Abstract
Spiracles are a general character of gnathostomes (jawed fishes), being present in antiarch placoderms, commonly regarded as the most basal gnathostome group. The presence of spiracular tubes in acanthodians has been deduced from grooves on the neurocranium of the derived acanthodiform Acanthodes bronni from the Permian of Germany, but until now these tubes were presumed to lack an external opening, rendering them non‐functional. Here we describe the external spiracular elements in specimens of the Middle Devonian acanthodiforms Cheiracanthus murchisoni, Cheiracanthus latus and Mesacanthus pusillus from northern Scotland, and the internal structure of these elements in C. murchisoni, demonstrating that the spiracle in acanthodiforms differed from all known extant and extinct fishes in having paired cartilage‐pseudobranch structures. This arrangement represents a transitional state between the presumed basal gnathostome condition with an unconstricted first gill slit (as yet not identified in any fossil) and the derived condition with a spiracle and a single pseudobranch derived from the posterior hemibranch of the mandibular arch. We identify the main tissue forming the pseudobranch as elastic cartilage, a tissue previously unrecorded in fossils.
Keywords: branchial skeleton, mineralised cartilage, pseudobranch, spiracular function, stem Chondrichthyes
In this study, we found the first evidence of a paired spiracular structure in the first gill arch position on ancient stem sharks, representing the only known example of a transitional state between the fully functional first gill postulated (but not yet identified) to have occurred in stem jawed fishes and the single, reduced pseudobranch present in more derived taxa. We have also identified the oldest record of elastic cartilage in the fossil record.

1. INTRODUCTION
Spiracles are a general character of gnathostomes; however, their presence or absence in agnathans (jawless fishes) was a controversial topic during the 20th century. Some amphiaspid heterostracan agnathans have bean‐shaped openings in the carapace immediately behind the eyes which have been interpreted as true spiracles (Halstead, 1971). However, it is unlikely that these paired openings are equivalent to the spiracles of gnathostomes. They probably had an inhalent function, as these amphiaspids were benthic animals (Janvier, 1996) living in a similar manner to modern rays, which lie half buried in the sediment of the seafloor and use their spiracles for intake of clear water. The enigmatic pituriaspid agnathans had comparable paired openings behind the orbits (Young, 1991). Osteostracan galeaspid agnathans had a single opening at the top of the carapace which has also been considered analogous, but not homologous, to the gnathostome spiracle (Gai et al. 2018), with this structure considered to have served the same function as the paired openings in the amphiaspids and modern rays. In summary, all these openings in agnathans are considered independent of the spiracle in crown gnathostomes (Miyashita, 2016).
The supposed ancestral condition for gnathostomes, with an unconstricted first gill slit, has never been identified in fossils. Zangerl and Williams (1975) claimed to have identified this arrangement in Upper Carboniferous symmoriiform sharks, but this is a highly derived group, and their proposal that this represented the most primitive gnathostome condition was dismissed (e.g. Maisey, 1980). Paired spiracles are present in arguably the most basal gnathostome group (Zhu et al. 2013), or equally most basal group (King et al. 2017), the antiarch placoderms (Stensiö, 1947; Young and Zhang, 1992). The spiracles are generally considered to represent the vestige of the first gill slit, reduced in size and displaced dorsally by the anterior migration of the hyoid arch to support the mandibular arch (i.e. the jaws). They comprise a tube behind the jaws, extending between a dorsal opening in the head (the spiracular cleft) and the oro‐pharyngeal cavity. In modern fish, they are present in most extant sharks, some rays, and basal actinopterygian lineages (Graham et al. 2014). The presence of spiracles in fossil vertebrates has typically been inferred from notches in skull roofing bones (e.g. Graham et al. 2014: fig. 4) and/or by a spiracular groove on the cranial bones, for example in placoderms (Young and Zhang, 1992) and actinopterygians (Gardiner, 1984).
The presence or absence of an unconstricted first gill slit in acanthodians was a vigorously debated topic in the mid‐20th century, with D. M. S. Watson the main proponent of its presence. Watson (1937) united the Acanthodii and Placodermi in the Class Aphetohyoidea, intermediate between the Cyclostomata and Pisces, based on their supposed unconstricted spiracular gill slit and a non‐suspensory hyoid arch. Contra to this interpretation, the hyoid arch in both acanthodians and placoderms has been shown to attach to the braincase and support the mandibular arch (Miles, 1964; Young, 1986), and the presence of spiracular tubes in acanthodians has long been advocated (e.g. Holmgren, 1942; Stensiö, 1947). Miles (1964) described a spiracular groove on the anterior basal ossification of Acanthodes, and this interpretation has been supported (or at least not debated) by subsequent authors. However, Miles (1965: p. 238) observed that ‘an external spiracular opening does not appear to have been present in Acanthodes, and evidence of its presence is also wanting in all other acanthodians, so that dorsally it is likely that the spiracular tube ended blindly’. Here we provide evidence to contradict this hypothesis, based on structures identified in acanthodian body fossils from the Middle Devonian of Scotland.
2. MATERIALS AND METHODS
Specimens are reposited in the fossil collections of the Natural History Museum, London (NHM UK PVP), National Museums Scotland, Edinburgh (NMS G), Natural History Museum of Sweden, Stockholm (NRM‐PZ), and Queensland Museum, Brisbane (QMF). Thin sections were made by J. d. B. by grinding down slices glued to glass slides, using various grain sizes of corundum grinding powder down to 4 μm. Sections were photographed using a Sony DSC‐H2 camera on a Nikon Eclipse E400 microscope.
3. DESCRIPTION
The dermal skeleton of the acanthodian head is micromeric, with most taxa having polygonal tesserae over the anterodorsal head region. Gill covers vary in form, comprising long branchiostegal rays in most taxa, and often with smaller dermal structures over the gills above the branchiostegal rays (Denison, 1979). Watson (1937: p. 87) described ‘two small sickle‐shaped bones’ above the main branchial cover that he identified as the upper end of the hyoid arch, on the Middle Devonian Cheiracanthus latus specimen NHMUK PVP.43273 (est. original length 170 mm) from the Orcadian Basin, northern Scotland (Figure 1a,b; We note that Watson referred this specimen to Cheiracanthus murchisoni, whereas it is labelled Cheiracanthus latus; the latter identification is confirmed by examination of the squamation). We re‐interpret these structures as a spiracular valve formed of paired elements. We have also identified other specimens showing evidence for spiracles. C. latus NHMUK PVP.3253 (Figure 1c) has remnants of the left and right side spiracles; an impression of a pseudobranch is preserved on C. murchisoni NRM‐PZ P1650 (Figure 1d; estimated total length of fish 140 mm); a fractured pseudobranch is visible on C. murchisoni NMS G.2019.105.15 (Figure 1e); paired spiracle elements are preserved on C. murchisoni QMF60005; and paired pseudobranchs were visible on C. murchisoni NMS G.2019.3.6, which was sacrificed for sectioning (Figures 1f and 2a–e; estimated original length of fish ca. 70 mm). The sections are presumed to be oblique through the structure (Figure 2f), based on their shape relative to the original surface appearance of the elements. One section (Figure 2a–d) shows the opposing sides of the valve in ‘life’ position as capsular elements with parallel, curving struts or plates formed of a dense acellular tissue, presumed to be bone, over a cartilage core, and a basal tissue we identify as elastic cartilage, characterised by round chondrocyte spaces in a fibrous ground substance (https://www.anatomyatlases.org/MicroscopicAnatomy/Section03/Plate0342.shtml). The other section through the end of the right capsule (Figure 2e) shows a section through the thicker terminal elastic cartilages, and narrow canals in the bone of the capsule. The elements were close to the posterodorsal edge of the palatoquadrate cartilage and a sensory line canal, the position expected for the spiracle based on its position on C. latus specimen NHMUK PVP.43273 (Figure 1a,b). Although the structure is estimated to have been less than half the size of the spiracle on the other Cheiracanthus specimens, the fish was also only half their length, or less, so the smaller spiracle size is commensurate with the size of the fish.
Figure 1.
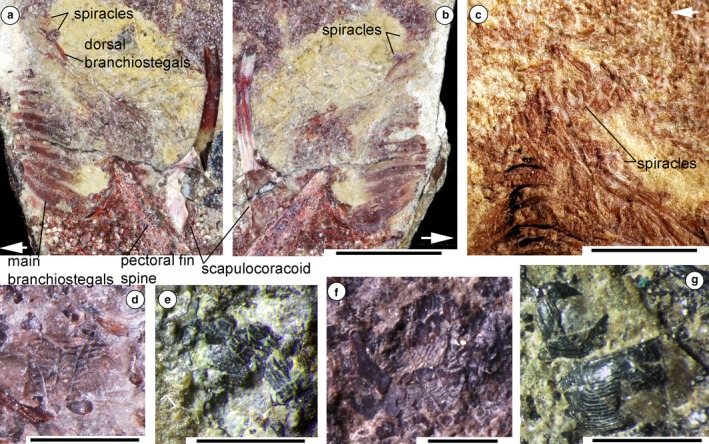
Spiracle structures in acanthodians from the Middle Devonian Orcadian Basin, northern Scotland. (a,b) Cheiracanthus latus NHMUK PVP.43273 from Tynet Burn, showing spiracles from left and right sides. (a) Part, branchial region, (b) counterpart, branchial region. br, branchiostegal rays; fs, fin spine. (c) C. latus NHMUK PVP.2353 from Tynet Burn. (d–f) C. murchisoni specimens (d) NRM‐PZ P1560 from Tynet Burn, impression of spiracular pseudobranch; (e) NMS G.2019.105.15 from Cromarty, crushed spiracular pseudobranch; (f) NMS G.2019.3.6 from Tynet Burn, right half pre‐sectioning, spiracular pseudobranchs. (g) Mesacanthus pusillus NMS G.2019.3.10, from Cromarty, Sutors. Scale bars: 10 mm (a,c), 2 mm (d,g). Arrows indicate anterior direction
Figure 2.
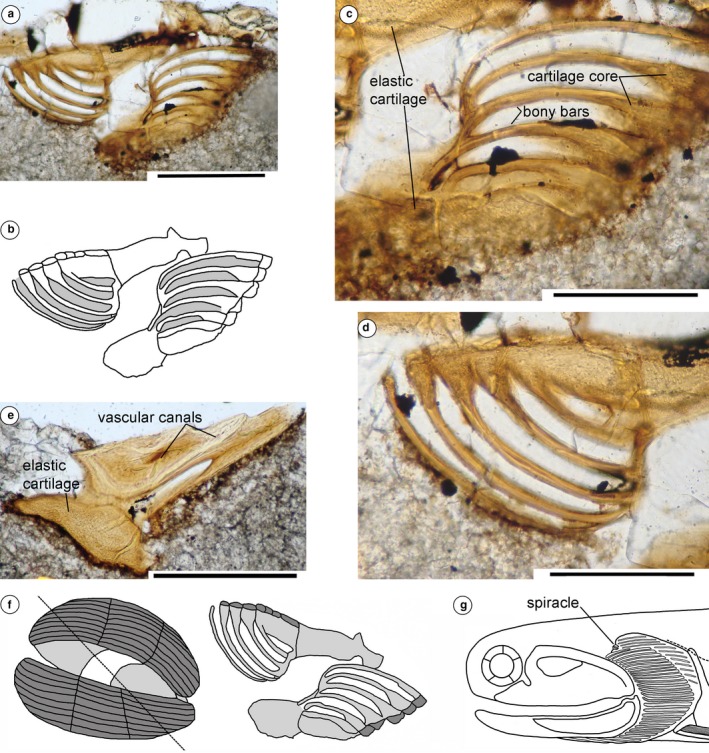
Cheiracanthus spiracle structure and reconstruction. (a–e) Thin section of spiracle elements on NMS G.2019.3.6, specimen sacrificed for serial sectioning, (a–d) NMS G.2019.3.6.26, oblique section through in situ spiracular valves; (e) NMS G.2019.3.6.25, oblique section through the end of the right valve. (f) Reconstruction of the spiracle in Cheiracanthus, showing orientation of thin section NMS G.2019.3.6.26. (g) Head and branchial region of Cheiracanthus showing position of spiracle. Scale bars: .2 mm (a,c–e)
In the Middle Devonian acanthodiform Mesacanthus pusillus, also from the Orcadian Basin (Cromarty, Sutors), the spiracle elements have a different shape, as exemplified by NMS G. 2019.3.10 (Figure 1g). In this specimen, only the outer cover of the paired elements is visible. These are subrectangular in outline, having a domed surface with close‐set parallel grooves running around the three outer sides. By comparison with the spiracle structure in Cheiracanthus, the ‘grooves’ are the gaps between the separate lamellae forming the pseudobranch. The internal structure is not visible.
4. DISCUSSION
4.1. Comparison with the spiracles of extant fishes
As acanthodians are now generally considered to be a group of stem chondrichthyans (e.g. Maisey et al. 2019), extant sharks are the best models for interpreting the acanthodian spiracular structure. Sharks lack any dermal mineralisations delimiting the spiracle, presumably including a lack of specialised scales (none is mentioned by Reif, 1985). Unfortunately, very few details have been published on the internal structure of the spiracle in chondrichthyans, with most investigations carried out in the 19th century (e.g. Ridewood, 1896) using standard dissection and serial sectioning techniques. Features that have been identified are the spiracular tube connecting the internal and external openings, dorsal and ventral caeca branching off the tube, a pre‐spiracular cartilage, and a pseudobranch on the wall of the tube close to the external opening. Recent work has focused on the spiracular sense organ, found in the dorsal caecum (Barry et al. 1988), but very little work has been done on the other features. Even in the Order Orectolobiformes, the spiracle shows wide variation in its external size, and the presence or absence of gill filaments (in the pseudobranch) and spiracular caeca (Goto, 2001). However, the only illustrations provided were rough sketches lacking details. An investigation of the spiracle in the Japanese Bull Shark (Tomita et al. 2018) used 3D computed tomography (CT) scanning to reproduce the exact shape and structure of the tube and its components. An epithelial pseudobranch with eight gill filaments is developed just inside the aperture, on the anterior side of the inner wall of the tube; the filaments lie parallel to the axis of the tube. The pseudobranch is supported anteriorly by a square cartilaginous plate, the pre‐spiracular cartilage (Tomita et al. 2018), which has also been described in Squalus acanthias and Scyliorhinus canicula (Ridewood, 1896). A pair of pre‐spiracular cartilages were identified in embryos of S. acanthias by El‐Toubi (1947), who considered them to be modified mandibular rays; and the pre‐spiracular cartilage in Rajiformes was identified as part of a movable valve (Goodrich, 1909).
The pseudobranch has usually been interpreted as the remnant of the posterior hemibranch of the mandibular arch (Wegner, 2015), although the common view that the spiracle represents a vestigial gill slit has been contradicted (Miyashita, 2016), with a suggestion that the spiracular epithelium secondarily acquired the folded structure forming the pseudobranch for non‐respiratory functions. However, the mineralised structure of the pseudobranch in Cheiracanthus and Mesacanthus supports the general view that the spiracle represents a vestigial gill.
As far as we can determine, the pre‐spiracular cartilage/s and the pseudobranch, if both are present, are separate elements in all extant chondrichthyans, and there is only one pseudobranch in each spiracular pouch. In Cheiracanthus, however, the paired mineralised spiracular capsules each combine both a cartilage plate and vestigial gill bars, indicating that remnants of two hemibranchs were preserved in each spiracular pouch. If the anteriormost one derived from the posterior hemibranch of the mandibular arch, then the second would likely be the anterior hemibranch of the hyoidean gill arch. The histological section of the pseudobranch and basal plate of C. murchisoni specimen NMS G.2019.3.6 (Figure 2a–e) shows that a tissue we identify as elastic cartilage forms the capsule, and the pseudobranch bars have a cartilage core inside a dense bone (the typical structure of perichondral bone). The spiracular tube evolved into the eustachian tube (Graham et al. 2014), and elastic cartilage is now found supporting the ears in many animals, perhaps representing a holdover from 400 Mya rather than coincidence (J. A. Long, pers. comm.).
In the one example of Mesacanthus that we were able to identify, only the outer layers of the spiracular structure appear to have been mineralised. As the chondrichthyan crown evolved from an ancestor within the climatiid acanthodian group, the latter must also have had a functional spiracle, but we have not yet identified one in institutional specimens. The spiracular structure we have identified in these acanthodiforms surely represents a transitional state between the ancestral, functional first gill arch of the (as yet unidentified) most basal gnathostome, and the separate spiracular cartilage and single pseudobranch in chondrichthyans. Current understanding of early vertebrate phylogeny places acanthodians, i.e. stem chondrichthyans, crownwards of placoderms, suggesting that this group also had paired structures in both spiracles, but these have not yet been identified.
4.2. Function
The spiracle in fishes serves two main functions. The basal actinopterygian Polypterus is the only extant osteichthyan known to use the spiracle for air‐breathing, but this function has also been proposed for fossil taxa including stem tetrapodomorphs (Clack, 2007). In extant sharks and rays, however, the spiracle is for intake of water, the opposite process to that of the functional gills through which water is expelled. It is not used for respiration, as it receives only oxygenated blood (Mallatt, 1996). One function is to sustain ventilatory flow to the gills when the mouth is obstructed by substrate or engaged in prey manipulation (Graham et al. 2014). The pseudobranch, if present, is for chemosensory, secretory, and/or thermoregulatory functions (Laurent and Dunel‐Erb, 1984).
The elaborate development of the mineralised spiracular structures in Cheiracanthus indicates the spiracle was functional (Figure 2f). The individual rays of the pseudobranch, with their elastic cartilage ends, could separate to allow water to flow through the capsule. The opposing pair of elements presumably also could be pressed together by muscles to form a valve, as in extant sharks and rays, closing the spiracular tube to prevent water flow. The ventilatory function associated with prey manipulation ascribed to the spiracle in extant fishes appears to be inapplicable in Cheiracanthus and Mesacanthus, which were nektonic fish lacking oral teeth and so unlikely to be predators. The ventral fin spines on the specimens lack any signs of abrasion from the substrate, and no fin spine drag trace fossils have been identified in the Orcadian Basin deposits, so it seems unlikely that these fish were even casually benthic, ruling out the likelihood of intake via the spiracle to avoid muddy water. The small size of the Cheiracanthus spiracle compared with the large size of the spiracle in benthic skates and rays is also an indicator that its function was more likely to be chemosensory than physical.
5. CONCLUSION
At least some acanthodian stem chondrichthyans possessed a functional spiracle, comprising a pair of opposed elements each formed of an encapsulating cartilage and a pseudobranch constructed of parallel bone gill bars with cartilaginous ends and cores. The elastic cartilage of the capsule facilitated opening and closing of inlet and outlet ducts between the gill bars for water flow through the pseudobranch. Cheiracanthid and mesacanthid acanthodiforms were nektonic fishes, and the spiracle and pseudobranch most likely had a chemosensory function.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Carole Jan Burrow: Conception of the study, interpretation of specimens, drafting article. Michael John Newman: Conception of the study, interpretation of specimens, drafting article. Jan L. den Blaauwen: Preparation and identification of structures, drafting article.
ACKNOWLEDGEMENTS
We thank John A. Long and an anonymous reviewer for their helpful comments, Sidney Johnston for donating specimen NMS G.2019.105.15, the Natural History Museum, London (NHMUK) and Swedish Museum of Natural History, Stockholm (NRM) staff for access to specimens, and Roger Jones for photographing NHMUK specimens. C.J.B. thanks the Queensland Museum (QM) for providing facilities.
Burrow CJ, Newman MJ, den Blaauwen JL. First evidence of a functional spiracle in stem chondrichthyan acanthodians, with the oldest known elastic cartilage. J. Anat. 2020;236:1154–1159. 10.1111/joa.13170
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- Barry, M.A. , Hall, D.H. and Bennett, M.V.L. (1988) The elasmobranch spiracular organ I. Morphological studies. Journal of Comparative Physiology A, 163, 85–92. [DOI] [PubMed] [Google Scholar]
- Clack, J.A. (2007) Devonian climate change, breathing, and the origin of the tetrapod stem group. Integrative and Comparative Biology, 47, 510–523. [DOI] [PubMed] [Google Scholar]
- Denison, R.H. (1979) Acanthodii In: Schultze H.‐P. (Ed.) Handbook of Paleoichthyology, Part 5. Stuttgart: Gustav Fischer Verlag, pp. 62. [Google Scholar]
- El‐Toubi, M.R. (1947) The development of the spiracular cartilages of the spiny dogfish, Acanthias vulgaris (Squalus acanthias). Biological Bulletin, 93, 287–295. [PubMed] [Google Scholar]
- Gai, Z. , Lu, L. , Zhao, W. and Zhu, M. (2018) New polybranchiaspiform fishes (Agnatha: Galeaspida) from the Middle Palaeozoic of China and their ecomorphological implications. PLoS ONE, 13, e0202217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner, B.G. (1984) The relationships of the palaeoniscid fishes, a review based on new specimens of Mimia and Moythomasia from the Upper Devonian of Western Australia. Bulletin of the British Museum (Natural History) Geology, 37, 173–428. [Google Scholar]
- Goodrich, E.S. (1909) Part IX. Vertebrata Craniata. Fascicule 1. Cyclostomes and fishes In: Lankester E.R. (Ed.) A Treatise on Zoology. London: Macmillan, pp. xvi+518. [Google Scholar]
- Goto, T. (2001) Comparative anatomy, phylogeny and cladistic classification of the order Orectolobiformes (Chondrichthyes, Elasmobranchii). Memoirs of the Graduate School of Fisheries Sciences, Hokkaido University, 48, 1–100. [Google Scholar]
- Graham, J.B. , Wegner, N.C. , Miller, L.A. , Jew, C.J. , Lai, N.C. , Berquist, R.M. et al (2014) Spiracular air breathing in polypterid fishes and its implications for aerial respiration in stem tetrapods. Nature Communications, 5, 6. [DOI] [PubMed] [Google Scholar]
- Halstead, L.B. (1971) The presence of a spiracle in the Heterostraci (Agnatha). Zoological Journal of the Linnean Society, 50, 195–197. [Google Scholar]
- Holmgren, N. (1942) Studies on the head of fishes: an embryological, morphological, and phylogenetical study. Part 3. The elasmobranch fishes. Acta Zoologica, 23, 129–261. [Google Scholar]
- Janvier, P. (1996) Early Vertebrates. Oxford: Oxford University Press. [Google Scholar]
- King, B. , Qiao, T. , Lee, M.S.Y. , Zhu, M. and Long, J.A. (2017) Bayesian Morphological Clock Methods resurrect placoderm monophyly and reveal rapid early evolution in jawed vertebrates. Systematic Biology, 66, 499–516. [DOI] [PubMed] [Google Scholar]
- Laurent, P. and Dunel‐Erb, S. (1984) The pseudobranch: Morphology and function In: Hoar W.S. and Randall D.J. (Eds.) Fish Physiology, Vol. 10B New York: Academic Press, pp. 285–323. [Google Scholar]
- Maisey, J.G. (1980) An evaluation of jaw suspension in sharks. American Museum Novitiates, 2706, 1–17. [Google Scholar]
- Maisey, J.G. , Janvier, P. , Pradel, A. , Denton, J.S.S. , Bronson, A.W. , Miller, R. et al (2019) Doliodus and pucapampellids: contrasting perspectives on stem chondrichthyan morphology In: Underwood C., Richter M. and Johanson Z. (Eds.) Evolution and Development of Fishes. Cambridge: Cambridge University Press, pp. 87–109. [Google Scholar]
- Mallatt, J. (1996) Ventilation and the origin of jawed vertebrates: A new mouth. Zoological Journal of the Linnean Society, 117, 329–404. [Google Scholar]
- Miles, R.S. (1964) A reinterpretation of the visceral skeleton of Acanthodes . Nature, 204, 457–459. [Google Scholar]
- Miles, R.S. (1965) Some features in the cranial morphology of acanthodians and the relationships of the Acanthodii. Acta Zoologica, 46, 233–255. [Google Scholar]
- Miyashita, T. (2016) Fishing for jaws in early vertebrate evolution: a new hypothesis of mandibular confinement. Biological Reviews, 91, 611–657. [DOI] [PubMed] [Google Scholar]
- Reif, W. (1985) Squamation and ecology of sharks. Courier Forschungsinstitut Senckenberg, 78, 1–255. [Google Scholar]
- Ridewood, W.G. (1896) On the spiracle and associated structures in elasmobranch fishes. Anatomischer Anzeiger, 11, 425–433. [Google Scholar]
- Stensiö, E. (1947) The sensory lines and dermal bones of the cheek in fishes and amphibians. Kongliga Svenska Vetenskaps Academiens Handlingar, 24, 1–195. [Google Scholar]
- Tomita, T. , Toda, M. , Miyamoto, K. , Ueda, K. and Nakaya, K. (2018) Morphology of a hidden tube: Resin injection and CT scanning reveal the three‐dimensional structure of the spiracle in the Japanese Bullhead Shark Heterodontus japonicus (Chondrichthyes; Heterodontiformes; Heterodontidae). The Anatomical Record, 301, 1336–1341. [DOI] [PubMed] [Google Scholar]
- Watson, D.M.S. (1937) The acanthodian fishes. Philosophical Transactions of the Royal Society B, 228, 49–146. [Google Scholar]
- Wegner, N.C. (2015) Elasmobranch gill structure. Fish Physiology, 34A, 101–151. [Google Scholar]
- Young, G.C. (1986) The relationships of placoderm fishes. Zoological Journal of the Linnean Society, 88, 1–56. [Google Scholar]
- Young, G.C. (1991) The first armoured agnathan vertebrates from the Devonian of Australia In: Chang M.‐M., Liu Y.‐H. and Zhang G.‐R. (Eds.) Early Vertebrates and Related Problems of Evolutionary Biology. Beijing: Science Press, pp. 67–85. [Google Scholar]
- Young, G.C. and Zhang, G. (1992) Structure and function of the pectoral joint and operculum in antiarchs, Devonian placoderm fishes. Palaeontology, 35, 443–464. [Google Scholar]
- Zangerl, R. and Williams, M.E. (1975) New evidence on the nature of the jaw suspension in Palaeozoic anacanthous sharks. Palaeontology, 18, 333–341. [Google Scholar]
- Zhu, M. , Yu, X. , Ahlberg, P.E. , Choo, B. , Lu, J. , Qiao, T. et al (2013) A Silurian placoderm with osteichthyan‐like marginal jaw bones. Nature, 502, 188–193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


