Abstract
The Hsp70 family of chaperones works with its co-chaperones, the nucleotide exchange factors and J-domain proteins, to facilitate a multitude of cellular functions. Central players in protein homeostasis, these jacks-of-many-trades are utilized in a variety of ways because of their ability to bind with selective promiscuity to regions of their client proteins that are exposed when the client is unfolded, either fully or partially, or visits a conformational state that exposes the binding region in a regulated manner. The key to Hsp70 functions is that their substrate binding is transient and allosterically cycles in a nucleotide-dependent fashion between high-and low-affinity states. In the past few years, structural insights into the molecular mechanism of this allosterically regulated binding have emerged and provided deep insight into the deceptively simple Hsp70 molecular machine that is so widely harnessed by nature for diverse cellular functions. In this review, these structural insights are discussed to give a picture of the current understanding of how Hsp70 chaperones work.
Introduction
Molecular chaperones play key roles in maintaining cellular protein health, facilitating protein targeting, and ensuring high-fidelity protein biosynthesis. Central players among molecular chaperones are the 70-kDa heat-shock proteins, or Hsp70s, which occur in virtually all organisms and all cellular locations. While these chaperones are widespread and perform highly diverse functions, they share a common fundamental mechanism of action. Intensive study over the past decade has led to a much deeper understanding of the structural basis for the molecular mechanism of Hsp70. In turn, this understanding is elucidating the functional roles of Hsp70s and the nature of their partnerships with co-chaperones in the cell. Failures in protein homeostasis are now implicated in many diseases, and the resulting advances in understanding Hsp70s offer promise that they can be therapeutic targets to treat protein homeostasis pathologies. Several reviews on Hsp70s have been published in the last 5 years [1–8], and we point the interested reader to these. Here, we focus on the great strides that have been made recently in the structure–function of Hsp70s and their interactions with co-chaperones and substrates.
Intramolecular allostery modulates Hsp70 substrate-binding affinities
Hsp70s are made up of an N-terminal 45-kDa actin-like nucleotide-binding domain (NBD) and a C-terminal 30-kDa substrate-binding domain (SBD). The SBD is composed of a β-sandwich subdomain (βSBD) with a canonical substrate-binding groove (which we will also refer to as the ‘canonical binding site’ for substrates), an α-helical lid subdomain (α-lid), and a disordered C-terminal region (Figure 1A). The NBD and SBD are connected by a conserved, largely hydrophobic interdomain linker. The functions of Hsp70s depend on an intramolecular allosteric mechanism that involves the binding and release of protein client modulated by ATP binding and hydrolysis. Much of the work elucidating the features of the allosteric mechanism of Hsp70s is based on a detailed study of the Escherichia coli Hsp70 DnaK. While recent work reveals evolutionary ‘tuning’ of allosteric properties of different Hsp70s [9], their overall mechanism is largely conserved, and so our general description is based on DnaK.
Figure 1. The structural arrangement of Hsp70 molecular chaperones.
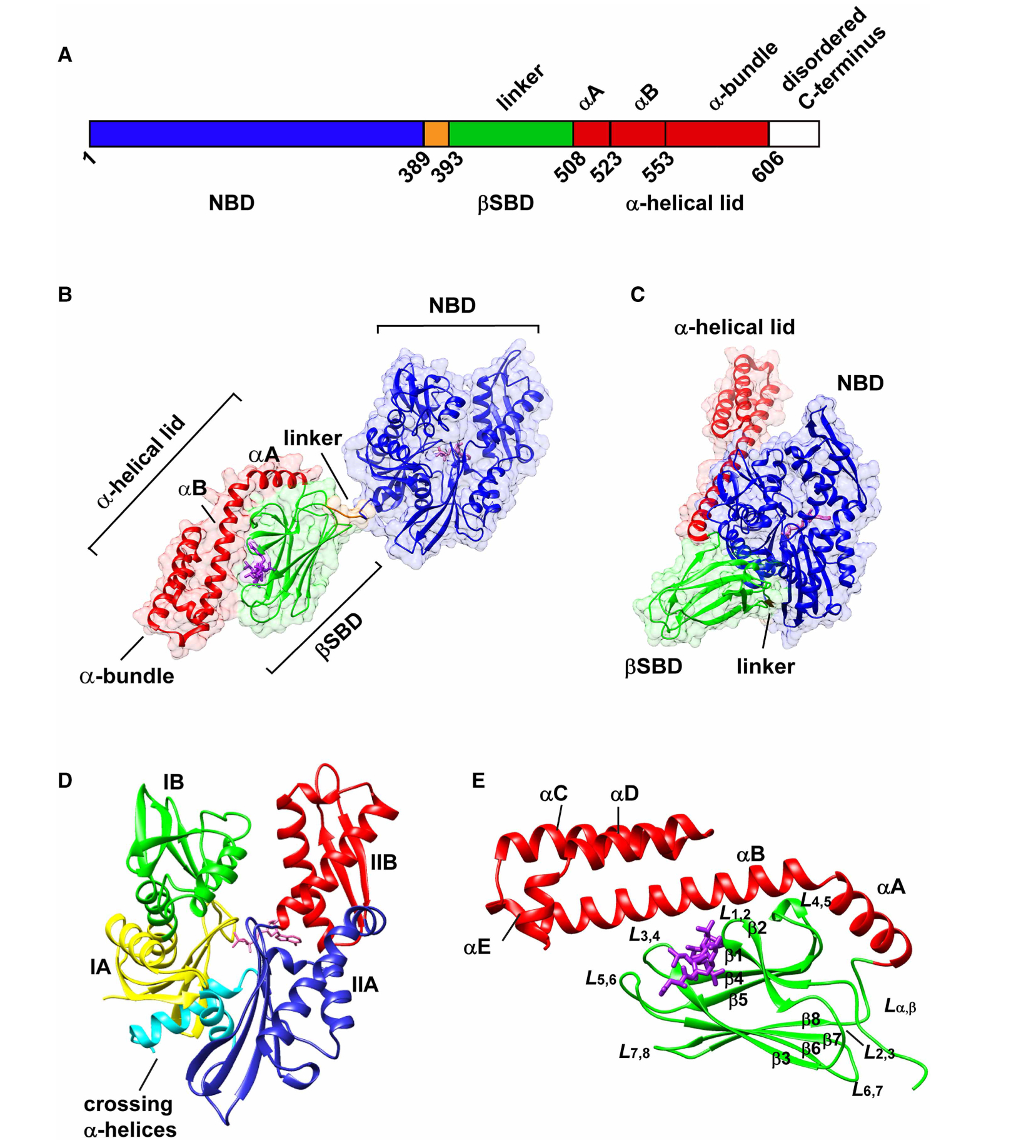
(A) Schematic representation of the structural domains of Hsp70s. The sequence numbers are based on the E. coli Hsp70, DnaK. Structures of DnaK showing (B) the canonical ADP-bound undocked state (PDB ID 2kho [12]), and (C) the canonical ATP-bound docked state (PDB ID 4b9q [17]). The NBD is colored in blue. In the substrate-binding domain (SBD), the βSBD is colored green, α-lid in red, and the linker in orange. The bound nucleotide and the peptide substrate NRLLLTG are shown in purple. (D) Subdomain organization of the NBD of a representative Hsp70. The structure shown is ADP-bound Hsc70 (PDB ID 3hsc [92]). Subdomains IA, IB, IIA, and IIB are colored in yellow, green, blue, and red, respectively. The crossing α-helices are shown in cyan. (E) Structure of the DnaK SBD bound to the peptide NRLLLTG (PDB 1dkz [39]). The binding mode of peptide substrate is illustrated in Figure 7.
Hsp70s display high substrate-binding affinity in the ADP-bound state. In the ADP-bound state of DnaK, the NBD and SBD are largely independent of each other (Figure 1B) with the interdomain linker relatively exposed and dynamic as shown by a variety of observations ranging from high proteolytic susceptibility of the linker [10,11] to NMR analysis [12,13]. The ADP-bound state is referred to as ‘domain undocked’. In this state, the SBD adopts a closed conformation with the α-lid packed against the βSBD to form a stable βSBD-α-lid interface, resulting in a low substrate on/off rate and high binding affinity. In this state, the ADP-bound NBD has a subdomain arrangement that does not favor interaction with the hydrophobic segment of the interdomain linker and is incompatible with ATP binding/hydrolysis. Upon ATP binding, the chaperone undergoes massive domain rearrangement according to many biochemical observations [13–16] and more recently to solved X-ray structures [17,18] (Figure 1C). The α-lid detaches from the βSBD, and both SBD subdomains become docked onto the NBD to form a new NBD–SBD interface. As a result, the SBD adopts an open conformation with the high substrate on/off rates and low affinity. By virtue of ATP hydrolysis and nucleotide exchange, the Hsp70 cycles between these ADP-and ATP-bound states, visiting the allosterically active state in between, which leads to the cycle of substrate binding and release that is harnessed for all of the functions of Hsp70s.
The NBD consists of four subdomains, IA, IB, IIA, and IIB, which are located on two lobes, I and II (Figure 1D). Nucleotide (ADP or ATP) binds at the cleft formed between subdomains IB and IIB, and all four subdomains are involved in nucleotide coordination. Several studies have focused on allosteric conformational changes within the NBD [19,20]. NMR chemical shift perturbation studies of the NBD of DnaK in different nucleotide-bound states revealed rotation of subdomain IIB upon nucleotide dissociation [21]. Binding of ATP causes re-orientations of the subdomains and long-range perturbation along with the NBD subdomain interfaces, resulting in the binding of the interdomain linker to lobe IIA [21]. An NMR residual dipolar coupling study of Hsc70 NBD showed that subdomains IA and IIA could move with respect to each other by a 10° relative shearing motion, which is expected to modulate their interaction with other domains and cofactors [20]. Additionally, a combined single-molecule force spectroscopy and theoretical simulation study suggests that the C-terminal helix of the NBD acts as molecular ‘glue’ mediating the coupling between lobes I and II [22]. Taken together, these results show that the subdomain re-orientations triggered by nucleotide binding play essential roles in NBD allostery.
In the high substrate affinity conformation of the SBD (when it is studied as an isolated domain or when the chaperone is in the undocked, ADP-bound state, Figure 1E), two antiparallel β-sheets and four upwards protruding loops (L1,2, L3,4, L4,5, and L5,6) make up the βSBD; the upper β-sheet consists of strands β1, β2, β4, and β5, and the lower one consists of strands β3, β6, β7, and β8. The substrate-binding groove is formed by β1, β2, L1,2, and L3,4. When ATP binds to the NBD, β8 dissociates from β7 in the lower sheet and hydrogen bonds with β5 of the upper sheet in the βSBD [17,18,23]. The βSBD undergoes a seesaw-like conformational change from a high substrate-binding affinity conformation to one with lower substrate affinity [24]. NMR chemical shift perturbation analysis revealed that the β5/β7/β8 hydrophobic cluster is a central hub that modulates the allosteric coupling within the SBD [24]. The α-lid subdomain is composed of helices α-A, α-B, and an α-bundle, which consists of three α-helices, α-C, α-D, and αE. Several studies show that the α-lid is highly dynamic and could adopt heterogeneous conformations [14,25,26]. The α-lid covers the substrate-binding groove in the βSBD with the signature kink between αA and αB in the isolated SBD and undocked SBD structures. In the recent structures of the ATP-bound docked state of DnaK [17,18], the α-lid is detached from the βSBD, and αA and αB fuse into one long helix, which interacts with the NBD. The conformational properties of the α-lid can also be modulated by the nature of substrates. While the α-lid remains in a ‘closed’ conformation tightly associated with the βSBD when a peptide substrate is bound, it is quite dynamic [26] and it can also detach, thus allowing the binding of folded or large substrates [27–29]. (See fuller discussion in the section below on substrate binding.)
Allosteric signal transmission between the Hsp70 NBD and SBD triggered by nucleotide or substrate binding has been extensively studied in recent years [26,30–34]. Allostery depends on an energetic balance among the interfaces formed between the α-lid and the βSBD, the α-lid and the NBD, the NBD and the βSBD, and the linker contacts with either the βSBD or the NBD. The allostery of Hsp70s thus results from an energetic tug-of-war between two orthogonal interfaces, the βSBD-α-lid interface formed in the undocked state, and the NBD–SBD interface formed in the docked state [34] (Figure 2A,B). Complementary to these observations, additional residues were identified to be part of an allosteric pathway between the NBD and SBD, based on mutagenesis results and details of the ATP-bound DnaK structure [35]. These authors proposed that binding of the interdomain linker to the NBD, and the rotation of the two lobes triggered by ATP-binding results in the interaction of two NBD residues, I168 and D328 with the βSBD; these residues act as triggers by their interaction with D481 and K414 in the SBD, which snaps the βSBD into a low substrate-binding affinity conformation [35].
Figure 2. Hsp70 allostery is governed by the energetic competition between domain interfaces.

(A) The βSBD-α-lid interface (red) formed in the undocked state of DnaK (PDB ID 2kho [12]) and (B) the NBD–SBD interface (blue) formed in the docked state of DnaK (PDB ID 4b9q [17]). (C) Schematic representation of the allosteric cycle of DnaK. Coloring follows the scheme described in Figure 1.
While the energetic tug-of-war of interdomain interfaces determines whether the docked or undocked state of an Hsp70 is favored, communication between the domains is mediated by the two structural components that act as ‘allosteric couplers’: the interdomain linker and the α-lid. Both are able to form favorable interactions with both the NBD and the SBD, and importantly, their tendency to interact with one or the other domain is modulated by the conformational state of the given domain. The interdomain linker contains a pre-dominantly hydrophilic N-terminal region, followed by a highly conserved hydrophobic sequence (389VLLL392 in DnaK). In the undocked state, NMR residual dipolar coupling and paramagnetic relaxation enhancement suggested that the NBD and SBD experience restricted rotation around a 35° cone with respect to each other [12]. While the linker in the ADP-bound state is relatively flexible, molecular dynamics simulations from our laboratory suggest that it is made up of relatively structured regions with hinges between them, which restricts both the relative orientations and distances between the NBD and SBD [31]. In addition, there is transient interaction between the C-terminal region of the linker and SBD, and the interaction site on SBD is only available when SBD is in high substrate-binding affinity conformation [31]. In the ATP-bound state, the hydrophobic sequence of the linker binds to subdomain IIA of NBD to form an antiparallel β-strand structure [23,36,37]. Binding of the linker to the NBD is sufficient to stimulate ATP hydrolysis activity [13,38]. Upon the association of the interdomain linker with the NBD triggered by ATP binding, the allosteric signal is further transmitted towards SBD by favoring the formation of NBD contacts with lynchpin sites on the βSBD, disengaging SBD strand β8 from strand β7, and disrupting the hydrophobic arch over the substrate-binding cleft [24]. Thus, the interdomain linker can interact with either domain, depending on the nucleotide-binding state of the NBD, and contribute to the allosteric energy landscape by favoring docked or undocked states: It is a true allosteric coupler.
The other allosteric coupler in Hsp70s is the α-lid. The α-lid can form strong and specific interactions with the βSBD loops when the SBD is in its high substrate affinity state (and more favorably, when a linear substrate is bound), or with the NBD when it is in the ATP-bound conformation. As in the case of the linker, the preferences of this structural entity for interaction with either domain are modulated by the conformational state of the domain, and the energy of the conformational state of the linker is engaged with is influenced by the interactions the α-lid participates in. Specifically, when the α-lid is bound to the βSBD, it forms three salt bridges (D526-R445, D540-R467, and K548-D431 in DnaK [39]), and when the α-lid coalesces onto the NBD, a large packing interface (1170 Å2) forms [18].
An ‘allosterically active’ state of DnaK, with both ATP and peptide substrate bound, has been observed by using an ATP hydrolysis defective mutant, DnaKT199A, so that ATP remains bound, and adding a peptide substrate [26,34]. This state must exist transiently during the allosteric cycle of wild-type DnaK because both ATP and substrate are bound when the ATPase activity is elevated, and the substrate off-rate is enhanced (Figure 2C). NMR studies showed that in this allosterically active state, the SBD and NBD are largely dissociated from one another, the interdomain linker remains bound to NBD thereby stimulating the ATP hydrolysis activity, and the α-lid/βSBD interface is at least partially formed [34].
While they share a common mechanism, Hsp70s display highly tunable allosteric landscapes [9,34,40]. Since allostery results from the energetic tug-of-war between the βSBD-α-lid interface and the NBD–SBD interface, evolutionary sequence changes at these two key interfaces shift the equilibrium between docked and undocked conformations thus modulating Hsp70 functions [34]. Two human cytoplasmic Hsp70s, HspA1, and Hsc70, which are 46% identical in sequence to DnaK, favor domain docking in all nucleotide-bound states more significantly than DnaK does (Figure 3). While DnaK adopts a docked conformation only when ATP-bound, significant domain docking is observed for both HspA1 and Hsc70 in their ADP-bound states [9]. As a result, HspA1 and Hsc70 exhibit lower substrate-binding affinities and reduced substrate stimulation of their ATP hydrolysis rates compared with DnaK. In contrast, the endoplasmic reticulum (ER) Hsp70, binding-immunoglobulin protein (BiP), shows less favorable domain docking than DnaK: its ATP-bound state is heterogeneous with only half of the molecules adopting a domain-docked conformation [40]. In addition to evolutionary tuning, the allosteric landscapes of Hsp70s are also modulated by post-translational modifications, and this tuning of their allosteric behaviors is directly related to their physiological functions [40–42]. For instance, AMPylation of T518 biases BiP towards the domain-docked state, subsequently inactivating BiP and limiting its interaction with substrates [40,42]. Phosphorylation of HspA1 at T66 promotes domain undocking and increases substrate-binding affinity in the ATP-bound state [41]. A physiological result of this modification is that the interactions of HspA1 with components of mitotic spindles are stabilized and the localization to the spindle is favored.
Figure 3. Evolutionary tuning of Hsp70 energy landscapes.

Schematic illustration of allosteric landscapes of HspA1, Hsc70 and DnaK in different ligand-bound states. ‘D’ represents the docked state; ‘U’ represents the undocked state; ‘P’ represents the partially docked state observed in ATP/peptide-bound DnaK, with the NBD and SBD largely undocked and with the linker still bound to NBD. The barrier heights and well depths are only qualitative. The figure is reproduced from [9].
Co-chaperones modulate the allosteric functions of Hsp70s
Hsp70s do not work alone. The allosteric functions of Hsp70s are under the influence of two partner classes of co-chaperones: J-domain proteins (JDPs) and nucleotide exchange factors (NEFs). While JDPs stimulate ATPase activity and drive multifunctionality of Hsp70s by targeting them to specific substrates and cellular locations, NEFs ensure the continuity of Hsp70s’ ATPase cycle by promoting ADP to ATP exchange and substrate release.
The prokaryotic JDP, DnaJ, facilitates substrate delivery and activates Hsp70 ATPase rate
JDPs form a large and diverse family of proteins consisting of over 50 members in humans. All of them share a ~70 amino-residue characteristic J-domain, which adopts a four-helical bundle structure with a conserved His–Pro–Asp (HPD) motif located between helices II and III that has been shown to be crucial for stimulation of Hsp70 ATPase activity [37,43–45] (Figure 4A). Considerable research over many years and many laboratories have shown that JDPs directly bind Hsp70s [36,45], but the structural details of the interaction remained elusive for a long time due to its transient and dynamic nature. Multiple research groups addressed this question using various methods and, in some instances, produced contradictory models of the complex [46,47]. A recently published crystal structure of E. coli DnaK in the ATP-bound state complexed with the J-domain of DnaJ (Figure 4B) and supporting mutagenesis analysis has revealed the mechanism of JDP action and explained previously published biophysical and biochemical observations [36]. In this structure, the J-domain binds at the interface between the NBD and the SBD of DnaK, forming direct contacts with both domains. The conserved HPD motif protrudes towards the interdomain linker, which plays such a crucial role in the allosteric cycle of DnaK (vide supra) [31,34,38], and also directly interacts with the βSBD. The authors propose that the way the J-domain interacts with the Hsp70 accounts for its favoring of substrate-induced undocking of the SBD from the NBD and the transmission of conformational changes in the substrate-bound βSBD to the NBD through the linker. These two complementary signals ensure the optimal positioning of the NBD lobes for ATP hydrolysis. Notably, residues of DnaK involved in the interaction with DnaJ are highly conserved, suggesting that the mechanism of JDP binding to Hsp70s is conserved from bacteria to humans.
Figure 4. Structural features of JDPs.
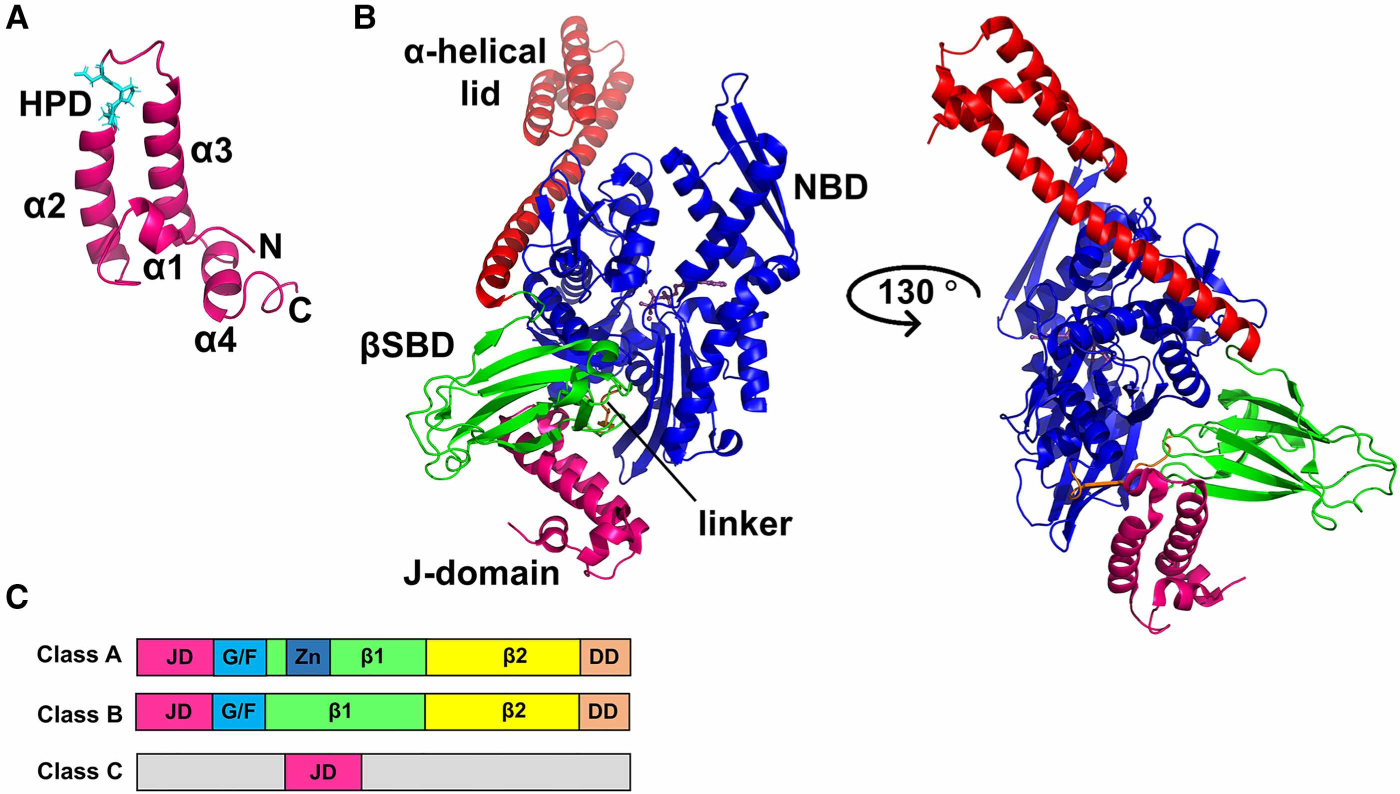
(A) NMR structure of the J-domain of E. coli DnaJ (PDB ID 1xbl [44]) indicating the four helices (α1, helix I; α2, helix II; α3, helix III; α4, helix IV) of the helical hairpin (in purple) and the His–Pro–Asp (HPD) motif (in cyan). (B) Structure of the ATP-bound open conformation of E. coli DnaK in complex with the J-domain of E. coli DnaJ (PDB ID 5nro [36]). The color scheme is the same as that for DnaK in Figure 1, and the J-domain is shown in magenta. The two orientations show that the J-domain binds at the interface between the NBD and the SBD of DnaK, forming direct contacts with both domains. (C) Domain organization and classification of J-proteins; JD, J-domain; G/F, Gly–Phe-rich region; Zn, Zn-finger; β1, first β-sandwich domain; β2, second β-sandwich domain; DD, dimerization domain. In class C JDPs, the functional J-domain can be present anywhere in the sequence and is not restricted to the N-terminus.
In addition to stimulating the DnaK ATP hydrolysis rates, DnaJ is proposed to help recruit substrates to its partner Hsp70. Like DnaK, DnaJ binds substrates through short sequences enriched in hydrophobic residues, but in addition, its substrates were observed to be enriched in aromatic and polar residues [48].
Eukaryotic JDPs mediate multiple functions
JDPs became highly complex during evolution, enabling them to perform highly complex functions. Because of their complex evolutionary history and functional diversification, the classification and nomenclature of JDPs are a matter of some debate (a comprehensive recapitulation of the classification and functions of JDPs can be found in [49,50]). Traditionally, JDPs are subdivided into three major classes (A, B, C) based on the ancestral DnaJ of E. coli (Figure 4C). Class A and class B both have the N-terminal J-domain adjacent to the Gly– Phe-rich region, followed by two topologically similar twisted β-sandwich domains and a C-terminal dimerization domain. The only difference between class A and class B JDPs is that the class A members have a Cys-rich Zn2+-finger inserted into the first of the two domains; this domain is important for client binding and delivery [51,52]. In contrast, in class C JDPs, the functional J-domain can be present anywhere in the sequence and not specifically at the N-terminus. Class C JDPs include a wide variety of other functional domains along with the J-domain [50,53] and often perform very specialized functions. Most organisms express more JDPs than Hsp70s [49,50,54–56], which suggests that many Hsp70s will have multiple JDPs partners.
Like DnaJ, the eukaryotic JDPs are implicated in substrate binding as well as activation of their partner Hsp70’s ATPase activity. Most JDPs, such as the JDPs of the ER lumen (ERdj4, ERdj5, and ERdj6) [57,58], display a broad substrate specificity (‘broad binders’). Other JDPs have exquisite specificity for binding (‘specific binders’) like the specialized J-protein auxilin, which helps in the removal of clathrin coats from endocytic vesicles [59,60]. Interestingly, some JDPs fall at the interface of the ‘specific binders’ and ‘broad binders’. For example, the metazoan JDPs, DnaJB6 and DnaJB8, bind Gln-rich sequences (polyQ, polyglutamine) [61]. In Huntington’s disease, the binding of JDPs to the polyQ stretches of huntingtin protein has been reported to inhibit aggregation [62].
Among the class A JDPs, sequence divergence is restricted to the SBD at the C-terminus, which has consequently developed the ability to bind to different client proteins and contribute to the specificity of J-protein function [63]. The most abundant cytosolic class A JDP in Saccharomyces cerevisiae is Ydj1, which is involved in protein folding, trafficking and degradation [64–66]. The functional relevance of Ydj1 is supported by the observation that deletion of Ydj1 or introduction of a mutation within its conserved HPD motif (H34Q) results in a null phenotype (cell death) [64–67]. One of the most important class B JDPs in yeast is Sis1, which plays a critical role in the remodeling of yeast prion, aggregates working in partnership with the Hsp70 Ssa1 and the AAA+ chaperone Hsp104 [68]. The remodeling of prion aggregates is dependent on the Sis1 J-domain. Importantly, studies have also shown that the mammalian JDPs, DnaJB6b and DnaJB8, are crucial for the remodeling of polyQ aggregates, again working with Hsp70 partners [69]. As in the yeast system, mutating the conserved HPD motif (H31Q) modulates the polyQ aggregation in vivo [61,70]. Class C JDPs are believed to interact with one or a small subset of Hsp70 substrates and perform more than one specialized function. The classic example is the ribosome-associated JDP of S. cerevisiae, zuotin (Zuo1). Zuo1 is a positively charged, 433 residue-long protein that contains a classic J-domain. The different domains of Zuo1 are responsible for its association with the ribosome’s 60S and 30S subunits, consistent with its proposed role of facilitating the folding of the nascent polypeptide [71]; this function is dependent on Hsp70 and relies on the Zuo1 J-domain. Zuo1 also plays an important role in imparting pleiotropic drug resistance to yeast cells in the ribosome-dissociated state. The C-terminal 69-residue domain of Zuo1 (Zuo1365–433) is sufficient for inducing pleiotropic drug resistance in yeast cells. However, this fragment is dispensable for the ribosome-associated chaperoning function of Zuo1 [72–74].
It has become increasingly evident that failure to maintain cellular proteostasis by the Hsp70 system results in many diseases, and in some cases, the defect resides in the JDP co-chaperone. The specialized functions of JDPs, in contrast with the more diverse roles of the Hsp70 family members, account for the observation that over 50% of known ‘chaperonopathies’ are linked to mutations of the JDPs [49]. Mutations in JDPs have been implicated in neuro-, cardiac-, and motor-neuropathies [75–77]. Also, the regulation of the precise expression pattern of JDPs is important for cellular health, as pointed by the altered expression levels of JDPs in several types of cancers. For example, recent studies on breast cancer samples showed high expression levels of DnaJB1, DnaJB6, and low expression levels of DnaJB4 and DnaJB12 [78]. Also, the dysfunction of JDPs located at neuronal synapsis is associated with human neurodegenerative diseases. For instance, defects in many of the class C JDPs are linked with ataxia, phenylketonuria-related neurodevelopmental deficits, autosomal dominant adult neuronal ceroid lipofuscinosis, and other disorders; mutations in DnaJB2 are associated with distal motor neuropathy as well as spinomuscular atrophy and Parkinsonism, while defects in DnaJB6 are linked with limbgirdle muscular dystrophy type 1D [79–82].
NEFs accelerate ADP release and ATP binding
The exchange of nucleotide from the NBD of all Hsp70s is facilitated by widely divergent NEFs. While these co-chaperones are diverse, both in terms of their specific functions and their structures, the impact of their action on Hsp70s is strikingly similar. There have been several excellent reviews on Hsp70 NEFs published recently [83–85]. Here, we focus on recent advances that have enabled the structures of NEFs to be related to their functional partnership with Hsp70s.
The prokaryotic NEF GrpE as a paradigm
The single bacterial NEF GrpE was originally discovered in E. coli as a factor essential for DNA replication of bacteriophage λ and for cell viability at all temperatures [86–88]. Subsequently, GrpE was shown to accelerate nucleotide exchange from ADP to ATP for the E. coli DnaK [89]. A crystal structure of GrpE in complex with the apo-NBD of DnaK was published in 1997 and revealed GrpE to be a homodimer consisting of two long N-terminal α-helices leading into a small four-helix bundle and two small β-domains [90] (Figure 5A). Although only one of the GrpE monomers directly interacts with a single NBD, dimerization of GrpE was shown to be essential for the stable association with DnaK [90,91]. Conformationally, the NBD in the complex with GrpE closely resembles the ADP-bound state with the exception of subdomain IIB’s position, which has rotated 14° away from the nucleotide-binding site [90,92] (Figure 5B,C). Based on this structural difference, a mechanism for nucleotide exchange was proposed in which GrpE binding causes rotation of subdomain IIB, facilitated by NBD hinge residues located at the IIA/IIB subdomain interface, which opens the nucleotide-binding site [90,93,94] (Figure 5C). As a result, GrpE binding lowers the energy barrier required for nucleotide exchange and ultimately stimulates the dissociation of ADP from DnaK up to 5000-fold [95]. This mechanism was later determined to be universal among prokaryotic and all classes of eukaryotic NEFs (vide infra), as all of them primarily bind to subdomain IIB of the NBD causing its displacement and subsequent nucleotide release.
Figure 5. The prokaryotic NEF GrpE.
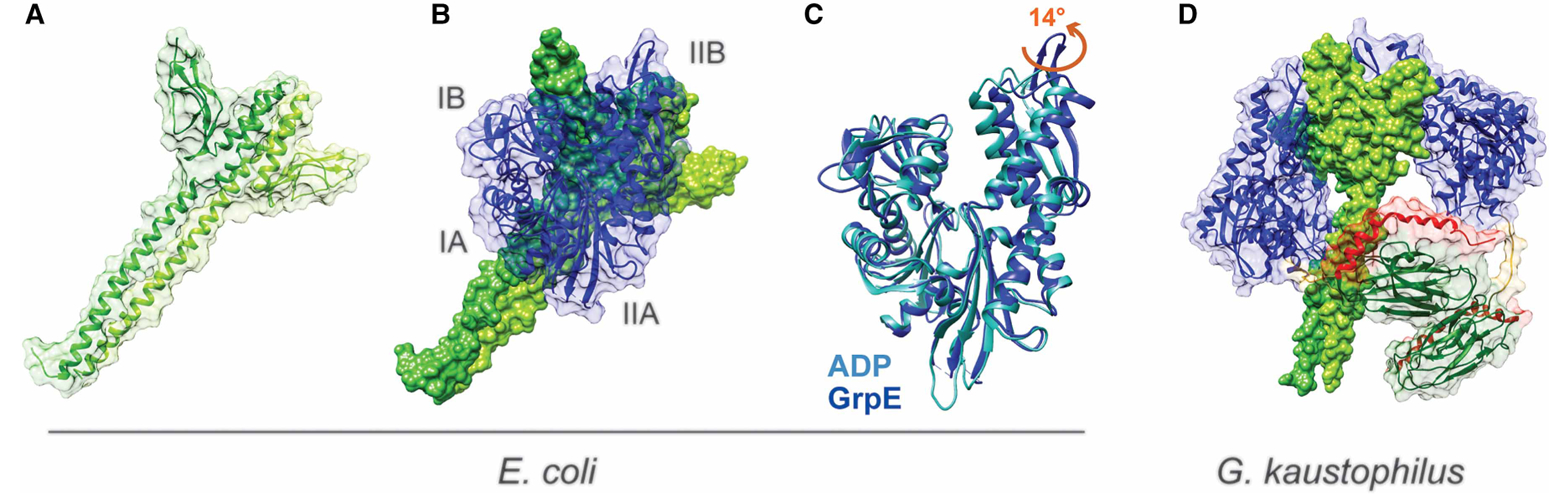
(A) Structure of E. coli DnaK GrpE (PDB ID: 1dkg [90]). (B) GrpE (green) in complex with the NBD of E. coli DnaK (blue). (C) Conformational changes in the NBD caused by GrpE binding. ADP-bound NBD is shown in light blue (homology model based on PDB ID 3hsc [92]) and GrpE-bound NBD in blue. The red arrow indicates the movement of subdomain IIB caused by GrpE binding. (D) Crystal structure of full-length DnaK from G. kaustophilus with its corresponding GrpE (PDB ID: 4ani [98]). The domains of DnaK are colored as in Figure 1.
It is worth mentioning that because the crystal structure of a complex of GrpE and the NBD of DnaK was determined in the absence of ADP and the SBD of the chaperone [90], there is some debate regarding the physiological relevance of the crystal structure. These concerns are amplified, considering that the accuracy of the interface between GrpE and the NBD in the crystal structure has previously been contested [96,97]. Studies have demonstrated that mutations of select NBD residues at the binding interface observed in the crystal structure have no effect on the ability of the NBD to form a complex with GrpE [96]. At the same time, mutations/deletions to several NBD or GrpE residues, not located on the binding interface, prevent the association between the chaperone and its NEF [93,96,97]. Of particular interest is a highly conserved loop in bacterial NBD, which is located at the tip of subdomain IIB and whose deletion was shown to abolish the interaction between GrpE and DnaK [96]. In contrast with the observed 2:1 stoichiometry between GrpE and the NBD of E. coli DnaK, a crystal structure of full-length G. kaustophilus DnaK complexed with its corresponding GrpE revealed 2:2 stoichiometry [98]. In this structure, the NBD of each DnaK interacts with one of the GrpE monomers in a manner similar to what was previously reported [90] (Figure 5D). However, the interdomain linker of one of DnaK molecules from the quaternary complex is inserted into the substrate-binding pocket of DnaK from the second complex present in the asymmetric unit, which raises doubt about the physiological relevance of this arrangement. Moreover, this 2:2 stoichiometry has yet to be observed for E. coli as previous studies have shown 2:1 ratio even in the context of full-length proteins [99].
In addition to its nucleotide exchange activity, GrpE has been proposed to participate in substrate release from Hsp70s. The precise mechanism behind this function is not fully understood; however, it was suggested that an N-terminal disordered region of GrpE may assist in the dissociation of the substrate by either acting as a pseudo-substrate to prevent substrate re-binding or by binding close to the substrate-binding cleft of the Hsp70 thereby changing its conformation [90,99,100]. A model derived from electron microscopic analysis suggests that the α-helical tail of GrpE tips towards the SBD of DnaK, which might modulate substrate dissociation [99]. Finally, GrpE is widely acknowledged for its role as a thermosensor within the Hsp70 chaperone system, as its conformational changes at physiologically relevant temperatures ranges regulate the chaperone system during heat shock [97,101,102]. The long α-helices of E. coli GrpE reversibly unfold with a melting transition midpoint of 48°C [103,104]. These temperature-induced conformational changes prevent the association between GrpE and DnaK, thus blocking the nucleotide exchange activity of the NEF and locking the chaperone in a high substrate affinity state [102]. In thermophilic bacteria, such as T. thermophilus, thermal transitions occur at higher temperatures to offset more extreme conditions [105].
Eukaryotic NEFs
In contrast with prokaryotes, eukaryotes have many NEFs, and they are associated with a variety of cellular functions. GrpE homologs in eukaryotes are found in mitochondria and chloroplasts [106–108]. There are multiple NEFs in the cytoplasm of eukaryotic cells, and they are classified into three structurally unrelated classes: BAGs, HspBP1/Fes1, and Hsp110s. Proteins from each class interact with both cytoplasmic Hsp70s, Hsc70, and HspA1, with no apparent preference (reviewed in [109]) and can be associated with specialized cellular processes (vide infra). In addition, two NEFS are localized to the ER where they regulate the functional cycle of the ER-resident Hsp70, BiP. One is an HspBP1 homolog called BiP-associated protein (Bap) in higher eukaryotes and Sil1 in yeast, and the other is an Hsp110-like protein called Grp170 (glucose-regulated protein of 170 kDa).
BAG (BCL-2-associated athanogene) proteins are the most structurally diverse family of NEFs. They contain a variety of domains that confer functional diversity to this family [110], but all share a characteristic BAG domain that was first described for BAG1 and is responsible for interaction with the NBD of Hsp70s [111]. Five out of the six BAG proteins in humans (BAG1–4 and BAG6) contain only one C-terminal BAG domain, while BAG5 has five BAG domains; only the most C-terminal has been shown to interact with Hsp70 [112]. Structurally, BAG domains form a three-helix bundle (Figure 6A) that directly interacts with subdomains IB and IIB of Hsp70s NBD leading to 14° rotation of the latter [111] (Figure 6D). Uniquely, the BAG domain of BAG2, called the ‘brand new BAG’ (BNB) domain, adopts a dimeric structure, and its interaction mode with the NBD is slightly different [113]. In addition to their interaction with the NBD, at least two BAG proteins, BAG1 and BAG3, have been reported to bind the SBD via a region that does not involve the BAG domain [114]. This interaction contributes to substrate release, similarly to the previous suggestion for prokaryotic NEF GrpE [100].
Figure 6. Structural features of eukaryotic NEFs.
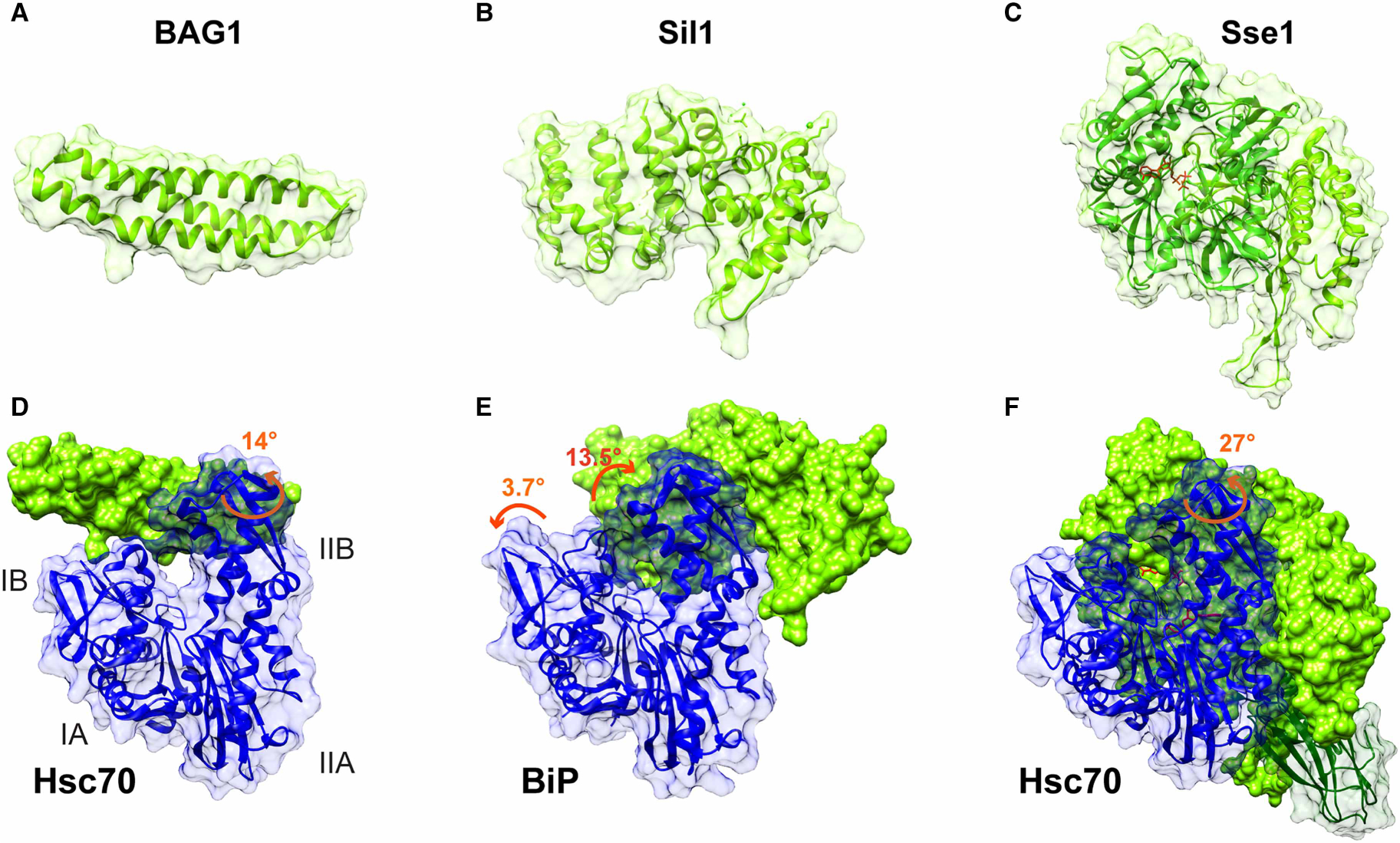
Structures of human BAG1 (A), yeast Sil1 (B), and the yeast Hsp110, Sse1 (C). Crystal structures of the complexes of these NEFs with their respective Hsp70s are shown below each isolated NEF structure, with an indication of what changes in the NBD conformation are caused by NEF binding: (D) bovine Hsc70 NBD-human BAG1 (PDB ID 1hx1 [111]); (E) yeast BiP NBD-Sil1 (PDB ID 3qml [71]); (F) yeast Hsc70–Hsp110 (PDB ID 3c7n [138]). The structure shown in (F) of the complex between Hsc70 and Hsp110 includes the SBD (shown in transparent surface and green ribbon). In (F), ADP*BeF3 bound to Hsp110 and ADP bound to Hsc70 are colored in red.
Presumably, an Hsp70 teams up with a given BAG depending on the particular function to be performed. BAG1, which has a ubiquitin-like domain, is involved in the degradation of misfolded proteins [115]. For example, BAG1 was found to promote binding of the leukemia-associated BCR-ABL protein to the proteasome [116] and, more recently, the Hsp70–BAG1 complex was shown to target mutant misfolded heart-specific potassium channels for proteasomal degradation [117]. BAG2, on the other hand, prevents uncontrolled degradation of proteins by inhibiting the E3 ubiquitin ligase CHIP (the carboxy terminus of Hsc70 interacting protein), which associates with Hsp70s and labels misfolded proteins for degradation [118,119]. A chaperone complex consisting of Hsp70, BAG3, and the small heat-shock protein (sHsp) HspB8 was found to target mutant misfolded superoxide dismutase SOD1 and polyQ-containing huntingtin, associated with familial amyotrophic lateral sclerosis and Huntington disease, respectively, for macroautophagy, a degradation process responsible for removing large protein complexes and portions of cytoplasm [120–122]. Recently, BAG3 was also shown to act as a sensor of proteotoxic stress during proteasome failure [123] and as a scaffolding platform bringing Hsp70s and sHsps together [124]. BAG4 and BAG5 inhibit apoptosis, albeit through different mechanisms: BAG4 blocks downstream signaling from receptors of the tumor necrosis factor family [125], while BAG5 migrates to the ER during ER stress where it interacts with BiP and prevents apoptosis [126]. Finally, BAG6 controls targeting of newly synthesized proteins to the ER and is involved in ER-associated degradation (ERAD) [127,128]. How individual BAGs are selected by Hsp70s is not entirely clear, but it was recently shown that the affinities of BAG proteins to Hsp70s are not all the same [129]. The results in this study suggest that a selection process might depend on tightly balanced concentrations of BAG proteins in a way that higher concentration of the particular BAG protein at certain cellular conditions compensates for differences in their affinities for Hsp70s.
The cytosolic NEF HspBP1 (Fes1 in yeast) and its ER-resident homologue, Bap (Sil1 in yeast), represent the smallest family of eukaryotic NEFs. Structurally, both of these proteins are characterized by a conserved domain consisting of four Armadillo-like repeats [130], each containing three α-helices arranged in a loose triangle, flanked by longer α-helices at both ends (Figure 6B). Overall, the conserved domain adopts an elongated curved shape characterized by a right-handed superhelical twist with a concave surface that makes direct contacts with the NBD of an Hsp70 [131,132]. Interestingly, two different modes of interaction were reported for HspBP1 and Sil1 complexes with HspA1A and BiP, respectively. According to the crystal structure of HspBP1 with a fragment of HspA1A containing only lobe II of the NBD, the Armadillo-like domain wraps around subdomain IIB in a way that would cause its N-terminal capping α-helices to clash with NBD subdomain IB, but the steric clash does not happen since the interaction with HspBP1 causes partial unfolding of lobe I [131]. The Armadillo-like domain of Sil1 also wraps around subdomain IIB of BiP; however, in contrast with HspBP1, its N-terminal capping α-helices make only a few direct contacts with subdomain IB [132]. Moreover, Sil1 binding does not cause local unfolding of lobe I instead pushing lobe I and lobe II away from the nucleotide-binding pocket by ~3.7° and 13.5°, respectively, and thus disturbing ADP contacts with the NBD (Figure 6E). In addition to their NEF activity, this family of NEFs has been recently found to promote substrate release from Hsp70s. Both HspBP1 and Bap have flexible N-terminal regions that have been shown to act as a pseudo-substrate by binding to the substrate-binding pocket of the SBD and in so doing, facilitating substrate release [133,134]. The fact that prokaryotic GrpE and two of three families of eukaryotic NEFs have been implicated in substrate release suggests that this function may be a conserved characteristic of several families of NEFs in addition to their nucleotide exchange activity.
The Hsp110s are the most abundant NEFs in eukaryotes. Hsp110s and Hsp70 share a common ancestor and are structurally homologous [135]. Like Hsp70s, Hsp110s consist of an N-terminal NBD and C-terminal SBD connected by a linker. While the NBD of Hsp110s closely resembles the NBD from Hsp70s, the SBD contains an insertion of unknown function between strands β7 and β8, and an extended C-terminus, both shown to be disordered [136] (Figure 6C). In the Hsp70–Hsp110 complex, NBDs of both proteins tightly interact with each other in a way that subdomain IIB of Hsp70 is embraced by NBD subdomains IB and IIB and additionally by the SBD α-helical lid of the Hsp110 (Figure 6F). These extensive contacts cause a 27° outward rotation of the Hsp70 subdomain IIB. Importantly, the βSBDs of both proteins are located outside of the interaction interface with their substrate-binding sites, facing away from one another, which leaves them available for substrate binding [137,138]. Interestingly, available evidence suggests that despite the similarity of Hsp110 domain organization to that of Hsp70s, Hsp110s do not cycle through docked and undocked states to perform their functions like Hsp70s do. First, although ATP binding to the NBD of Hsp110s is required for their interaction with Hsp70 [139], ATP hydrolysis is not needed for their activity [140,141]. Second, the hydrophobic linker in Hsp70s is replaced by a positively charged linker in Hsp110s. This difference in sequences might prevent Hsp110’s linker from sampling a set of conformations crucial for interdomain allosteric communication in Hsp70s [31]. Finally, peptide binding by the SBD of Hsp110s is characterized by extremely fast on and off rates, indicating that the SBD does not adopt a closed state and interacts with substrates only transiently [142].
Besides their NEF activity, Hsp110s have also been reported to act as ‘holdases’ by binding proteins and preventing their aggregation [143]. Indeed, as seen in the crystal structure, the substrate-binding pocket present in Hsp70s is preserved in Hsp110s [136] although with specificity for peptides enriched in aromatic residues [142]. How this holdase activity is achieved, however, is unknown since there is no evidence that Hsp110s can adopt a closed high-affinity state.
Most strikingly, Hsp110s are unique among other eukaryotic NEFs in that they facilitate protein disaggregation by Hsp70s in metazoans, which lack Hsp104, the Hsp70 disaggregation partner in yeast [140,144]. In fact, it was recently found that even when Hsp104 is present it requires an Hsp70–Hsp110 complex for both its recruitment to protein aggregates and its disaggregation activity [145]. In metazoans, the precise mechanism by which Hsp110s assist Hsp70s in disaggregation remains unknown. Since Hsp110s contain the SBD domain known to bind substrates, it is plausible that Hsp110s interact with client proteins along with Hsp70s to assist in the process. Conversely, a recent study showed that only their NEF activity is necessary for disaggregation [146], in which case the question arises why other classes of NEFs are unable to assist Hsp70s in protein disaggregation [147]. In addition, one study showed that Hsp110 could use ATP to unfold proteins independently from Hsp70s, but these results have not been confirmed or reproduced to date [148].
The guts of the Hsp70 allosteric machine: Hsp70 substrate interactions and how they relate to their cellular functions
What characteristics define an Hsp70 substrate?
As early as in 1985, it was proposed that Hsp70s bind to hydrophobic surfaces exposed in non-native proteins: Pelham et al. [149] observed that upon heat-shock, ribosomal proteins aggregate through hydrophobic interactions, and Hsp70, by binding tightly to the resulting exposed hydrophobic surfaces, helped to disrupt the aggregates and release their substrates in a strictly ATP hydrolysis-dependent fashion. The hydrophobic surfaces proposed to be Hsp70 substrate interaction sites were later defined more precisely to be short sequences that we now know are typical sequences that bind to the SBD pocket. Peptide arrays and phage display studies identified stretches of hydrophobic residues and positive charges as the main sequence features of Hsp70 substrates [150–154]. Even though there was not a specific consensus sequence for binding identified, these sequence features allowed the development of algorithms that can predict putative binding sites within a proteins primary structure [153,154]. When the relevant algorithms are applied to the E. coli proteome, the frequency of appearance of the predicted DnaK-binding sites is remarkably high (one site every 40 residues, on average).
For the chaperone to bind, however, the sites on any given protein must be exposed, and accessibility constitutes the second major criterion for Hsp70 substrate selection. There are general situations where both criteria are met, and understanding these situations offers insight into the diverse functions of Hsp70s: (i) Polypeptide chains emerging from the ribosome during biosynthesis or emerging from translocation sites in membranes present potential binding sites until they are sequestered by structure formation during folding. (ii) Proteins that are partially unfolded or transiently visit the unfolded state, most often as a result of temperature stress, mutation, or lack of an obligatory partner in a complex. In some cases, folded proteins may display fluctuating accessible binding sites in their ensemble of populated states. (iii) Proteins that are largely folded but contain unfolded regions, often on termini or loops; in this class, the binding motifs generally serve as specific recognition sites (vide infra for examples of different types of Hsp70 protein substrates). Given these criteria, how many Hsp70 substrates are there in the cell? This point was extensively studied in the literature using a wide array of genetic and proteomics approaches. The results allowed not only the identification of multiple cellular substrates of the chaperone but also the characterization of the cellular effect of Hsp70 depletion, mutation, or overexpression in E. coli [155–157]. More recently, two studies sought to identify all physiological substrates bound to DnaK at a particular time. In one case, substrates were identified by pulling down the complexes with DnaK from E. coli and subjecting them to mass spectrometry [158]. In another case, chaperone effects on the folding of hundreds of cytosolic E. coli proteins were assayed using a reconstituted chaperone-free translation system. Addition of the DnaK system significantly augmented the amount of soluble protein produced for many of those tested [159]. These two reports show that numerous proteins with diverse functions, including both newly synthesized and pre-existing proteins, are substrates of DnaK.
The substrate-binding site of Hsp70s is promiscuously selective
The molecular details of the interaction between the canonical binding site of Hsp70s and substrates have been extensively characterized in vitro using short peptides as model substrates, which avoids the experimental challenges of working with large, unfolded proteins. This strategy has been exploited to obtain atomic-resolution structures of peptides bound to the canonical binding site of Hsp70. The first crystal structure [39] of the SBD of E. coli DnaK showed that the model peptide substrate NR (NRLLLTG) sits in a cleft in the βSBD, with the helical subdomain acting as a lid and covering the substrate (Figure 7). Subsequently, multiple structural studies of DnaK/substrate peptide complexes by NMR and crystallography have established the generality of this mode of binding, regardless of the sequence of the bound peptides (see [2] for a summary). Moreover, structures of model substrates bound to Hsp70s from other organisms have revealed the same basic arrangement, pointing to the high conservation of this binding mode.
Figure 7. The SBD of E. coli DnaK contains the canonical binding site.

(A) Representation of the crystal structure of the SBD of E. coli (gray) (PDB ID: 1dkz [39]) with bound NR peptide (NRLLLTG, in purple sticks). (B) Top view of the SBD canonical binding site, where the α-lid and residues 404–406 and 429–431 have been removed to visualize the bound NR peptide (residues labeled 1–7). The surfaces on the SBD contacted by the substrate peptide are colored according to the residue position in the substrate, and the numbers (−2 to +2) indicate the ‘positions’ relative to the ‘0th residue’, which corresponds to the SBD site that contacts the central residue of the peptide [39].
In Hsp70s, the binding groove is made up of five pockets in the βSBD, where the central pocket (termed the ‘0th position’) has the highest stringency, and thus invokes the highest selectivity [15] (Figure 7). This position optimally accommodates Leu, and Ile, and less favorably Val and Phe. The other pockets in the groove can accommodate various substrate residues, although there is a bias against negatively charged residues, and there are slight preferences at each pocket. Taken together, the properties of the binding site lead to intriguing selective promiscuity. This feature provides Hsp70s with the ability to bind to various protein clients in the cell and perform a wide spectrum of functions.
The backbone of the bound substrate takes up an extended conformation with a gentle twist and extensive hydrogen bonding. While these features are remarkably common among all structures solved to date, there are some intriguing variabilities: For example, the NR model peptide can bind in a different register, hence shifting which pocket each residue occupies, some antimicrobial peptides can bind to the βSBD despite their lack of positive charges, and a few peptides have been observed to bind in the opposite orientation to the lion’s share of examples [160,161].
A few studies have proposed that sites on the Hsp70 away from the canonical substrate-binding groove may be implicated in substrate binding, although the supporting evidence has usually been indirect, for example, by testing the impact of mutation or truncation of Hsp70s on its activities [162–166].
Protein substrates utilize the same binding mode as peptide models
The study of the atomic details of the binding interactions between Hsp70s and peptide substrates provided a necessary first step in understanding how Hsp70s bind to their cellular targets, which are generally full-length substrates. Recent advances in many experimental techniques, such as single-molecule fluorescence and high-resolution NMR, have allowed our structural investigation of substrate binding to Hsp70s to transition from peptides to larger proteins [2,5]. All cases studied to date show that Hsp70 utilizes the canonical binding site in the SBD to bind to accessible Hsp70 binding motifs in their client proteins, regardless of the overall conformation of the particular substrate [29,167–169].
A completely unfolded protein should expose one or more Hsp70 binding sequences that can be recognized and bound by the chaperone in the same manner as a peptide substrate. Indeed, several studies have identified and characterized by different methods Hsp70 chaperones bound to a variety of unfolded substrates. In some cases, the protein was engineered to be an unfolded model Hsp70 substrate; for example, a fragment of the staphylococcal nuclease that unfolds at 37°C [170], a slow-folding mutant of RNase H [171], truncated fragments of apomyoglobin [172,173], or rhodanese refolded in the presence of Hsp70 [174]. In other experiments, the whole conformational ensemble of a substrate under conditions where unfolded states were in equilibrium with natively folded species was probed for Hsp70 binding: namely, the N-terminal domain of the D. melanogaster adaptor protein drk (drkN SH3) [175] or the small 53-residue human telomere repeat binding factor 1 (hTRF1) [176–178]. In all of these cases, the substrates were observed to be globally unfolded in the Hsp70-bound state [168,170,173–175,179–181] and to lack long-range interactions that might have been established in the unfolded state of the substrate in the absence of the chaperone [178]. Detailed analysis shows that the binding of the chaperone does alter the conformational ensemble of some substrates, like loss of some residual structure and long-range interactions [172,178], expansion of the unfolded state [174], or formation of the residual secondary structure away from the chaperone-binding site on the substrate [168,180]. Not surprisingly given the presence of multiple binding sites in several of the unfolded protein substrates, evidence has been presented that more than one chaperone-binding sites in the substrate can be simultaneously bound to Hsp70s [174,176,177]. The weak, transient, and cyclical nature of chaperone binding combined with the possibility of multiple binding sites generates a highly heterogeneous complex ensemble in these cases of fully or largely unfolded substrates in complex with Hsp70s.
Hsp70s can also bind to exposed chaperone-binding motifs on partially folded or near-native substrates [2,5] and in some cases, the substrate remains partially folded while bound [28,29,167]. There are fewer in-depth structural studies of these interactions, but it is clear that folded proteins can expose chaperone-binding sequences as they sample multiple conformations, in exposed unstructured loops or termini, and in sparsely and transiently populated unstructured states. These types of binding interaction have been described, for example, for the native DnaK substrate, σ32 [167], and the eukaryotic Hsp70 substrate, the glucocorticoid receptor (GR) [182] (see below for a detailed description of these substrates). In all cases, the result of the initial binding of Hsp70 to the exposed short binding motifs on partially unfolded substrates is the acquisition of more unfolded or less stable conformations of the clients that are then allowed to refold [183–185]), be targeted for degradation [167], or handed to other chaperone systems [182,186]. This type of binding is also used by Hsp70s to actively unfold substrates in an ATP-dependent fashion acting as an ‘unfoldase’ (for a comprehensive review see [3]). Nevertheless, whether by conformational selection or active unfolding, the consequence of Hsp70 binding in these cases is a shift to more unfolded states of the substrate.
One general finding is that Hsp70s accommodate more folded substrates by exploiting the dynamic nature of the α-lid, which can detach from the βSBD [17,18,26–29]. Moreover, the α-lid can directly interact with regions of the bound client. Interactions with the α-lid, together with the βSBD binding cleft, were shown to stabilize the DnaK-bound partially folded maltose-binding protein (MBP) and a monomeric variant of the replication initiation protein repE (RepE54), as the stabilization of these DnaK-bound substrates is abolished when the α-lid is truncated [28].
The last type of Hsp70 binding interaction with a protein substrate occurs when a binding motif is present on a well-folded substrate essentially as a tag to mediate chaperone binding, for example, at a terminus of the protein. The consequences of binding, in this case, are more straightforward, in that the ensemble of folded states of the substrate may not be perturbed. Instead, the substrate may be delivered to a downstream chaperone or partner, or, as is postulated for clathrin (vide infra), disassembly of the substrate from a complex is facilitated by chaperone binding [187–189].
Hsp70 binding to protein substrates effectuates multiple biological outcomes
In the cell, Hsp70s perform a myriad of highly diverse functions by binding to their protein clients as described. In all cases, the ability of the chaperone to bind transiently (the residence time determined by allosteric cycling) to sequences that are usually sequestered within the folded state of a protein in some way confers an advantage to the given physiological system. This transient chaperone binding thus affects the substrate, whether it be delay of folding to prevent misfolding and enable translocation across a membrane, facilitation of productive folding, inhibition of aggregation and dissociation of aggregates, maintenance of an unfolded state for translocation across a membrane, hand-off to a downstream chaperone or degradation machine, and disassembly of specific complexes. In this section, we wish to paint a picture of how this simple binding/unbinding machine has been harnessed evolutionarily in such a wide array of functions. We will not give an encyclopedic description of Hsp70 functions, but rather select illustrative examples and relate them to the structural and mechanistic properties of Hsp70s described in the preceding sections (Figure 8).
Figure 8. Examples of modes of Hsp70 binding to protein substrates.
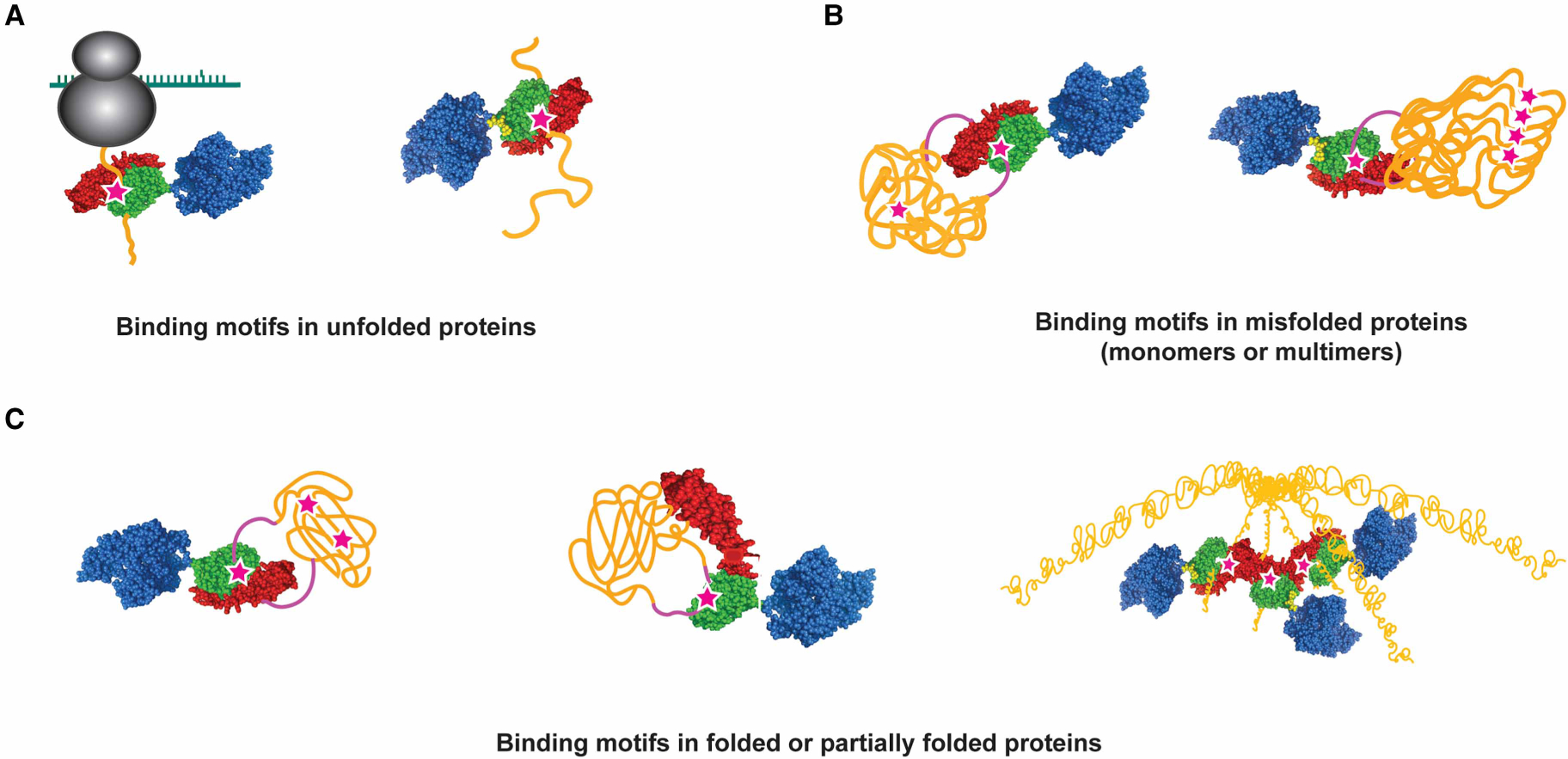
(A) Hsp70s may bind to motifs exposed in unfolded proteins to facilitate their proper folding as they emerge from the ribosome, to keep them unfolded for translocation into organelles, to enable hand-off to downstream chaperones, or to prevent aggregation. (B) Hsp70s may bind to motifs exposed at the surface of misfolded or aggregated substrates. (C) Hsp70 may bind motifs that are available on folded or near-native proteins. This may occur when the protein transiently exposes the binding (left panel, for example, when DnaJ opens the DnaK binding site on σ32 [167], center panel, for example, when Hsp70 binds to an exposed site in the glucocorticoid receptor [182]) or when the binding site resides on in an unstructured region (right panel, for example, Hsc70 binding to the C-terminal motif of native clathrin in triskelion structures [187]). In all panels, the substrate is depicted in orange, the Hsp70 binding motif is shown as purple stars, and the Hsp70 colored as in Figure 1.
Delay of folding to prevent misfolding and enable translocation across a membrane
Hsp70s have been implicated in the chaperoning of nascent chains in both prokaryotes [156,190] and eukaryotes [191]. In the case of eukaryotes, specialized Hp70s have evolved that are dedicated to assisting protein biogenesis [191–193]. The S. cerevisiae system is particularly well studied: The Hsp70 homolog, Ssb, works together with a dedicated ribosome-associated complex (RAC) made up of the J-protein zuotin (Zuo2) and a specialized Hsp70 homolog, Ssz1 to greet nascent chains while they are emerging from the ribosome and ensure the fidelity of protein folding while diminishing the risk of aggregation [194]. The association of Ssb with the ribosome is critical to its ability to perform a dedicated task in nascent chain folding [195]. Recently, in vivoHsp70 has been reported to inhibi selective ribosome profiling has revealed exquisite details about the Ssb interactome and how specific substrate interactions integrate actions at the ribosome to modulate translation rates, facilitate folding, and support targeting to membranes [196].
Another critical cellular function that relies on the interaction of Hsp70s with unfolded polypeptides is the chaperoning of chains that are destined to cross membranes [197]. On the entry side of the membrane to be traversed, the targeted polypeptide must be unfolded, while on the exit side, the chain must be held to resist back-sliding and guided to avoid premature folding and prepare it for downstream chaperone interactions. These roles have been described for protein translocation into mitochondria [198], chloroplasts [199], and the ER [200].
Facilitation of productive folding
As described above, Hsp70s bind to unfolded substrates and generate a heterogeneous ensemble of conformations where substrates may undergo conformational sampling while chaperone-bound. In this way, substrates may be helped to find an optimal folding pathway and to search a conformational space, also avoiding misfolded energy traps [175–177]. Additionally, simultaneous substrate binding to multiple DnaK molecules should lead to considerable substrate expansion as a way to convey the substrate through a productive folding pathway [174].
Details of the implications of partially folded or near-native substrates bound to Hsp70 were provided by a recent study that used optical tweezers to mechanically unfold MBP and RepE54 to test the impact of their interaction with DnaK [28]. While native MBP did not bind to DnaK, near-native states were stabilized against forced unfolding by binding to DnaK. Conversely, when MBP bound DnaK in a more unfolded state, the chaperone impaired substrate refolding. These provocative data were explained through a kinetic competition mechanism where unfolded DnaK-locked chains could not refold unless folding was initiated before chaperone binding, in which case they were then subsequently stabilized by DnaK and more resistant to mechanical unfolding.
To date, there is no direct evidence that the rate of folding of substrates is altered by Hsp70 binding. For example, the measured rate of refolding of both staphylococcal nuclease [170] and luciferase [181] has been reported to be the same in the absence of Hsp70 and after ATP-induced release from the chaperone. As the proposed influence of the chaperone on the unfolded ensemble suggests that it disfavors kinetically trapped misfolded states, acceleration of folding might occur. New observations from the Hartl laboratory indeed support this possibility (F. U. Hartl and M. Hayer-Hartl, personal communication, 2019).
Inhibition of aggregation and dissociation of aggregates
Not surprisingly, predicted Hsp70 binding sequences correspond closely with those that are predicted to be highly aggregation-prone. Thus, the sequestering of these short sequences by Hsp70s in unfolded, partially folded or misfolded proteins should inhibit aggregation (acting as a ‘holdase’) [201]. The early observation (described above) that deletion of DnaK caused widespread aggregation in E. coli [155] established that this Hsp70 plays a key role in preventing aggregation in vivo. In addition, in vitro studies have demonstrated inhibition of aggregation by the Hsp70 system using model substrates such as firefly luciferase, β-galactosidase, or rhodanese [202–205]. More recently, the ability of Hsp70s to inhibit the aggregation of disease-related proteins has been investigated [1,206,207]. For example, Hsp70 has been reported to inhibit α-synuclein aggregation in vitro by binding prefibrillar species [208,209], to block the early stages of Tau aggregation by suppressing the formation of critical nuclei [210], and to suppress the aggregation of the Aβ peptide [211]; both Tau protein and Aβ peptide aggregation have been associated with Alzheimer’s disease.
In addition, Hsp70 binding has been implicated in the dissociation of protein aggregates [212]. Hsp70s are envisioned to bind an aggregated substrate via an exposed favorable binding sequence at the surface of the aggregate [3,213–215]. Here, by ATP-fueled clamping of the substrate in the canonical binding site, local unfolding propagates and extracts the substrate from the aggregate’s surface. While the Hsp70 system has been reported to perform this function alone [216], the efficiency of disaggregation is greatly augmented by cooperation with other unfolding chaperones, like Hsp104/Hsp100/Hsp110 [3,212–214]. In the case of a polypeptide that is part of a large aggregate or refractory removal from the aggregate, a mechanism called ‘entropic pulling’ has been invoked to explain the action of the Hsp70 system on the substrate [217,218]. This same model can be applied to the facilitation of polypeptide translocation across membranes and the tugging on nascent chains out of the ribosome exit tunnel [219,220]. By this model, the large Hsp70 molecules bound to a polypeptide on the surface of an aggregate (or at the exit of a channel) move with increased freedom away from the anchoring site by repulsion/collision with the aggregate (or the channel) creating an effective pulling force that removes the substrate [184,217,218].
Hand-off to a downstream chaperone or degradation machine
In vivo functions of Hsp70s frequently involve the partnering with downstream cellular machinery. A prime example is the hand-off of substrates from DnaK to the GroEL/ES chaperonin complex in E. coli [221]. Also, Hsp70s are known to bind to substrate proteins that display their chaperone-binding sequences as part of a regulatory process, or as they need to be processed in the cell (see [2,5]). The regulation of the stability of the E. coli σ32 by DnaK and DnaJ constitutes a paradigm for the interaction of Hsp70 chaperones with natively folded substrates and illustrates how such binding can be exploited physiologically to modulate the activity of a cellular factor [222–225]. The σ32 subunit of the RNA polymerase binds to the core enzyme in response to cellular stress and targets it to the promoters of heat-shock genes [226–228]. Under physiological conditions, the native state of σ32 is a substrate of DnaJ and DnaK [222–225], and chaperone binding renders σ32 susceptible to degradation by the protease FtsH. Upon heat shock, when DnaK/J are less abundant, σ32 is stabilized and is able to bind the RNA polymerase to mount a stress response. Many details of the interaction between DnaK with native σ32 as well as the impact of chaperone binding on σ32 structure were mapped at residue-level by Rodriguez et al. [167]. This work revealed that in native σ32 DnaJ and DnaK bind to different (proximal) sites that are transiently exposed in the substrate when it is not bound to the RNA polymerase. Initial binding of DnaJ to σ32 destabilizes the DnaK-binding site and facilitates its binding. Tight binding of DnaK destabilizes a region in the N-terminal domain of σ32, which is the primary target for FtsH-mediated degradation.
In another well-characterized example, the ligand-dependent activation of the GR in the cell occurs a result of the cooperation between the Hsp70 and Hsp90 systems [182,229,230]. The work by Kirschke et al. revealed the molecular details by which Hsp70 (in the presence of Hsp40 and ATP) binds to a folded form of the ligand-binding domain of the GR (LBDGR) hindering ligand binding, or actively removing it from its binding site, stabilizing an inactive form of the receptor. Hsp70 binding (but not Hsp40 alone) causes a local unfolding of LBDGR, enough to prevent ligand binding, and only after this ‘priming’ step, the Hsp90 system (Hop, p23) is able to rescue LBDGR from the Hsp70-mediated inactivation and promote ligand binding [182].
Disassembly of specific complexes
As described earlier, disassembly of the clathrin-coated vesicles during endocytosis is mediated by Hsc70 [183]. Clathrin in the lattice is first recognized by the specialized J-protein auxilin [185], which recruits Hsc70 to the site. Hsc70 binding to the QLMLT sequence at the C-terminal of clathrin results in the extraction of clathrin from the lattice and disassembly of the vesicle coat. Evidence supports the action of Hsc70 on the clathrin triskelion to be a destabilization by binding to sites exposed by conformational distortions [187,188]. The ‘conformational selection’ and ‘entropic pulling’ mechanisms have been invoked to explain the molecular mechanism of action of Hsc70 in a disassembly process [184,189].
Final thoughts on the multifunctional Hsp70 molecular machine
It is impressive that the simple two-domain Hsp70 molecular chaperone has been recruited to participate in such a wide array of physiological functions throughout prokaryotes, archaea, and eukaryotes. The capacity to bind with nucleotide-modulated affinity to segments of polypeptide chain using sequences that discriminate fully folded proteins from partially folded species has endowed Hsp70s with a highly useful mechanism. The duality of their action implicates them in a multitude of cellular pathways: as holdases, they hold substrates for a residence time that can be modulated by ATP/ADP ratios and by co-chaperone availability, and as unfoldases, they cause their bound substrates to unfold by virtue of the geometric consequence of retaining a 7-residue segment in a clamped SBD binding site. Their binding site shows promiscuity, such that many, many client proteins can be recognized. Nonetheless, they are selective for hydrophobic stretches that would normally be sequestered, which is key to Hsp70 functions.
As we have discussed in this Review, Hsp70s are a result of evolutionary opportunism: the combination of an actin fold, which provides nucleotide switchability, and a novel fold that cradles the polypeptide under a regulatable lid. The deep understanding that has emerged from structural and biochemical studies of Hsp70s has provided mechanistic insight into how the allosteric communication between the two ligand-binding domains of Hsp70s is achieved by energetic competition between interfaces formed in the two alternative conformations accessible to each domain. A focus of future research will be the relationship of the tunable allosteric mechanism of Hsp70s to their functional diversification: are some Hsp70s tuned for specific functions, perhaps because of specific substrate repertoires?
This is indeed an exciting time to witness synergistic advances in the cell biology of Hsp70 functions, new discoveries of their relationship to many pathologies, and structural revelations about their molecular mechanism. While there have been major advances in recent years in our understanding of the molecular mechanism of Hsp70 chaperones, as described in this review, major questions remain about the physiological roles of these key players in protein homeostasis. Answering these questions will require a deeper understanding of the interactions of Hsp70s with their co-chaperones and substrates. The knowledge that eukaryotic Hsp70s may partner with multiple NEFs and JDPs adds to the complexity of the Hsp70 system and its capacity to be specialized for particular functions. Hsp70s themselves are tunable by sequence modification or through partnerships with diverse co-chaperones. In addition, in a cellular milieu, Hsp70s will see an array of potential protein substrates, the affinity of which may vary through a wide range. The result is that the selection of client interactions is non-random, and the dwell time of a given substrate on an Hsp70 will vary. Putting Hsp70 networks back into the complex, cellular environment will rely on clever methods of determining substrate partitioning.
From the intense research effort on Hsp70 mechanisms and cellular roles in the recent past have emerged many opportunities to design or screen for modulators of Hsp70 function. We have not reviewed this body of work here, as there have been several recent reviews [231–238]. We direct the interested reader to these reviews, and we want to emphasize that the rich knowledge gained on the structure and mechanism of Hsp70s as well as the enhanced understanding of their functions have enabled either the rational design of inhibitors/activators as well as the design of assays to screen libraries for Hsp70 modulators. As a result, there are now numerous small molecules that can be used as leads for clinical therapies and as tools for dissection of Hsp70 functions.
Funding
The writing of this review was supported by a grant to L.M.G. from the National Institutes of Health (GM118161).
Abbreviations
- βSBD
β-sandwich subdomain
- Bap
BiP-associated protein
- BAG
BCL-2-associated athanogene
- BiP
binding-immunoglobulin protein
- CHIP
carboxy terminus of Hsp70-interacting protein
- drkN
terminal domain of the D. melanogaster adaptor protein drk
- ER
endoplasmic reticulum
- Fes1
factor exchange for SSA1 protein 1
- GR
glucocorticoid receptor
- hERG
human Ether a Go-go-Related Gene potassium channel
- HspB8
heat shock protein B-8
- HspBP1
Hsp70 binding protein 1
- Hsp104
heat shock protein 104
- Hsp110
heat shock proteins 110
- Hsc70
heat shock cognate 71-kDa protein
- JDPs
J-domain proteins
- LBDGR
ligand-binding domain of the GR
- MBP
maltose-binding protein
- NBD
nucleotide-binding domain
- NEF
nucleotide exchange factors
- NMR
nuclear magnetic resonance
- polyQ
polyglutamine
- RAC
ribosome-associated complex
- RepE54
monomeric variant of the replication initiation protein
- SBD
substrate-binding domain
- SOD1
superoxide dismutase
- Zuo1
zuotin
Footnotes
This multipronged research activity on Hsp70s is exciting, and we look forward to a very fruitful future for research on Hsp70s.
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Balchin D, Hayer-Hartl M and Hartl FU (2016) In vivo aspects of protein folding and quality control. Science 353, aac4354 10.1126/science.aac4354 [DOI] [PubMed] [Google Scholar]
- 2.Clerico EM, Tilitsky JM, Meng W and Gierasch LM (2015) How hsp70 molecular machines interact with their substrates to mediate diverse physiological functions. J. Mol. Biol 427, 1575–1588 10.1016/j.jmb.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finka A, Mattoo RU and Goloubinoff P (2016) Experimental milestones in the discovery of molecular chaperones as polypeptide unfolding enzymes. Annu. Rev. Biochem 85, 715–742 10.1146/annurev-biochem-060815-014124 [DOI] [PubMed] [Google Scholar]
- 4.Gestwicki JE and Shao H (2019) Inhibitors and chemical probes for molecular chaperone networks. J. Biol. Chem 294, 2151–2161 10.1074/jbc.TM118.002813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer MP and Gierasch LM (2018) Recent advances in the structural and mechanistic aspects of Hsp70 molecular chaperones. J. Biol. Chem 294, 2085–2097 10.1074/jbc.REV118.002810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer MP and Kityk R (2015) Insights into the molecular mechanism of allostery in Hsp70s. Front. Mol. Biosci 2, 58 10.2741/s425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wruck F, Avellaneda MJ, Koers EJ, Minde DP, Mayer MP, Kramer G et al. (2018) Protein folding mediated by trigger factor and Hsp70: new insights from single-molecule approaches. J. Mol. Biol 430, 438–449 10.1016/j.jmb.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 8.Zuiderweg ERP, Hightower LE and Gestwicki JE (2017) The remarkable multivalency of the Hsp70 chaperones. Cell Stress Chaperones 22, 173–189 10.1007/s12192-017-0776-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng W, Clerico EM, McArthur N and Gierasch LM (2018) Allosteric landscapes of eukaryotic cytoplasmic Hsp70s are shaped by evolutionary tuning of key interfaces. Proc. Natl Acad. Sci. U.S.A 115, 11970–11975 10.1073/pnas.1811105115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchberger A, Theyssen H, Schroder H, McCarty JS, Virgallita G, Milkereit P et al. (1995) Nucleotide-induced conformational changes in the ATPase and substrate binding domains of the DnaK chaperone provide evidence for interdomain communication. J. Biol. Chem 270, 16903–16910 10.1074/jbc.270.28.16903 [DOI] [PubMed] [Google Scholar]
- 11.Kamath-Loeb AS, Lu CZ, Suh WC, Lonetto MA and Gross CA (1995) Analysis of three DnaK mutant proteins suggests that progression through the ATPase cycle requires conformational changes. J. Biol. Chem 270, 30051–30059 10.1074/jbc.270.50.30051 [DOI] [PubMed] [Google Scholar]
- 12.Bertelsen EB, Chang L, Gestwicki JE and Zuiderweg ER (2009) Solution conformation of wild-type E. coli Hsp70 (DnaK) chaperone complexed with ADP and substrate. Proc. Natl Acad. Sci. U.S.A 106, 8471–8476 10.1073/pnas.0903503106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swain JF, Dinler G, Sivendran R, Montgomery DL, Stotz M and Gierasch LM (2007) Hsp70 chaperone ligands control domain association via an allosteric mechanism mediated by the interdomain linker. Mol. Cell 26, 27–39 10.1016/j.molcel.2007.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mapa K, Sikor M, Kudryavtsev V, Waegemann K, Kalinin S, Seidel CA et al. (2010) The conformational dynamics of the mitochondrial Hsp70 chaperone. Mol. Cell 38, 89–100 10.1016/j.molcel.2010.03.010 [DOI] [PubMed] [Google Scholar]
- 15.Marcinowski M, Rosam M, Seitz C, Elferich J, Behnke J, Bello C et al. (2013) Conformational selection in substrate recognition by Hsp70 chaperones. J. Mol. Biol 425, 466–474 10.1016/j.jmb.2012.11.030 [DOI] [PubMed] [Google Scholar]
- 16.Wilbanks SM, Chen L, Tsuruta H, Hodgson KO and McKay DB (1995) Solution small-angle X-ray scattering study of the molecular chaperone Hsc70 and its subfragments. Biochemistry 34, 12095–12106 10.1021/bi00038a002 [DOI] [PubMed] [Google Scholar]
- 17.Kityk R, Kopp J, Sinning I and Mayer MP (2012) Structure and dynamics of the ATP-bound open conformation of Hsp70 chaperones. Mol. Cell 48, 863–874 10.1016/j.molcel.2012.09.023 [DOI] [PubMed] [Google Scholar]
- 18.Qi R, Sarbeng EB, Liu Q, Le KQ, Xu X, Xu H et al. (2013) Allosteric opening of the polypeptide-binding site when an Hsp70 binds ATP. Nat. Struct. Mol. Biol 20, 900–907 10.1038/nsmb.2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharya A, Kurochkin AV, Yip GNB, Zhang Y, Bertelsen EB and Zuiderweg ERP (2009) Allostery in Hsp70 chaperones is transduced by subdomain rotations. J. Mol. Biol 388, 475–490 10.1016/j.jmb.2009.01.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y and Zuiderweg ERP (2004) The 70-kDa heat shock protein chaperone nucleotide-binding domain in solution unveiled as a molecular machine that can reorient its functional subdomains. Proc. Natl Acad. Sci. U.S.A 101, 10272–10277 10.1073/pnas.0401313101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuravleva A and Gierasch LM (2011) Allosteric signal transmission in the nucleotide-binding domain of 70-kDa heat shock protein (Hsp70) molecular chaperones. Proc. Natl Acad. Sci. U.S.A 108, 6987–6992 10.1073/pnas.1014448108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer D, Merz DR, Pelz B, Theisen KE, Yacyshyn G, Mokranjac D et al. (2015) Nucleotides regulate the mechanical hierarchy between subdomains of the nucleotide binding domain of the Hsp70 chaperone DnaK. Proc. Natl Acad. Sci. U.S.A 112, 10389–10394 10.1073/pnas.1504625112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Nune M, Zong Y, Zhou L and Liu Q (2015) Close and allosteric opening of the polypeptide-binding site in a human Hsp70 chaperone BiP. Structure 23, 2191–2203 10.1016/j.str.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhuravleva A and Gierasch LM (2015) Substrate-binding domain conformational dynamics mediate Hsp70 allostery. Proc. Natl Acad. Sci. U.S.A 112, E2865–E2873 10.1073/pnas.1506692112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee R, Jayaraj GG, Peter JJ, Kumar V and Mapa K (2016) Monitoring conformational heterogeneity of the lid of DnaK substrate-binding domain during its chaperone cycle. FEBS J. 283, 2853–2868 10.1111/febs.13769 [DOI] [PubMed] [Google Scholar]
- 26.Lai AL, Clerico EM, Blackburn ME, Patel NA, Robinson CV, Borbat PP et al. (2017) Key features of an Hsp70 chaperone allosteric landscape revealed by ion-mobility native mass spectrometry and double electron-electron resonance. J. Biol. Chem 292, 8773–8785 10.1074/jbc.M116.770404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcinowski M, Höller M, Feige MJ, Baerend D, Lamb DC and Buchner J (2011) Substrate discrimination of the chaperone BiP by autonomous and cochaperone-regulated conformational transitions. Nat. Struct. Mol. Biol 18, 150–158 10.1038/nsmb.1970 [DOI] [PubMed] [Google Scholar]
- 28.Mashaghi A, Bezrukavnikov S, Minde DP, Wentink AS, Kityk R, Zachmann-Brand B et al. (2016) Alternative modes of client binding enable functional plasticity of Hsp70. Nature 539, 448–451 10.1038/nature20137 [DOI] [PubMed] [Google Scholar]
- 29.Schlecht R, Erbse AH, Bukau B and Mayer MP (2011) Mechanics of Hsp70 chaperones enables differential interaction with client proteins. Nat. Struct. Mol. Biol 18, 345–351 10.1038/nsmb.2006 [DOI] [PubMed] [Google Scholar]
- 30.Chiappori F, Merelli I, Milanesi L, Colombo G and Morra G (2016) An atomistic view of Hsp70 allosteric crosstalk: from the nucleotide to the substrate binding domain and back. Sci. Rep 6, 23474 10.1038/srep23474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.English CA, Sherman W, Meng W and Gierasch LM (2017) The Hsp70 interdomain linker is a dynamic switch that enables allosteric communication between two structured domains. J. Biol. Chem 292, 14765–14774 10.1074/jbc.M117.789313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.General IJ, Liu Y, Blackburn ME, Mao W, Gierasch LM and Bahar I (2014) ATPase subdomain IA is a mediator of interdomain allostery in Hsp70 molecular chaperones. PLoS Comp. Biol 10, e1003624 10.1371/journal.pcbi.1003624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicolai A, Delarue P and Senet P (2013) Decipher the mechanisms of protein conformational changes induced by nucleotide binding through free-energy landscape analysis: ATP binding to Hsp70. PLoS Comp. Biol 9, e1003379 10.1371/journal.pcbi.1003379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhuravleva A, Clerico EM and Gierasch LM (2012) An interdomain energetic tug-of-war creates the allosterically active state in Hsp70 molecular chaperones. Cell 151, 1296–1307 10.1016/j.cell.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kityk R, Vogel M, Schlecht R, Bukau B and Mayer MP (2015) Pathways of allosteric regulation in Hsp70 chaperones. Nat. Commun 6, 8308–8319 10.1038/ncomms9308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kityk R, Kopp J and Mayer MP (2018) Molecular mechanism of J-domain-triggered ATP hydrolysis by Hsp70 chaperones. Mol. Cell 69, 227–237 10.1016/j.molcel.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 37.Qian YQ, Patel D, Hartl FU and McColl DJ (1996) Nuclear magnetic resonance solution structure of the human Hsp40 (HDJ-1) J-domain. J. Mol. Biol 260, 224–235 10.1006/jmbi.1996.0394 [DOI] [PubMed] [Google Scholar]
- 38.Vogel M, Bukau B and Mayer MP (2006) Allosteric regulation of Hsp70 chaperones by a proline switch. Mol. Cell 21, 359–367 10.1016/j.molcel.2005.12.017 [DOI] [PubMed] [Google Scholar]
- 39.Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME et al. (1996) Structural analysis of substrate binding by the molecular chaperone DnaK. Science 272, 1606–1614 10.1126/science.272.5268.1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wieteska L, Shahidi S and Zhuravleva A (2017) Allosteric fine-tuning of the conformational equilibrium poises the chaperone BiP for post-translational regulation. eLife 6, pii:e29430 10.7554/eLife.29430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukherjee M, Sabir S, O’Regan L, Sampson J, Richards MW, Huguenin-Dezot N et al. (2018) Mitotic phosphorylation regulates Hsp72 spindle localization by uncoupling ATP binding from substrate release. Sci. Signal 11, pii:eaao2464 10.1126/scisignal.aao2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preissler S, Rohland L, Yan Y, Chen R, Read RJ and Ron D (2017) AMPylation targets the rate-limiting step of BiP’s ATPase cycle for its functional inactivation. eLife 6, pii:e29428 10.7554/eLife.29428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laufen T, Mayer MP, Beisel C, Klostermeier D, Mogk A, Reinstein J et al. (1999) Mechanism of regulation of hsp70 chaperones by DnaJ cochaperones. Proc. Natl Acad. Sci. U.S.A 96, 5452–5457 10.1073/pnas.96.10.5452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pellecchia M, Szyperski T, Wall D, Georgopoulos C and Wuthrich K (1996) NMR structure of the J-domain and the Gly/Phe-rich region of the Escherichia coli DnaJ chaperone. J. Mol. Biol 260, 236–250 10.1006/jmbi.1996.0395 [DOI] [PubMed] [Google Scholar]
- 45.Suh WC, Burkholder WF, Lu CZ, Zhao X, Gottesman ME and Gross CA (1998) Interaction of the Hsp70 molecular chaperone, DnaK, with its cochaperone DnaJ. Proc. Natl Acad. Sci. U.S.A 95, 15223–15228 10.1073/pnas.95.26.15223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alderson TR, Kim JH and Markley JL (2016) Dynamical structures of Hsp70 and Hsp70-Hsp40 complexes. Structure 24, 1014–1030 10.1016/j.str.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malinverni D, Lopez AJ, Rios PDL, Hummer G and Barducci A (2017) Modeling Hsp70/Hsp40 interaction by multi-scale molecular simulations and co-evolutionary sequence analysis. eLife 6, e23471 10.7554/eLife.23471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rudiger S, Schneider-Mergener J and Bukau B (2001) Its substrate specificity characterizes the DnaJ co-chaperone as a scanning factor for the DnaK chaperone. EMBO J. 20, 1042–1050 10.1093/emboj/20.5.1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kampinga HH, Andreasson C, Barducci A, Cheetham ME, Cyr D, Emanuelsson C et al. (2019) Function, evolution, and structure of J-domain proteins. Cell Stress Chaperones 24, 7–15 10.1007/s12192-018-0948-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kampinga HH and Craig EA (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol 11, 579–592 10.1038/nrm2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheetham ME and Caplan AJ (1998) Structure, function and evolution of DnaJ: conservation and adaptation of chaperone function. Cell Stress Chaperones 3, 28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kota P, Summers DW, Ren HY, Cyr DM and Dokholyan NV (2009) Identification of a consensus motif in substrates bound by a Type I Hsp40. Proc. Natl Acad. Sci. U.S.A 106, 11073–11078 10.1073/pnas.0900746106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Craig EA and Marszalek J (2017) How do J-proteins get Hsp70 to do so many different things? Trends Biochem. Sci 42, 355–368 10.1016/j.tibs.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qiu XB, Shao YM, Miao S and Wang L (2006) The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell. Mol. Life Sci 63, 2560–2570 10.1007/s00018-006-6192-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajan VB and D’Silva P (2009) Arabidopsis thaliana J-class heat shock proteins: cellular stress sensors. Funct. Integr. Genomics 9, 433–446 10.1007/s10142-009-0132-0 [DOI] [PubMed] [Google Scholar]
- 56.Walsh P, Bursac D, Law YC, Cyr D and Lithgow T (2004) The J-protein family: modulating protein assembly, disassembly and translocation. EMBO Rep. 5, 567–571 10.1038/sj.embor.7400172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Behnke J, Mann MJ, Scruggs FL, Feige MJ and Hendershot LM (2016) Members of the Hsp70 family recognize distinct types of sequences to execute ER quality control. Mol. Cell 63, 739–752 10.1016/j.molcel.2016.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pobre KFR, Poet GJ and Hendershot LM (2019) The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: getting by with a little help from ERdj friends. J. Biol. Chem 294, 2098–2108 10.1074/jbc.REV118.002804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fotin A, Cheng Y, Grigorieff N, Walz T, Harrison SC and Kirchhausen T (2004) Structure of an auxilin-bound clathrin coat and its implications for the mechanism of uncoating. Nature 432, 649–653 10.1038/nature03078 [DOI] [PubMed] [Google Scholar]
- 60.Fotin A, Cheng Y, Sliz P, Grigorieff N, Harrison SC, Kirchhausen T et al. (2004) Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature 432, 573–579 10.1038/nature03079 [DOI] [PubMed] [Google Scholar]
- 61.Hageman J, Rujano MA, van Waarde MA, Kakkar V, Dirks RP, Govorukhina N et al. (2010) A DNAJB chaperone subfamily with HDAC-dependent activities suppresses toxic protein aggregation. Mol. Cell 37, 355–369 10.1016/j.molcel.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 62.Kakkar V, Mansson C, de Mattos EP, Bergink S, van der Zwaag M, van Waarde M et al. (2016) The S/T-Rich Motif in the DNAJB6 chaperone delays polyglutamine aggregation and the onset of disease in a mouse model. Mol. Cell 62, 272–283 10.1016/j.molcel.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 63.Sahi C, Kominek J, Ziegelhoffer T, Yu HY, Baranowski M, Marszalek J et al. (2013) Sequential duplications of an ancient member of the DnaJ-family expanded the functional chaperone network in the eukaryotic cytosol. Mol. Biol. Evol 30, 985–998 10.1093/molbev/mst008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caplan AJ, Cyr DM and Douglas MG (1992) YDJ1p facilitates polypeptide translocation across different intracellular membranes by a conserved mechanism. Cell 71, 1143–1155 10.1016/S0092-8674(05)80063-7 [DOI] [PubMed] [Google Scholar]
- 65.Caplan AJ and Douglas MG (1991) Characterization of YDJ1: a yeast homologue of the bacterial DnaJ protein. J. Cell Biol 114, 609–621 10.1083/jcb.114.4.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levy EJ, McCarty J, Bukau B and Chirico WJ (1995) Conserved ATPase and luciferase refolding activities between bacteria and yeast Hsp70 chaperones and modulators. FEBS Lett. 368, 435–440 10.1016/0014-5793(95)00704-D [DOI] [PubMed] [Google Scholar]
- 67.Cyr DM (1995) Cooperation of the molecular chaperone Ydj1 with specific Hsp70 homologs to suppress protein aggregation. FEBS Lett. 359, 129–132 10.1016/0014-5793(95)00024-4 [DOI] [PubMed] [Google Scholar]
- 68.Lopez N, Aron R and Craig EA (2003) Specificity of class II Hsp40 Sis1 in maintenance of yeast prion [RNQ+]. Mol. Biol. Cell 14, 1172–1181 10.1091/mbc.e02-09-0593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chai Y, Koppenhafer SL, Bonini NM and Paulson HL (1999) Analysis of the role of heat shock protein (Hsp) molecular chaperones in polyglutamine disease. J. Neurosci 19, 10338–10347 10.1523/JNEUROSCI.19-23-10338.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gillis J, Schipper-Krom S, Juenemann K, Gruber A, Coolen S, van den Nieuwendijk R et al. (2013) The DNAJB6 and DNAJB8 protein chaperones prevent intracellular aggregation of polyglutamine peptides. J. Biol. Chem 288, 17225–17237 10.1074/jbc.M112.421685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan W, Schilke B, Pfund C, Walter W, Kim S and Craig EA (1998) Zuotin, a ribosome-associated DnaJ molecular chaperone. EMBO J. 17, 4809–4817 10.1093/emboj/17.16.4809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ducett JK, Peterson FC, Hoover LA, Prunuske AJ, Volkman BF and Craig EA (2013) Unfolding of the C-terminal domain of the J-protein Zuo1 releases autoinhibition and activates Pdr1-dependent transcription. J. Mol. Biol 425, 19–31 10.1016/j.jmb.2012.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eisenman HC and Craig EA (2004) Activation of pleiotropic drug resistance by the J-protein and Hsp70-related proteins, Zuo1 and Ssz1. Mol. Microbiol 53, 335–344 10.1111/j.1365-2958.2004.04134.x [DOI] [PubMed] [Google Scholar]
- 74.Gautschi M, Mun A, Ross S and Rospert S (2002) A functional chaperone triad on the yeast ribosome. Proc. Natl Acad. Sci. U.S.A 99, 4209–4214 10.1073/pnas.062048599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kakkar V, Meister-Broekema M, Minoia M, Carra S and Kampinga HH (2014) Barcoding heat shock proteins to human diseases: looking beyond the heat shock response. Dis. Model. Mech 7, 421–434 10.1242/dmm.014563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Linxweiler M, Schick B and Zimmermann R (2017) Let’s talk about Secs: Sec61, Sec62 and Sec63 in signal transduction, oncology and personalized medicine. Signal Transduct. Target. Ther 2, 17002 10.1038/sigtrans.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zarouchlioti C, Parfitt DA, Li W, Gittings LM and Cheetham ME (2018) DNAJ proteins in neurodegeneration: essential and protective factors. Philos. Trans. R. Soc. Lond. B Biol. Sci 373, pii:20160534 10.1098/rstb.2016.0534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wawrzynow B, Zylicz A and Zylicz M (2018) Chaperoning The Guardian of the genome. The two-faced role of molecular chaperones in p53 tumor suppressor action. Biochim. Biophys. Acta Rev. Cancer 1869, 161–174 10.1016/j.bbcan.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 79.Anikster Y, Haack TB, Vilboux T, Pode-Shakked B, Thony B, Shen N et al. (2017) Biallelic mutations in DNAJC12 cause hyperphenylalaninemia, dystonia, and intellectual disability. Am. J. Hum. Genet 100, 257–266 10.1016/j.ajhg.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burgoyne RD and Morgan A (2015) Cysteine string protein (CSP) and its role in preventing neurodegeneration. Semin. Cell Dev. Biol 40, 153–159 10.1016/j.semcdb.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koutras C and Braun JE (2014) J protein mutations and resulting proteostasis collapse. Front. Cell. Neurosci 8, 191 10.3389/fncel.2014.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sanchez E, Darvish H, Mesias R, Taghavi S, Firouzabadi SG, Walker RH et al. (2016) Identification of a large DNAJB2 deletion in a family with spinal muscular atrophy and Parkinsonism. Hum. Mutat 37, 1180–1189 10.1002/humu.23055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bracher A and Verghese J (2015) The nucleotide exchange factors of Hsp70 molecular chaperones. Front. Mol. Biosch 2, 10 10.3389/fmolb.2015.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sturner E and Behl C (2017) The role of the multifunctional BAG3 protein in cellular protein quality control and in disease. Front. Mol. Neurosci 10, 177 10.3389/fnmol.2017.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yakubu UM and Morano KA (2018) Roles of the nucleotide exchange factor and chaperone Hsp110 in cellular proteostasis and diseases of protein misfoldin. Biol. Chem 399, 1215–1221 10.1515/hsz-2018-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ang D and Georgopoulos C (1989) The heat-shock-regulated grpE gene of Escherichia coli is required for bacterial growth at all temperatures but is dispensable in certain mutant backgrounds. J. Bacteriol 171, 2748–2755 10.1128/jb.171.5.2748-2755.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson C, Chandrasekhar GN and Georgopoulos C (1989) Escherichia coli DnaK and GrpE heat shock proteins interact both in vivo and in vitro. J. Bacteriol 171, 1590–1596 10.1128/jb.171.3.1590-1596.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saito H and Uchida H (1977) Initiation of the DNA replication of bacteriophage lambda in Escherichia coli K12. J. Mol. Biol 113, 1–25 10.1016/0022-2836(77)90038-9 [DOI] [PubMed] [Google Scholar]
- 89.Liberek K, Marszalek J, Ang D, Georgopoulos C and Zylicz M (1991) Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc. Natl Acad. Sci. U.S.A 88, 2874–2878 10.1073/pnas.88.7.2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Harrison CJ, Hayer-Hartl M, Di Liberto M, Hartl F and Kuriyan J (1997) Crystal structure of the nucleotide exchange factor GrpE bound to the ATPase domain of the molecular chaperone DnaK. Science 276, 431–435 10.1126/science.276.5311.431 [DOI] [PubMed] [Google Scholar]
- 91.Wu B, Wawrzynow A, Zylicz M and Georgopoulos C (1996) Structure-function analysis of the Escherichia coli GrpE heat shock protein. EMBO J. 15, 4806–4816 10.1002/j.1460-2075.1996.tb00861.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Flaherty KM, DeLuca-Flaherty C and McKay DB (1990) Three-dimensional structure of the ATPase fragment of a 70 K heat-shock cognate protein. Nature 346, 623–628 10.1038/346623a0 [DOI] [PubMed] [Google Scholar]
- 93.Chang L, Thompson AD, Ung P, Carlson HA and Gestwicki JE (2010) Mutagenesis reveals the complex relationships between ATPase rate and the chaperone activities of Escherichia coli heat shock protein 70 (Hsp70/DnaK). J. Biol. Chem 285, 21282–21291 10.1074/jbc.M110.124149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ung PM, Thompson AD, Chang L, Gestwicki JE and Carlson HA (2013) Identification of key hinge residues important for nucleotide-dependent allostery in E. coli Hsp70/DnaK. PLoS Comp. Biol 9, e1003279 10.1371/journal.pcbi.1003279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Packschies L, Theyssen H, Buchberger A, Bukau B, Goody RS and Reinstein J (1997) Grpe accelerates nucleotide exchange of the molecular chaperone DnaK with an associative displacement mechanism. Biochemistry 36, 3417–3422 10.1021/bi962835l [DOI] [PubMed] [Google Scholar]
- 96.Brehmer D, Rüdiger S, Gässler CS, Klostermeier D, Packschies L, Reinstein J et al. (2001) Tuning of chaperone activity of Hsp70 proteins by modulation of nucleotide exchange. Nat. Struct. Mol. Biol 8, 427–432 10.1038/87588 [DOI] [PubMed] [Google Scholar]
- 97.Grimshaw JP, Siegenthaler RK, Zuger S, Schonfeld HJ, Z’Graggen BR and Christen P (2005) The heat-sensitive Escherichia coli grpE280 phenotype: impaired interaction of GrpE(G122D) with DnaK. J. Mol. Biol 353, 888–896 10.1016/j.jmb.2005.08.069 [DOI] [PubMed] [Google Scholar]
- 98.Wu C-C, Naveen V, Chien C-H, Chang Y-W and Hsiao C-D (2012) Crystal structure of DnaK protein complexed with nucleotide exchange factor GrpE in DnaK chaperone system: insight into intermolecular communication. J. Biol. Chem 287, 21461–21470 10.1074/jbc.M112.344358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Melero R, Moro F, Pérez-Calvo MÁ, Perales-Calvo J, Quintana-Gallardo L, Llorca O et al. (2015) Modulation of the chaperone DnaK allosterism by the nucleotide exchange factor GrpE. J. Biol. Chem 290, 10083–10092 10.1074/jbc.M114.623371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brehmer D, Gassler C, Rist W, Mayer MP and Bukau B (2004) Influence of GrpE on DnaK-substrate interactions. J. Biol. Chem 279, 27957–27964 10.1074/jbc.M403558200 [DOI] [PubMed] [Google Scholar]
- 101.Grimshaw JP, Jelesarov I, Schönfeld HJ and Christen P (2001) Reversible thermal transition in GrpE, the nucleotide exchange factor of the DnaK heat-shock system. J. Biol. Chem 276, 6098–6104 10.1074/jbc.M009290200 [DOI] [PubMed] [Google Scholar]
- 102.Siegenthaler RK and Christen P (2006) Tuning of DnaK chaperone action by nonnative protein sensor DnaJ and thermosensor GrpE. J. Biol. Chem 281, 34448–34456 10.1074/jbc.M606382200 [DOI] [PubMed] [Google Scholar]
- 103.Gelinas AD, Langsetmo K, Toth J, Bethoney KA, Stafford WF and Harrison CJ (2002) A structure-based interpretation of E. coli GrpE thermodynamic properties. J. Mol. Biol 323, 131–142 10.1016/S0022-2836(02)00915-4 [DOI] [PubMed] [Google Scholar]
- 104.Gelinas AD, Toth J, Bethoney KA, Langsetmo K, Stafford WF and Harrison CJ (2003) Thermodynamic linkage in the GrpE nucleotide exchange factor, a molecular thermosensor. Biochemistry 42, 9050–9059 10.1021/bi034416b [DOI] [PubMed] [Google Scholar]
- 105.Groemping Y and Reinstein J (2001) Folding properties of the nucleotide exchange factor GrpE from Thermus thermophilus: GrpE is a thermosensor that mediates heat shock response. J. Mol. Biol 314, 167–178 10.1006/jmbi.2001.5116 [DOI] [PubMed] [Google Scholar]
- 106.Miao B, Davis JE and Craig EA (1997) Mge1 functions as a nucleotide release factor for Ssc1, a mitochondrial Hsp70 of Saccharomyces cerevisiae. J. Mol. Biol 265, 541–552 10.1006/jmbi.1996.0762 [DOI] [PubMed] [Google Scholar]
- 107.Schlicher T and Soll J (1997) Chloroplastic isoforms of DnaJ and GrpE in pea. Plant Mol. Biol 33, 181–185 10.1023/A:1005784115363 [DOI] [PubMed] [Google Scholar]
- 108.Srivastava S, Savanur MA, Sinha D, Birje A, V R, Saha PP et al. (2017) Regulation of mitochondrial protein import by the nucleotide exchange factors GrpEL1 and GrpEL2 in human cells. J. Biol. Chem 292, 18075–18090 10.1074/jbc.M117.788463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gierasch LM (2016) Structure and action of molecular chaperones In Structure and Action of Molecular Chaperones (Horwich AL, ed.), World Scientific Publishing, Singapore [Google Scholar]
- 110.Behl C (2016) Breaking BAG: the co-chaperone BAG3 in health and disease. Trends Pharmacol. Sci 37, 672–688 10.1016/j.tips.2016.04.007 [DOI] [PubMed] [Google Scholar]
- 111.Sondermann H, Scheufler C, Schneider C, Hohfeld J, Hartl FU and Moarefi I (2001) Structure of a Bag/Hsc70 complex: convergent functional evolution of Hsp70 nucleotide exchange factors. Science 291, 1553–1557 10.1126/science.1057268 [DOI] [PubMed] [Google Scholar]
- 112.Arakawa A, Handa N, Ohsawa N, Shida M, Kigawa T, Hayashi F et al. (2010) The C-terminal BAG domain of BAG5 induces conformational changes of the Hsp70 nucleotide-binding domain for ADP-ATP exchange. Structure 18, 309–319 10.1016/j.str.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 113.Xu Z, Page RC, Gomes MM, Kohli E, Nix JC, Herr AB et al. (2008) Structural basis of nucleotide exchange and client binding by the Hsp70 cochaperone Bag2. Nat. Struct. Mol. Biol 15, 1309–1317 10.1038/nsmb.1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rauch JN, Zuiderweg ER and Gestwicki JE (2016) Non-canonical interactions between Heat Shock Cognate Protein 70 (Hsc70) and Bcl2-associated Anthanogene (BAG) co-chaperones are important for client release. J. Biol. Chem 291, 19848–19857 10.1074/jbc.M116.742502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luders J, Demand J and Hohfeld J (2000) The ubiquitin-related BAG-1 provides a link between the molecular chaperones Hsc70/Hsp70 and the proteasome. J. Biol. Chem 275, 4613–4617 10.1074/jbc.275.7.4613 [DOI] [PubMed] [Google Scholar]
- 116.Tsukahara F and Maru Y (2010) Bag1 directly routes immature BCR-ABL for proteasomal degradation. Blood 116, 3582–3592 10.1182/blood-2009-10-249623 [DOI] [PubMed] [Google Scholar]
- 117.Hantouche C, Williamson B, Valinsky WC, Solomon J, Shrier A and Young JC (2017) Bag1 co-chaperone promotes TRC8 E3 ligase-dependent degradation of misfolded human ether a go-go-related gene (hERG) potassium channels. J. Biol. Chem 292, 2287–2300 10.1074/jbc.M116.752618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arndt V, Daniel C, Nastainczyk W, Alberti S and Hohfeld J (2005) BAG-2 acts as an inhibitor of the chaperone-associated ubiquitin ligase CHIP. Mol. Biol Cell 16, 5891–5900 10.1091/mbc.e05-07-0660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dai Q, Qian SB, Li HH, McDonough H, Borchers C, Huang D et al. (2005) Regulation of the cytoplasmic quality control protein degradation pathway by BAG2. J. Biol. Chem 280, 38673–38681 10.1074/jbc.M507986200 [DOI] [PubMed] [Google Scholar]
- 120.Carra S, Seguin SJ, Lambert H and Landry J (2008) Hspb8 chaperone activity toward poly(Q)-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. J. Biol. Chem 283, 1437–1444 10.1074/jbc.M706304200 [DOI] [PubMed] [Google Scholar]
- 121.Crippa V, Sau D, Rusmini P, Boncoraglio A, Onesto E, Bolzoni E et al. (2010) The small heat shock protein B8 (HspB8) promotes autophagic removal of misfolded proteins involved in amyotrophic lateral sclerosis (ALS). Hum. Mol. Genet 19, 3440–3456 10.1093/hmg/ddq257 [DOI] [PubMed] [Google Scholar]
- 122.Minoia M, Boncoraglio A, Vinet J, Morelli FF, Brunsting JF, Poletti A et al. (2014) BAG3 induces the sequestration of proteasomal clients into cytoplasmic puncta: implications for a proteasome-to-autophagy switch. Autophagy 10, 1603–1621 10.4161/auto.29409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meriin AB, Narayanan A, Meng L, Alexandrov I, Varelas X, Cisse II et al. (2018) Hsp70-Bag3 complex is a hub for proteotoxicity-induced signaling that controls protein aggregation. Proc. Natl Acad. Sci. U.S.A 115, E7043–E7052 10.1073/pnas.1803130115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rauch JN, Tse E, Freilich R, Mok SA, Makley LN, Southworth DR et al. (2017) BAG3 is a modular, scaffolding protein that physically links Heat Shock Protein 70 (Hsp70) to the small heat shock proteins. J. Mol. Biol 429, 128–141 10.1016/j.jmb.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miki K and Eddy EM (2002) Tumor necrosis factor receptor 1 is an ATPase regulated by silencer of death domain. Mol. Cell. Biol 22, 2536–2543 10.1128/MCB.22.8.2536-2543.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bruchmann A, Roller C, Walther TV, Schafer G, Lehmusvaara S, Visakorpi T et al. (2013) Bcl-2 associated athanogene 5 (Bag5) is overexpressed in prostate cancer and inhibits ER-stress induced apoptosis. BMC Cancer 13, 96 10.1186/1471-2407-13-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hessa T, Sharma A, Mariappan M, Eshleman HD, Gutierrez E and Hegde RS (2011) Protein targeting and degradation are coupled for elimination of mislocalized proteins. Nature 475, 394–397 10.1038/nature10181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang X, Olberding KE, White C and Li C (2011) Bcl-2 proteins regulate ER membrane permeability to luminal proteins during ER stress-induced apoptosis. Cell Death Differ. 18, 38–47 10.1038/cdd.2010.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rauch JN and Gestwicki JE (2014) Binding of human nucleotide exchange factors to heat shock protein 70 (Hsp70) generates functionally distinct complexes in vitro. J. Biol. Chem 289, 1402–1414 10.1074/jbc.M113.521997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huber AH, Nelson WJ and Weis WI (1997) Three-dimensional structure of the armadillo repeat region of beta-catenin. Cell 90, 871–882 10.1016/S0092-8674(00)80352-9 [DOI] [PubMed] [Google Scholar]
- 131.Shomura Y, Dragovic Z, Chang H-C, Tzvetkov N, Young JC, Brodsky JL et al. (2005) Regulation of Hsp70 function by HspBP1: structural analysis reveals an alternate mechanism for Hsp70 nucleotide exchange. Mol. Cell 17, 367–379 [DOI] [PubMed] [Google Scholar]
- 132.Yan M, Li J and Sha B (2011) Structural analysis of the Sil1-Bip complex reveals the mechanism for Sil1 to function as a nucleotide-exchange factor. Biochem. J 438, 447–455 10.1042/BJ20110500 [DOI] [PubMed] [Google Scholar]
- 133.Gowda NKC, Kaimal JM, Kityk R, Daniel C, Liebau J, Ohman M et al. (2018) Nucleotide exchange factors Fes1 and HspBP1 mimic substrate to release misfolded proteins from Hsp70. Nat. Struct. Mol. Biol 25, 83–89 10.1038/s41594-017-0008-2 [DOI] [PubMed] [Google Scholar]
- 134.Rosam M, Krader D, Nickels C, Hochmair J, Back KC, Agam G et al. (2018) Bap (Sil1) regulates the molecular chaperone BiP by coupling release of nucleotide and substrate. Nat. Struct. Mol. Biol 25, 90–100 10.1038/s41594-017-0012-6 [DOI] [PubMed] [Google Scholar]
- 135.Easton DP, Kaneko Y and Subjeck JR (2000) The hsp110 and Grp1 70 stress proteins: newly recognized relatives of the Hsp70s. Cell Stress Chaperones 5, 276–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu Q and Hendrickson WA (2007) Insights into Hsp70 chaperone activity from a crystal structure of the yeast Hsp110 Sse1. Cell 131, 106–120 10.1016/j.cell.2007.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Polier S, Dragovic Z, Hartl FU and Bracher A (2008) Structural basis for the cooperation of Hsp70 and Hsp110 chaperones in protein folding. Cell 133, 1068–1079 10.1016/j.cell.2008.05.022 [DOI] [PubMed] [Google Scholar]
- 138.Schuermann JP, Jiang J, Cuellar J, Llorca O, Wang L, Gimenez LE et al. (2008) Structure of the Hsp110:Hsc70 nucleotide exchange machine. Mol. Cell 31, 232–243 10.1016/j.molcel.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shaner L, Sousa R and Morano KA (2006) Characterization of Hsp70 binding and nucleotide exchange by the yeast Hsp110 chaperone Sse1. Biochemistry 45, 15075–15084 10.1021/bi061279k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rampelt H, Kirstein-Miles J, Nillegoda NB, Chi K, Scholz SR, Morimoto RI et al. (2012) Metazoan Hsp70 machines use Hsp110 to power protein disaggregation. EMBO J. 31, 4221–4235 10.1038/emboj.2012.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shaner L, Trott A, Goeckeler JL, Brodsky JL and Morano KA (2004) The function of the yeast molecular chaperone Sse1 is mechanistically distinct from the closely related hsp70 family. J. Biol. Chem 279, 21992–22001 10.1074/jbc.M313739200 [DOI] [PubMed] [Google Scholar]
- 142.Xu X, Sarbeng EB, Vorvis C, Kumar DP, Zhou L and Liu Q (2012) Unique peptide substrate binding properties of 110-kDa heat-shock protein (Hsp110) determine its distinct chaperone activity. J. Biol. Chem 287, 5661–5672 10.1074/jbc.M111.275057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Oh HJ, Chen X and Subjeck JR (1997) Hsp110 protects heat-denatured proteins and confers cellular thermoresistance. J. Biol. Chem 272, 31636–31640 10.1074/jbc.272.50.31636 [DOI] [PubMed] [Google Scholar]
- 144.Shorter J (2011) The mammalian disaggregase machinery: Hsp110 synergizes with Hsp70 and Hsp40 to catalyze protein disaggregation and reactivation in a cell-free system. PloS ONE 6, e26319 10.1371/journal.pone.0026319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kaimal JM, Kandasamy G, Gasser F and Andreasson C (2017) Coordinated Hsp110 and Hsp104 activities power protein disaggregation in Saccharomyces cerevisiae. Mol. Cell. Biol 37, pii: e00027–17 10.1128/MCB.00027-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Garcia VM, Nillegoda NB, Bukau B and Morano KA (2017) Substrate binding by the yeast Hsp110 nucleotide exchange factor and molecular chaperone Sse1 is not obligate for its biological activities. Mol. Biol. Cell 28, 2066–2075 10.1091/mbc.e17-01-0070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gao X, Carroni M, Nussbaum-Krammer C, Mogk A, Nillegoda NB, Szlachcic A et al. (2015) Human Hsp70 disaggregase reverses Parkinson’s-linked alpha-synuclein amyloid fibrils. Mol. Cell 59, 781–793 10.1016/j.molcel.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mattoo RU, Sharma SK, Priya S, Finka A and Goloubinoff P (2013) Hsp110 is a bona fide chaperone using ATP to unfold stable misfolded polypeptides and reciprocally collaborate with Hsp70 to solubilize protein aggregates. J. Biol. Chem 288, 21399–21411 10.1074/jbc.M113.479253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lewis MJ and Pelham HR (1985) Involvement of ATP in the nuclear and nucleolar functions of the 70 kd heat shock protein. EMBO J. 4, 3137–3143 10.1002/j.1460-2075.1985.tb04056.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Blond-Elguindi S, Fourie AM, Sambrook JF and Gething MJ (1993) Peptide-dependent stimulation of the ATPase activity of the molecular chaperone BiP is the result of conversion of oligomers to active monomers. J. Biol. Chem 268, 12730–12735 [PubMed] [Google Scholar]
- 151.Flynn GC, Chappell TG and Rothman JE (1989) Peptide binding and release by proteins implicated as catalysts of protein assembly. Science 245, 385–390 10.1126/science.2756425 [DOI] [PubMed] [Google Scholar]
- 152.Fourie AM, Sambrook JF and Gething MJ (1994) Common and divergent peptide binding specificities of hsp70 molecular chaperones. J. Biol. Chem 269, 30470–30478 [PubMed] [Google Scholar]
- 153.Rudiger S, Germeroth L, Schneider-Mergener J and Bukau B (1997) Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 16, 1501–1507 10.1093/emboj/16.7.1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Van Durme J, Maurer-Stroh S, Gallardo R, Wilkinson H, Rousseau F and Schymkowitz J (2009) Accurate prediction of DnaK-peptide binding via homology modelling and experimental data. PLoS Comp. Biol 5, e1000475 10.1371/journal.pcbi.1000475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Deuerling E, Patzelt H, Vorderwulbecke S, Rauch T, Kramer G, Schaffitzel E et al. (2003) Trigger factor and DnaK possess overlapping substrate pools and binding specificities. Mol. Microbiol 47, 1317–1328 10.1046/j.1365-2958.2003.03370.x [DOI] [PubMed] [Google Scholar]
- 156.Deuerling E, Schulze-Specking A, Tomoyasu T, Mogk A and Bukau B (1999) Trigger factor and DnaK cooperate in folding of newly synthesized proteins. Nature 400, 693–696 10.1038/23301 [DOI] [PubMed] [Google Scholar]
- 157.Wild J, Altman E, Yura T and Gross CA (1992) Dnak and DnaJ heat shock proteins participate in protein export in Escherichia coli. Genes Dev. 6, 1165–1172 10.1101/gad.6.7.1165 [DOI] [PubMed] [Google Scholar]
- 158.Calloni G, Chen T, Schermann S, Chang H-C, Genevaux P, Agostini F et al. (2012) Dnak functions as a central hub in the E. coli chaperone network. Cell Rep. 1, 251–264 10.1016/j.celrep.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 159.Niwa T, Kanamori T, Ueda T and Taguchi H (2012) Global analysis of chaperone effects using a reconstituted cell-free translation system. Proc. Natl Acad. Sci. U.S.A 109, 8937–8942 10.1073/pnas.1201380109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cupp-Vickery JR, Peterson JC, Ta DT and Vickery LE (2004) Crystal structure of the molecular chaperone HscA substrate binding domain complexed with the IscU recognition peptide ELPPVKIHC. J. Mol. Biol 342, 1265–1278 10.1016/j.jmb.2004.07.025 [DOI] [PubMed] [Google Scholar]
- 161.Zahn M, Berthold N, Kieslich B, Knappe D, Hoffmann R and Strater N (2013) Structural studies on the forward and reverse binding modes of peptides to the chaperone DnaK. J. Mol. Biol 425, 2463–2479 10.1016/j.jmb.2013.03.041 [DOI] [PubMed] [Google Scholar]
- 162.Aponte RA, Zimmermann S and Reinstein J (2010) Directed evolution of the DnaK chaperone: mutations in the lid domain result in enhanced chaperone activity. J. Mol. Biol 399, 154–167 10.1016/j.jmb.2010.03.060 [DOI] [PubMed] [Google Scholar]
- 163.Gong W, Hu W, Xu L, Wu H, Wu S, Zhang H et al. (2018) The C-terminal GGAP motif of Hsp70 mediates substrate recognition and stress response in yeast. J. Biol. Chem 293, 17663–17675 10.1074/jbc.RA118.002691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hu SM and Wang C (1996) Involvement of the 10-kDa C-terminal fragment of Hsc70 in complexing with unfolded protein. Arch. Biochem. Biophys 332, 163–169 10.1006/abbi.1996.0328 [DOI] [PubMed] [Google Scholar]
- 165.Smock RG, Blackburn ME and Gierasch LM (2011) Conserved, disordered C terminus of DnaK enhances cellular survival upon stress and DnaK in vitro chaperone activity. J. Biol. Chem 286, 31821–31829 10.1074/jbc.M111.265835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tsai MY and Wang C (1994) Uncoupling of peptide-stimulated ATPase and clathrin-uncoating activity in deletion mutant of hsc70. J. Biol. Chem 269, 5958–5962 [PubMed] [Google Scholar]
- 167.Rodriguez F, Arsène-Ploetze F, Rist W, Rüdiger S, Schneider-Mergener J, Mayer MP et al. (2008) Molecular basis for regulation of the heat shock transcription factor sigma32 by the DnaK and DnaJ chaperones. Mol. Cell 32, 347–358 10.1016/j.molcel.2008.09.016 [DOI] [PubMed] [Google Scholar]
- 168.Sekhar A, Rosenzweig R, Bouvignies G and Kay LE (2015) Mapping the conformation of a client protein through the Hsp70 functional cycle. Proc. Natl Acad. Sci. U.S.A 112, 10395–10400 10.1073/pnas.1508504112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vega CA, Kurt N, Chen Z, Rudiger S and Cavagnero S (2006) Binding specificity of an alpha-helical protein sequence to a full-length Hsp70 chaperone and its minimal substrate-binding domain. Biochemistry 45, 13835–13846 10.1021/bi061432a [DOI] [PubMed] [Google Scholar]
- 170.Palleros DR, Shi L, Reid KL and Fink AL (1994) hsp70-protein complexes. Complex stability and conformation of bound substrate protein. J. Biol. Chem 269, 13107–13114 [PubMed] [Google Scholar]
- 171.Sekhar A, Santiago M, Lam HN, Lee JH and Cavagnero S (2012) Transient interactions of a slow-folding protein with the Hsp70 chaperone machinery. Prot. Sci 21, 1042–1055 10.1002/pro.2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen Z, Kurt N, Rajagopalan S and Cavagnero S (2006) Secondary structure mapping of DnaK-bound protein fragments: chain helicity and local helix unwinding at the binding site. Biochemistry 45, 12325–12333 10.1021/bi0612263 [DOI] [PubMed] [Google Scholar]
- 173.Kurt N, Rajagopalan S and Cavagnero S (2006) Effect of hsp70 chaperone on the folding and misfolding of polypeptides modeling an elongating protein chain. J. Mol. Biol 355, 809–820 10.1016/j.jmb.2005.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kellner R, Hofmann H, Barducci A, Wunderlich B, Nettels D and Schuler B (2014) Single-molecule spectroscopy reveals chaperone-mediated expansion of substrate protein. Proc. Natl Acad. Sci. U.S.A 111, 13355–13360 10.1073/pnas.1407086111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee JH, Zhang D, Hughes C, Okuno Y, Sekhar A and Cavagnero S (2015) Heterogeneous binding of the SH3 client protein to the DnaK molecular chaperone. Proc. Natl Acad. Sci. U.S.A 112, 4206–4215 10.1073/pnas.1505173112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rosenzweig R, Sekhar A, Nagesh J and Kay LE (2017) Promiscuous binding by Hsp70 results in conformational heterogeneity and fuzzy chaperone-substrate ensembles. eLife 6, pii: e28030 10.7554/eLife.28030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sekhar A, Nagesh J, Rosenzweig R and Kay LE (2017) Conformational heterogeneity in the Hsp70 chaperone-substrate ensemble identified from analysis of NMR-detected titration data. Prot. Sci 26, 2207–2220 10.1002/pro.3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sekhar A, Rosenzweig R, Bouvignies G and Kay LE (2016) Hsp70 biases the folding pathways of client proteins. Proc. Natl Acad. Sci. U.S.A 113, 2794–2801 10.1073/pnas.1601846113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen X, Araç D, Wang T-M, Gilpin CJ, Zimmerberg J and Rizo J (2006) SNARE-mediated lipid mixing depends on the physical state of the vesicles. Biophys. J 90, 2062–2074 10.1529/biophysj.105.071415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kurt N and Cavagnero S (2008) Nonnative helical motif in a chaperone-bound protein fragment. Biophys. J 94, L48–L50 10.1529/biophysj.107.127647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sharma SK, De los Rios P, Christen P, Lustig A and Goloubinoff P (2010) The kinetic parameters and energy cost of the Hsp70 chaperone as a polypeptide unfoldase. Nat. Chem. Biol 6, 914–920 10.1038/nchembio.455 [DOI] [PubMed] [Google Scholar]
- 182.Kirschke E, Goswami D, Southworth D, Griffin PR and Agard DA (2014) Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell 157, 1685–1697 10.1016/j.cell.2014.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schlossman DM, Schmid SL, Braell WA and Rothman JE (1984) An enzyme that removes clathrin coats: purification of an uncoating ATPase. J. Cell Biol 99, 723–733 10.1083/jcb.99.2.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sousa R, Liao HS, Cuellar J, Jin S, Valpuesta JM, Jin AJ et al. (2016) Clathrin-coat disassembly illuminates the mechanisms of Hsp70 force generation. Nat. Struct. Mol. Biol 23, 821–829 10.1038/nsmb.3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ungewickell E, Ungewickell H, Holstein SE, Lindner R, Prasad K, Barouch W et al. (1995) Role of auxilin in uncoating clathrin-coated vesicles. Nature 378, 632–635 10.1038/378632a0 [DOI] [PubMed] [Google Scholar]
- 186.Luengo M, Kityk T, Mayer R, and Rudiger MP and D SG (2018) Hsp90 breaks the deadlock of the Hsp70 chaperone system. Mol. Cell 70, 545–552e9 10.1016/j.molcel.2018.03.028 [DOI] [PubMed] [Google Scholar]
- 187.Bocking T, Aguet F, Harrison SC and Kirchhausen T (2011) Single-molecule analysis of a molecular disassemblase reveals the mechanism of Hsc70-driven clathrin uncoating. Nat. Struct. Mol. Biol 18, 295–301 10.1038/nsmb.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bocking T, Aguet F, Rapoport I, Banzhaf M, Yu A, Zeeh JC et al. (2014) Key interactions for clathrin coat stability. Structure 22, 819–829 10.1016/j.str.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xing Y, Bocking T, Wolf M, Grigorieff N, Kirchhausen T and Harrison SC (2010) Structure of clathrin coat with bound Hsc70 and auxilin: mechanism of Hsc70-facilitated disassembly. EMBO J. 29, 655–665 10.1038/emboj.2009.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Teter SA, Houry WA, Ang D, Tradler T, Rockabrand D, Fischer G et al. (1999) Polypeptide flux through bacterial Hsp70: DnaK cooperates with trigger factor in chaperoning nascent chains. Cell 97, 755–765 10.1016/S0092-8674(00)80787-4 [DOI] [PubMed] [Google Scholar]
- 191.Frydman J (2001) Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem 70, 603–647 10.1146/annurev.biochem.70.1.603 [DOI] [PubMed] [Google Scholar]
- 192.Albanese V, Yam AY, Baughman J, Parnot C and Frydman J (2006) Systems analyses reveal two chaperone networks with distinct functions in eukaryotic cells. Cell 124, 75–88 10.1016/j.cell.2005.11.039 [DOI] [PubMed] [Google Scholar]
- 193.Gloge F, Becker AH, Kramer G and Bukau B (2014) Co-translational mechanisms of protein maturation. Curr. Opin. Struct. Biol 24, 24–33 10.1016/j.sbi.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 194.Zhang Y, Sinning I and Rospert S (2017) Two chaperones locked in an embrace: structure and function of the ribosome-associated complex RAC. Nat. Struct. Mol. Biol 24, 611–619 10.1038/nsmb.3435 [DOI] [PubMed] [Google Scholar]
- 195.Hanebuth MA, Kityk R, Fries SJ, Jain A, Kriel A, Albanese V et al. (2016) Multivalent contacts of the Hsp70 Ssb contribute to its architecture on ribosomes and nascent chain interaction. Nat. Commun 7, 13695 10.1038/ncomms13695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Doring K, Ahmed N, Riemer T, Suresh HG, Vainshtein Y, Habich M et al. (2017) Profiling Ssb-nascent chain interactions reveals principles of Hsp70-assisted folding. Cell 170, 298–311 10.1016/j.cell.2017.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Craig EA (2018) Hsp70 at the membrane: driving protein translocation. BMC Biol. 16, 11 10.1186/s12915-017-0474-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Voos W and Rottgers K (2002) Molecular chaperones as essential mediators of mitochondrial biogenesis. Biochim. Biophys. Acta 1592, 51–62 10.1016/S0167-4889(02)00264-1 [DOI] [PubMed] [Google Scholar]
- 199.Flores-Perez U and Jarvis P (2013) Molecular chaperone involvement in chloroplast protein import. Biochim. Biophys. Acta 1833, 332–340 10.1016/j.bbamcr.2012.03.019 [DOI] [PubMed] [Google Scholar]
- 200.Wu X, Cabanos C and Rapoport TA (2019) Structure of the post-translational protein translocation machinery of the ER membrane. Nature 566, 136–139 10.1038/s41586-018-0856-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tyedmers J, Mogk A and Bukau B (2010) Cellular strategies for controlling protein aggregation. Nat. Rev. Mol. Cell Biol 11, 777–788 10.1038/nrm2993 [DOI] [PubMed] [Google Scholar]
- 202.Buchberger A, Valencia A, McMacken R, Sander C and Bukau B (1994) The chaperone function of DnaK requires the coupling of ATPase activity with substrate binding through residue E171. EMBO J. 13, 1687–1695 10.1002/j.1460-2075.1994.tb06433.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Freeman BC and Morimoto RI (1996) The human cytosolic molecular chaperones hsp90, hsp70 (hsc70) and hdj-1 have distinct roles in recognition of a non-native protein and protein refolding. EMBO J. 15, 2969–2979 10.1002/j.1460-2075.1996.tb00660.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Minami Y, Hohfeld J, Ohtsuka K and Hartl FU (1996) Regulation of the heat-shock protein 70 reaction cycle by the mammalian DnaJ homolog, Hsp40. J. Biol. Chem 271, 19617–19624 10.1074/jbc.271.32.19617 [DOI] [PubMed] [Google Scholar]
- 205.Schumacher RJ, Hansen WJ, Freeman BC, Alnemri E, Litwack G and Toft DO (1996) Cooperative action of Hsp70, Hsp90, and DnaJ proteins in protein renaturation. Biochemistry 35, 14889–14898 10.1021/bi961825h [DOI] [PubMed] [Google Scholar]
- 206.Ciechanover A and Kwon YT (2017) Protein quality control by molecular chaperones in neurodegeneration. Front. Neurosci 11, 185 10.3389/fnins.2017.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pratt WB, Gestwicki JE, Osawa Y and Lieberman AP (2015) Targeting Hsp90/Hsp70-based protein quality control for treatment of adult onset neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol 55, 353–371 10.1146/annurev-pharmtox-010814-124332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Aprile FA, Arosio P, Fusco G, Chen SW, Kumita JR, Dhulesia A et al. (2017) Inhibition of alpha-synuclein fibril elongation by hsp70 is governed by a kinetic binding competition between alpha-synuclein species. Biochemistry 56, 1177–1180 10.1021/acs.biochem.6b01178 [DOI] [PubMed] [Google Scholar]
- 209.Dedmon MM, Christodoulou J, Wilson MR and Dobson CM (2005) Heat shock protein 70 inhibits alpha-synuclein fibril formation via preferential binding to prefibrillar species. J. Biol. Chem 280, 14733–14740 10.1074/jbc.M413024200 [DOI] [PubMed] [Google Scholar]
- 210.Kundel F, De S, Flagmeier P, Horrocks MH, Kjaergaard M, Shammas SL et al. (2018) Hsp70 inhibits the nucleation and elongation of tau and sequesters tau aggregates with high affinity. ACS Chem. Biol 13, 636–646 10.1021/acschembio.7b01039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Evans CG, Wisen S and Gestwicki JE (2006) Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1–42) aggregation in vitro. J. Biol. Chem 281, 33182–33191 10.1074/jbc.M606192200 [DOI] [PubMed] [Google Scholar]
- 212.Nillegoda NB, Wentink AS and Bukau B (2018) Protein disaggregation in multicellular organisms. Trends Biochem. Sci 43, 285–300 10.1016/j.tibs.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 213.Fernandez-Fernandez MR, Gragera M, Ochoa-Ibarrola L, Quintana-Gallardo L and Valpuesta JM (2017) Hsp70 — a master regulator in protein degradation. FEBS Lett. 591, 2648–2660 10.1002/1873-3468.12751 [DOI] [PubMed] [Google Scholar]
- 214.Mogk A, Bukau B and Kampinga HH (2018) Cellular handling of protein aggregates by disaggregation machines. Mol. Cell 69, 214–226 10.1016/j.molcel.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 215.Zwirowski S, Klosowska A, Obuchowski I, Nillegoda NB, Pirog A, Zietkiewicz S et al. (2017) Hsp70 displaces small heat shock proteins from aggregates to initiate protein refolding. EMBO J. 36, 783–796 10.15252/embj.201593378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Diamant S, Ben-Zvi AP, Bukau B and Goloubinoff P (2000) Size-dependent disaggregation of stable protein aggregates by the DnaK chaperone machinery. J. Biol. Chem 275, 21107–21113 10.1074/jbc.M001293200 [DOI] [PubMed] [Google Scholar]
- 217.De Los Rios P, Ben-Zvi A, Slutsky O, Azem A and Goloubinoff P (2006) Hsp70 chaperones accelerate protein translocation and the unfolding of stable protein aggregates by entropic pulling. Proc. Natl. Acad Sci. U.S.A 103, 6166–6171 10.1073/pnas.0510496103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Goloubinoff P and De Los Rios P (2007) The mechanism of Hsp70 chaperones: (entropic) pulling the models together. Trends Biochem. Sci 32, 372–380 10.1016/j.tibs.2007.06.008 [DOI] [PubMed] [Google Scholar]
- 219.Liu B, Han Y and Qian SB (2013) Cotranslational response to proteotoxic stress by elongation pausing of ribosomes. Mol. Cell 49, 453–463 10.1016/j.molcel.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shalgi R, Hurt JA, Krykbaeva I, Taipale M, Lindquist S and Burge CB (2013) Widespread regulation of translation by elongation pausing in heat shock. Mol. Cell 49, 439–452 10.1016/j.molcel.2012.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Langer T, Lu C, Echols H, Flanagan J, Hayer MK and Hartl FU (1992) Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature 356, 683–689 10.1038/356683a0 [DOI] [PubMed] [Google Scholar]
- 222.Gamer J, Bujard H and Bukau B (1992) Physical interaction between heat shock proteins DnaK, DnaJ, and GrpE and the bacterial heat shock transcription factor sigma 32. Cell 69, 833–842 10.1016/0092-8674(92)90294-M [DOI] [PubMed] [Google Scholar]
- 223.Gamer J, Multhaup G, Tomoyasu T, McCarty JS, Rudiger S, Schonfeld HJ et al. (1996) A cycle of binding and release of the DnaK, DnaJ and GrpE chaperones regulates activity of the Escherichia coli heat shock transcription factor sigma32. EMBO J. 15, 607–617 10.1002/j.1460-2075.1996.tb00393.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Liberek K and Georgopoulos C (1993) Autoregulation of the Escherichia coli heat shock response by the DnaK and DnaJ heat shock proteins. Proc. Natl Acad. Sci. U.SA 90, 11019–11023 10.1073/pnas.90.23.11019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Liberek K, Galitski TP, Zylicz M and Georgopoulos C (1992) The DnaK chaperone modulates the heat shock response of Escherichia coli by binding to the sigma 32 transcription factor. Proc. Natl Acad. Sci. U.S.A 89, 3516–3520 10.1073/pnas.89.8.3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bukau B (1993) Regulation of the Escherichia coli heat-shock response. Mol. Microbiol 9, 671–680 10.1111/j.1365-2958.1993.tb01727.x [DOI] [PubMed] [Google Scholar]
- 227.Gross CA, Chan CL and Lonetto MA (1996) A structure/function analysis of Escherichia coli RNA polymerase. Philos. Trans. R. Soc. Lond. B Biol. Sci 351, 475–482 10.1098/rstb.1996.0045 [DOI] [PubMed] [Google Scholar]
- 228.Yura T and Nakahigashi K (1999) Regulation of the heat-shock response. Curr. Opin. Microbiol 2, 153–158 10.1016/S1369-5274(99)80027-7 [DOI] [PubMed] [Google Scholar]
- 229.Alvira S, Cuellar J, Rohl A, Yamamoto S, Itoh H, Alfonso C et al. (2014) Structural characterization of the substrate transfer mechanism in Hsp70/Hsp90 folding machinery mediated by Hop. Nat. Commun 5, 5484–5499 10.1038/ncomms6484 [DOI] [PubMed] [Google Scholar]
- 230.Morgner N, Schmidt C, Beilsten-Edmands V, Ebong IO, Patel NA, Clerico EM et al. (2015) Hsp70 forms antiparallel dimers stabilized by post-translational modifications to position clients for transfer to Hsp90. Cell Rep. 11, 759–769 10.1016/j.celrep.2015.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Assimon VA, Gillies AT, Rauch JN and Gestwicki JE (2013) Hsp70 protein complexes as drug targets. Curr. Pharm. Des 19, 404–417 10.2174/138161213804143699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Repalli J and Meruelo D (2015) Screening strategies to identify HSP70 modulators to treat Azheimer’s disease. Drug Des. Devel. Ther 9, 321–331 10.2147/DDDT.S72165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fontaine SN, Martin MD and Dickey CA (2016) Neurodegeneration and the Heat Shock Protein 70 machinery: Implications for therapeutic development. Curr. Top. Med. Chem 16, 2741–2752 10.2174/1568026616666160413140741 [DOI] [PubMed] [Google Scholar]
- 234.Kumar S, Stokes J 3rd, Singh UP, Scissum Gunn K, Acharya A, Manne U et al. (2016) Targeting Hsp70: a possible therapy for cancer. Cancer Lett 374, 156–166 10.1016/j.canlet.2016.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li X, Shao H, Taylor IR and Gestwicki JE (2016) Targeting allosteric control mechanisms in heat shock protein 70 (Hsp70). Curr. Top. Med. Chem 16, 2729–2740 10.2174/1568026616666160413140911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lackie RE, Maciejewski A, Ostapchenko VG, Marques-Lopes J, Choy WY, Duennwald ML et al. (2017) The Hsp70/Hsp90 chaperone machinery in neurodegenerative diseases. Front. Neurosci 11, 254 10.3389/fnins.2017.00254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Reis SD, Pinho BR and Oliveira JMA (2017) Modulation of molecular chaperones in Huntington’s disease and other polyglutamine disorders. Mol. Neurobiol 54, 5829–5854 10.1007/s12035-016-0120-z [DOI] [PubMed] [Google Scholar]
- 238.Ferraro M, D’Annessa I, Moroni E, Morra G, Paladino A, Rinaldi S et al. (2019) Allosteric modulators of HSP90 and HSP70: Dynamics meets function through structure-based drug design. J. Med. Chem 62, 60–87 10.1021/acs.jmedchem.8b00825 [DOI] [PubMed] [Google Scholar]


