Abstract
Background
Aggressive posterior retinopathy of prematurity (APROP), which has a poor visual prognosis, is common in low- and middle-income countries (LMICs) as a result of suboptimal oxygen monitoring (primary prevention). The purpose of this study was to compare outcomes in APROP eyes treated with laser to eyes treated with antivascular endothelial growth factor (anti-VEGF) therapy.
Methods
The medical records of a cohort of APROP eyes treated with anti-VEGF (2010–2018) and another of eyes treated with laser photocoagulation (2002–2010) at the same institution in South India were reviewed retrospectively and compared. The main outcome was the proportion of eyes developing retinal detachment during resolution of acute ROP.
Results
A total of 398 eyes of 199 preterm babies with APROP were included: 168 eyes were treated with photocoagulation; 230, with anti-VEGF. From 2002 to 2010, compared to the more recent cohort, babies diagnosed with APROP tended to be heavier (P < 0.001), older (P < 0.001), and exposed to fewer days of oxygen (P = 0.02). In the laser-treated cohort, 17 of 168 eyes (10%) developed retinal detachment (7, stage 5; 12, stage 4), compared with 3 of 230 (1%) in the anti-VEGF cohort (all stage 4 [P = 0.002]).
Conclusions
The incidence of retinal detachment was significantly lower in eyes treated with anti-VEGF compared with laser-.treated eyes In the absence of a randomized trial, these data suggest that anti-VEGF may lead to better anatomic outcomes, although questions remain concerning dosage, timing, and risks.
There is an epidemic of retinopathy of prematurity (ROP) among premature babies in low- and middle-income countries (LMICs) that differs from ROP as typically seen in the United States.1 In 2005 the International Classification for ROP (ICROP)2 identified aggressive posterior ROP (APROP), a severe form of ROP with a high risk for progression to retinal detachment.2 In the era of modern oxygen regulation, APROP is rare (<5% of ROP) and predominantly seen in the youngest and smallest neonates.2 In LMICs, however, where resource constraints limit precise oxygen monitoring, APROP is both more prevalent and more severe. Since APROP that develops in older babies is usually directly related to excessive oxygen exposure (eg, unfiltered 100% oxygen), and because the phenotypic characteristics of this disease are described as similar to the oxygen-induced retinopathy mouse model, some authors have attempted to distinguish between “oxygen-induced” ROP (OI-ROP), often seen in older and heavier babies, and APROP, typically seen in the United States in the most premature infants. We will use the term APROP in this report, although we recognize that the phenotype of APROP seen in South India may not be the same as that seen in other populations.3,4 Because improved primary prevention (oxygen management) reduced the prevalence of APROP in North America prior to any of the existing randomized clinical trials in ROP,5–8 and there having been no such trials in APROP, the optimal management of APROP, especially in LMICs, is not well-studied.
The Pediatric Retina Division of the Aravind Eye Hospital at Coimbatore (AEH) has seen this epidemic unfold over the last 20 years in Southern India. In 2010, a therapeutic transition occurred at AEH wherein all eyes with APROP were treated primarily with antivascular endothelial growth factor (anti-VEGF) therapy because of anecdotal reports of better anatomic outcomes compared to laser panretinal photocoagulation in these most aggressive cases. In this study, we compare the demographics and outcomes of APROP eyes treated in the 8 years since 2010 with a control cohort from the prior 8 years of eyes treated with photocoagulation.
Subjects and Methods
The Aravind Eye Hosptial Institutional Review Board approved this study, which adhered to the tenets of the Declaration of Helsinki. All patients diagnosed with APROP at AEH between July 2002 and August 2018 were identified through retrospective review of medical records. Ophthalmoscopic examination was performed by 2 ophthalmologists (PS and NV) during the study period. Patients were diagnosed with APROP based on the ophthalmoscopic examination using clinical criteria from the ICROP, which defines APROP as being notable for posterior disease, prominence of plus disease, and ill-defined “retinopathy” (lack of traditional progression of peripheral stage).2 The following data were extracted from the records: sex, gestational age (GA), birth weight (BW), number of days on oxygen (mode of delivery was not recorded), postmenstrual age (PMA), and postnatal age (PNA) at first examination and treatment. All patients were followed until the disease was fully regressed or, in the case of anti-VEGF, the retina was felt to be fully vascularized, or until disease recurred. Fluorescein angiography (FA) was performed at the discretion of the investigator, and PRP or repeat anti-VEGF was administered at investigator discretion for disease recurrence.
Summary statistics and t tests were used to compare the means of the populations. Data analysis was performed using Stata v12.0 (College Station, Texas).
Results
The demographics of the two cohorts are summarized in Table 1. A total of 398 eyes of 199 preterm babies with APROP were identified and included in this analysis; 168 (84 patients) were treated with PRP and 230 (115 patients) with anti-VEGF. During the same study period, a total of 532 non-APROP treatment-requiring cases were seen, thus APROP represented 28% of the cases treated during this study period.
Table 1.
Demographics of patients with aggressive posterior retinopathy at the Aravind Eye Hospital, Coimbatore, 2002–2018
| Study parameter | Treatment groupa |
P value |
|
|---|---|---|---|
| Laser (n = 84; 168 eyes) |
Anti-VEGF (n = 115; 230 eyes) |
||
| BW, g | 1532 ± 363 | 1298 ± 371 | <0.001 |
| GA, weeks | 31.5 ± 2.1 | 30.1 ± 2.7 | <0.001 |
| Sex, no. (% male) | 51 (61) | 78 (68) | 0.3 |
| Days on oxygen prior to diagnosis | 9.8 ± 8.3 | 13.8 ± 14.7 | 0.01 |
| PMA at first exam, weeks | 34.1 ± 2.2 | 34.6 ± 2.6 | 0.19 |
| PNA at first exam, weeks | 2.7 ± 1.5 | 4.4 ± 1.7 | <0.001 |
| PMA at treatment, weeks | 35.6 ± 2.2 | 35.0 ± 2.6 | 0.06 |
| PNA at treatment, weeks | 4.1 ± 1.4 | 4.8 ± 1.7 | 0.001 |
| No. eyes with RD (%) | 17 (10) | 3 (1) | 0.002 |
BW, birth weight; GA, gestational age; PMA, postmenstrual age; PNA, postnatal age; RD, retinal detachment.
All values mean with standard deviation except as noted.
The underlying demographics of patients being diagnosed with APROP changed over time. In 2002–2010, compared to the more recent cohort, babies diagnosed with APROP tended to be heavier (P < 0.001), older (P < 0.001), and exposed to fewer days of oxygen (P = 0.02). In the laser-treated cohort, 17 of 168 eyes (10%) in 10 patients developed retinal detachment (7 eyes with stage 5; 12 with stage 4), compared with 3 of 230 eyes (1%) in 2 patients in the anti-VEGF cohort (all stage 4 [P = 0.002]). In the anti-VEGF treated cohort, 26 of 117 patients (22%) treated primarily with anti-VEGF developed disease recurrence at a mean (with standard deviation) of 3 ± 1.5 months (range, 1–8) in one or both eyes and were retreated at investigator discretion. Twenty-one of these babies were retreated with laser, and 5 were retreated with anti-VEGF. Of the 5, 4 had subsequent laser treatment, and 1 fully vascularized without further treatment. One baby (<1%) in the anti-VEGF group died prior to discharge and 16 (8% overall; 4 laser and 12 anti-VEGF) were lost to follow-up after 1 month.
Discussion
This study compared eyes with APROP treated with anti-VEGF with control eyes treated with laser photocoagulation. There are several key findings. First, the cohort treated with anti-VEGF had fewer retinal detachments than the laser-treated eyes, despite being smaller and more premature overall. Second, eyes with APROP treated with anti-VEGF frequently required additional treatment. Third, babies in India develop APROP at a BW, GA, and PNA that would confer almost no risk of ROP in a North American cohort, reinforcing the need for improved primary prevention protocols and region-specific screening criteria.1
Current North American practice guidelines allow the use of both laser and anti-VEGF for treatment of type 1 ROP (tertiary prevention).9 However, as a result of the poor anatomic outcomes often seen in zone I ROP and growing clinical data favoring anti-VEGF in more aggressive and posterior disease, practice patterns have changed over the last 15 years toward primary use of anti-VEGF in APROP, both in the United States and in LMICs.3,6,10–12
Our data suggests that anti-VEGF therapy may lead to improved anatomic outcomes in these severe cases. There are no randomized clinical treatment trials for eyes with APROP, which, according to the ICROP, have up to a 50% risk of retinal detachment. Given the therapeutic transition toward anti-VEGF worldwide for these cases and the low incidence of APROP in most populations, a randomized trial to better answer this question may not be feasible. However, important questions remain concerning optimal dose, medication, and timing, and the potential for systemic adverse events from neonatal anti-VEGF use.9,13
In our study, disease recurrence was not uncommon following anti-VEGF, with 22% of patients requiring retreatment at a mean of 3 months after primary treatment. This is higher than some reports in the literature but consistent with the trend toward higher reported rates in eyes with more posterior disease. Close follow-up is essential for these patients.9,13
Finally, it is notable that the mean gestational age of the APROP infants in these cohorts is roughly 30 weeks’ PMA, as in Figure 1, a cutoff which in the United States generally implies almost no risk of any ROP (in the absence of other risk factors), let alone APROP. This suggests that the majority of APROP in India is preventable with improved primary prevention. Toward that end, AEH has implemented an intensive educational curriculum for nurses, parents, and neonatologists and has noted reduced incidence of the most severe cases of APROP at established neonatal units (unpublished data from AEH).We note that the temporal trend between the two cohorts does suggest an improvement in primary prevention over this time period, since the eyes with APROP in the later cohort were younger and smaller on average.
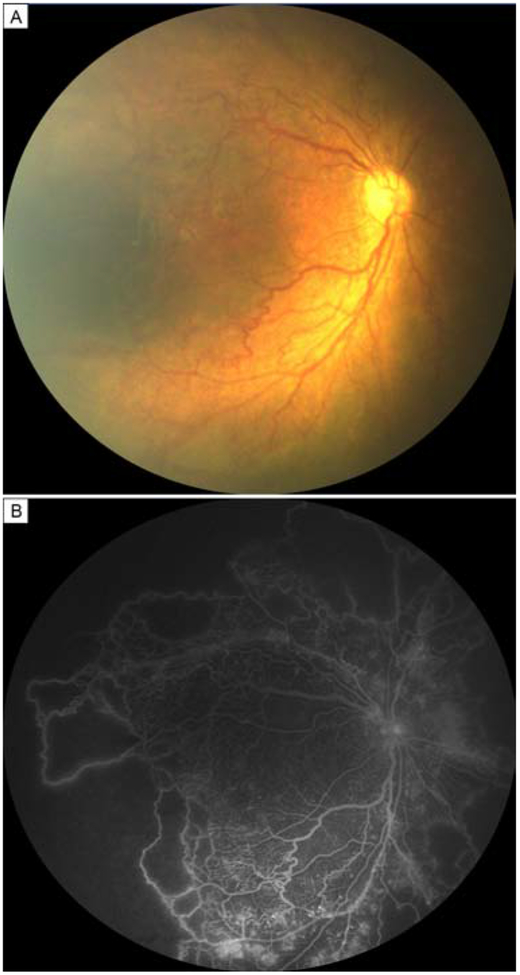
FIG 1.
Patient diagnosed with aggressive posterior retinopathy of prematurity. Fundus photograph (A) and fluorescein angiogram (B) of a 3-week-old boy demonstrating posterior disease, nonperfusion, and vascular dilation and tortuosity. The boy was born at 31 weeks’ postmenstrual age, with a birthweight of 1418 g.
This study has several limitations to this retrospective, historically controlled, cohort study. APROP is a subjective diagnosis, and these cases were identified based on a single examiner’s diagnosis. There could have been other temporal differences that limit comparison between the two cohorts at different points in time, such as improved screening, earlier detection, improved treatment efficacy. We note, however that the mean PMA at time of first examination did not change over the two cohorts, and the mean PNA at time of first examination and treatment were later in the anti-VEGF group. No babies in the laser group were retreated with photocoagulation. It is unclear whether repeated (or more) laser could have reduced the rate of retinal detachment in this cohort; however, it is notable that the 10% rate of retinal detachment in the laser group is less than the estimated 50% of retinal detachment in APROP noted in ICROP. Finally, 10% of the babies in the anti-VEGF group were lost to follow-up after 1 month, and thus their final anatomic outcomes are unknown. If all of the patients who were lost to follow-up developed retinal detachments, the outcome of the study could be different.
Improvements in neonatal care led to the end of the so-called first epidemic of ROP in the twentieth century.1 These data remind us that the low-hanging fruit for ROP blindness prevention in the world today is translation of this existing knowledge into evidence-based primary, secondary, and tertiary ROP prevention programs in LMICs that are tailored to the local disease epidemiology and pathophysiology.1 Implementation research focused on improvements in primary prevention may require coordinated efforts between neonatalogy, ophthalmology, and healthcare policy makers. As neonatology continues to improve and lower the age of viability for preterm births, APROP will remain a clinically relevant disease entity everywhere, although with the improvements in oxygen monitoring, it could become less common in regions where failure of primary prevention is leading to a generation of avoidable blindness.
Acknowledgments
This project was supported by grants R01EY19474, K12EY027720, and P30EY10572 from the National Institutes of Health (Bethesda, MD), and by unrestricted departmental funding and a Career Development Award (JPC) from Research to Prevent Blindness (New York, NY). None of the funding agencies had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Disclosures: Michael F. Chiang is an unpaid member of the Scientific Advisory Board for Clarity Medical Systems (Pleasanton, CA), and a consultant for Novartis (Basel, Switzerland), and an initial member of Inteleretina (Honolulu, HI). R. V. Paul Chan is on the Scientific Advisory Board for Visunex Medical Systems (Pleasanton, CA), and a consultant for Genentech (South San Francisco, CA). Michael Chiang and J. Peter Campbell receive research support from Genentech.
References
- 1.Gilbert C, Rahi J, Eckstein M, O’Sullivan J, Foster A. Retinopathy of prematurity in middle-income countries. Lancet 1997;350:12–14. [DOI] [PubMed] [Google Scholar]
- 2.International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005;123;991–9. [DOI] [PubMed] [Google Scholar]
- 3.Shah PK, Narendran V, Kalpana N. Aggressive posterior retinopathy of prematurity in large preterm babies in South India. Arch Dis Child Fetal Neonatal Ed 2012;97:F371–5. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Castellanos MA, Velez-Montoya R, Price K, et al. Vascular changes on fluorescein angiography of premature infants with low risk of retinopathy of prematurity after high oxygen exposure. Int J Retina Vitreous 2017;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 2003;121:1684–94. [DOI] [PubMed] [Google Scholar]
- 6.Mintz-Hittner HA, Kennedy KA, Chuang AZ; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011;364:603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: preliminary results. Arch Ophthalmol 1988;106:471–9. [DOI] [PubMed] [Google Scholar]
- 8.RAINBOW Study: a Randomized, Controlled Study Evaluating the Efficacy and Safety of RAnibizumab Compared With Laser Therapy for the Treatment of INfants BOrn Prematurely With Retinopathy of Prematurity. https://clinicaltrials.gov/ct2/show/NCT02375971. Updated November 14, 2018.
- 9.VanderVeen DK, Melia M, Yang MB, Hutchinson AK, Wilson LB, Lambert SR. Anti-vascular endothelial growth factor therapy for primary treatment of type 1 retinopathy of prematurity: A report by the American Academy of Ophthalmology. Ophthalmology 2017;124:619–33. [DOI] [PubMed] [Google Scholar]
- 10.Tong Q, Yin H, Zhao M, Li X, Yu W. Outcomes and prognostic factors for aggressive posterior retinopathy of prematurity following initial treatment with intravitreal ranibizumab. BMC Ophthalmol 2018;18:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yetik H, Gunay M, Sirop S, Salihoglu Z. Intravitreal bevacizumab monotherapy for type-1 prethreshold, threshold, and aggressive posterior retinopathy of prematurity—27-month follow-up results from Turkey. Graefes Arch Clin Exp Ophthalmol 2015;253:1677–83. [DOI] [PubMed] [Google Scholar]
- 12.Yoon JM, Shin DH, Kim SJ, et al. Outcomes after laser versus combined laser and bevacizumab treatment for type 1 retinopathy of prematurity in zone 1. Retina 2017;37:88–96. [DOI] [PubMed] [Google Scholar]
- 13.Mintz-Hittner HA, Geloneck MM, Chuang AZ. Clinical management of recurrent retinopathy of prematurity after intravitreal vevacizumab monotherapy. Ophthalmology 2016;123:1845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]