Abstract
Therapeutic development and monitoring require demonstration of effects on disease phenotype. However, due to the complexity of measuring clinically-relevant effects in rare multisystem diseases, robust biomarkers are essential. For the mucopolysaccharidoses (MPS), the measurement of glycosaminoglycan levels is relevant as glycosaminoglycan accumulation is the primary event that occurs due to reduced lysosomal enzyme activity. Traditional dye-based assays that measure total glycosaminoglycan levels have a high background, due to a normal, baseline glycosaminoglycan content in unaffected individuals. An assay that selectively detects the disease-specific non-reducing ends of heparan sulfate glycosaminoglycans that remain undegraded due to deficiency of a specific enzyme in the catabolic pathway avoids the normal background, increasing sensitivity and specificity. We evaluated glycosaminoglycan content by dye-based and non-reducing end methods using urine, serum, and cerebrospinal fluid from MPS I human samples before and after treatment with intravenous recombinant human alpha-l-iduronidase. We found that both urine total glycosaminoglycans and serum heparan sulfate derived non-reducing end levels were markedly decreased compared to baseline after 26 weeks and 52 weeks of therapy, with a significantly greater percentage reduction in serum non-reducing end (89.8% at 26 weeks and 81.3% at 52 weeks) compared to urine total glycosaminoglycans (68.3% at 26 weeks and 62.4% at 52 weeks, p<0.001). Unexpectedly, we also observed a decrease in non-reducing end levels in cerebrospinal fluid in all five subjects for whom samples were collected (mean 41.8% reduction, p=0.01). The non-reducing ends in cerebrospinal fluid showed a positive correlation with serum non-reducing end levels in the subjects (r2 = 0.65, p=0.005). Results suggest utility of the non-reducing end assay in evaluating a therapeutic response in MPS I.
Keywords: Mucopolysaccharidosis, lysosomal storage disorder, enzyme replacement therapy, Hurler, glycosaminoglycan
1. INTRODUCTION
Mucopolysaccharidosis I (MPS I) is a lysosomal storage disease caused by deficiency of the enzyme α-l-iduronidase (EC 3.2.1.76) leading to heparan sulfate (HS) and dermatan sulfate glycosaminoglycan accumulation throughout the body and dysfunction of multiple organ systems [1]. Intravenous (IV) enzyme replacement therapy with recombinant human α-l-iduronidase (rhIDU) has been available for MPS I since 2003 [2]. Administered as a weekly infusion, IV rhIDU has been shown to reduce lysosomal glycosaminoglycan storage and improve many clinical symptoms including liver and spleen size, range of motion and functional status measures [2,3]. However, IV rhIDU does not effectively treat neurologic manifestations of central nervous system glycosaminoglycan storage in MPS I patients [4]. Hematopoietic stem cell transplantation is also used in MPS I patients and can result in prevention or stabilization of central nervous system disease, possibly by the ability of donor cells to enter the brain and secrete enzyme [4,5]. Patients must be transplanted before significant central nervous system disease has occurred, and even then learning disabilities may persist [6]. Finally, intrathecal rhIDU is currently being studied for the treatment of central nervous system disease in attenuated MPS I patients as well as in severely affected MPS I patients undergoing hematopoietic stem cell transplantation [7].
One of the challenges to optimizing all of these therapies is the lack of specific biomarkers that can be effectively correlated with clinical findings. Urinary glycosaminoglycan levels measured by a dye-binding assay have been used to demonstrate effectiveness of treatment with IV rhIDU [8,9]. This type of assay measures total glycosaminoglycans including both stored lysosomal (pathologic) and normal (physiologic) glycosaminoglycans, by nonspecific binding to negative charged molecules [10,11]. Since normal physiological glycosaminoglycan levels and composition change with age and are highly variable between unaffected people, the resulting high and variable background complicates assessment of changes in lysosomal glycosaminoglycan storage in response to enzyme therapy [12,13]. Serum glycosaminoglycan levels cannot be measured with the dye-binding method. Furthermore, cerebrospinal fluid (CSF) glycosaminoglycans measured using dye-based methods show little elevation in MPS I patients over normal values [7]. A novel approach has been developed for measuring biomarkers derived from the non-reducing ends (NRE) of glycosaminoglycans that selectively accumulate due to the deficiency of a specific enzyme in the catabolic pathway [14,15]. This assay can be used to detect and quantitate disease specific pathologic glycosaminoglycans in various types of samples including blood, CSF and tissues. In contrast to the dye-based total glycosaminoglycan measurement, this method demonstrated a greater difference in glycosaminoglycan levels in the brains of MPS I dogs compared to normal dogs in studies of intrathecal rhIDU treatment [16]. Thus the NRE glycosaminoglycan detection method provides an alternative means of monitoring the efficacy of enzyme replacement or other therapies.
In this report we compared HS NRE measurement of MPS I specific pathologic glycosaminoglycans to total glycosaminoglycan measurements in monitoring the response to enzyme therapy in MPS I patients. Samples of serum, urine and CSF were previously collected from MPS I subjects treated with IV rhIDU in a completed clinical trial [2]. We measured total glycosaminoglycans in patient urine using a dye-based assay and HS NRE method in patient serum and CSF. We compared the correlation of each type of glycosaminoglycan measurement with clinical outcome measures accessed from case report forms.
2. MATERIALS AND METHODS
2.1. Samples
This study involved review of case report forms and biochemical testing of patient samples originally obtained with written informed consent [2]. The study protocol was reviewed and approved by the John Wolf Human Subjects Committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
Samples and clinical outcome data were available from 10 MPS I subjects with biochemically confirmed leukocyte α-l-iduronidase deficiency who were treated with IV rhIDU infusions once weekly for 52 weeks as part of a previously published clinical trial [2]. Eight subjects were clinically classified as Hurler-Scheie, one subject as Hurler, and one subject as Scheie subtype of MPS I. Urine, blood and CSF were collected from each patient during the treatment course and de-identified using a numerical code only. Clinical outcome data were recorded on case report forms and the data we specifically accessed for this study included: spleen and liver volume quantitated by magnetic resonance imaging; shoulder flexion, and knee and elbow extension quantitated by goniometry. We compared these clinical outcome data with biochemical assays performed on subject samples that were collected during the course of the trial.
2.2. Biochemical analysis
Numerically coded samples in 200-300 microliter aliquots were sent to Zacharon Pharmaceuticals (San Diego, CA) and the analysis performed in 2009 and 2010. HS NRE analysis was performed on all samples in a blinded fashion using the fluorescent UPLC Sensi-Pro™ Substrate Assay. This assay labels and measures the NRE of glycosaminoglycan fragments, employing high performance liquid chromatography (HPLC) to quantify the abundance of only those glycosaminoglycans that have accumulated due to reduced activity of a specific lysosomal enzyme [14]. In MPS I, these HS markers are unique NRE-derived disaccharides that terminate in iduronic acid due to the lysosomal deficiency in α-l-iduronidase [14].
Briefly, samples were prepared by normalizing each to equivalent volumes using phosphate buffered saline. Glycosaminoglycans were extracted and purified by DEAE chromatography and digested with heparin lyases (IBEX Technologies, Montreal, Canada) which degrade all forms of HS but do not cleave any other glycosaminoglycan class [14]. Finally, the glycosaminoglycans were tagged with AMAC (2-aminoacridone) fluorescent dye and analyzed by HPLC. The MPS I NRE structures (β-D-idopyranosyluronate-(1→4)-2-N-sulfamino-6-sulfo-2-deoxy-α/β-D-glucopyranoside, I0S6, [17]) were quantified based on standard curves of saturated HS derived disaccharides (Iduron, UK). HS standards were analyzed with each set of samples. At the time these samples were assayed, the coefficient of variance of the method was 13%. The current commercially available clinical diagnostic Sensi-Pro™ HS-NRE assay is based on LC-MS (ARUP) quantitation with NRE standards. For comparison with HS NRE measurements, urine total glycosaminoglycans were analyzed using an Alcian blue dye-based assay as previously described [18].
2.3. Statistical analysis
We compared pre-treatment with post-treatment glycosaminoglycan levels after 26 weeks and 52 weeks of therapy. Means and standard deviations were calculated with SigmaPlot 12 (Systat Software, Chicago, IL) and data were analyzed using repeated measures ANOVA. A p-value of less than 0.05 was considered statistically significant. We also performed linear regression analysis and computed Pearson correlation coefficients to assess the relation of glycosaminoglycan level measurements to quantitative clinical outcome measures at these time points.
3. RESULTS
At baseline prior to treatment, there was a qualitatively similar profile of glycosaminoglycan levels in the 10 MPS I subjects studied as measured by urine total glycosaminoglycans (Fig. 1a) or serum HS NRE (Fig. 1b). After 26 weeks and 52 weeks of treatment with rhIDU, each method demonstrated a noticeable reduction in the respective glycosaminoglycan levels (Tables 1 and 2, and Fig. 1a,b). Regression analysis failed to demonstrate a significant correlation between percentage changes in glycosaminoglycan levels or absolute glycosaminoglycan levels measured by each method (Fig. 2). There was an appreciable residual urinary total glycosaminoglycan level in all 10 subjects after 26 weeks and 52 weeks of treatment (Fig. 1a, grey and white bars), while in contrast serum HS NRE levels dropped nearly to zero (Fig. 1b, grey and white bars). This observation was accompanied by a significantly greater percentage reduction in glycosaminoglycan levels from baseline when measured as serum HS NRE as compared to urine total glycosaminoglycans during the course of IV rhIDU therapy (Fig. 1c). After 26 weeks of treatment the average percentage reduction in serum HS NRE was 89.8% while that in urine total glycosaminoglycans was 68.3% (p<0.001). After 52 weeks of IV rhIDU treatment the average percentage reduction in serum HS NRE was 81.3% while that in urine total glycosaminoglycans was 62.4% (p<0.001).
Fig 1. Urine glycosaminoglycan (GAG) and serum HS NRE values in MPS I subjects before and during treatment with weekly IV rhIDU.
Individual values for (a) urine total GAG and (b) serum HS NRE levels in MPS I subjects pre-treatment (black bars), after 26 weeks (grey bars), and after 52 weeks (white bars) of treatment. (c) Mean percent reduction in urine GAG and serum HS NRE after 26 weeks (89.8% vs 68.2%, p<0.001) and after 52 weeks (81.3% vs 62.4%, p<0.001) of treatment. Error bars represent standard deviation.
Table 1:
Individual subject data at week 26 of treatment with weekly IV rhIDU*
| Subject | Urine GAG mg/mL (% reduction) |
Serum HS NRE pmol/mL (% reduction) |
CSF HS NRE pmol/mL |
Reduction in liver volume |
Reduction in spleen volume |
Increase in shoulder flexion |
|---|---|---|---|---|---|---|
| 1 | 57.7 (62.8%) |
2.41 (85.9%) |
22.2% | 30.29% | 1% | |
| 2 | 84.9 (67.8%) |
1.27 (92.9%) |
34.3 | 24.3% | 36.4% | 7.4% |
| 3 | 47.9 (57.2%) |
0 (100%) |
73.2 | 25% | 18.2% | 10% |
| 4 | 59.2 (68.2%) |
1.03 (93.6%) |
26.4% | 18.95% | 9.6% | |
| 5 | 73.7 (62.8%) |
0.92 (96.4%) |
42.9 | 29.3% | 3.3% | 16.3% |
| 6 | 30.5 (85.0%) |
2.02 (86.2%) |
31.3% | 26.9% | −5.8% | |
| 7 | 21.6 (69.5%) |
0.98 (83.1%) |
17% | –18% | 0.4% | |
| 8 | 181 (64.4%) |
6.74 (80.3%) |
70.4 | 34.2% | 37.2% | −11.7% |
| 9 | 94.7 (64.7%) |
3.25 (79.3%) |
−1.7% | −104% | 21.3% | |
| 10 | 41.3 (80.3%) |
0 (100%) |
23.4% | 19.8% | 47.2% |
Dose of IV rhIDU was 0.58 mg/kg body weight weekly. Urine glycosaminoglycans (GAG) was measured by Alcian blue dye-binding method and expressed in mg/L. Serum and CSF HS NRE were measured by Sensi-Pro™ assay and expressed in pmol/mL.
Table 2:
Individual subject data at week 52 of treatment with weekly IV rhIDU*
| Subject | Urine GAG mg/mL (% reduction) |
Serum HS NRE pmol/mL (% reduction) |
CSF HS NRE pmol/mL |
Reduction in liver volume |
Reduction in spleen volume |
Increase in shoulder flexion |
|---|---|---|---|---|---|---|
| 1 | 85.6 (45.4%) |
3.61 (78.9%) |
19.1% | 24.3% | 8% | |
| 2 | 69.8 (73.6%) |
2.64 (85.3%) |
13.1% | 25.3% | −1.1% | |
| 3 | 40.0 (64.3%) |
1.28 (96.1%) |
29.5% | 21.5% | 11.0% | |
| 4 | 48.3 (74.0%) |
1.07 (93.4%) |
48.4 | 33.4% | 4.6% | 33.7% |
| 5 | 85.6 (56.8%) |
7.64 (69.8%) |
29.1% | 10.6% | 27.4% | |
| 6 | 54.0 (73.4%) |
4.19 (71.3%) | 31.4% | 33.2% | 7.1% | |
| 7 | 23.0 (66.4%) |
1.21 (79.2%) |
17.5% | 15.9% | −2.6% | |
| 8 | 294 (42.7%) |
8.71 (74.5%) |
36.6% | 37.2% | 71.5% | |
| 9 | 94.0 (64.9%) |
3.75 (76.1%) |
27.5% | 24.4% | 45.3% | |
| 10 | 79.1 (62.3%) |
2.16 (88.3%) |
22.6% | 11.0% | 41.7% |
Dose of IV rhIDU was 0.58 mg/kg body weight weekly. Urine glycsoaminoglycans (GAG) was measured by Alcian blue dye-binding method and expressed in mg/L. Serum and CSF HS NRE were measured by Sensi-Pro™ assay and expressed in pmol/mL.
Fig. 2. Relationship between serum HS NRE and urine glycosaminoglycans (GAG) by Alcian blue dye-binding method.
(a) Absolute values in MPS I subjects pretreatment, and at 26 weeks and 52 weeks of weekly IV rhIDU treatment. (b) Relationship between percent reduction in serum HS NRE values and urinary GAG in the subjects. Symbols represent individual subjects. Linear regression coefficients (least squares) shown (p=NS for all).
The total glycosaminoglycan assay is measuring the sum of pathological and physiological glycosaminoglycans so it is not surprising that the levels fall but do not reach zero because rhIDU is not expected to alter the level of normal physiological glycosaminoglycans. In contrast, since the NRE is a structure that is undetectable in samples from iduronidase sufficient people, NRE levels should approach zero when rhIDU is completely restored.
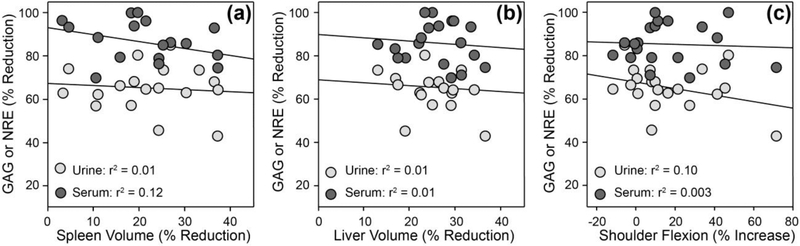
To compare the relative sensitivities of serum HS NRE or urine total glycosaminoglycan levels as biomarkers for clinical outcome measures we performed linear regression analyses of these data with measurements of spleen and liver volume, shoulder flexion, and knee and elbow extension. The individual subject data for these outcome measures are shown in Tables 1 and 2. There was no significant correlation between changes in either serum HS NRE or urine total glycosaminoglycan levels and changes in spleen volume as measured by MRI (Fig. 3a), changes in liver volume as measured by MRI (Fig. 3b) or changes in shoulder flexion as measured with goniometry (Fig. 3c). The lack of correlation is not surprising given the complexity of the disease, unknown reversibility of clinical measures, and the likely time-dependent effects of elevated substrate burden. Larger and longer-term studies will be required to fully characterize the correlation of biomarkers to clinical measures of disease.
Fig. 3. Relationship between urine glycosaminoglycans (GAG) or serum HS NRE and clinical endpoints.
Comparison between reductions in spleen volume (a) or liver volume (b) as measured by MRI, or increases in shoulder flexion (c) as measured by goniometry. Symbols represent individual subjects. Linear regression coefficients (least squares) shown (p=NS for all).
CSF glycosaminoglycans could in principle serve as a marker for central nervous system disease. CSF was available at baseline and after treatment with IV rhIDU for five of the ten subjects. The post-treatment sample used was the week 26 sample except for subject 4, for whom no week 26 sample was available and week 52 was used instead. Enzyme replacement therapy with IV rhIDU is not expected to cross the blood-brain barrier to a substantial degree. Surprisingly, we observed a significant reduction in CSF HS NRE levels in the MPS I subjects studied following 26 weeks of IV rhIDU treatment (Fig. 4a,b). There was a 41.8% reduction in the average CSF HS NRE level after 26 or 52 weeks of IV rhIDU compared to the average pre-treatment level (p=0.01). Linear regression analysis showed a significant positive correlation between CSF HS NRE and serum HS NRE measurements during the treatment course (p=0.005, Fig. 4c) though there was no significant correlation between CSF HS NRE and urine total glycosaminoglycan levels.
Fig. 4. CSF HS NRE in MPS I subjects after IV rhIDU treatment.
Mean and standard deviation from week 0 (n=5) to week 26 (n=4) shown in (a) (mean decrease 41.8%, p=0.01). Individual subjects are shown in (b). Subject codes correspond to those published [2]. There was no week 26 CSF sample for subject 4; week 52 is shown. (c) Scatterplot and correlation by linear regression between CSF and serum HS NRE levels in these subjects (p=0.005).
4. DISCUSSION
We found that both urine total glycosaminoglycans and serum HS NRE levels were markedly decreased compared to baseline after 26 weeks and 52 weeks of IV rhIDU therapy. There was a significantly greater percentage reduction in serum HS NRE compared to urine total glycosaminoglycans in response to IV rhIDU. However, changes in urine glycosaminoglycans and serum HS NRE measured at 26 and 52 weeks of therapy did not correlate significantly with changes in joint range of motion and liver or spleen volume. While there was a positive correlation between absolute serum HS NRE and urine total glycosaminoglycan levels there was no correlation between percentage reductions in these levels.
Unexpectedly, we observed a significant decrease in CSF HS NRE levels in the study subjects, with reduction of HS NRE levels in the CSF by 2-fold. This result is surprising because IV rhIDU is not expected to cross the blood-brain barrier in sufficient amounts to correct central nervous system disease [19]. Despite the reduction in CSF HS NRE, the level remained in the MPS I disease range. One possibility is that small amounts of rhIDU enter the brain. It has previously been shown that systemically administered rhIDU has little penetration across the blood-brain barrier and diffusion into the meninges and brain in adult MPS I animals [19,20]. However, MPS I dogs treated with weekly IV rhIDU beginning in the neonatal period showed normal brain glycosaminoglycan levels at one year of age [21]. While the newborn blood-brain barrier may have mannose 6-phosphate receptors that could partially explain these results [22,23], it seems unlikely that this degree of biochemical efficacy would be sustained over a one-year period in the absence of continued supply of rhIDU to the brain. Indeed, the Dickson laboratory has found iduronidase activity at 2-4% of carrier levels in the brain of MPS I dogs treated as adults with IV rhIDU, which while low resulted in normal brain glycosaminoglycan levels by Alcian blue dye-binding assay [24]. Another possibility is that there is some non-CNS driven influence on CSF NRE levels. The origin of CSF HS is not known. As CSF is produced in choroid plexus utilizing fluid and electrolytes from the systemic circulation, it is not beyond the realm of possibility that systemic glycosaminoglycans could somehow enter CSF. Perhaps supporting this suggestion, there was a positive correlation between CSF HS NRE and serum HS NRE levels in these subjects. At this point it is unclear what the mechanism is. In terms of the implications of the findings, it is important to note that if treatment with IV rhIDU lowers CSF NRE levels by 2-fold, and MPS I patients treated with IV rhIDU continue to show cognitive decline over time, then therapy that results in a reduction in CSF NRE but does not normalize them may not be sufficient to correspond to a disease modifying effect. This supports the notion that potential therapeutics should aim to bring the patient NRE levels to the normal range.
However, if one accepts the hypothesis that low levels of IV rhIDU reach the brain and that CSF glycosaminoglycan reduction correlates with clinical impact (which, it is important to note, has not thus far been demonstrated, [25]), an alternative explanation for continued cognitive decline in MPS I patients who are treated long-term with IV rhIDU may be that therapy was not initiated sufficiently early in the course of disease. Studies from MPS I patients with the severe (Hurler) phenotype who are treated with hematopoietic stem cell transplantation show it cannot arrest cognitive deterioration if this intervention is initiated after the age of three years, with better outcomes among patients transplanted prior to twelve months of age [4,26]. There are little long-term data on MPS I patients treated with IV rhIDU beginning at an early age. One report describes a patient with the Hurler form of MPS I who did not receive hematopoietic stem cell transplant but was instead treated with IV rhIDU beginning at two years of age. This patient had a milder neurological course than would be expected for the natural history of MPS I Hurler disease, but nonetheless developed cognitive impairment beginning at age ten years and hydrocephalus at eleven years [27].
Both serum HS NRE and urine total glycosaminoglycan measurements demonstrated a reduction in response to IV rhIDU treatment. The clinical utility of such glycan-based disease biomarker measurements depends on the magnitude of effect that can be observed and the presumptive source of glycosaminoglycans observed in each fluid or tissue [28]. Apart from NRE assays, there are mass spectrometry-based assays for measurement of glycosaminoglycans that avoid some of the issues that plague the dye-based assay. However, the main limitation of the urine total glycosaminoglycan measurement is that a significant amount of glycosaminoglycan is normally excreted in unaffected people, creating a high residual background [29]. In contrast, pathologic lysosomal glycosaminoglycan storage is expected to be zero in unaffected people, thus giving the highly sensitive NRE assay a higher disease specificity compared to the urine total glycosaminoglycan measurement [28]. The serum HS NRE measurement also exhibited a much larger dynamic range with a greater than 12-fold difference in levels observed when comparing affected MPS I and normal dogs [16]. Urinary glycosaminoglycans have previously been reported to show poor correlation with blood glycosaminoglycans, with serum HS and dermatan sulfate non-reducing end markers showing better correlation with tissue levels in liver and brain in MPS I mice [30,31]. Based on these observations it was hypothesized that the serum HS NRE levels would correlate with liver or spleen glycosaminoglycans which are reduced by 84 and 83%, respectively, in IV rhIDU treated MPS I dogs [21]. In contrast to the MPS I dogs previously studied, there was a respective 26% and 21% reduction in liver and spleen volumes in the MPS I subjects whose clinical outcome data we reviewed for this study. We did not observe a significant correlation between these changes in organ volume and changes in serum HS NRE levels during the rhIDU treatment course. This may be an effect of the small number of subjects we studied or a reflection of the fact that these subjects with attenuated disease did not have massive hepatosplenomegaly at the start of the treatment course. We did not measure other glycosaminoglycans such as dermatan sulfate that may be more relevant to physical disease than HS. We also did not study patients receiving hematopoietic stem cell transplantation. Another potential confounder in determining treatment efficacy are IgG antibodies against rhIDU, which may be associated with less post-treatment reduction in urinary glycosaminoglycans [32,33]. While anti-IDU antibody data were not accessed for this study, the published report from these subjects indicates that while titers developed in four of the ten subjects by week eight, they became undetectable over time, suggesting that anti-IDU antibodies may not have substantially affected our results [9].
Regression analysis failed to demonstrate a significant correlation between percentage changes in glycosaminoglycan levels or absolute glycosaminoglycan levels measured by each method. Part of that lack of correlation is likely the complexity of what each assay is measuring. Dye-binding assays measure some complex mixture of all sulfated glycosaminoglycans including HS, chondroitin sulfate, dermatan sulfate, and maybe keratan sulfate in a non-linear way. In practice, a HS standard, usually porcine HS, is used to assign a quantitative value to the colorometric change. However, the composition of glycosaminoglycans including their sulfation, epimerization, and length, can change the sensitivity of the assay. In addition, these compositional features change in disease states and can vary between individuals, challenging the concept that a true standard is possible. In contrast, the Sensi-Pro NRE assay evaluates only the HS-derived NRE that exists due to the specific lysosomal deficiency in iduronidase. To make a more complete comparison between these methods, it would be important to measure both the HS and chondroitin/dermatan sulfate-derived NRE.
With the recent progress in developing LCMS-based assays to more specifically quantify glycosaminoglycans in MPS [34-36], it will be critical to understand and carefully interpret what each assay is measuring. In contrast to the historical dye-binding method, the newer LCMS based “total HS” methods have the distinct advantage of directly measuring the quantity and composition of HS. The key difference between the NRE and total HS is that the NREs are unique glycan structures that are not present in samples from unaffected people. Their disease specificity is due to the fact that the NRE structure is only generated in the lysosome due to the deficiency of the lysosomal enzyme. In the case of MPS I, the structure that cannot be degraded (a terminal iduronic acid) is not synthesized at the end of an HS polymer due to the substrate preferences of the HS biosynthetic enzymes including the 2-O sulfotransferase and C5 epimerase [37,38]. This feature gives the NRE its high specificity for MPS I. In contrast, the total HS methods will quantify the pathological lysosomal HS in addition to the normal physiological HS. Regardless of the method used, these modern glycosaminoglycan quantification methods are all robust ways to measure the increase of glycosaminoglycans in disease and the reduction in response to therapy. Since every assay is slightly different, it is critical that the healthy normal ranges are reported to allow for full interpretation of the data.
5. Conclusions:
In summary, the HS NRE measurement is more specific than dye-binding methods for lysosomal glycosaminoglycan storage and can detect low levels of glycosaminoglycans in multiple tissues and fluids [14,15]. The increased sensitivity and specificity of the NRE assays allow for routine measurement of potentially more clinically relevant samples such as serum, plasma, and CSF, compared to standard approaches that measure urinary glycosaminoglycans. The absence of a high residual background, as observed with the urine total glycosaminoglycan measurement, could have advantages in monitoring response to therapy. For example, in cases where patients begin to develop an anti-enzyme antibody response, small increases in HS NRE could signal reduced enzyme efficacy owing to antibody effect. This is suggested by our observations that low titers of anti-rhIDU antibodies prevent normalization of brain HS NRE in MPS I dogs treated with intrathecal enzyme replacement, while the standard dye-binding assay could not distinguish between low and high-titer groups, instead finding brain glycosaminoglycan levels “normal” in all MPS I, intrathecal-treated dogs regardless of anti-rhIDU antibody titer [16], Meanwhile the absence of a humoral immune response to IV rhIDU improves efficacy in treating systemic disease in animal models [21,39-41]. Finally, we were surprised to observe a 2-fold reduction in CSF HS NRE in subjects receiving IV rhIDU without concomitant central nervous system-directed therapy. Since MPS I patients treated with IV rhIDU develop cognitive impairment despite treatment, one should exercise caution in predicting the outcome of CNS therapies based on relatively small reductions in CSF glycosaminoglycans.
ACKNOWLEDGMENTS:
We thank Thomas Lester, Jim Beitel, and Charles Glass for thoughtful discussions. We thank the patients and their families whose participation made this work possible.
Funding: This work was supported by Zacharon Pharmaceuticals, the National Institute of Neurological Disorders and Stroke (SBIR R43 NS077513), and the Lysosomal Disease Network. The Lysosomal Disease Network (LDN) U54-NS065768 is a part of the National Institute of Health (NIH) Rare Diseases Clinical Research Network, supported through collaboration between the NIH Office of Rare Diseases Research at the National Center for Advancing Translational Science, the National Institute of Neurological Disorders and Stroke (NINDS), and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- rhIDU, EC 3.2.1.76
Recombinant human alpha-l-idurondiase
- MPS
mucopolysaccharidosis
- GAG
glycosaminoglycan
- HS
heparan sulfate
- NRE
non-reducing end
- ELISA
enzyme-linked immunosorbent assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Neufeld EF, Muenzer J, The mucopolysaccharidoses, in: Scriver CR, Beaudet AL, Valle D, Sly WS (Eds.), Metab. Mol. Bases Inherit. Dis, McGraw-Hill, New York, 2001: pp. 3421–3452. [Google Scholar]
- [2].Kakkis ED, Muenzer J, Tiller GE, Waber L, Belmont J, Passage M, Izykowski B, Phillips J, Doroshow R, Walot I, Hoft R, Neufeld E, Enzyme-replacement therapy in mucopolysaccharisosis I, N. Engl. J. Med 344 (2001) 182–188. [DOI] [PubMed] [Google Scholar]
- [3].Wraith JE, Clarke LA, Beck M, Kolodny EH, Pastores GM, Muenzer J, Rapoport DM, Berger KI, Swiedler SJ, Kakkis ED, Braakman T, Chadbourne E, Walton-Bowen K, Cox GF, Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human α-L-iduronidase (laronidase), J. Pediatr 144 (2004) 581–588. [DOI] [PubMed] [Google Scholar]
- [4].Shapiro EG, Nestrasil I, Rudser K, Delaney K, Kovac V, Ahmed A, Yund B, Orchard PJ, Eisengart J, Niklason GR, Raiman J, Mamak E, Cowan MJ, Bailey-Olson M, Harmatz P, Shankar SP, Cagle S, Ali N, Steiner RD, Wozniak J, Lim KO, Whitley CB, Neurocognition across the spectrum of mucopolysaccharidosis type I: Age, severity, and treatment, Mol. Genet. Metab 116 (2015) 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kunin-Batson AS, Shapiro EG, Rudser KD, Lavery CA, Bjoraker KJ, Jones SA, Wynn RF, Vellodi A, Tolar J, Orchard PJ, Wraith JE, Long-term cognitive and functional outcomes in children with mucopolysaccharidosis (MPS)-IH (Hurler syndrome) treated with hematopoietic cell transplantation, JIMD Rep. 29 (2016) 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aldenhoven M, Boelens J, de Koning TJ, The clinical outcome of Hurler syndrome after stem cell transplantation, Biol. Blood Marrow Transplant 14 (2008) 485–498. [DOI] [PubMed] [Google Scholar]
- [7].Dickson PI, Kaitila I, Harmatz P, Mlikotic A, Chen AH, Victoroff A, Passage MB, Madden J, Le SQ, Naylor DE, Mucopolysaccharidosis I Intrathecal Research Collaborative, Safety of laronidase delivered into the spinal canal for treatment of cervical stenosis in mucopolysaccharidosis I, Mol. Genet. Metab 116 (2015) 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Laraway S, Mercer J, Jameson E, Ashworth J, Hensman P, Jones SA, Outcomes of long-term treatment with laronidase in patients with mucopolysaccharidosis type I, J. Pediatr 178 (2016) 219–226. [DOI] [PubMed] [Google Scholar]
- [9].Sifuentes M, Doroshow R, Hoft R, Mason G, Walot I, Diament M, Okazaki S, Huff K, Cox GF, Swiedler SJ, Kakkis ED, A follow-up study of MPS I patients treated with laronidase enzyme replacement therapy for 6 years, Mol. Genet. Metab 90 (2007) 171–180. [DOI] [PubMed] [Google Scholar]
- [10].de Jong JG, Wevers RA, Laarakkers C, Poorthuis BJ, Dimethylmethylene blue-based spectrophotometry of glycosaminoglycans in untreated urine: a rapid screening procedure for mucopolysaccharidoses, Clin. Chem 35 (1989) 1472–7. [PubMed] [Google Scholar]
- [11].de Jong JGN, Heijs WMJ, Wevers RA, Mucopolysaccharidoses screening: dimethylmethylene blue versus Alcian blue, Ann. Clin. Biochem. An Int. J. Biochem. Lab. Med 31 (1994) 267–271. [DOI] [PubMed] [Google Scholar]
- [12].Auray-Blais C, Lavoie P, Tomatsu S, Valayannopoulos V, Mitchell JJ, Raiman J, Beaudoin M, Maranda B, Clarke JTR, UPLC-MS/MS detection of disaccharides derived from glycosaminoglycans as biomarkers of mucopolysaccharidoses, Anal. Chim. Acta 936 (2016) 139–148. [DOI] [PubMed] [Google Scholar]
- [13].Wei W, Niñonuevo MR, Sharma A, Danan-Leon LM, Leary JA, A comprehensive compositional analysis of heparin/heparan sulfate-derived disaccharides from human serum, Anal. Chem 83 (2011) 3703–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lawrence R, Brown JR, Al-Mafraji K, Lamanna WC, Beitel JR, Boons GJ, Esko JD, Crawford BE, Disease-specific non-reducing end carbohydrate biomarkers for mucopolysaccharidoses, Nat. Chem. Biol 8 (2012) 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aoyagi-Scharber M, Crippen-Harmon D, Lawrence R, Vincelette J, Yogalingam G, Prill H, Yip BK, Baridon B, Vitelli C, Lee A, Gorostiza O, Adintori EG, Minto WC, Van Vleet JL, Yates B, Rigney S, Christianson TM, Tiger PMN, Lo MJ, Holtzinger J, Fitzpatrick PA, LeBowitz JH, Bullens S, Crawford BE, Bunting S, Clearance of heparan sulfate and attenuation of CNS pathology by intracerebroventricular BMN 250 in Sanfilippo type B mice, Mol. Ther. - Methods Clin. Dev 6 (2017) 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dickson PI, Ellinwood NM, Brown JR, Witt RG, Le SQ, Passage MB, Vera MU, Crawford BE, Specific antibody titer alters the effectiveness of intrathecal enzyme replacement therapy in canine mucopolysaccharidosis I, Mol. Genet. Metab 106 (2012) 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lawrence R, Olson SK, Steele RE, Wang L, Warrior R, Cummings RD, Esko JD, Evolutionary differences in glycosaminoglycan fine structure detected by quantitative glycan reductive isotope labeling., J. Biol. Chem 283 (2008) 33674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kakkis ED, McEntee MF, Schmidtchen A, Neufeld EF, Ward DA, Gompf RE, Kania S, Bedolla C, Chien SL, Shull RM, Long-term and high-dose trials of enzyme replacement therapy in the canine model of mucopolysaccharidosis I, Biochem. Mol. Med 58(1996) 156–167. [DOI] [PubMed] [Google Scholar]
- [19].Kakkis E, McEntee M, Vogler C, Le S, Levy B, Belichenko P, Mobley W, Dickson P, Hanson S, Passage M, Intrathecal enzyme replacement therapy reduces lysosomal storage in the brain and meninges of the canine model of MPS I, Mol Genet Metab. 83 (2004) 163–174. [DOI] [PubMed] [Google Scholar]
- [20].Kakkis ED, Schuchman E, He X, Wan Q, Kania S, Wiemelt S, Hasson CW, O’Malley T, Weil MA, Aguirre GA, Brown DE, Haskins ME, Enzyme replacement therapy in feline mucopolysaccharidosis I, Mol. Genet. Metab 72 (2001) 199–208. [DOI] [PubMed] [Google Scholar]
- [21].Dierenfeld AD, McEntee MF, Vogler CA, Vite CH, Chen AH, Passage M, Le S, Shah S, Jens JK, Snella EM, Kline KL, Parkes JD, Ware WA, Moran LE, Fales-Williams AJ, Wengert JA, Whitley RD, Betts DM, Boal AM, Riedesel EA, Gross W, Ellinwood NM, Dickson PI, Replacing the enzyme α-l-iduronidase at birth ameliorates symptoms in the brain and periphery of dogs with mucopolysaccharidosis type I, Sci. Transl. Med 2 (2010) 60ra89–60ra89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vogler C, Sands MS, Levy B, Galvin N, Birkenmeier EH, Sly WS, Enzyme replacement with recombinant beta-glucuronidase in murine mucopolysaccharidosis type VII: impact of therapy during the first six weeks of life on subsequent lysosomal storage, growth, and survival, Pediatr. Res 39 (1996) 1050–1054. [DOI] [PubMed] [Google Scholar]
- [23].Urayama A, Grubb JH, Sly WS, Banks WA, Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier, Proc. Natl. Acad. Sci. U. S. A 101 (2004) 12658–12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dickson P, Vogler C, Levy B, McEntee M, Passage M, Le S, Snider S, Manuel H, Kakkis E, Treatment of CNS lysosomal storage with high-dose intravenous enzyme replacement therapy in tolerant MPS I dogs, Abstract, American Society of Human Genetics annual meeting, New Orleans, LA (2006) 2351A. [Google Scholar]
- [25].Wijburg FA, Whitley CB, Muenzer J, Gasperini S, del Toro M, Muschol N, Cleary M, Sevin C, Shapiro E, Bhargava P, Kerr D, Alexanderian D, Intrathecal heparan-N-sulfatase in patients with Sanfilippo syndrome type A: A phase IIb randomized trial, Mol. Genet. Metab 126 (2019) 121–130. [DOI] [PubMed] [Google Scholar]
- [26].Whitley CB, Belani KG, Chang PN, Summers CG, Blazar BR, Tsai MY, Latchaw RE, Ramsay NKC, Kersey JH, Long-term outcome of Hurler syndrome following bone marrow transplantation, Am. J. Med. Genet 46(1993) 209–218. [DOI] [PubMed] [Google Scholar]
- [27].Eisengart JB, Jarnes J, Ahmed A, Nestrasil I, Ziegler R, Delaney K, Shapiro E, Whitley C, Long-term cognitive and somatic outcomes of enzyme replacement therapy in untransplanted Hurler syndrome, Mol. Genet. Metab. Reports 13 (2017) 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lawrence R, Brown JR, Lorey F, Dickson PI, Crawford BE, Esko JD, Glycan-based biomarkers for mucopolysaccharidoses, Mol. Genet. Metab 112 (2014) 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Komosinska-Vassev K, Blat D, Olczyk P, Szeremeta A, Jura-Półtorak A, Winsz-Szczotka K, Klimek K, Olczyk K, Urinary glycosaminoglycan (uGAG) excretion in healthy pediatric and adolescent population, Clin. Biochem 47 (2014) 1341–1343. [DOI] [PubMed] [Google Scholar]
- [30].Erickson RP, Sandman R, Epstein CJ, van Robertson WB, Lack of relationship between blood and urine levels of glycosaminoglycans and lysomal enzymes, Biochem. Med 12 (1975) 331–339. [DOI] [PubMed] [Google Scholar]
- [31].Saville JT, McDermott BK, Fuller M, Glycosaminoglycan fragments as a measure of disease burden in the mucopolysaccharidosis type I mouse, Mol. Genet. Metab 123 (2018) 112–117. [DOI] [PubMed] [Google Scholar]
- [32].Giugliani R, Rojas VM, Martins AM, Valadares ER, Clarke JTR, Góes JEC, Kakkis ED, Worden MA, Sidman M, Cox GF, A dose-optimization trial of laronidase (Aldurazyme) in patients with mucopolysaccharidosis I, Mol. Genet. Metab 96 (2009) 13–19. [DOI] [PubMed] [Google Scholar]
- [33].Clarke LA, Wraith JE, Beck M, Kolodny EH, Pastores GM, Muenzer J, Rapoport DM, Berger KI, Sidman M, Kakkis ED, Cox GF, Long-term efficacy and safety of laronidase in the treatment of mucopolysaccharidosis I, Pediatrics 123 (2009) 229–240. [DOI] [PubMed] [Google Scholar]
- [34].Volpi N, Maccari F, Galeotti F, Zampini L, Santoro L, Padella L, Galeazzi T, Gabrielli O, Coppa GV, Plasmatic dermatan sulfate and chondroitin sulfate determination in mucopolysaccharidoses, J. Pharm. Biomed. Anal 85 (2013) 40–45. [DOI] [PubMed] [Google Scholar]
- [35].Tomatsu S, Montaño AM, Oguma T, Dung VC, Oikawa H, Gutiérrez ML, Yamaguchi S, Suzuki Y, Fukushi M, Barrera LA, Kida K, Kubota M, Orii T, Validation of disaccharide compositions derived from dermatan sulfate and heparan sulfate in mucopolysaccharidoses and mucolipidoses II and III by tandem mass spectrometry, Mol. Genet. Metab 99 (2010) 124–131. [DOI] [PubMed] [Google Scholar]
- [36].Nielsen TC, Rozek T, Hopwood JJ, Fuller M, Determination of urinary oligosaccharides by high-performance liquid chromatography/electrospray ionization–tandem mass spectrometry: Application to Hunter syndrome, Anal. Biochem 402 (2010) 113–120. [DOI] [PubMed] [Google Scholar]
- [37].Debarnot C, Monneau YR, Roig-Zamboni V, Delauzun V, Le Narvor C, Richard E, Hénault J, Goulet A, Fadel F, Vivès RR, Priem B, Bonnaffé D, Lortat-Jacob H, Bourne Y, Substrate binding mode and catalytic mechanism of human heparan sulfate d-glucuronyl C5 epimerase, Proc. Natl. Acad. Sci 116 (2019) 6760–6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sheng J, Xu Y, Dulaney SB, Huang X, Liu J, Uncovering biphasic catalytic mode of C5-epimerase in heparan sulfate biosynthesis., J. Biol. Chem 287 (2012) 20996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dickson P, Peinovich M, McEntee M, Lester T, Le S, Krieger A, Manuel H, Jabagat C, Passage M, Kakkis E, Immune tolerance improves the efficacy of enzyme replacement therapy in the canine model of mucopolysaccharidosis I, J. Clin. Invest 118 (2008) 2868–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Le SQ, Kan S-H, Clarke D, Sanghez V, Egeland M, Vondrak KN, Doherty TM, Vera MU, Iacovino M, Cooper JD, Sands MS, Dickson PI, A humoral immune response alters the distribution of enzyme replacement therapy in murine mucopolysaccharidosis type I, Mol. Ther. – Methods Clin. Dev 8 (2018) 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Baldo G, Mayer FQ, Martinelli BZ, de Carvalho TG, Meyer FS, de Oliveira PG, Meurer L, Tavares A, Matte U, Giugliani R, Tavares Â, Matte U, Giugliani R, Enzyme replacement therapy started at birth improves outcome in difficult-to-treat organs in mucopolysaccharidosis I mice, Mol. Genet. Metab 109 (2013) 33–40. [DOI] [PubMed] [Google Scholar]