomach capacity that prevents them from consuming large amounts at one time, and they are typically advised to consume liquids and solids separately. This counseling can lead to different meal patterns, with more frequent grazing. Furthermore, bariatric surgery patients need to consume sufficient protein to preserve lean body mass while in a hypocaloric state (Moize et al., 2013; Schollenberger et al., 2016).
We aimed to determine to what extent our weight loss maintenance intervention, evaluated for patients who underwent behavioral weight loss, requires modification for implementation among bariatric surgery patients. We describe an early-phase study to evaluate whether the intervention is ready for more rigorous testing in a subsequent, adequately powered RCT. We sought to establish recruitment, retention, and fidelity monitoring procedures; evaluate feasibility of utilizing weight from the electronic medical record (EMR) as an outcome; observe improvement in behavioral risk factors; and evaluate treatment acceptability.
Methods
Study Design
We ground our approach in the ORBIT model of intervention development (Czajkowski et al., 2015), which comprises four phases: (1) Phase Ia: define basic elements; (2) Phase Ib refine: refine for strength and efficiency; (3) Phase IIa proof of concept: establish a clinically significant signal; and (4) Phase IIb pilot testing: establish clinically significant signal over noise. Because our study funding and timing parameters precluded stepwise progression through these phases, we utilized a hybrid approach that combined goals from multiple ORBIT phases into a single study. Our goals consistent with Phase Ib were to (1) ensure that we have the appropriate treatment components and (2) assess treatment acceptability. Our goals consistent with Phase IIa were to (1) develop the treatment manual, training materials, and fidelity monitoring procedures, and (2) evaluate whether the intervention can produce a clinically significant improvement in behavioral risk factors. Our goals consistent with Phase IIb were to (1) evaluate our recruitment and retention methods and (2) evaluate feasibility of using weight from the electronic medical record (EMR) as an outcome. To achieve these goals, we conducted a single-group, pre-post design study applied to a small sample, supplemented with post-intervention qualitative interviews. The pre-post design is consistent with Phase IIa of the ORBIT model.
To achieve our study goals in the funding period of one year, we designed an abbreviated version of the intervention that was 16 weeks in duration. Quantitative outcomes were assessed at baseline and 16 weeks, and qualitative interviews were conducted within one week of the 16-week outcome assessment.
Setting
This study was conducted in the Veterans Affairs Healthcare System (VA), which provides bariatric surgery to approximately 500 patients per year across 21 sites (Gunnar, 2017). The bariatric surgery programs offer four to five follow-up in-person visits in the first post-operative year and annual visits thereafter. Many veterans live a great distance from the facility at which they undergo surgery, providing a barrier to receiving the recommended follow-up care from the surgical team (Eisenberg et al., 2017). Even if patients are willing to travel a great distance, the frequency (i.e., annually after the first year) of follow-up may be insufficient to provide adequate behavioral skills counseling for patients to initiate and maintain the recommended, life-long lifestyle changes. To increase access and enable more frequent contact, we designed this intervention to be delivered by telephone.
This early-phase study was conducted from the VA hospital in Madison, WI. Approval was obtained from the University of Wisconsin Institutional Review Board (#2016–1433). Four VA hospitals with at least 20 bariatric surgery cases annually were selected for recruitment: Ann Arbor, MI; West Roxbury, MA; Long Beach, CA; and Palo Alto, CA.
The first participant was enrolled in January of 2018, and the final outcome assessment was conducted for the last enrolled participant in June of 2018. No changes were made to eligibility criteria or measurements after study commencement. There were no interim analyses or stopping rules.
Intervention Refinement
We identified the didactic content needed to address the unique dietary requirements of this patient population and the optimal time to begin our intervention in two ways: 1) We held a conference call with the bariatric surgeon and at least one other bariatric team member (e.g., registered dietitian (RD), nurse practitioner) at each participating site to inquire about post-operative usual care for bariatric surgery patients; and 20 We utilized data from a separate qualitative study to evaluate patient and provider experiences with severe obesity treatment decision making in the VA healthcare system (Jolles et al., 2019).
Intervention materials developed for this study include a patient workbook and an interventionist manual. Patient workbooks were mailed to participants prior to their first intervention call. The intervention manual included instructions and telephone scripts for the interventionist. This abbreviated intervention comprised four weight loss initiation calls delivered weekly followed by five weight loss maintenance calls delivered biweekly; each call had a planned duration of 30–45 minutes. The number and frequency of calls were feasible to deliver within the study funding period and allowed us to establish fidelity monitoring procedures.
Based on our conversations with the bariatric teams, we assumed that participants had received didactic content on, and initiated, lifestyle changes since surgery. However, because our conceptual model of weight loss maintenance specifies that participants should re-initiate weight loss skills if they exceed a pre-specified weight threshold, we taught participants weight loss behavioral skills, including goal setting, action planning, and self-monitoring. These skills were imparted during four calls addressing post-surgical lifestyle changes (see Supplemental Information 1 for content): meal timing and hydration (call 1); protein intake and carbohydrate quality (call 2); exercise and lifestyle physical activity (call 3); and vitamin and mineral supplements (call 4). During each call, the RD probed the participants for recommendations they remembered receiving from their bariatric teams and for current habits related to that call’s topics. The RD then provided key concepts in a conversation format. After the RD felt the participant understood the didactic content, she invited the participant to create a goal, action plan, and monitoring plan related to the topic and to write them in their workbook. The RD reviewed goal progress and invited participants to update their goals at the beginning of each subsequent call before introducing new content.
The remaining five calls—delivered every two weeks—focused on weight loss maintenance constructs based on our conceptual model (Voils, Gierisch, et al., 2014): satisfaction with outcomes of weight loss, relapse prevention, weight self-monitoring, and social support. During each maintenance call, the RD reviewed responses from the previous call and asked participants if they wanted to update the information, such as by formulating a new relapse prevention plan.
Training, Intervention Delivery, and Fidelity
At study start, the study bariatric surgeon (LF) delivered a one-hour didactic session with accompanying slides to the entire study team. This instruction included the types of bariatric procedures, complications associated with each, and recommended post-surgical lifestyle changes. A registered dietitian (RD) was chosen to deliver this intervention given the considerable knowledge needed to instruct patients about macronutrients, vitamins, and minerals. RDs are also aware of the importance of physical activity and can be taught how to deliver physical activity intervention. The RD obtained and reviewed a list of vitamin and mineral supplements, methods of obtaining them through VA pharmacy, and side effects from a VA bariatric pharmacist. The RD received training in the behavioral principles underlying the intervention from a social psychologist (CV).
To promote intervention fidelity, the telephone scripts were programmed into the VA Research Electronic Data Capture (REDCap) system. This allowed keyboard entry and enabled the interventionist to review responses from previous calls. The RD audiorecorded all calls. Each week, the study investigators and RD met for one hour to review a subset of recorded calls. The investigators completed fidelity checklists corresponding to each call’s content and provided feedback to the RD. After the first few participants were enrolled, the team made small changes to the telephone scripts to improve participant comprehension of intervention content.
Participant Recruitment and Enrollment
Veterans were eligible for the study if they had bariatric surgery (RYGB or laparoscopic sleeve gastrectomy; SG) between January 1, 2016 and June 30, 2017 at one of the four recruitment sites. Patients were targeted for enrollment approximately one year post-surgery as the VA bariatric surgery teams and previous studies identify this as a high-risk time point for beginning weight regain (Courcoulas et al., 2013; Voils, Adler, et al., 2017). Furthermore, patients are likely to lose weight in the year following surgery regardless of adherence to recommended lifestyle changes due to the anatomical changes resulting from surgery.
To identify potentially eligible patients, we applied an algorithm including International Classification of Diseases (ICD-9, ICD-10) and Current Procedural Terminology (CPT) codes from a previous study (Maciejewski et al., 2016) to data from the VA Corporate Data Warehouse (CDW). Study personnel conducted chart review to confirm that patients underwent bariatric surgery for the purpose of weight loss. The four bariatric teams were asked to provide patient lists for the recruitment timeframe for comparison to the data pull and chart review; this information provided information on the best source of potential participants for a future RCT. For recruitment into this early-phase study, study personnel determined patient eligibility using data pull and subsequent chart review rather than the site operative lists.
The study team mailed recruitment letters to patients, targeting an equal number of patients per site. At least one week later, the study team placed a screening and recruitment telephone call. Eligibility criteria assessed by telephone included English speaking, regular access to a telephone, no current cancer, no hearing impairment, and willingness to have calls audiorecorded. At the end of the recruitment call, patients provided verbal consent and were scheduled for a baseline assessment call. Within one week of consent, a research team member placed a separate telephone call to complete baseline measures. Approximately one week later, the RD delivered the first of nine intervention calls. For the weekly intervention calls, the call window was +/− four days from the target date; for the biweekly maintenance calls, the window was +/− seven days. If a participant could not complete a call within the call window, the content was covered during the next call window. Approximately 16 weeks from baseline (two weeks following the target date for the last intervention telephone call), study personnel conducted an outcome assessment by telephone.
Measures
Demographics and disordered eating habits were obtained during the baseline call; all other measures were obtained at baseline and 16 weeks. Participants received $25 for each assessment (up to $50 total). Because this was an early-phase study for which EMR weight was considered the primary outcome, assessors were not blinded.
We assessed disordered eating habits to determine if we would need to address this issue in a future RCT (e.g., by making referrals to psychology or including appropriate intervention content). We administered the 16-item Eating Disorder Examination (Beglin, 1994; Grilo, Henderson, Bell, & Crosby, 2013), which has been validated in a bariatric surgery population (see Supplemental Information 2 for content).
Dietary intake was assessed by a single-day, multiple-pass, 24-hour diet recall (Davis et al., 2015; Jonnalagadda et al., 2000; Steinfeldt, Anand, & Murayi, 2013). A study team member conducted the diet recalls, which consisted of five sets of questions designed to obtain detailed information on foods consumed the prior day. Dietary information collected included type of food, amount, brand, and preparation method. Daily physical activity was assessed with the International Physical Activity Questionnaire – Short Form for Telephone (Lee, Macfarlane, Lam, & Stewart, 2011) (Supplemental Information 2).
Maintenance and recovery self-efficacy for diet and physical activity were assessed following the methods of Schwarzer (Schwarzer, 2008) and using items that we created in a previous study (Voils, Olsen, et al., 2017) (Supplemental Information 2). Satisfaction with outcomes was assessed with a 9-item measure developed by Baldwin (Baldwin, Rothman, & Jeffery, 2009), and social support for diet and physical activity were assessed with measures developed by Ball and Crawford (Ball & Crawford, 2006) and adapted for this study (Supplemental Information 2).
Due to lack of a valid, reliable method for measuring frequency of weight self-monitoring, we created a single item for this study: “During the last four months, how often did you weigh yourself?” Response options were daily, nearly every day, 3 or 4 times a week, once or twice a week, 2 or 3 times a month, once a month, less than once a month, or never. To evaluate the feasibility of using EMR weight as an outcome, we ascertained all available EMR weights from six months prior to consent through six months post consent via a query of the VA national CDW.
Due to the minimal risk nature of this study, adverse events were not systematically assessed. Instead, participants had the opportunity to mention any health concerns during their intervention calls, outcome assessment, or qualitative interview. No participant reported an adverse event.
Qualitative Interviews
At the end of the 16-week assessment telephone call, participants were asked to participate in an optional qualitative interview to discuss their experience in the study. Within two weeks of the 16-week assessment call, the principal investigator (CV) or a study team member trained in qualitative data collection conducted structured interviews with a subset of participants to assess intervention acceptability. Questions addressed: frequency and content of follow-up appointments with the bariatric team and primary care provider; needs unmet by clinical care; workbook content and mode of delivery; recall and relevance of the four maintenance constructs (i.e., satisfaction with outcomes, weight monitoring, relapse prevention, social support); and intervention dose (i.e., duration, frequency, and amount). The interviewers recorded all conversations. To facilitate rapid analysis, the interviewers took notes on a standardized form that was organized by topic; these notes served as data for analysis, with audiorecordings consulted as needed.
Analysis Plan
Our analysis plan included: validation of the bariatric surgical case identification data pull algorithm; calculation of recruitment, retention, and adherence rates; calculation of pre-post differences in outcomes; and content-analysis of qualitative interview notes. Our predetermined target sample size was 30, which we determined would be sufficient to evaluate feasibility of study processes and treatment acceptability (Leon, Davis, & Kraemer, 2011). All calculations were performed using SAS version 9.4 software (Cary, NC).
To determine how to identify patients who received surgery for weight loss accurately, we compared the data pull algorithm to 1) operative lists provided by two bariatric teams and 2) manual chart review by a study team member. When discrepancies were present, we utilized chart review to make a final determination.
We calculated recruitment rate as the number of participants who provided consent divided by the number called. Our a priori criterion was 25%, which we deemed sufficient considering that ~500 patients receive surgery per year in the VA healthcare system and that we would likely need to recruit 150–180 patients over an 18-month period in a future RCT. We tabulated reasons for refusal and ineligibility to inform eligibility criteria or recruitment processes for a future RCT.
We calculated the retention rate for survey data as the number of participants who completed 16-week assessments divided by the number who started the intervention. We did not call participants for 16-week outcome assessments if they did not receive at least one intervention telephone call. Because our goal was not to infer efficacy, we did not follow standard RCT methods in which outcomes are sought from everyone who provides baseline measures and are analyzed as intent-to-treat (ITT), regardless of intervention adherence. Our a priori criterion for follow-up outcome assessments was 80%, a common criterion in weight management studies (Yancy Jr et al., 2010; Yancy et al., 2015).
Retention for the weight outcome was defined as the number of participants who had both a baseline and a 16-week follow-up EMR weight out of all participants with a baseline EMR weight. Baseline weight was defined as the closest EMR weight measured prior to baseline. The 16-week follow up weight was defined as the post-baseline weight measured closest to the date of follow-up. Feasibility of using EMR weight as an outcome of a future RCT requires participants to have at least one weight measurement in each six-month period (i.e., pre- and post-consent). We removed clinically implausible and same-day duplicate weights prior to analysis. Means and standard deviations were calculated from these observed weights.
For self-report measures, we followed standard protocols for calculating total and subscale scores and addressing outliers. We calculated IPAQ scores for weekly metabolic minutes of walking, moderate-intensity physical activity, vigorous-intensity physical activity, and total physical activity.
For self-reported weight, behavioral risk factors, and conceptual model constructs, we calculated means and standard deviations at baseline and 16 weeks for all participants with data at that time point. To determine evidence of clinically significant outcomes, we calculated the effect size of the pre-post difference and 95% confidence intervals. Effects were considered significant if the CI did not include 0. We performed these calculations using the SAS %effect_size macro for the one group pretest-posttest design and pooled standard deviations of raw scores (Kadel & Kip, 2012). We did not calculate inferential statistics because statistics based on small sample sizes are unlikely to reflect population-based estimates (Kraemer, Mintz, Noda, Tinklenberg, & Yesavage, 2006; Leon et al., 2011). Because weight loss of >3% is considered clinically significant (Jensen et al., 2014), we considered regain of ≤3% as evidence of weight loss maintenance. We calculated the proportion of participants who regained ≤3% of their baseline weight. Our goal was that ≥ 50% of participants would regain ≤ 3% of their baseline weight, acknowledging that this magnitude of regain would be unusual over four months. There is no clinical guideline or published literature suggesting an appropriate threshold for this short time period. For the behavioral risk factors (diet and physical activity) and conceptual model constructs, we aimed for effect sizes that differed from 0.
For intervention adherence, we calculated the mean and standard deviation of the number of intervention calls completed. Intervention adherence is one metric for treatment acceptability, as high nonadherence might signal that the dose is suboptimal or content is inappropriate (Voils, King, et al., 2014).
We conducted rapid analysis of the qualitative interview data, content-analyzing the notes from the structured interview templates (Gale et al., 2019). Two coders generated a priori codes to represent themes corresponding to the template sections. Using five completed templates, they also generated emergent codes to summarize any data not captured by the a priori coding scheme. The two investigators discussed the codes and their definitions until they achieved consensus. One investigator applied the a priori and emergent codes to the remaining templates, consulting to clarify notes when necessary. The investigators generated a summary of each section of the template to reflect representation of participant responses.
Results
Validation of Data Pull Algorithm
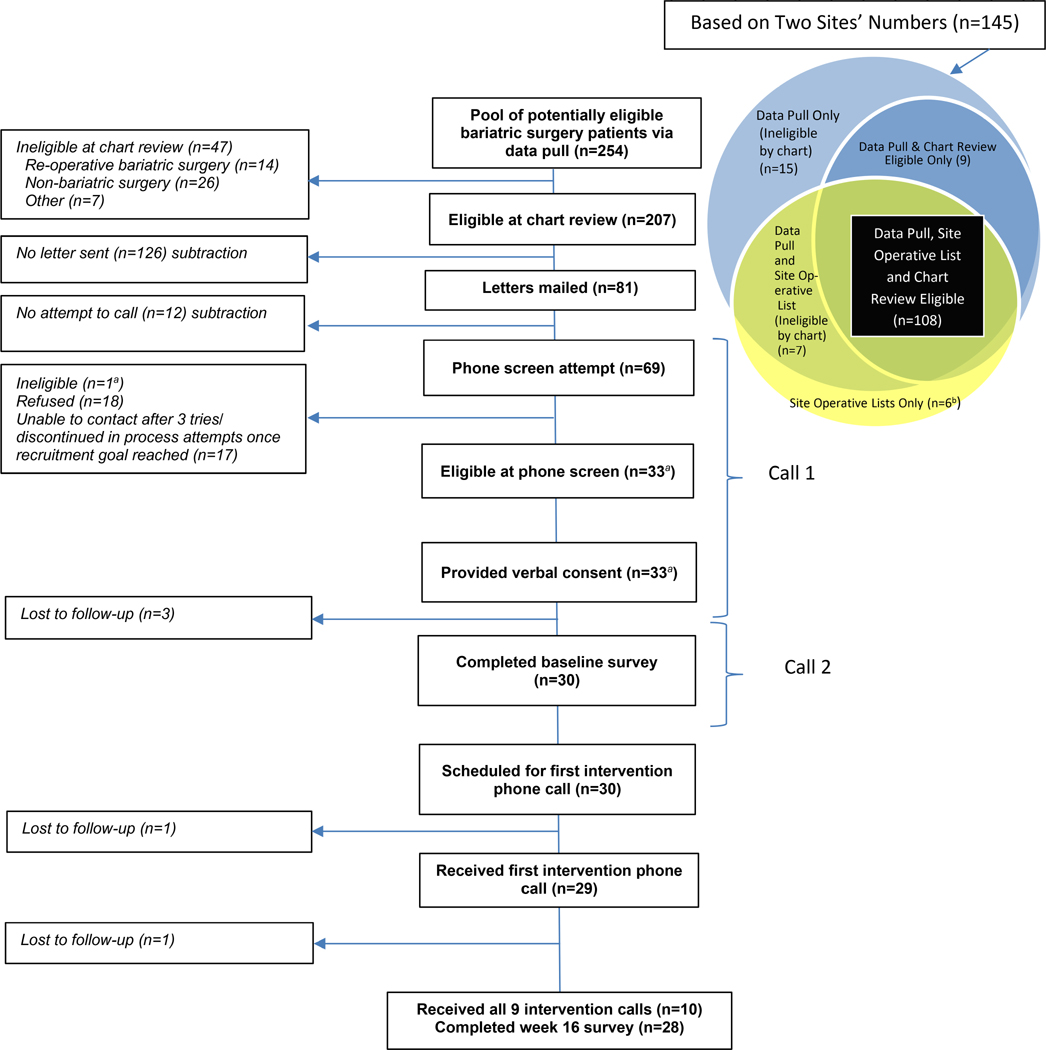
The EMR data pull yielded 254 patients. After performing chart review of these patients, we excluded 47 (19%): 14 due to re-operative bariatric surgery, 28 due to surgery for reasons other than weight loss, and 7 for other reasons (see left side of Figure 1). The two site operative lists identified additional patients (n=6) who received surgery but were not captured by the data pull (Venn diagram on right side of Figure 1). The data pull identified patients eligible for the study that did not appear on the two site operative lists (n=9); the eligibility of these patients was confirmed via chart review, and these patients were considered appropriate for recruitment.
Figure 1.
MAINTAIN-B CONSORT Flow Diagram
aOne patient was initially treated as eligible and consented, but because of uncertainty related to an exclusionary criteria, was ultimately classified as ineligible. This patient is not counted in the “Eligible at phone screen” and “Provided verbal consent” boxes of this figure.
b Not Chart Reviewed
Recruitment
We mailed recruitment letters to 81 patients (Figure 1); we stopped at 81 and did not attempt to call 12 of these 81 patients because we had achieved our target sample size. Of the 69 patients for whom telephone screening was attempted, we obtained consent from 33 (48%) and baseline measures from 30 (43%). We anticipated recruiting over a four-month period but condensed to a six-week period due to a regulatory delay. Among the 30 participants with baseline measures, the average age was nearly 57 years, 73% were White, and 80% were male. Other baseline characteristics are presented in Table 1.
Table 1.
Baseline Demographic and Clinical Characteristicsa
| Characteristic |
(N=30) |
|---|---|
| Age, years, M(SD) | 56.9 (10.0) |
| Race | |
| White, N(%) | 22 (73.3%) |
| Black, N(%) | 4 (13.3%) |
| Multiracial/Other, N(%) | 2 (6.7%) |
| Hispanic ethnicity (N%) | 2 (6.7%) |
| Female, N(%) | 6 (20.0%) |
| High school graduate or above, N(%) | 30 (100.0%) |
| Employment Status | |
| Working full or part-time, N(%) | 12 (40.0%) |
| Retired, N(%) | 12 (40.0%) |
| Unemployed, N(%) | 1 (3.3%) |
| Other/Disabled, N(%) | 5 (16.7%) |
| Nearest VA hospital/clinic very or somewhat convenient, N(%) | 27 (90.0%) |
| Disordered Eating Habits | |
| Dietary restraint | 3.6 (2.3) |
| Shape/Weight overvaluation | 1.5 (2.1) |
| Body dissatisfaction | 1.4 (2.1) |
| Weight at surgery, lb, M(SD)b | 284.3 (59.0) |
| Surgical weight loss (bariatric surgery-baseline), lb, M(SD)b | 69.4 (35.8) |
| Surgical weight loss (bariatric surgery-baseline), %, M(SD)b | 23.7 (9.4) |
| BMI at surgery, kg/m2, M(SD)b | 43.1 (7.6)` |
| BMI at baseline, kg/m2, M(SD)b | |
| Based on medical record weight and heightb | 32.7 (6.1) |
| Based on self-reported weighta and medical record heightb | 31.9 (6.0) |
Note. BMI=body mass index.
Baseline is the point of study enrollment (9–15 months after surgery). All data points, except weight and height (used in calculation of BMI) from the electronic medical record, were collected during the baseline telephone call, which was 1–8 days after the screening telephone call.
Based on weight(s) obtained from the electronic medical record.
Missing data: Baseline BMI based on self-report (n=1); race (n=2); ethnicity (n=1); convenience of nearest VA hospital (n=1).
Retention for Outcomes
We reached 28/30 participants for 16-week follow-up telephone assessments (93% retention for survey outcomes). All participants had a least one weight measured in the six months prior to consent and in the six months post-consent. From six months prior to consent to six months post-consent, all participants had multiple weights (mean=8.1 and median=7) recorded in the EMR, with a mean of 4 (median=3) during the six months after baseline. The closest weight prior to consent was measured a median of 32 (interquartile range 11–56) days prior to baseline. The post-baseline weight closest to the 16-week telephone follow-up was measured a median of 107 (interquartile range 91–134 days) post-consent.
Intervention Delivery and Adherence
After listening to the recorded intervention calls for the first few participants, we recognized that participants had been overexerting while exercising, leading to subsequent periods of inactivity We thus changed the physical activity portion of the intervention by adding language to the script to teach the concept of activity pacing, which involves setting a specific time limit on activity, stopping before pain or other symptoms emerge, and taking a rest before engaging in further activity.
We made two additional observations that did not result in changes in the current study but will inform a future RCT. The first was that one call was insufficient to address each lifestyle topic. For example, some participants knew neither the goal for daily protein intake nor how much they were consuming. Similarly, we learned that one call was insufficient to address carbohydrate quality and challenges incurred with physical activity pacing.
A second observation was that some participants listed medication and pain reduction as well as physical health (e.g., blood pressure and blood sugar) and mental health (e.g., depression) improvements as outcomes with which they were satisfied. These outcomes are not reflected in the satisfaction with outcomes measure that we used, which was developed in a non-surgical population (Baldwin et al., 2009).
Twenty-nine participants participated in the first intervention telephone call. Among these participants, the mean number of calls received (out of a maximum of 9) was 7.8 (SD=1.3, median=8, min=4, max=9). The mean duration of the four initiation calls was 31.8 min (SD=9.1 min) and of maintenance calls was 21.6 min (SD=7.6 min). All participants who received < 9 calls missed calls due to the difficulty of scheduling the weekly initiation calls in a narrow window (+/− 4 days) rather than dropout/withdrawal from the intervention.
Signal of Effect
Table 2 presents means at baseline and at the 16-week follow-up for EMR and self-reported weight, macronutrients, physical activity, and constructs related to our conceptual model. Participants maintained their weight loss during the short intervention: 73% (22 of 30) regained less than 3% of their baseline weight over the 16-week study period. Participants also reported increasing their walking, moderate, and total physical activity as measured by the IPAQ (ES 0.38 to 0.52), presented in Table 2 in metabolic minutes per week. Participants reported increases in family support for diet and physical activity (ES 0.53 and 0.46, respectively). Frequency of self-weighing increased, stayed the same, and decreased for approximately 47%, 30%, and 17% of participants, respectively. Daily nutrient intake did not change significantly from baseline, nor did self-efficacy or friend support for diet and physical activity.
Table 2.
Baseline and 16-Week Follow-up Means and Effect Sizes for Outcomes
| Outcome |
Reliabilitya |
Baseline M (SD) |
16-Week Follow-up M (SD) |
Effect Size (95% CI)b |
|---|---|---|---|---|
| Medical record weight (lb)c | n/a | 214.9 (44.3) | 213.7 (43.1) | −0.03 ( −0.13, 0.07) |
| Self-reported weight (lb) | n/a | 206.3 (35.7) | 208.3 (44.3) | 0.06 (−0.04, 0.16) |
| Daily nutrientsd | n/a | |||
| Protein intake (g/day) | 71.5 (26.2) | 71.6 (22.8) | 0.00 (−0.40, 0.40) | |
| Carbohydrate intake (g/day) | 144.4 (74.3) | 141.6 (67.2) | −0.07 (−0.51, 0.37) | |
| Dietary fiber intake (g/day) | 4.3 (3.6) | 3.2 (4.7) | −0.28 (−0.81, 0.24) | |
| Total fat intake (g/day) | 62.8 (31.7) | 74.6 (48.3) | 0.30 (−0.25, 0.85) | |
| Physical activity (metabolic minutes/week)e | n/a | |||
| Walking | 821.4 (965.5) | 1271.7 (1221.0) | 0.38 (0.07, 0.69) | |
| Moderate intensity physical activity | 342.7 (450.0) | 893.4 (1371.3) | 0.52 (0.06, 0.97) | |
| Vigorous intensity physical activity | 1124.0 (1445.2) | 1424.3 (1636.9) | 0.23 (−0.25, 0.72) | |
| Total physical activity | 2288.0 (1942.7) | 3589.4 (3237.8) | 0.49 (0.11, 0.86) | |
| Recovery self-efficacy | ||||
| Diet | .68 | 3.3 (0.6) | 3.4 (0.6) | 0.25 (−0.36, 0.85) |
| Physical activity | .73 | 3.3 (0.5) | 3.3 (0.7) | 0.10 (−0.36, 0.57) |
| Maintenance self-efficacy | ||||
| Diet | .93 | 3.3 (0.5) | 3.4 (0.5) | 0.26 (−0.13, 0.65) |
| Physical activity | .93 | 3.3 (0.6) | 3.4 (0.5) | 0.25 (−0.10, 0.60) |
| Satisfaction with outcomes | .92 | 2.5 (1.5) | 2.6 (1.3) | 0.22 (−0.02, 0.46) |
| Social supportf | ||||
| Family support-diet | .83 | 3.1 (1.1) | 3.6 (1.0) | 0.53 (0.22, 0.84) |
| Family sabotage-diet | .49 | 2.1 (0.8) | 2.0 (0.9) | −0.18 (−0.69, 0.32) |
| Family support- physical activity | .72 | 2.7 (0.8) | 3.0 (0.9) | 0.46 (0.13, 0.78) |
| Family sabotage- physical activity | .38 | 2.0 (0.7) | 2.0 (0.7) | 0.06 (−0.38, 0.49) |
| Friend support-diet | .82 | 2.7 (1.0) | 2.9 (0.9) | 0.16 (−0.29, 0.62) |
| Friend sabotage-diet | .50 | 2.1 (0.8) | 2.3 (1.1) | 0.28 (−0.22, 0.78) |
| Friend support-physical activity | .89 | 2.5 (1.1) | 2.8 (1.0) | 0.24 (−0.16, 0.63) |
| Friend sabotage- physical activity | .52 | 1.8 (0.7) | 2.1 (0.7) | 0.38 (−0.23, 0.99) |
| Frequency of weight monitoring (n (%)) | n/a | |||
| Daily | 8 (26.7%) | 11 (39.3%) | -- | |
| Nearly every day | 9 (30.0%) | 3 (10.7%) | -- | |
| 3 or 4 times a week | 3 (10.0%) | 6 (21.4%) | -- | |
| Once or twice a week | 2 (6.7%) | 6 (21.4%) | -- | |
| 2 or 3 times a month | 5 (16.7%) | 1 (3.6%) | -- | |
| Once a month | 1 (3.3%) | 1 (3.6%) | -- | |
| Less than once a month | 2 (6.7%) | 0 (0.0)% | ||
Abbreviations: M=mean, SD=standard deviation, CI=confidence interval, n/a=not applicable
Note. N=30 participants completed the baseline survey and, of those, N=28 completed 16-week follow-up surveys. Outcome data on self-reported weight were missing at baseline (n=1). At 16 weeks, there were missing data (n=1) for each daily nutrient intake outcome.
Reliability was assessed with Cronbach’s alpha.
The SAS macro %effect_size for one group pretest-posttest design was used to calculate effect sizes and 95% confidence intervals (Kadel & Kip, 2012). Calculations were restricted to participants with both baseline and 16-week data and utilized standard deviations of raw scores (rather than standard deviations of change scores).
N=30 for baseline and 16-week electronic medical record (EMR) weight. Baseline EMR weight represents the mean of participants’ weights measured closest, but prior, to baseline. 16-week EMR weight represents the mean of participants’ weights measured closest to 16 weeks post-baseline.
Obtained via Food Processor Nutrition Analysis software.
Metabolic minutes per week estimated from the Short Form of the International Physical Activity Questionnaire-Short for Telephone (Lee et al., 2011).
N=29 (baseline) and N=25 (16-week follow-up) participants reported having family in which to confide. N=22 (baseline) and N=14 (16-week follow-up) participants reported having a friend in which to confide. There were additional baseline missing values (n=1) for each of the four friend social support subscales. Reported means and standard deviations for the social support measures are based on these reduced sample sizes.
Treatment Acceptability
Qualitative interviews were conducted with 22 participants. Participants appreciated the opportunity to relearn instructions provided by the bariatric team members; some participants were particularly appreciative of the intervention because they did not attend all recommended follow-up visits with the bariatric teams due to travel distance. Some participants found that the calls began as they were beginning to fall into old unhealthy habits and thus thought the timing was appropriate, whereas others would have appreciated earlier intervention (6–9 months post-surgery) before they started to slip into old habits. Most participants reported that they found the calls on protein, hydration, and vitamin and mineral supplementation most helpful. In the initiation calls, participants appreciated learning how to set short-term behavioral goals to help them achieve or maintain their long-term weight loss goals. In the maintenance calls, participants found it helpful to think about satisfaction with outcomes because “these things are more important than the number itself.” They increased their frequency of self-weighing and navigated their high-risk situations successfully as the result of pre-planning. A few participants did not find it helpful to plan for high-risk situations because they were entrenched in new healthy habits and did not perceive risk of relapsing. All but one person identified a support person. Although a few participants selected an adult child, coworker, or friend as a support person, most participants selected a spouse/partner. Many participants consulted the study materials between intervention calls, and all reviewed them with the RD during calls. Some participants reported that they would appreciate receiving the materials electronically instead of as a hard copy by mail. Regarding dosing, participants appreciated more frequent contact initially but would have preferred less frequent long-term follow-up. They found the call duration to be adequate. A few participants expressed the desire for longer call windows due to scheduling difficulties.
Discussion
Through this early-phase study, we evaluated the feasibility of several study processes, including recruitment, retention, intervention delivery, and using weight from the EMR as an outcome. We were able to enroll patients in a relatively short time period (30 patients over six weeks) and met our a priori criterion of 25%. Investigators often must balance the number of people available to recruit, the number of full-time employees available, and the need to make up for unforeseen regulatory delays. Although we were able to shorten our recruitment period from four months to six weeks to adhere to our funding timeline, the recruitment rate likely would not be sustainable in a larger study for two reasons. First, VA surgeons perform ~500 cases per year, and the number of cases varies by month, limiting the pool of patients available for recruitment each month. Second, with a more aggressive recruitment rate, one interventionist would need to make more intervention calls than would be possible in one month. By enrolling a smaller number of participants per month at a steady rate throughout a trial (e.g., 10), the workload would be feasible for a single interventionist throughout the RCT.
We also met our a priori criterion for retention of 80%, achieving 93% for survey outcomes and 100% for EMR weight. A caveat of these promising results is that the recruitment or retention rates may differ in a randomized pilot RCT. Lower recruitment and retention rates may be observed in an RCT study because participants may be disappointed at their randomization assignment, particularly for control participants. However, in our previous trial of weight loss maintenance following behavioral weight loss (Voils, Olsen, et al., 2017), retention was higher for usual care than intervention (90% vs. 80%), perhaps because intervention participants who were less successful did not want to disappoint study personnel.
We also observed high adherence to the intervention and received positive feedback from participants in qualitative interviews, indicating high treatment acceptability. Participants were able to implement the behavioral skills and desired contact that is more frequent initially and then reduces in frequency as they successfully maintain their weight loss. Because several participants desired contact sooner after surgery than we provided, investigators should consider recruiting participants earlier (e.g., 6–9 months post-surgery).
Descriptive differences in the pre- and post-measures were as expected for weight, physical activity, and family social support for diet and physical activity. The improvement in physical activity is promising, as physical activity is important for weight loss maintenance (Schoeller, Shay, & Kushner, 1997). Whether changes in these outcomes occur more among intervention than control participants, and whether they mediate the effect of the intervention on weight regain, needs to be evaluated in an adequately powered RCT. Dietary intake, particularly protein intake, did not increase as expected. As we learned, many participants focused on measuring but not increasing protein intake. Furthermore, we conducted a 24-hour dietary assessment on only one day instead of the recommended three days, which may not capture dietary intake accurately (Jonnalagadda et al., 2000). In a future RCT it will be important to address dietary intake over a longer period and to assess dietary recall over three days.
In addition to supporting feasibility, acceptability, and signal of an effect, this early-phase study taught us several lessons to inform a larger, adequately powered RCT. First, the data pull from the EMR yielded 19% of patients who were ineligible because they received bariatric surgery for reasons other than weight loss. This suggests that investigators attempting to recruit from this population will need to supplement EMR data pull with at least one other method, such as manual chart review, as we utilized, or patient screening questions. Review of surgical notes in the EMR will ensure accuracy of information.
A second lesson learned is that we need to allow more time to teach recommended lifestyle behaviors and allow participants to practice behavioral skills. Mastery of education and behavioral skills related to weight loss (i.e., goal setting and self-monitoring of dietary intake) is important so that patients can re-engage in weight loss efforts when they start to regain weight.
A third lesson learned was that, despite our high intervention adherence rate, we need to schedule all calls further apart and with longer windows (e.g., +/− one week) to facilitate scheduling and allow patients sufficient time to practice newly learned behavioral skills. In our previous trials that used telephone as a primary mode of delivery, contact was made monthly or every two months, and adherence was high [mean of 7.3 out of 8 calls received in one trial (Voils, Olsen, et al., 2017); mean 7.9 out of 9 in another (Voils et al., 2013)]. Key to these high adherence rates, we believe, is scheduling flexibility and having a scheduling window of +/− one week around the target date for each intervention contact.
A fourth lesson relates to a variety of issues around construct measurement. One is that the Short IPAQ does not capture the variety of activities in which the bariatric surgery population engages. Alternative measurements include the long IPAQ or a more objective method such as an accelerometer. Investigators may consider budgetary constraints and specification of physical activity as a primary versus secondary outcome when making this decision. A second measurement issue is that a few social support subscales produced reliability estimates below conventional standards for acceptability (i.e., <.70). We observed similar coefficients in previous research involving weight management in a non-surgical population (Voils et al., 2016). Further psychometric work is needed to ensure that the measures are reliable and valid in these populations. A third measurement issue is that the satisfaction with outcomes measure does not include issues specific to this population, including medication, pain reduction, and physical and mental health improvements; items should be added to capture these outcomes. A fourth measurement issue is that a single 24-hour recall is not optimal for estimating dietary intake. An optimal protocol would include at least one weekday and weekend day at each assessment period (Moshfegh et al., 2008). A final measurement issue is that, although use of EMR-captured weight is consistent with a pragmatic trial approach, reviewers may be unwilling to accept it as a primary outcome due to concerns about missing data, incorrect entries, or patients leaving the healthcare system. For shorter-term studies, alternative outcomes might include obesity-specific quality of life, either as a total score or a specific subscale such as physical function. For longer-term studies, alternatives might include remission of obesity-related comorbidities, mortality, or healthcare utilization.
Lessons from our early-phase study may not generalize to other settings. First, the method for identifying patients who had bariatric surgery may not generalize to different EMR systems. Second, the feasibility and acceptability of delivering a telephone-based intervention may not generalize to bariatric surgery programs that have a small catchment area where frequent, in-person follow-up is possible and preferred. Third, the VA bariatric surgery patient population is mostly male, many of whom have psychiatric comorbidities. Predominantly female patient populations may react differently to our intervention.
Taken together, results of this early-phase study inform plans to seek funding for an adequately powered RCT. One possible design is a two-arm trial comparing the intervention to a usual care comparator; this would be consistent with the research question and appropriate given that no alternatives are currently implemented in clinical care (Freedland et al., 2019). Stratifying randomization by surgical weight loss will be important as early surgical weight loss predicts long-term weight loss (Hindle, de la Piedad Garcia, & Brennan, 2017). Although we evaluated the feasibility of using weight as a primary outcome, other constructs could be considered as primary outcomes, such as physical function given its responsiveness to change in the bariatric surgery population (Kolotkin, Crosby, Gress, Hunt, & Adams, 2009). Given participants’ desire to have more frequent contact initially and the amount of education and skills training needed after surgery to promote life-long behavior changes, the intervention could be delivered more frequently initially (e.g., biweekly) and then decrease in frequency (e.g., monthly and then bimonthly) as participants master behavioral weight loss maintenance skills.
In summary, this non-randomized, early-phase study allowed us to develop an accurate method of identifying eligible patients, evaluate recruitment and retention processes, refine intervention content, establish treatment acceptability to participants, and establish feasibility of collecting an outcome from the EMR. An adequately powered RCT is warranted to determine if the intervention yields clinically meaningful reductions in weight regain among patients who receive bariatric surgery compared to a comparator group who does not receive this intervention. With promising results, such an intervention could be implemented in clinical care to maximize long-term weight loss and associated outcomes resulting from bariatric surgery.
Supplementary Material
Acknowledgments
The authors thank Lisa Nackers, PhD for comments on early versions of the intervention scripts. The authors also thank Katherine Cronin, MPH, Elizabeth Jeanes, MPH, and Kerui Xu, PhD for assistance with data collection, Lesa Powell for conducting the electronic medical record data pull, and Aviel Alkon for programming the tracking database. This study was funded by a grant (PPO 16–331) to Dr. Voils. Effort on this grant was also made possible by a VA Health Services Research & Development (HSR&D) Research Career Scientist award (RCS 14–443) to Dr. Voils, a career development award from the National Heart, Lung, and Blood Institute to Dr. McVay (K23 HL127334-01), a VA HSR&D Career Development Award (CDA 15–060) to Dr. Funk, and resources at the Durham Center of Innovation to Accelerate Discovery and Practice Transformation (ADAPT; CIN 13–410).
References
- Baldwin AS, Rothman AJ, & Jeffery RW (2009). Satisfaction with weight loss: Examining the longitudinal covariation between people’s weight-loss-related outcomes and experiences and their satisfaction. Annals of Behavioral Medicine, 38(3), 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, & Crawford D (2006). An investigation of psychological, social, and environmental correlates of obesity and weight gain in young women. International Journal of Obesity, 30, 1240–1249. [DOI] [PubMed] [Google Scholar]
- Beglin F. a. (1994). Eating Disorder Examination Questionnaire (EDE-Q 6.0) In Fairburn CG (Ed.), Cognitive Behavior Therapy and Eating Disorders. New York: Guildford Press. [Google Scholar]
- Benson-Davies S, Davies ML, & Kattelmann K (2013). Understanding eating and exercise behaviors in post Roux-en-Y gastric bypass patients: A quantitative and qualitative study. Bariatr Surg Pract Patient Care, 8(2), 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TC, Simmons EB, Webb K, Burns JL, & Kushner RF (2015). Trends in weight regain following Roux-en-Y Gastric Bypass (RYGB) bariatric surgery. Obesity Surgery, 25(8), 1474–1481. [DOI] [PubMed] [Google Scholar]
- Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, … Longitudinal Assessment of Bariatric Surgery, C. (2013). Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. Journal of the American Medical Association, 310(22), 2416–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowski SM, Powell LH, Adler N, Naar-King S, Reynolds KD, Hunter CM, … Charlson ME (2015). From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychology, 34(10), 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KK, Tate DF, Lang W, Neiberg RH, Polzien K, Rickman AD, … Jakicic JM (2015). Racial differences in weight loss among adults in a behavioral weight loss intervention: Role of diet and physical ctivity. Journal of Physical Activity and Health, 12(12), 1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efferdinger C, Konig D, Klaus A, & Jagsch R (2017). Emotion regulation and mental well-being before and six months after bariatric surgery. Eating and Weight Disorders, 22(2), 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D, Lohnberg JA, Kubat EP, Bates CC, Greenberg LM, & Frayne SM (2017). Systems innovation model: an integrated interdisciplinary team approach pre- and post-bariatric surgery at a veterans affairs (VA) medical center. Surgery for obesity and related diseases, 13(4), 600–606. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, & Ogden CL (2016). Trends in obesity among adults in the United States, 2005 to 2014. Journal of the American Medical Association, 315(21), 2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland KE, King AC, Ambrosius WT, Mayo-Wilson E, Mohr DC, Czajkowski SM, … Riley WT (2019). The selection of comparators for randomized controlled trials of health-related behavioral interventions: recommendations of an NIH expert panel. Journal of Clinical Epidemiology, 110, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire RH, Borges MC, Alvarez-Leite JI, & Toulson Davisson Correia MI (2012). Food quality, physical activity, and nutritional follow-up as determinant of weight regain after Roux-en-Y gastric bypass. Nutrition, 28(1), 53–58. [DOI] [PubMed] [Google Scholar]
- Funk LM, Gunnar W, Dominitz JA, Eisenberg D, Frayne S, Maggard-Gibbons M, … Maciejewski ML (2017). A health services research agenda for bariatric surgery within the Veterans Health Administration. Journal of General Internal Medicine, 32(Suppl 1), 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale RC, Wu J, Erhardt T, Bounthavong M, Reardon CM, Damschroder LJ, & Midboe AM (2019). Comparison of rapid vs in-depth qualitative analytic methods from a process evaluation of academic detailing in the Veterans Health Administration. Implementation Science, 14(1), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global BMIMC, Di Angelantonio E, Bhupathiraju Sh N, Wormser D, Gao P, Kaptoge S, … Hu FB (2016). Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet, 388(10046), 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, … Nordmann AJ (2013). Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. British Medical Journal, 347, f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzarand M, Toolabi K, & Farid R (2017). The bariatric surgery and weight losing: A meta-analysis in the long- and very long-term effects of laparoscopic adjustable gastric banding, laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy on weight loss in adults. Surgical Endoscopy. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Henderson KE, Bell RL, & Crosby RD (2013). Eating disorder examination-questionnaire factor structure and construct validity in bariatric surgery candidates. Obesity Surgery, 23(5), 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar W (2017). Bariatric surgery provided by the Veterans Health Administration: Current state and a look to the future. Journal of General Internal Medicine, 32(1), 4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman KM, Carver TE, Christou NV, & Andersen RE (2014). Keeping the weight off: physical activity, sitting time, and weight loss maintenance in bariatric surgery patients 2 to 16 years postsurgery. Obesity Surgery, 24(7), 1064–1072. [DOI] [PubMed] [Google Scholar]
- Hindle A, de la Piedad Garcia X, & Brennan L (2017). Early post-operative psychosocial and weight predictors of later outcome in bariatric surgery: A systematic literature review. Obesity Reviews, 18(3), 317–334. [DOI] [PubMed] [Google Scholar]
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, … Yanovski SZ (2014). 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Journal of the American College of Cardiology, 63(25 Pt B), 2985–3023. [DOI] [PubMed] [Google Scholar]
- Jolles SA, Alagoz E, Liu N, Voils CI, Shea G, & Funk LM (2019). Motivations of males with severe obesity who pursue medical weight management or bariatric surgery. Journal of Laparoendoscopic & Advanced Surgical Techniques Part A, 29(6), 730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonnalagadda SS, Mitchell DC, Smiciklas-Wright H, Meaker KB, Heel NV, Karmally W, … Kris-Etherton PM(2000). Accuracy of energy intake data estimated by a multiplepass, 24-hour dietary recall technique. Journal of the American Dietetic Association, 100(3), 303–311. [DOI] [PubMed] [Google Scholar]
- Kadel R, & Kip K (2012). A SAS macro to compute effect size (Cohen’s d) and its confidence internal from raw survey data. . Paper presented at the Annual Southeastern SAS Users Group Conference, Raleigh/Durham, NC. [Google Scholar]
- Kiernan M, Brown SD, Schoffman DE, Lee K, King AC, Taylor CB, … Perri MG (2012). Promoting healthy weight with “stability skills first”: A randomized trial. Journal of Consulting and Clinical Psychology, 81(2), 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman NL, Melkonian A, Borden S. t., Rohrbacker N, Lynch WD, & Gardner HH (2009). The impact of morbid obesity and bariatric surgery on comorbid conditions: a comprehensive examination of comorbidities in an employed population. Journal of Occupational and Environmental Medicine, 51(2), 170–179. [DOI] [PubMed] [Google Scholar]
- Kolotkin RL, & Andersen JR (2017). A systematic review of reviews: Exploring the relationship between obesity, weight loss and health-related quality of life. Clin Obes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolotkin RL, Crosby RD, Gress RE, Hunt SC, & Adams TD (2009). Two-year changes in health-related quality of life in gastric bypass patients compared with severely obese controls. Surgery for obesity and related diseases, 5(2), 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer H, Mintz J, Noda A, Tinklenberg K, & Yesavage J (2006). Caution regarding the use of pilot studies to guide power calculations for study proposals. Archives of General Psychiatry, 63, 484–489. [DOI] [PubMed] [Google Scholar]
- Lee PH, Macfarlane DJ, Lam TH, & Stewart SM (2011). Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. The International Journal of Behavioral Nutrition and Physical Activity, 8, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon AC, Davis LL, & Kraemer HC (2011). The role and interpretation of pilot studies in clinical research. Journal of Psychiatric Research, 45(5), 626–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewski ML, Arterburn DE, Van Scoyoc L, Smith VA, Yancy WS Jr., Weidenbacher HJ, … Olsen MK (2016). Bariatric surgery and long-term durability of weight loss. JAMA Surg, 151(11), 1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moize V, Andreu A, Rodriguez L, Flores L, Ibarzabal A, Lacy A, … Vidal J (2013). Protein intake and lean tissue mass retention following bariatric surgery. Clinical Nutrition, 32(4), 550–555. [DOI] [PubMed] [Google Scholar]
- Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, … Cleveland LE (2008). The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. American Journal of Clinical Nutrition, 88(2), 324–332. [DOI] [PubMed] [Google Scholar]
- Rothman AJ (2000). Toward a theory-based analysis of behavioral maintenance. Health Psychology, 19(1, Suppl), 64–69. [DOI] [PubMed] [Google Scholar]
- Rudolph A, & Hilbert A (2013). Post-operative behavioural management in bariatric surgery: A systematic review and meta-analysis of randomized controlled trials. Obesity Reviews, 14(4), 292–302. [DOI] [PubMed] [Google Scholar]
- Schoeller DA, Shay K, & Kushner RF (1997). How much physical activity is needed to minimize weight gain in previously obese women? American Journal of Clinical Nutrition, 66(3), 551–556. [DOI] [PubMed] [Google Scholar]
- Schollenberger AE, Karschin J, Meile T, Kuper MA, Konigsrainer A, & Bischoff SC (2016). Impact of protein supplementation after bariatric surgery: A randomized controlled double-blind pilot study. Nutrition, 32(2), 186–192. [DOI] [PubMed] [Google Scholar]
- Schwarzer R (2008). Modeling health behavior change: How to predict and modify the adoption and maintenance of health behaviors. Applied Psychology, 57(1), 1–29. [Google Scholar]
- Schwarzer R, & Renner B (2000). Social-cognitive predictors of health behavior: action self-efficacy and coping self-efficacy. Health Psychology, 19(5), 487–495. [PubMed] [Google Scholar]
- Sherwood NE, Crain AL, Martinson BC, Anderson CP, Hayes MG, Anderson JD, … Jeffery RW (2013). Enhancing long-term weight loss maintenance: 2 year results from the Keep It Off randomized controlled trial. Preventive Medicine, 56(3–4), 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom L (2013). Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. Journal of Internal Medicine, 273(3), 219–234. [DOI] [PubMed] [Google Scholar]
- Steinfeldt L, Anand J, & Murayi T (2013). Food Reporting Patterns in the USDA Automated Multiple-Pass Method. Procedia Food Science, 2, 145–156. [Google Scholar]
- Stewart F, & Avenell A (2016). Behavioural interventions for severe obesity before and/or after bariatric surgery: A systematic review and meta-analysis. Obesity Surgery, 26(6), 1203–1214. [DOI] [PubMed] [Google Scholar]
- Sundbom M, Hedberg J, Marsk R, Boman L, Bylund A, Hedenbro J, … Naslund E (2017). Substantial decrease in comorbidity 5 years after gastric bypass: A population-based study from the Scandinavian Obesity Surgery Registry. Annals of Surgery, 265(6), 1166–1171. [DOI] [PubMed] [Google Scholar]
- Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, … for the Weight Loss Maintenance Collaborative Research, G. (2008). Comparison of strategies for sustaining weight loss: The Weight Loss Maintenance randomized controlled trial. Journal of the American Medical Association, 299(10), 1139–1148. [DOI] [PubMed] [Google Scholar]
- Voils CI, Adler R, Liu N, & Funk LM (2017). Understanding weight regain and the need for life-long follow-up after bariatric surgery. Current Surgery Reports, 5(33), 1–10. [Google Scholar]
- Voils CI, Coffman CJ, Yancy WS Jr., Weinberger M, Jeffreys AS, Datta S, … Bosworth HB (2013). A randomized controlled trial to evaluate the effectiveness of CouPLES: A spouse-assisted lifestyle change intervention to improve low-density lipoprotein cholesterol. Preventive Medicine, 56(1), 46–52. [DOI] [PubMed] [Google Scholar]
- Voils CI, Gierisch JM, Yancy WS Jr, Sandelowski M, Smith R, Bolton J, & Strauss JL (2014). Differentiating behavior initiation and maintenance: Theoretical framework and proof of concept. Health Education & Behavior, 41(3), 325–336. [DOI] [PubMed] [Google Scholar]
- Voils CI, Grubber JM, McVay MA, Olsen MK, Bolton J, Gierisch JM, … Yancy WS Jr (2016). Recruitment and retention for a weight loss maintenance trial involving weight loss prior to randomization. Obesity Science & Practice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voils CI, King HA, Maciejewski ML, Allen KD, Yancy WS Jr., & Shaffer JA (2014). Approaches for informing optimal dose of behavioral interventions. Annals of Behavioral Medicine, 48(3), 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voils CI, Olsen MK, Gierisch JM, McVay MA, Grubber JM, Gaillard L, … Yancy WS Jr. (2017). Maintenance of Weight Loss After Initiation of Nutrition Training: A randomized trial. Annals of Internal Medicine, 166(7), 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KV, & Ogden J (2015). Patients’ long-term experiences following obesity surgery with a focus on eating behaviour: A qualitative study. Journal of Health Psychology. [DOI] [PubMed] [Google Scholar]
- Yancy WS Jr, Westman E, McDuffie J, Grambow S, Jeffreys A, Bolton J, … Oddone E (2010). A randomized trial of a low-carbohydrate, ketogenic diet versus orlistat plus a low-fat diet for weight loss. Archives of Internal Medicine, 170(2), 136–145. [DOI] [PubMed] [Google Scholar]
- Yancy WS Jr., Mayer SB, Coffman CJ, Smith VA, Kolotkin RL, Geiselman PJ, ‥ Voils CI (2015). Effect of allowing choice of diet on weight loss: A randomized trial. Annals of Internal Medicine, 162(12), 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.