Abstract
Background and Purpose:
Few studies have examined the separate contributions of systolic and diastolic blood pressures (SBP, DBP) on subclinical cerebrovascular disease, especially using the 2017 American College of Cardiology (ACC) / American Heart Association (AHA) Blood Pressure Guidelines. Further, associations with region-specific white matter hyperintensity volume (WMHV) are underexplored.
Methods:
Using data from the Northern Manhattan Study, a prospective cohort study of stroke risk and cognitive aging, we examined associations between SBP and DBP, defined by the 2017 ACC/AHA guidelines, and regional WMHV. We used a linear mixed model approach to account for the correlated nature of regional brain measures.
Results:
The analytic sample (N=1205; mean age 64±8 years) consisted of 61% women and 66% Hispanics/Latinos. DBP levels were significantly related to WMHV differentially across regions (P for interaction<0.05). Relative to those with DBP 90+ mmHg, participants with DBP <80 mmHg had 13% lower WMHV in the frontal lobe (95% CI: −21%, −3%), 11% lower WMHV in the parietal lobe (95% CI: −19%, −1%), 22% lower WMHV in the anterior periventricular region (95% CI: −30%, −14%), and 16% lower WMHV in the posterior periventricular region (95% CI: −24%, −6%). Participants with DBP 80–90 mmHg also exhibited about −12% (95% CI: −20%, −3%) lower WMHV in the anterior periventricular region and −9% (95% CI: −18%, −0.4%) lower WMHV in the posterior periventricular region, relative to participants with DBP 90+ mmHg. Post-hoc pairwise t-tests showed that estimates for periventricular WMHV were significantly different from estimates for temporal WMHV (Holms stepdown-adjusted P<0.05). SBP was not strongly related to regional WMHV.
Conclusions:
Lower DBP levels, defined by the 2017 ACC/AHA guidelines, were related to lower WM lesion load, especially in the periventricular regions relative to the temporal region.
INTRODUCTION
Blood pressure (BP) management has been recommended as a means to prevent cognitive decline.1 In 2017, the American College of Cardiology (ACC) and American Heart Association (AHA) released new BP guidelines, including <120/80 mmHg defining normal BP2, largely due to data from the Systolic BP Intervention Trial (SPRINT).3 Whether these new BP guidelines are associated with brain aging outcomes remains unclear. SPRINT investigators recently found that participants who underwent intensive BP control exhibited lower increases in white matter (WM) lesion load compared to those in the standard therapy arm.4 However, participants in clinical trials represent a selected group, and thus generalizability to community samples may be limited.
The separate contributions of systolic and diastolic BPs (SBP, DBP) to subclinical cerebrovascular disease are unclear, since many studies have focused only on SBP5. Studies examining both have found that DBP is also associated with greater WM lesion load6–9. Furthermore, most studies have only examined global burden of WM lesions without considering region-specific associations, which may provide insight into mechanisms by which hypertension affects cerebrovascular disease burden.
Previous work from the Northern Manhattan Study (NOMAS) has shown that greater DBP was related to greater global WM lesion load.6,10 We aimed to extend this work by examining associations between BP levels, defined by the 2017 ACC/AHA guidelines, and regional WM lesion load. We hypothesized that lower levels of BP, especially SBP <120 mmHg, would be related to lower WM lesion load and that this effect is different across regions. In secondary analyses, we also examined pulse pressure (PP) and mean arterial pressure (MAP) to elucidate potential mechanisms, similar to our previous work.10
MATERIALS AND METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Cohort Description and Analytic Sample
Participants were recruited for NOMAS between 1993 and 2001, as previously described.11 Briefly, random digit-dialing was used to identify participants living in Northern Manhattan with the following eligibility criteria: 1) clinically stroke-free, 2) aged >40 years old, and 3) lived in Northern Manhattan for ≥3 months in a household with a telephone. Enrolled participants underwent a full clinical and demographic interview with trained research assistants in English or Spanish (N=3298).
As previously described,12 between 2003 and 2008, 1091 participants from the original cohort were recruited into the NOMAS Magnetic Resonance Imaging (MRI) Sub-Study with the following eligibility criteria (33% of original cohort): 1) clinically stroke-free, 2) aged >50 years old, and 3) no contraindications to MRI. An additional 199 household members were enrolled, and these participants underwent full clinical and demographic interviews, similar to the original cohort.
Our analytic sample consisted of MRI Sub-Study participants who had BP and regional WM hyperintensity volume (WMHV) data available (N=1205). Institutional review boards from the University of Miami and Columbia University approved the study, and all participants provided informed consent. Covariate measurement (done at study entry) is outlined in the Supplemental Material. This study is a cross-sectional analysis of data from a prospective cohort study.
Primary Exposures of Interest: Systolic and Diastolic Blood Pressure Levels
Our exposures of interest were SBP and DBP levels, defined by the ACC/AHA 2017 Guidelines.2 Given these variables are highly correlated, and given our interest in their separate effects, we ran separate models for SBP and DBP. As previously described6, BP was measured at study entry (at baseline for original NOMAS participants, at MRI visit for household members) with a calibrated, random-zero sphyngomanometer after 5 minutes of relative immobility in a seated position. Two measurements at the right brachial artery 10 minutes apart were taken and averaged. SBP was categorized into: <120 mmHg, 120–129 mmHg, 130–139 mmHg, and 140+ mmHg (reference group). DBP was categorized into: <80 mmHg, 80–89 mmHg, and 90+ mmHg (reference group). Hypertension status was defined as normal (SBP<120 mmHg and DBP<80 mmHg), elevated (SBP=120–129 mmHg and DBP<80 mmHg), stage 1 hypertension (SBP=130–139 mmHg or DBP=80–89 mmHg), and stage 2 hypertension (SBP=140+ mmHg or DBP=90+ mmHg).
Secondary Exposures of Interest: Pulse Pressure and Mean Arterial Pressure
PP and MAP were calculated as previously described10 and modeled as z-scores. Briefly, PP was calculated subtracting DBP from SBP. MAP was calculated with the following formula: .
Outcome of Interest: Regional White Matter Lesion Hyperintensity Volume
Participants underwent a cerebral MRI between 2003–2008 at Columbia University Medical Center on a 1.5T Philips Intera scanner.12 Regional WMHVs were measured as previously described.13 Briefly, 85 participants were excluded from regional analysis due to lack of fluid-attentuated inversion recovery (FLAIR) images, image artifact, or failure of the registration method. From T2-weighted FLAIR sequences, regional WMHVs were measured using customized protocols from the FSL software package (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki). Skull stripping was performed using the FSL-BET tool,14 and whole brain segmentation was performed with the FSL-FAST algorithm after correction for nonuniformity.15 WMH voxels were defined as voxels with an intensity >3.5 standard deviations above the mean intensity. Regional WMHVs were automatically measured bilaterally across 4 lobar regions (frontal, temporal, parietal, occipital) and 2 periventricular regions (within 1 cm of the lateral ventricular wall,16 anterior and posterior), and calculated using the MNI structural atlas as a reference (Supplemental Figure I). Images were registered nonlinearly to the atlas template and mapped using the FSL-FNIRT tool.17 A representative figure highlighting regions of interest can be found in the Supplement. Bilateral measurements were summed, and we natural log-transformed these variables after adding a small constant to achieve normality and homoscedasticity of errors in linear models.
To measure total intracranial volume (TIV), images were sent to the University of California, Davis for analysis as previously described.12 TIV was added as a covariate in our models to account for differences in head size.
Statistical Analysis
Covariate distributions were compared across hypertension groups using one-way ANOVAs for normally distributed variables, Kruskal-Wallis tests for non-normally distributed variables, and chi-squared tests for categorical variables.
We modeled the associations of interest using multi-level random intercept linear mixed models to account for the correlation among regional WMHVs (see Supplemental Material for model parameterization). All models exhibited an intraclass correlation coefficient >0.10, indicating that there is substantial variation in the outcome of interest explained by the clustering within individuals, justifying the mixed model approach. To test for differences in the association of predictors across regions, we included a multiplicative interaction term between brain region and the predictors of interest. We chose known confounders as covariates, measured at study entry. We also tested two-way multiplicative interactions between brain region and covariates, and terms with p-values <0.05 were kept in the model. Though we were concerned about differential effects of covariates on WMHV by region, we were not interested in 3-way interactions because they are not very interpretable and they do not seem biologically or sociodemographically plausible. The model selection procedure is outlined in the Supplemental Material. Our final models were adjusted for: age, sex, race/ethnicity, TIV, BMI, brain region, smoking status, anti-hypertensive medication use, any physical activity, moderate alcohol consumption, diabetes, hyperlipidemia, years between baseline and MRI, and two-way multiplicative interaction terms between brain region and age, sex, race/ethnicity, TIV, anti-hypertensive medication use, smoking status, diabetes, and years between baseline and MRI.
For predictors of interest that were significant at P<0.05, we wanted to test whether regional estimates were different from each other. We compared “significant” regional estimates and estimates from the other regions using a series of contrast statements. For each set of hypothesis tests per “significant” region, we corrected p-values for multiple comparisons using the Holm stepdown procedure.18
To assess potential selection bias, we compared covariate distributions between original cohort members and household members. We re-ran analyses weighted for the inverse probability of selection into the MRI Sub-Study in the subsample of participants recruited from the original NOMAS cohort (N=1025) (procedure outlined in the Supplemental Material)19. We also reanalyzed excluding these household members.
Beta coefficients and 95% confidence intervals were back-transformed using the following formula: (, such that one unit increase in the predictor is associated with a beta-unit percent change in WMHV.20 Analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC). Figures were generated in R (https://www.r-project.org/) using the ggplot221 and forestplot packages.22
RESULTS
Participant Characteristics
Participant characteristics are presented in Table 1, stratified by hypertension status. Overall, the analytic sample (N=1205) consisted of 61% women, 66% Hispanics/Latinos, 15% current smokers, 55% reporting any physical activity, and 41% reporting moderate alcohol consumption. The sample had a mean (SD) age of 64 (8) years, and the mean (SD) time lag was 6 (3) years between baseline and MRI visits. The distribution of age, race/ethnicity, years between baseline and MRI, BMI, diabetes diagnosis, physical activity, total intracranial volume, and total WMHV significantly differed across hypertension status (Table 1). Figure 1 illustrates the median (1st and 3rd quartiles) WMHV in each region, stratified by hypertension status. For all regions except temporal and occipital, WMHV significantly related to hypertension status (P<0.05) in bivariate (i.e. unadjusted) analyses.
Table 1.
Sample Characteristics
| All (N=1205) | Normal (N=120) | Elevated (N=95) | Stage 1 HTN (N=311) | Stage 2 HTN (N=679) | P | |
|---|---|---|---|---|---|---|
| Sociodemographic variables | ||||||
| Women, n (%) | 739 (61) | 72 (60) | 60 (63) | 176 (57) | 431 (63) | 0.214 |
| Age (years), mean (SD) | 64 (8) | 61 (9) | 64 (8) | 63 (9) | 65 (8) | <.0001 |
| Age categories, n (%) | ||||||
| <65 years old | 659 (55) | 84 (70) | 49 (52) | 189 (61) | 337 (50) | <.0001 |
| 65+ years old | 546 (45) | 36 (30) | 46 (48) | 122 (39) | 342 (50) | |
| Race/ethnicity, n (%) | ||||||
| Non-Hispanic White | 173 (14) | 26 (22) | 23 (24) | 37 (12) | 87 (13) | 0.0006 |
| Non-Hispanic Black | 209 (17) | 18 (15) | 15 (16) | 47 (15) | 129 (19) | |
| Hispanic/Latino | 797 (66) | 69 (58) | 54 (57) | 221 (71) | 453 (67) | |
| Other | 26 (2) | 7 (6) | 3 (3) | 6 (2) | 10 (1) | |
| Years between Baseline and MRI, mean (SD) | 6 (3) | 5 (4) | 5 (3) | 6 (4) | 7 (3) | <.0001 |
| Clinical variables | ||||||
| Systolic blood pressure (SBP), mmHg, mean (SD) | 140 (20) | 110 (6) | 123 (3) | 129 (7) | 152 (16) | <.0001 |
| SBP categories | ||||||
| <120 mmHg, n (%) | 141 (12) | 120 (100) | 0 (0) | 17 (5) | 4 (1) | <.0001 |
| 120–129 mmHg, n (%) | 212 (18) | 0 (0) | 95 (100) | 95 (31) | 22 (3) | |
| 130–139 mmHg, n (%) | 266 (22) | 0 (0) | 0 (0) | 199 (64) | 67 (10) | |
| 140+ mmHg, n (%) | 586 (49) | 0 (0) | 0 (0) | 0 (0) | 586 (86) | |
| Diastolic blood pressure (DBP), mmHg, mean (SD) | 83 (11) | 69 (6) | 72 (5) | 80 (5) | 88 (10) | <.0001 |
| DBP categories | ||||||
| <80 mmHg, n (%) | 390 (32) | 120 (100) | 95 (100) | 78 (25) | 97 (14) | <.0001 |
| 80–90 mmHg, n (%) | 441 (37) | 0 (0) | 0 (0) | 223 (75) | 208 (31) | |
| 90+ mmHg, n (%) | 374 (31) | 0 (0) | 0 (0) | 0 (0) | 374 (55) | |
| Pulse pressure, mmHg, mean (SD) | 57 (16) | 41 (6) | 51 (6) | 49 (9) | 64 (17) | <.0001 |
| Mean arterial pressure, mmHg, mean (SD) | 102 (12) | 83 (5) | 89 (3) | 96 (4) | 109 (9) | <.0001 |
| Anti-hypertensive medication use, n (%) | 490 (41) | 18 (15) | 22 (23) | 90 (29) | 360 (53) | <.0001 |
| Body mass index, kg/m2, mean (SD) | 28 (5) | 26 (5) | 27 (4) | 28 (5) | 29 (5) | <.0001 |
| Diabetes mellitus, n (%) | 230 (19) | 17 (14) | 13 (14) | 52 (17) | 148 (22) | 0.045 |
| Hypercholesterolemia, n (%) | 781 (65) | 79 (66) | 56 (59) | 204 (66) | 442 (65) | 0.661 |
| Health behaviors | ||||||
| Smoking status, n (%) | ||||||
| Current | 182 (15) | 23 (19) | 12 (13) | 52 (17) | 95 (14) | 0.065 |
| Former | 450 (37) | 56 (47) | 38 (40) | 113 (36) | 243 (36) | |
| Never | 573 (48) | 41 (34) | 45 (47) | 146 (47) | 341 (50) | |
| Any physical activity, n (%) | 654 (55) | 78 (67) | 50 (53) | 156 (51) | 370 (55) | 0.033 |
| Moderate alcohol consumption, n (%) | 489 (41) | 58 (48) | 42 (44) | 133 (43) | 256 (38) | 0.091 |
| Brain MRI variables | ||||||
| Total intracranial volume, mL, mean (SD) | 1151 (122) | 1181 (124) | 1161 (134) | 1157 (116) | 1141 (121) | 0.004 |
| Total WMHV, mL, median (q1, q3) | 4 (2, 9) | 3 (2, 6) | 3 (2, 6) | 3 (2, 7) | 5 (3, 10) | <.0001 |
HTN=hypertension. P-values obtained from chi-squared tests for categorical variables, one-way ANOVAs for normally distributed variables, and Kruskal-Wallis tests for non-normally distributed variables.
Figure 1. Median Regional White Matter Hyperintensity Volume, Stratified by Hypertension Status.
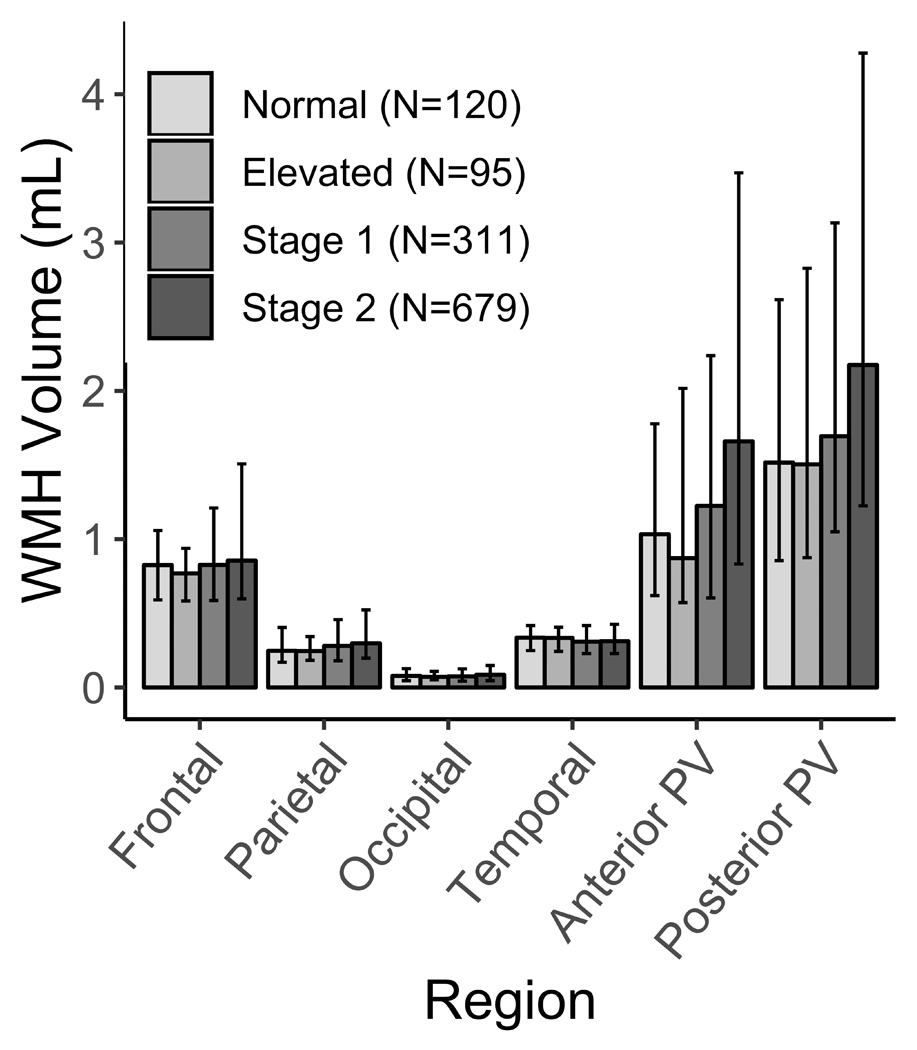
WMH=white matter hyperintensity. PV=periventricular. Bars=median WMHV. Error bars=first and third quartiles.
Generally, the household members group had a significantly greater proportion of Hispanics/Latinos, anti-hypertensive medication use, hypercholesterolemia diagnosis, and moderate alcohol consumption as well as greater BMI on average. Household members also exhibited a lower proportion of reported physical activity, and lower SBP, DBP, PP, MAP, and WMHV on average (Supplemental Table I).
Associations Between SBP, DBP, and Regional WMHV
SBP levels were not related to differences in regional WMHV (Figure 2, P for interaction>0.05). Those with lower SBP levels exhibited smaller frontal WMHV relative to those with SBP 140+ mmHg, though confidence limits overlapped the null (Figure 2).
Figure 2. Associations Between Systolic Blood Pressure Levels at Study Entry and Regional White Matter Hyperintensity Volume at MRI Visit.
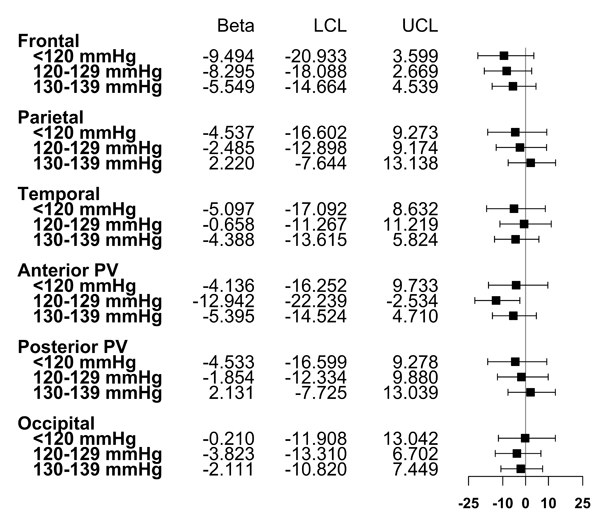
Points represent beta coefficients; error bars represent 95% confidence intervals. Estimates are transformed such that they represent the expected percent change in WMHV for each category, compared to SBP 140+ mmHg. Model adjusted for age, sex, race/ethnicity, TIV, BMI, brain region, smoking status, anti-hypertensive medication use, any physical activity, moderate alcohol consumption, diabetes, hypercholesterolemia, years between baseline and MRI, and two-way multiplicative interaction terms between brain region and age, sex, race/ethnicity, TIV, anti-hypertensive medication use, smoking status, diabetes, and years between baseline and MRI.
DBP levels were significantly related to WMHV differentially across regions (Figure 3, P for interaction<0.05). Relative to those with DBP 90+ mmHg, participants with DBP <80 mmHg had 13% smaller WMHV in the frontal lobe (95% CI: −21%, −3%), 11% smaller WMHV in the parietal lobe (95% CI: −19%, −1%), 22% smaller WMHV in the anterior periventricular region (95% CI: −30%, −14%), and 16% smaller WMHV in the posterior periventricular region (95% CI: −24%, −6%). Participants with DBP 80–90 mmHg also exhibited about 12% smaller WMHV in the anterior periventricular region (95% CI: −20%, −3%) and 9% smaller WMHV in the posterior periventricular region (95% CI: −18%, −0.4%), relative to participants with DBP 90+ mmHg. A DBP level of 80–90 mmHg was not significantly related to frontal or parietal lobe WMHV, and point estimates approached the null. Finally, neither DBP level was significantly related to temporal or occipital lobe WMHV, and point estimates approached the null. In post-hoc analyses, we identified the anterior and posterior periventricular areas as significant regions. Differences in effect estimates for both regions were largest in comparison to temporal WMHV (Holm stepdown-adjusted P<0.05).
Figure 3. Associations Between Diastolic Blood Pressure Levels at Study Entry and Regional White Matter Hyperintensity Volume at MRI Visit.
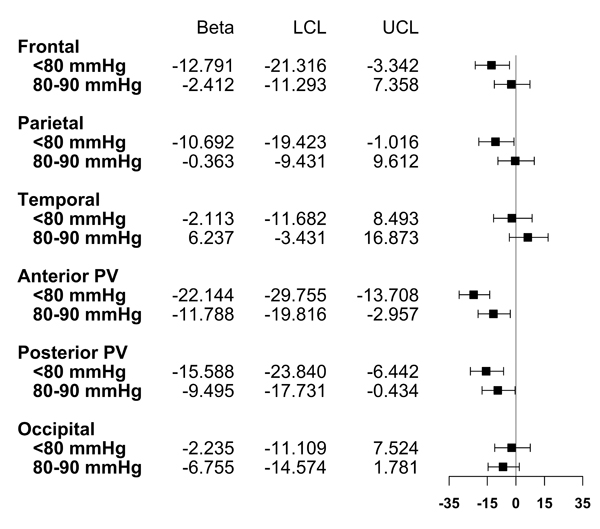
Points represent beta coefficients; error bars represent 95% confidence intervals. LCL=lower 95% confidence limit. UCL=upper 95% confidence limit. Estimates are transformed such that they represent the expected percent change in WMHV for each category, compared to DBP 90+ mmHg. Model adjusted for age, sex, race/ethnicity, TIV, BMI, brain region, smoking status, anti-hypertensive medication use, any physical activity, moderate alcohol consumption, diabetes, hypercholesterolemia, years between baseline and MRI, and two-way multiplicative interaction terms between brain region and age, sex, race/ethnicity, TIV, anti-hypertensive medication use, smoking status, diabetes, and years between baseline and MRI
Associations Between MAP, PP, and Regional WMHV
We found that PP was not significantly related to differences in regional WMHV (Table 2, P for interaction >0.05), and effect estimates approached the null. However, MAP was significantly related to differences in regional WMHV (Table 2, P for interaction <0.05). Per 1 SD increase in MAP, participants exhibited about 7% greater frontal WMHV (95% CI: 2%, 11%), about 5% greater parietal WMHV (95% CI: 0.5%, 9%), about 11% greater anterior periventricular WMHV (95% CI: 6%, 16%), and about 8% greater posterior periventricular WMHV (95% CI: 3%, 12%). In contrast, MAP was not significantly related to WMHV in the temporal or occipital regions, and effect estimates approached the null (Table 2). In post-hoc analyses for MAP, we identified the anterior and posterior periventricular, frontal, and parietal regions as significant. Differences in effect estimates were largest when comparing the effects of MAP on parietal and temporal WMHV vs. anterior periventricular WMHV, and temporal WMHV vs. posterior periventricular WMHV (Holm stepdown-adjusted P<0.05).
Table 2.
Associations Between Pulse Pressure (PP) and Mean Arterial Pressure (MAP) at Study Entry and Regional White Matter Hyperintensity Volume at MRI Visit
| Beta | LCL | UCL | |
|---|---|---|---|
| Frontal | |||
| PP (z-score) | 1.014 | −3.207 | 5.419 |
| MAP (z-score) | 6.670 | 2.293 | 11.233 |
| Parietal | |||
| PP (z-score) | −1.097 | −5.230 | 3.215 |
| MAP (z-score) | 4.787 | 0.488 | 9.271 |
| Temporal | |||
| PP (z-score) | 1.671 | −2.577 | 6.105 |
| MAP (z-score) | 1.408 | −2.752 | 5.747 |
| Anterior PV | |||
| PP (z-score) | 0.728 | −3.481 | 5.120 |
| MAP (z-score) | 10.909 | 6.359 | 15.654 |
| Posterior PV | |||
| PP (z-score) | 0.130 | −4.054 | 4.496 |
| MAP (z-score) | 7.681 | 3.263 | 12.288 |
| Occipital | |||
| PP (z-score) | 0.674 | −3.197 | 4.700 |
| MAP (z-score) | 2.219 | −1.645 | 6.235 |
PP=pulse pressure and MAP=mean arterial pressure. PP and MAP expressed as z-score units. Estimates are transformed such that they represent the expected percent change in WMHV per z-score unit of PP and MAP. Model adjusted for age, sex, race/ethnicity, TIV, BMI, brain region, smoking status, anti-hypertensive medication use, any physical activity, moderate alcohol consumption, diabetes, hyperlipidemia, years between baseline and MRI, and two-way multiplicative interaction terms between brain region and age, sex, race/ethnicity, TIV, anti-hypertensive medication use, smoking status, diabetes, and years between baseline and MRI.
Sensitivity Analyses Accounting for Selection Bias
Compared to our main analyses, inferences from our sensitivity analyses weighted for inverse probability of selection were similar, and estimates were generally stronger in magnitude (Supplemental Table II). For example, in the original analysis, participants with SBP<120 mmHg had about 9% smaller WMHV (95% CI: −21%, 4%) compared to those with SBP 140+ mmHg. After weighting for inverse probability of selection, participants with SBP<120 mmHg had about 17% smaller WMHV (95% CI: −31%, −0.2%) compared to those with SBP 140+ mmHg. See Supplemental Table II for the rest of the results. Compared to our main analyses, inferences and estimates from a re-analysis among only original NOMAS members were similar (Supplemental Table III).
DISCUSSION
Contrary to our initial hypothesis, we found that lower DBP levels – and not SBP levels – as defined by the 2017 ACC/AHA guidelines, were associated with less WM lesion load differentially across regions. The strongest associations were found in the periventricular regions, and differences in effect estimates were largest compared to the temporal region. Further, greater MAP was related to greater region-specific WMHV, especially the periventricular regions. Sensitivity analyses accounting for potential selection bias indicate that selective survival into the MRI sub-study most likely attenuated our results.
One study showed no significant differences between the old and new guidelines with regard to predicting global WM lesion load23. Recent data suggest that SBP control to these new targets was more strongly related to cardiac versus cerebrovascular imaging outcomes24. Previous studies from NOMAS have shown that greater DBP was related to greater global WM lesion load.6,10 Consistent with these previous NOMAS studies, we found that SBP targets were not related to global WM hyperintensity volume.24 The present study extends previous NOMAS work in two ways: first, by examining ACC/AHA guidelines that have become especially relevant for brain aging outcomes as evidenced by the SPRINT MIND findings; and second, by examining region-specific WMHV.6,10,25
Consistent with previous NOMAS studies, studies have shown that greater DBP is associated with greater global WM lesion load.6–9 However, similar analyses using data with repeated MRI measurements suggest that associations of BP with WM lesion progression are not significant after accounting for baseline WM lesion load.26,27 Further, studies with repeated BP measures and earlier in the lifecourse are necessary to evaluate whether BP measured in old age reflects cumulative BP exposure throughout adulthood or BP characteristic of older adults.
These data suggest that lower DBP, in the range of the intensive BP target, was related to less WM lesion load in the periventricular, frontal, and parietal regions, especially in comparison to temporal WMHV. The differential regional susceptibility to hypertensive damage explains the varied distribution of regional WM lesion load, and thus, our findings support these pathological differences across regions28. Vessels supplying watershed (i.e. periventricular) regions are especially susceptible to hypertensive damage, which likely drives downstream clinical outcomes. This explains, at least partially, why periventricular WMHV has been related to increased risk of stroke29, functional decline13, and specific depressive symptomology.30 However, damage in other regions, like the frontal and parietal lobes, may still have clinical consequences. For example, previous work has shown that greater parietal lobe WMHV is associated with increased risk of dementia31, while both frontal and parietal lobe WMHV have been related to worsening domain-specific cognition.32 More work is warranted to examine whether region-specific effects of these BP levels on WM lesion load contribute to the manifestation of these clinical outcomes.
Brain pathology studies have documented venous collagenosis in periventricular WM lesions,33 and therefore, WM lesions in this region may be due to changes in peripheral vascular resistance indicated by elevated DBP. However, greater SBP could also cause arteriolar changes and subsequent increases in peripheral vascular resistance. Alternatively, our results showing that MAP was also related to periventricular WMHV is consistent with the idea that DBP and MAP act as indicators of steady blood flow. Thus, sustained diastolic hypertension may lead to arterial remodeling or negative effects on cerebral autoregulation, which may especially impact the periventricular region. Further, our findings are in contrast to evidence suggesting that PP is associated with vascular remodeling in chronic hypertension34. In our sample with a high average SBP, but relatively normal average DBP, elevated DBP might be more predictive of cerebrovascular injury. Focusing on elevated DBP might be especially relevant in populations with high hypertension rates, such as in racial/ethnic minorities.
There are limitations to this study. First, causation cannot be inferred from this cross-sectional study. Second, survival bias may have attenuated our results, as our sensitivity analyses suggest. Third, we lack other measures of WM integrity that may better inform mechanisms. Fourth, though we found statistically significant associations, these may not translate to clinically significant outcomes. Fifth, though we adjusted for potential confounders of interest, unexplained variance in WMHV may be due to unmeasured and residual confounding and measurement error. Sixth, lack of data on BP measures and medication use between baseline and MRI limits our ability to examine patterns of BP over time. Sixth, though multi-level models account for correlation between our repeated measures of WMH, other methods can be used to examine these associations, such as general linear models for each region with adjustment for multiple testing. Lastly, WMHs are a non-specific finding and could be due to other etiologies besides cerebral small vessel disease, such as blood-brain barrier disruption, inflammation, or demyelinating processes.
There are also several strengths. First, our results are generalizable to other aging, mostly Hispanic/Latino populations. Second, mixed models account for the correlation of regional brain metrics data and allows us to test multiple hypotheses within one model. Third, our sensitivity analyses specifically addressed possible selection bias, including the use of inverse probability of selection weights.
In conclusion, DBP levels, defined by the 2017 ACC/AHA guidelines, were related to lower WM lesion load differentially across brain regions. Future studies using multiple MRI and BP measurements should be conducted to strengthen causal inference.
Supplementary Material
SOURCES OF FUNDING
This work was funded by NINDS (R01NS29993, F30NS103462) and the Evelyn F. McKnight Brain Institute.
Footnotes
DISCLOSURES
Dr. Elkind receives compensation for providing consultative services for BioTelemetry/Cardionet, Bristol–Myers Squibb-Pfizer Partnership, Boehringer Ingelheim, Daiichi-Sankyo, Janssen Pharmaceuticals, and Sanofi-Regeneron Partnership; receives research support from diaDexus, Inc, Bristol–Myers Squibb/Sanofi Pharmaceuticals Partnership, Roche, and the National Institutes of Health/ National Institute of Neurological Disorders and Stroke; has given expert legal opinions on behalf of Organon (NuvaRing and stroke litigation) and Hi-Tech; and serves on the National, Founders Affiliate, and New York City Chapter Boards of the American Heart Association/American Stroke Association. He receives royalties from UpToDate for chapters related to stroke. Dr. Sacco receives federal grant support (R01 NS 29993), private foundation support (American Heart Association Bugher Center, Evelyn F. McKnight Brain Institute), and pharma research support (Boehringer Ingelheim). Dr. Wright receives royalties for 2 chapters on Vascular Dementia from UpToDate. Dr. Caunca receives federal grant support (F30NS103462) and private foundation support (Evelyn F. McKnight Brain Institute). The other authors report no disclosures.
REFERENCES
- 1.National Academies of Sciences, Engineering, and Medicine. Preventing cognitive decline and dementia: a way forward. Downey A, Stroud C, Landis S, Leshner AI, eds. Washington, DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DEJ, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task F. J. Am. Coll. Cardiol. 2018;71:2199–2269.29146533 [Google Scholar]
- 3.Wright JTJ, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco M V, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The SPRINT Research Group. A randomized trial of intensive versus standard systolic blood pressure control on brain structure: results from SPRINT MIND MRI. In: Alzheimer’s Association International Conference Chicago, IL: 2018. [Google Scholar]
- 5.Gottesman RF, Coresh J, Catellier DJ, Sharrett AR, Rose KM, Coker LH, et al. Blood pressure and white-matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) study. Stroke 2010;41:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcus J, Gardener H, Rundek T, Elkind MS, Sacco RL, Decarli C, et al. Baseline and longitudinal increases in diastolic blood pressure are associated with greater white matter hyperintensity volume: the Northern Manhattan Study. Stroke. 2011;42:2639–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNeil CJ, Myint PK, Sandu A-L, Potter JF, Staff R, Whalley LJ, et al. Increased diastolic blood pressure is associated with MRI biomarkers of dementia-related brain pathology in normative ageing. Age Ageing. 2018;47:95–100. [DOI] [PubMed] [Google Scholar]
- 8.Aribisala BS, Morris Z, Eadie E, Thomas A, Gow A, Valdes Hernandez MC, et al. Blood pressure, internal carotid artery flow parameters, and age-related white matter hyperintensities. Hypertens. (Dallas, Tex. 1979). 2014;63:1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shokouhi M, Qiu D, Samman Tahhan A, Quyyumi AA, Hajjar I. Differential Associations of Diastolic and Systolic Pressures with Cerebral Measures in Older Individuals with Mild Cognitive Impairment. Am. J. Hypertens. 2018;13:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutierrez J, Elkind MS V, Cheung K, Rundek T, Sacco RL, Wright CB. Pulsatile and steady components of blood pressure and subclinical cerebrovascular disease: the Northern Manhattan Study. J. Hypertens. 2015;33:2115–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacco RL, Anand K, Lee HS, Boden-Albala B, Stabler S, Allen R, et al. Homocysteine and the risk of ischemic stroke in a triethnic cohort: the Northern Manhattan Study. Stroke. 2004;35:2263–2269. [DOI] [PubMed] [Google Scholar]
- 12.Dong C, Nabizadeh N, Caunca M, Cheung YK, Rundek T, Elkind MS, et al. Cognitive correlates of white matter lesion load and brain atrophy: the Northern Manhattan Study. Neurology. 2015;85:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhamoon MS, Cheung Y-K, Bagci A, Alperin N, Sacco RL, Elkind MS V, et al. Periventricular white matter hyperintensities and functional decline. J. Am. Geriatr. Soc. 2018;66:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith SM. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. [DOI] [PubMed] [Google Scholar]
- 16.DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson J, Smith S, Jenkinson M. Fnirt—fmrib’s non-linear image registration tool. Annu. Meet. Organ. Hum Brain Mapp. 2008; [Google Scholar]
- 18.Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979;6:65–70. [Google Scholar]
- 19.Ganguli M, Lee C-W, Hughes T, Snitz BE, Jakubcak J, Duara R, et al. Who wants a free brain scan? Assessing and correcting for recruitment biases in a population-based sMRI pilot study. Brain Imaging Behav. 2015;9:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.UCLA: Statistical Consulting Group. How Can I Interpret Log Transformed Variables In Terms Of Percent Change In Linear Regression? | SAS FAQ [Internet]. [cited 2018 Feb 16]; Available from: https://stats.idre.ucla.edu/sas/faq/how-can-i-interpret-log-transformed-variables-in-terms-of-percent-change-in-linear-regression/
- 21.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2016. [Google Scholar]
- 22.Gordon M, Lumley T. forestplot: advanced forest plot using “grid” graphics. R package version 1.7.2. 2017. [Google Scholar]
- 23.Del Brutto OH, Mera RM. Neuroimaging Signatures of Cerebral Small Vessel Disease at Blood Pressure Cutoff Levels of 130/80 and 140/90 mmHg: A Population-Based Study in Community-Dwellers Aged >/= 60 Years. High Blood Press. Cardiovasc. Prev. 2018;25:203–208. [DOI] [PubMed] [Google Scholar]
- 24.Nakanishi K, Jin Z, Homma S, Elkind MS V, Rundek T, Tugcu A, et al. Association of Blood Pressure Control Level With Left Ventricular Morphology and Function and With Subclinical Cerebrovascular Disease. J. Am. Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, et al. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA. 2019;321:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickie DA, Ritchie SJ, Cox SR, Sakka E, Royle NA, Aribisala BS, et al. Vascular risk factors and progression of white matter hyperintensities in the Lothian Birth Cohort 1936. Neurobiol. Aging. 2016;42:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verhaaren BFJ, Vernooij MW, de Boer R, Hofman A, Niessen WJ, van der Lugt A, et al. High blood pressure and cerebral white matter lesion progression in the general population. Hypertens. (Dallas, Tex. 1979). 2013;61:1354–1359. [DOI] [PubMed] [Google Scholar]
- 28.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H1598–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaffashian S, Tzourio C, Zhu Y-C, Mazoyer B, Debette S. Differential Effect of White-Matter Lesions and Covert Brain Infarcts on the Risk of Ischemic Stroke and Intracerebral Hemorrhage. Stroke. 2016;47:1923–1925. [DOI] [PubMed] [Google Scholar]
- 30.Tully PJ, Debette S, Mazoyer B, Tzourio C. White Matter Lesions are Associated with Specific Depressive Symptom Trajectories among Incident Depression and Dementia Populations: Three-City Dijon MRI Study. Am. J. Geriatr. Psychiatry. 2017;25:1311–1321. [DOI] [PubMed] [Google Scholar]
- 31.Brickman AM, Zahodne LB, Guzman VA, Narkhede A, Meier IB, Griffith EY, et al. Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer’s disease incidence. Neurobiol. Aging. 2015;36:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lampe L, Kharabian-Masouleh S, Kynast J, Arelin K, Steele CJ, Loffler M, et al. Lesion location matters: The relationships between white matter hyperintensities on cognition in the healthy elderly. J. Cereb. Blood Flow Metab. 2017;39:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moody DM, Brown WR, Challa VR, Ghazi-Birry HS, Reboussin DM. Cerebral microvascular alterations in aging, leukoaraiosis, and Alzheimer’s disease. Ann. N. Y. Acad. Sci. 1997;826:103–116. [DOI] [PubMed] [Google Scholar]
- 34.AlGhatrif M, Lakatta EG. The conundrum of arterial stiffness, elevated blood pressure, and aging. Curr. Hypertens. Rep. 2015;17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


