Abstract
Mammalian pregnancy involves remodelling of the uterine epithelium to enable placentation. In marsupials, such remodelling has probably played a key role in the transition from ancestral invasive placentation to non‐invasive placentation. Identifying uterine alterations that are unique to marsupials with non‐invasive placentation can thus elucidate mechanisms of marsupial placental evolution. We identified apical alterations to uterine epithelial cells prior to implantation in Monodelphis domestica, a member of the least derived living marsupial clade (Didelphidae) with invasive (endotheliochorial) placentation. We then compared these traits with those of Macropus eugenii (Macropodidae) and Trichosurus vulpecula (Phalangeridae), both with non‐invasive placentation, to identify which alterations to the uterine epithelium are ancestral and which facilitate secondarily evolved non‐invasive placentation. In M. domestica, remodelling of the uterine epithelium involves reduced cellular heterogeneity and development of uterodome‐like cells, suggesting that similar alterations may also have occurred in the marsupial common ancestor. These alterations also overlap with those of both T. vulpecula and Ma. eugenii, suggesting that the placental shift from invasive to non‐invasive placentation in marsupials involves essential, conserved characteristics, irrespective of placental mode. However, unique apical alterations of both T. vulpecula and Ma. eugenii, relative to M. domestica, imply that lineage‐specific alterations underpin the evolutionary shift to non‐invasive placentation in marsupials.
Keywords: marsupial, morphology, placentation, plasma membrane transformation
Invasive placentation in Monodelphis domestica (A, this study) requires a homogeneous epithelium with uterodome‐like cells that may also have formed in the marsupial common ancestor. Comparison with secondarily evolved non‐invasive placentation in Trichosurus vulpecula (B, Laird et al., 2017b) and Macropus eugenii (C, Freyer et al., 2002) reveals that both conserved and lineage‐specific traits, relative to M. domestica, underpin the evolutionary shift to non‐invasive placentation in marsupials.

1. INTRODUCTION
Amniote viviparity often involves formation of a placenta during pregnancy to meet the physiological needs of a developing embryo in utero (Murphy et al., 2000; Thompson et al., 2002). In mammals, viviparity has evolved once in the common ancestor of eutherian mammals and marsupials (Lillegraven, 1975; Zeller and Freyer, 2001). Placental morphology in mammals is highly diverse (Mossman, 1987; Murphy, 1998) and invasion of the uterus by the embryo at implantation occurs along a penetrative spectrum (Wildman, 2016), from non‐invasive (epitheliochorial) placentation (Ferner and Mess, 2011), involving relatively superficial maternal‐embryonic contact, through to highly invasive (haemochorial) placentation with direct transfer between maternal and embryonic blood streams (Mess, 2014). Remodelling of the uterine epithelium, termed the plasma membrane transformation, is required for uterine receptivity to the embryo, irrespective of placental type (Murphy, 2004). In eutherian mammals, many aspects of remodelling facilitate embryonic invasion at implantation, including reduction of baso‐lateral adhesion of uterine epithelial cells, such that uterine cells are more readily displaced by invasive embryonic cells (Enders and Schlafke, 1967; Schlafke and Enders, 1975; Preston et al., 2004; Kaneko et al., 2008). By contrast, remodelling in marsupial pregnancy involves maternal defences against the implanting embryo, including reinforcement of the basal connections of the uterine epithelium that potentially reduce or prevent invasion (Laird et al., 2017a, 2018). Lack of similar defences in eutherian placentae, which are typically more invasive than those of marsupials (Amoroso, 1952; Renfree et al., 2013), may reflect decidualisation of fibroblasts in the uterine stroma during pregnancy. This uniquely eutherian phenomenon serves to regulate stromal invasion by the embryo after the uterine epithelium has been breached (Kin et al., 2014; Wagner et al., 2014). Since marsupials lack decidualisation, and thus a stromal mechanism of regulating embryonic invasion, the uterine epithelium itself may thus play a greater role in regulating marsupial implantation than it does in eutherian mammals, resulting in maternal reinforcement of the existing epithelial barrier (Laird, 2017).
Phylogenetic distribution of placental types in mammals suggests that the eutherian common ancestor had invasive placentation that was either haemochorial or endotheliochorial (Carter and Mess, 2007; Elliot and Crespi, 2009). Recent molecular evidence suggests that invasive placentation is also ancestral for marsupials (Mess and Ferner, 2010); therefore, non‐invasive placentation in both eutherian mammals (Elliot and Crespi, 2009; Carter and Enders, 2013) and marsupials (Mess and Ferner, 2010; Ferner and Mess, 2011) is likely to be secondarily derived (Carter and Mess, 2007). Accumulation of maternal defences to the embryo may be a mechanism by which non‐invasive placentation has evolved secondarily in marsupials (Crespi and Semeniuk, 2004; Vogel, 2005; Carter and Mess, 2007; Laird et al., 2017a). Hence, unique aspects of remodelling involved in non‐invasive marsupial placentation can identify how the uterus has been modified to support this placental type.
Non‐invasive placentation occurs in both phalangerid and macropodid marsupials (Figure 1; Pilton and Sharman, 1962; Tyndale‐Biscoe and Renfree, 1987; Freyer et al., 2003; Mess and Ferner, 2010; Laird et al., 2018), and has probably evolved independently in these two families. The uterine epithelium of both Macropus eugenii (tammar wallaby; Macropodidae) and Trichosurus vulpecula (brushtail possum; Phalangeridae) undergoes similar morphological and molecular modifications during pregnancy (Freyer et al., 2002; Laird et al., 2017b, 2018), yet apical remodelling of the uterine epithelium also involves species‐specific differences. For example, cellular diversity of the uterine epithelium increases before embryonic attachment in T. vulpecula (Laird et al., 2017b), yet decreases in Ma. eugenii as the uterine epithelium becomes more uniform (Freyer et al., 2002). In addition, cells of T. vulpecula develop extremely domed and elongated apices during the implantation period (Laird et al., 2017b), but these cells do not develop in Ma. eugenii (Freyer et al., 2002).
Figure 1.
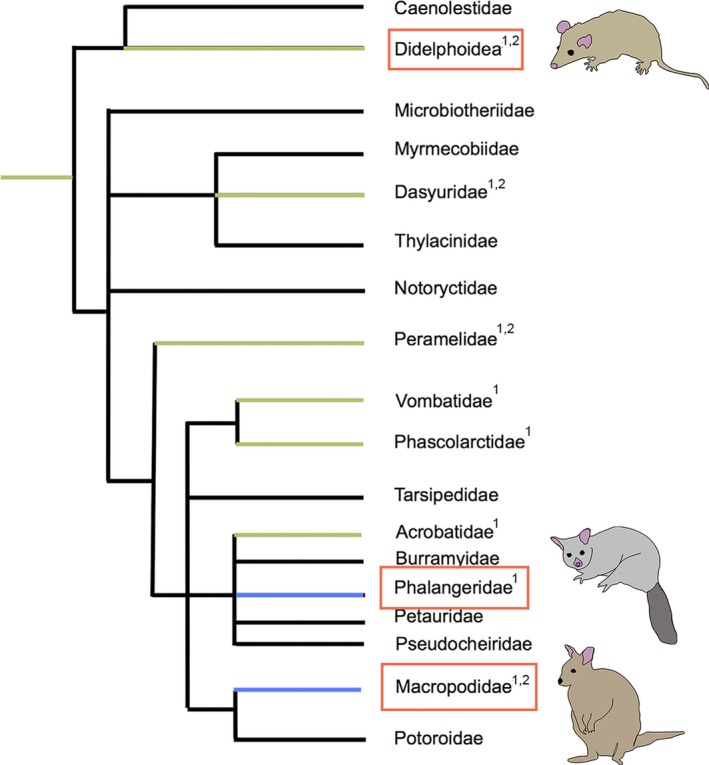
Phylogenetic distribution of placental types in Metatheria (redrawn from Freyer et al. (2003) and Laird et al. (2018)). Endotheliochorial placentation (green); epitheliochorial placentation (blue); unknown placentation (black). Clades compared in this studied are indicated by red boxes. 1Mess and Ferner (2010); 2Freyer et al. (2003)
Members of Didelphidae, the least derived clade of living marsupials (Nilsson et al., 2010), represent ancestral marsupial reproductive traits (Harder et al., 1993; Freyer et al., 2003; Freyer and Renfree, 2009; Kin et al., 2014; Hansen et al., 2016, 2017; Griffith et al., 2017) and are therefore ideal for testing evolutionary theories of how and when marsupial placental modes arose (Freyer et al., 2002; Hansen et al., 2016). The didelphid Monodelphis domestica (grey short‐tailed opossum), in which embryonic implantation is invasive (Harder et al., 1993; Kin et al., 2014), can identify the ancestral states of remodelling characteristics, and thus the mechanisms involved in the evolutionary transition from ancestrally invasive to non‐invasive embryonic attachment.
In the present paper, we report the first detailed investigation of the uterine surface alterations required for receptivity to the embryo in M. domestica, using scanning electron microscopy. We compare these alterations with those of T. vulpecula (Laird et al., 2017b) and Ma. eugenii (Freyer et al., 2002) to identify which apical alterations are ancestral and which have developed secondarily for non‐invasive embryonic attachment in marsupials.
2. METHODS
2.1. Study species
Monodelphis domestica has a gestation period of 14.5–15 days (Harder et al., 1993; Mate et al., 1994; Zeller and Freyer, 2001). Unlike other marsupial species, females of M. domestica do not undergo a true (spontaneous) oestrous cycle as oestrus is induced by male pheromones (Fadem, 1985; Hinds et al., 1992; Harder et al., 1993). Ovulation occurs approximately 20–24 hr post‐copulation (Baggott and Moore, 1990; Mate et al., 1994; Zeller and Freyer, 2001). The shell coat ruptures 12 days post‐copulation, followed by invasive (endotheliochorial) implantation of the trophoblastic syncytium of the trilaminar yolk sac (Zeller and Freyer, 2001), which persists until birth.
2.2. Animal husbandry and tissue collection
This study was approved under protocol numbers 13‐100920‐MCC and 15‐200334‐B‐MC from the University of New Mexico Institutional Animal Care and Use Committee. Animals used in this study were from a captive‐bred research colony housed at the Department of Biology Animal Research Facility at the University of New Mexico and housed as per Hansen et al. (2016). Adult males and females were housed separately when not breeding. Oestrus was induced by introducing the female into a cage with a male. Pregnancies were timed once mating was observed. Pregnant animals were killed by inhaled isofluorane overdose (>5%) until breathing had ceased for >1 min. Uterine tissues were collected from 15 pregnant females between 7 days 0 hr and 14 days 0 hr post‐conception. Females were grouped into three reproductive stages over the period of implantation (Mate et al., 1994): Stage 1 (pre‐implantation, between 7 days 0 hr and 10 days 12 hr post‐copulation; n = 6); Stage 2 (implantation, between 11 days 1 hr–12 days 2 hr post‐copulation; n = 5); Stage 3 (post‐implantation; between 13 days 0 hr and 14 days 0 hr post‐copulation; n = 4). The morphology of the non‐pregnant (cycling) uterine epithelium is described in Wick and Kress (2002).
2.3. Tissue processing for scanning electron microscopy
Uterine horns were excised and opened to expose the internal surface, and embryos and amnions were removed. Tissue samples were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (PB) for 1 hr, followed by rinsing in 0.1 M PB. Samples were then post‐fixed in 4% osmium tetroxide for 1 hr, followed by further rinsing in 0.1 M PB and gradual dehydration to 100% ethanol. These samples were then dried with a Leica EM CPD300 Critical Point Dryer (Leica) using carbon dioxide as the drying agent. Dried tissue was mounted onto aluminium stubs and coated with gold (15 nm). Images were captured on a JEOL NeoScope JCM‐600 Tabletop scanning electron microscope and a Zeiss Sigma HD VP STEM (Zeiss).
2.4. Tissue processing for transmission electron microscopy
For transmission electron microscopy, a small portion of excised uterus from each animal was fixed in glutaraldehyde as per the scanning electron microscopy samples, then post‐fixation was carried out for 1 hr in 4% osmium tetroxide with 0.8% potassium ferrocyanide [K4Fe(CN)6] to enhance membrane contrast (Hulstaert et al., 1983). Samples were rinsed in 0.1 M PB, then 50%, 70% and 100% ethanol. Larger pieces of tissue were cut to ~0.5 mm3. Ethanol was replaced by Spurrs resin (Agar Scientific) in increments of 25%, and tissue pieces were transferred to individual BEEM capsules and polymerized at 62°C overnight. Semi‐thin (200‐µm) and ultra‐thin (70‐µm) sections were cut using a Leica Ultrastat 7 cryostat (Leica) and ultra‐thin sections were transferred to 200‐µm mesh copper grids (ProSci Tech). Grids were post‐stained by floating each on a drop of 2% uranyl acetate for 10 min, rinsing in warm water, then floating on a drop of Reynolds lead citrate surrounded by sodium hydroxide (NaOH) pellets for 10 min (Hayat, 1986). Grids were rinsed and air dried before imaging. Similar to the process for scanning electron microscopy, images were captured using a Zeiss Sigma HD VP STEM (Zeiss).
2.5. Morphometrics
Cellular heterogeneity of the uterine epithelium of M. domestica was determined by comparing the relative abundance of distinct cell types over the three pregnancy stages (Laird et al., 2017b). The entire uterine surface was examined using scanning electron microscopy to confirm uniformity. At least five high magnification images were randomly selected for morphometric analysis per animal (500× and 3,000×; ~100–400 cells per image), with most at ~1,400–1,700× (200–300 cells per image). Cells were counted systematically using an overlaid grid. Each cell in an image was allocated to one of five cell types: bare surface; sparse microvilli; dense microvilli; budding cells and ciliated cells (as per Laird et al., 2017b; see Results for examples of cell types). Damaged cells, or cells that could not be clearly identified, were excluded from the count. Proportions were obtained by dividing the total number of each cell type by the total number of cells in the image. Proportions for each animal were arcsine‐transformed (Dytham, 2011). Cells were counted and the analysis was applied until SEM < 0.05 for each cell type for each animal (Aherne and Dunhill, 1982). Arcsine‐transformed proportions were log‐transformed to satisfy assumptions of normality. These values were analysed using a generalized linear model with respect to cell type by anova in statistica v9.0 (Statsoft), with pregnancy stage as a covariate.
3. RESULTS
3.1. Scanning electron microscopy
3.1.1. Stage 1: Pre‐implantation (7 days 0 hr–10 days 12 hr post‐copulation)
The uterine surface is folded at ~7 days post‐conception (Figure 2a). Cell apices are slightly domed and are densely covered by short blunt microvilli, and ciliated cells are interspersed with microvillous cells (Figure 2a,b). Small secretory droplets occur in the uterine lumen. By ~10 days post‐conception, microvilli are spiky, rather than blunt, and plaques of glycocalyx occur on the uterine surface (Figure 2c). Additionally, throughout Stage 1, cells in some uterine regions are flattened with raised borders, and are uniformly arranged in a ‘honeycomb’ pattern (Figure 2d). Ciliated cells are evenly distributed in these regions (Figure 2d).
Figure 2.
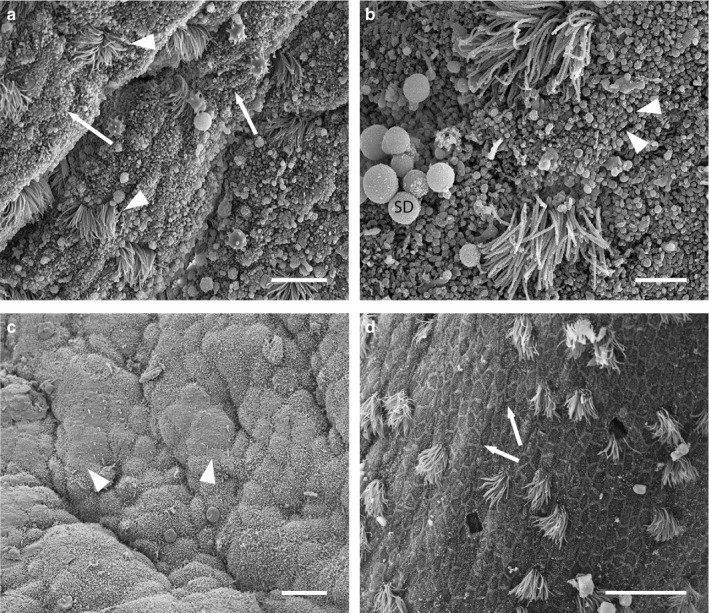
Scanning electron micrographs of the uterine surface of Monodelphis domestica at Stage 1 of pregnancy (7 days 0 hr–10 days 12 hr post‐copulation). (a) Ciliated cells (arrowheads) are interspersed with densely microvillous cells (arrows). (b) Microvillous cells are densely covered in short blunt microvilli (arrowheads). Small secretory droplets (SD) also occur in the uterus. (c) Plaques of glycocalyx (arrowheads) occur on the uterine surface. (d) In some uterine regions, uterine epithelial cells are flattened, with raised borders, and arranged in a ‘honeycomb’ pattern (arrows). Scale bars = 10 μm (a), 4 μm (b), 15 μm (c), 20 μm (d)
3.1.2. Stage 2: Implantation (11 days 1 hr–12 days 2 hr post‐copulation)
Uterine epithelial cells at Stage 2 possess spiky microvilli, and cell apices are more domed than for Stage 1 (Figure 3a). Many microvillous cells have a ‘dimple’ on the apical surface (Figure 3b), as well as clumps of glycocalyx. Cells with longer microvilli occur at this stage (Figure 3c), alongside the ciliated cells, which have become fewer by ~12 days 2 hr post‐conception. Similarly to Stage 1, regions of the uterus retain a uniform ‘honeycomb’ appearance that is morphologically distinct throughout Stage 2 (Figure 3d). These ‘honeycomb’ regions are restricted to the crests of uterine folds (Figure 3e), whereas regions of uterine epithelium with domed cells are restricted to the bases of these folds (Figure 3e).
Figure 3.
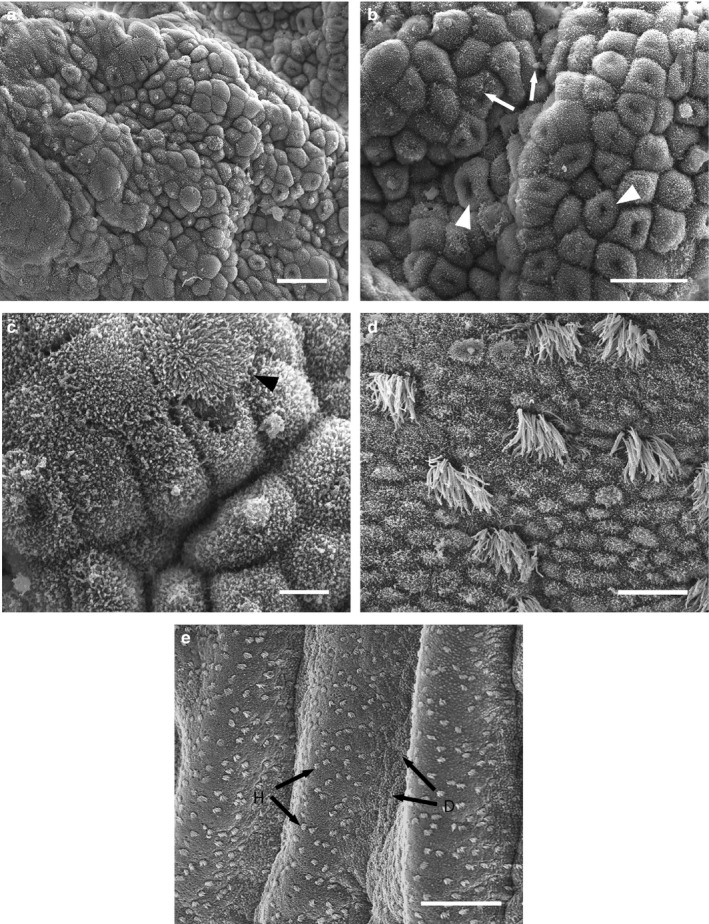
Scanning electron micrographs of the uterine surface of Monodelphis domestica at Stage 2 of pregnancy (11 days 1 hr–12 days 2 hr post‐copulation). (a) Uterine region consisting of cells with domed apices. (b) Higher magnification of (a): depicts apical ‘dimples’ (arrowheads) and clumps of glycocalyx (arrows). (c) Cells with elongated microvilli (arrowhead) occur. (d) Uterine region consisting of uniform ‘honeycomb’ cells. (e) Domed uterine epithelial cells (d, arrows) are restricted to the bases of uterine folds, while flattened ‘honeycomb’ regions (h, arrows) occur on the crests. Scale bars = 25 μm (a), 20 μm (b), 5 μm (c), 10 μm (d), 100 μm (e)
3.1.3. Stage 3: Post‐implantation (13 days 0 hr–14 days 0 hr post‐copulation)
Uterine epithelial cells are domed and irregular by Stage 3, with sparse spiky apical microvilli (Figure 4a). In some regions, cells are extremely domed (budding cells; Figure 4b) and lack apical projections. Glycocalyx plaques cover large regions of uterine epithelium (Figure 4b). Ciliated cells are few, except in regions of regularly arranged ‘honeycomb’ cells, which still occur at the crests of uterine folds (Figure 4c).
Figure 4.
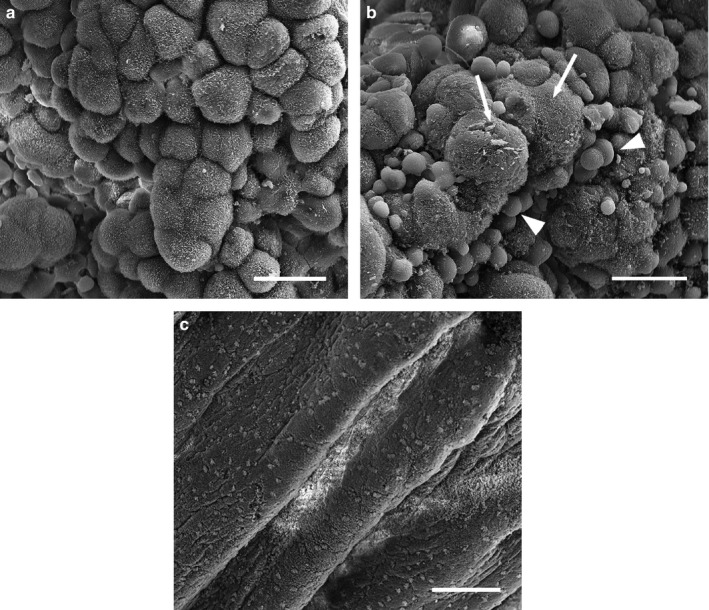
Scanning electron micrographs of the uterine surface of Monodelphis domestica at Stage 3 of pregnancy (13 days 0 hr–14 days 0 hr post‐copulation). The uterine epithelium consists of domed and irregular cells (a), and budding cells (b, arrowheads) with large glycocalyx plaques (b, arrows). Regions of honeycomb cells also occur (c). Scale bars = 20 μm (a, b), 100 μm (c)
3.2. Transmission electron microscopy
At Stage 1 (pre‐implantation), both uterine epithelial cells and trophoblastic cells are densely covered in microvilli (Figure 5a–c). Uterine epithelial cells are elongated and form a single columnar layer, underlain by large blood vessels close to their basal membranes (Figure 5b). Trophoblastic cells are large and domed and underlain by a thin layer of mesenchyme (Figure 5c). By Stage 2 (implantation), uterine epithelial cells are less uniform and have apices that project into the uterine lumen (Figure 5d). These projections are more pronounced by Stage 3 (post‐implantation) when the uterine surface is highly folded, and rounded budding cells extend into the lumen (Figure 5e).
Figure 5.
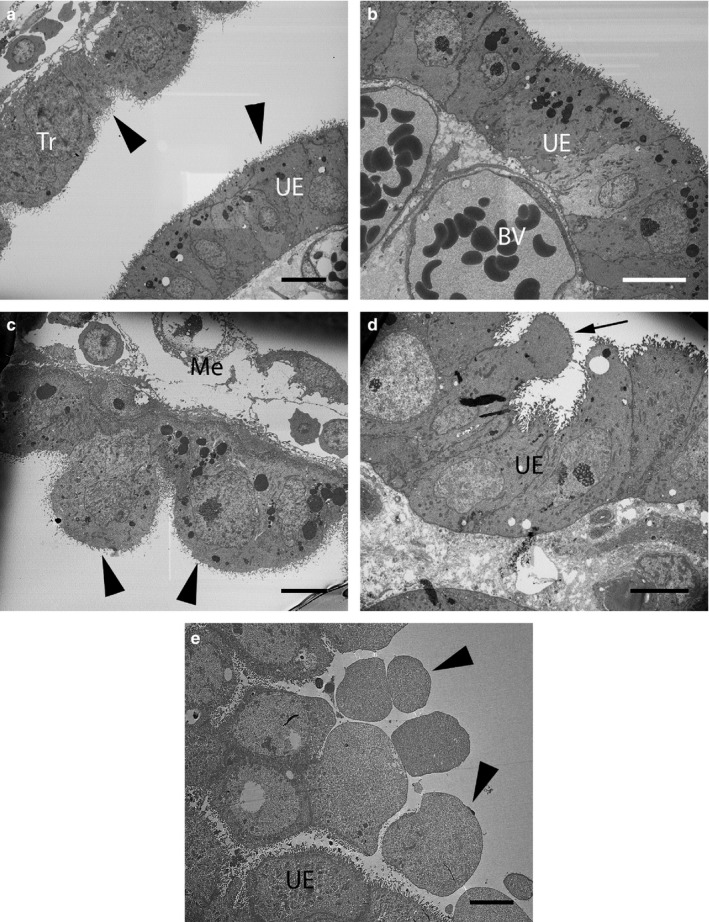
Transmission electron micrographs of the uterus of Monodelphis domestica. (a–c) Stage 1 (Pre‐implantation), (d) Stage 2 (Implantation) and (e) Stage 3 (Post‐implantation). (a) Both uterine epithelial cells (UE) and trophoblastic cells (Tr) have elongated microvilli (arrowheads). (b) Higher magnification of (a), depicting single layer of columnar UE underlain by maternal blood vessels (BV). (c) Higher magnification of (a), depicting large trophoblastic cells (arrowheads) underlain by mesenchyme (Me). (d) UE cells at implantation are less regular and project into the uterine lumen (arrow). (e) Budding cells (arrowheads) are prominent post‐implantation. Scale bars = 10 µm (a–c), 6 µm (d), 5 µm (e)
3.3. Morphometrics
Densely microvillous cells dominate the uterine surface across all three stages (Figure 6). Budding cells are significantly more abundant at Stage 3 (F = 11.6649, df = 2; p < .001) than at other stages, while bare surface cells appear to be more abundant at Stage 1. A reduction in abundance of ciliated cells occurs between Stages 1 and 3. Overall cellular diversity reduces after Stage 1 and the uterine epithelium is least diverse at Stage 2, after which diversity increases (Figure 6).
Figure 6.
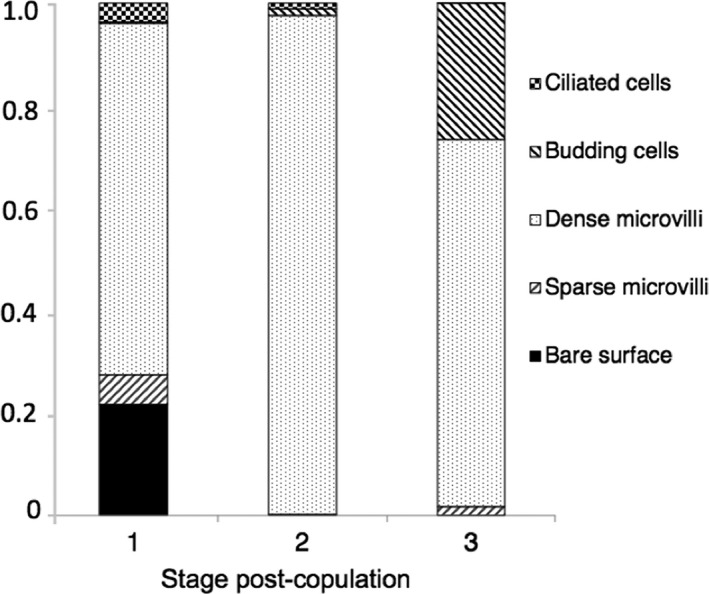
Cellular composition of the uterine epithelium of Monodelphis domestica. Graph depicts relative proportions of each of five distinct cell types on the uterine surface over the three pregnancy stages. Data are presented as means of arcsine‐transformed proportions for each stage of pregnancy (SEM < 0.05)
4. DISCUSSION
The uterine epithelium of M. domestica undergoes substantial apical remodelling during the implantation period that is consistent with a plasma membrane transformation (Murphy et al., 2000; Murphy, 2004). Short, blunt microvilli are replaced by spiky microvilli prior to embryonic implantation in M. domestica, corresponding to a peak in plasma progesterone ~9–10 days post‐copulation (Fadem and Rayve, 1985; Hinds et al., 1992; Bradshaw and Bradshaw, 2011), as also occurs in rats and mice (Ljungkvist, 1972; Murphy, 2000). This change in microvilli also corresponds to a shortening of microvilli on the trophectodermal surface, enabling subsequent interdigitation of trophoblastic and endometrial microvilli and reducing the barrier between fetal and maternal blood (Zeller and Freyer, 2001). Ciliated cells are reduced in the uterine epithelium by Stage 3 in M. domestica, similar to post‐oestrus deciliation in the cycling non‐pregnant uterus in response to oestrogen reduction (Wick and Kress, 2002). Loss of ciliated cells is typical of remodelling in a range of eutherian mammals (Barberini et al., 1978; Stroband et al., 1986; Murphy, 2004) and marsupials (Enders and Enders, 1969; Ward and Renfree, 1988; Rose et al., 1999; Laird et al., 2014). Since M. domestica most closely resembles the reproductive traits of the most recent marsupial common ancestor (Harder et al., 1993; Freyer et al., 2002; Kin et al., 2014; Hansen et al., 2016; Griffith et al., 2017), similarities in apical cell remodelling during pregnancy in M. domestica and eutherian mammals suggest that these alterations are ubiquitous in mammalian pregnancy and that a plasma membrane transformation may also have occurred in the therian common ancestor.
Interestingly, the uterine epithelium of M. domestica is not uniformly remodelled. Regions of flattened ‘honeycomb’ cells with raised borders, interspersed with ciliated cells, are retained throughout pregnancy and occur at the crests of uterine folds. In contrast, remodelled cells occur only at the bases of these folds. This morphological difference between remodelled and non‐remodelled regions could relate to the high functional differentiation of embryos of M. domestica, which have an invasive trilaminar yolk sac and a non‐invasive bilaminar yolk sac (Freyer et al., 2002), resulting in a mixed epitheliochorial‐endotheliochorial placenta (Zeller and Freyer, 2001; Carter and Enders, 2016). Implantation of the trilaminar yolk sac side forms a trophectodermic syncytium (Freyer et al., 2002) and placental cells closely interdigitate with those of the uterine epithelium, enabling the syncytium to erode the uterine epithelium at regular intervals (Zeller and Freyer, 2001). In contrast, microvillous cells of the bilaminar side interact non‐invasively with microvilli of uterine epithelial cells at implantation (Zeller and Freyer, 2001). Hence, in M. domestica, remodelled regions in the bases of uterine folds may facilitate invasive embryonic interactions (Enders and Schlafke, 1967) with the trilaminar side, while non‐remodelled regions may facilitate non‐invasive contact with the bilaminar yolk sac. The receptive uterus of Sminthopsis crassicaudata (fat‐tailed dunnart) also has remodelled and non‐remodelled sites (Laird et al., 2014), and embryos of this species show similar functional differentiation to those of M. domestica (Roberts and Breed, 1994). In contrast, the uterus is uniformly remodelled in both T. vulpecula (Laird et al., 2017b) and Ma. eugenii (Freyer et al., 2002), two species for which embryos are non‐invasive. Hence, uniform remodelling of the entire uterine surface may be a modification to facilitate non‐invasive embryonic attachment in marsupials.
An alternate explanation is that restriction of remodelled cells to certain regions may be a maternal innovation to reduce parent–offspring conflict at the embryonic–maternal interface. Invasive trophoblasts are probably ancestral for marsupials (Mess and Ferner, 2010). Since microvilli greatly increase the surface area for active transport, the conflict hypothesis predicts that maternal microvillous regions are smaller than those of embryonic surfaces to restrict transfer of resources (Kazemian et al., 2019). Confinement of domed and densely microvillous cells to discrete regions in M. domestica and S. crassicaudata may thus regulate embryonic invasion by limiting physical contact between maternal and embryonic microvillous surfaces. This hypothesis also accounts for uniform remodelling in T. vulpecula and Ma. eugenii, as non‐invasive placentation affords greater maternal control over resource allocation (Kazemian et al., 2019). Indeed, the plasma membrane transformation itself may also be a maternal mechanism to regulate the timing of placentation by restricting uterine receptivity to a brief window. Embryonic attachment in M. domestica, and potentially in the marsupial stem species, thus appears to be regulated both temporally, via a strict receptivity window, and spatially, by constricting remodelled epithelia to discrete regions.
Cellular diversity of the receptive uterine epithelium decreases prior to implantation in M. domestica (Figure 7a). A similar decrease occurs in a range of eutherian mammal species (Potts and Racey, 1971; Winterhager and Denker, 1990; Murphy et al., 2000), as well as in S. crassicaudata (Laird et al., 2014) and potentially Ma. eugenii (Freyer et al., 2002). In these species, a uniform uterine epithelium facilitates physical maternal‐embryonic contact (Schlafke and Enders, 1975), as well as transfer of essential substances and signalling molecules between uterine and trophoblastic cells. In contrast, cell heterogeneity peaks at implantation for T. vulpecula, suggesting that a diverse uterine epithelium may be required for non‐invasive embryonic attachment in this species (Laird et al., 2017b), but not Ma. eugenii (Figure 7b,c). Alternately, maternal recognition of pregnancy occurs in both Ma. eugenii (Renfree, 2000) and M. domestica (Griffith et al., 2019), but not T. vulpecula (Pilton and Sharman, 1962; Renfree, 2000; Laird et al., 2017b). Hence decreased cell diversity in M. domestica and Ma. eugenii may form part of an endometrial response to the presence of an embryo.
Figure 7.
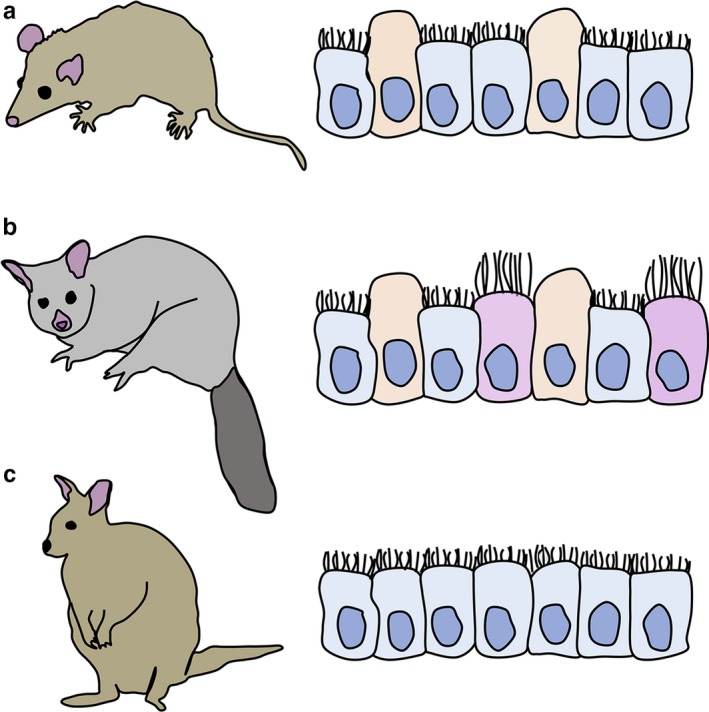
Diagrammatic representation of receptive uterine epithelial cell morphology in marsupials. (a) Monodelphis domestica (present study; invasive endotheliochorial placentation). (b) Trichosurus vulpecula [non‐invasive epitheliochorial placentation (Laird et al., 2017b)]. (c) Macropus eugenii [non‐invasive epitheliochorial placentation (Freyer et al., 2002)]. Blue cells = microvillous cells; orange cells = pinopods/uterodomes; pink/mauve cells = ciliated cells
Elongated budding cells appear in the uterine epithelium of M. domestica ~12 days post‐conception, and are abundant throughout Stage 3 (Figure 7a). These budding cells are consistent with previous observations of M. domestica (Griffith et al., 2017), and are similar in appearance to the domed uterine epithelial cells present at pro‐oestrus (Wick and Kress, 2002). However, unlike budding cells of the pregnant uterus, domed cells at pro‐oestrus are interspersed with solitary cilia, lack an apical ‘dimple’ and occur in the absence of uterine folds. Hence domed cells pro‐oestrus and budding cells at implantation, while morphologically similar, probably serve different functions. Domed apices during pregnancy in M. domestica are also morphologically similar to pinopods in rats and mice (Nilsson, 1958; Murphy, 2000) and uterodomes in other mammals (Murphy, 2000; Murphy et al., 2000; Stavreus‐Evers, 2005; Nikzad et al., 2010) and viviparous lizards (Hosie et al., 2003; Adams et al., 2005). In rodents, these cells are indicative of a uterus which is becoming receptive (Murphy, 2000; Stavreus‐Evers, 2005). However, appearance of these cells is coincident with embryonic attachment in M. domestica (Zeller and Freyer, 2001; Freyer et al., 2002; Hansen et al., 2017), suggesting they play a role in later pregnancy in this species. Similar cells develop prior to implantation in T. vulpecula (Figure 7b; Laird et al., 2017b) and are thought to facilitate secretion of haemotrophes from the maternal blood circulation across the uterine epithelium for uptake by the embryo (Laird et al., 2017b). A similar role is likely for M. domestica as a shift in embryonic nutrition, from uterine secretions to haemotrophe from the maternal circulation, occurs around the time of attachment (Zeller and Freyer, 2001; Freyer et al., 2007; Hansen et al., 2017), as also occurs for most marsupials. In contrast, uterodome‐like cells do not develop during pregnancy in either S. crassicaudata (Laird et al., 2014) or Ma. eugenii (Figure 7c; Freyer et al., 2002), suggesting that these cells are not related to a particular placental form. Hence uterodome‐like cells may have formed part of the receptive epithelium in the therian common ancestor, and have been subsequently lost in Dasyuridae and Macropodidae, or may have arisen independently in both M. domestica and T. vulpecula. Unusually, embryonic development post‐implantation in Ma. eugenii is supported by both histotrophy and haemotrophy (Freyer et al., 2002, 2003). Hence, nutrient transfer via both mechanisms in Ma. eugenii may result in fewer or different uterine mechanisms to facilitate haemotrophic nutrient transfer, potentially accounting for the lack of uterodome‐like cells in the receptive uterus.
Uterine alterations in M. domestica prior to implantation, including decreased cellular heterogeneity of the uterine epithelium, reduction of ciliated cells and development of cells with spiky microvilli and uterodome‐like cells, are likely to most closely represent those of the marsupial stem species. Conservation of these traits in more derived marsupial clades suggests that they are essential for marsupial pregnancy, irrespective of placental type. In contrast, Ma. eugenii and T. vulpecula undergo patterns of apical remodelling that differ both from those of each other and of M. domestica, including absence of uterodome‐like cells in Ma. eugenii (Freyer et al., 2002) and increased cell diversity at implantation in T. vulpecula (Laird et al., 2017b). Molecular differences in the uterus also occur, including different localization patterns of the lateral plasma membrane protein, desmoglein‐2, in Ma. eugenii and T. vulpecula, suggesting that desmoglein‐2 plays different roles in these species (Laird et al., 2018) to eutherian mammals (Preston et al., 2004) and S. crassicaudata (Dudley et al., 2015). Hence, successful non‐invasive embryonic attachment in marsupials requires lineage‐specific alterations to the uterine epithelium, consistent with the independent evolution of non‐invasive attachment in Macropodidae and Phalangeridae (Freyer et al., 2003; Laird et al., 2017b). Nevertheless, aspects of apical remodelling of both Ma. eugenii and T. vulpecula that overlap with those of M. domestica demonstrate that the transition from ancestrally invasive to non‐invasive placentation in marsupials has not required innovation in all aspects of uterine remodelling, as some appear to be essential for placentation in therian mammals.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
VH collected and fixed samples. MKL processed samples for microscopy, collected the data and wrote the manuscript. All authors contributed to experimental design, technical advice, image interpretation and manuscript preparation and revision.
ACKNOWLEDGEMENTS
The authors thank K. S. Richardson for assistance with statistical analysis. and acknowledge the facilities, and the scientific and technical assistance of the Australian Microanalysis Research Facility at the Australian Centre for Microscopy and Microanalysis at the University of Sydney. We particularly acknowledge the assistance of N. Gokoolparsadh and P. Trimby.
Laird MK, Hansen VL, McAllan BM, Murphy CR, Thompson MB. Uterine epithelial remodelling during pregnancy in the marsupial Monodelphis domestica (Didelphidae): Implications for mammalian placental evolution. J. Anat. 2020;236:1126–1136. 10.1111/joa.13162
Funding information
This project was funded by an ARC Discovery Project Grant no. DP130101589 awarded to CR Murphy, MB Thompson and BM McAllan, and by The Ann Macintosh Foundation of the Discipline of Anatomy and Histology and the Murphy Laboratory.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Adams, S.M. , Biazik, J.M. , Thompson, M.B. and Murphy, C.R. (2005) Cytoepitheliochorial placenta of the viviparous lizard Pseudemoia entrecasteauxii: a new placental morphotype. Journal of Morphology, 264, 264–276. 10.1002/jmor.10314 [DOI] [PubMed] [Google Scholar]
- Aherne, W.A. and Dunhill, M.S. (1982) Morphometry. London, UK: Arnold. [Google Scholar]
- Amoroso, E.C. (1952) Placentation In: Parkes A.S. (Ed.) Marshall’s Physiology of Reproduction. New York, NY: Longmans Green, pp. 127–311. [Google Scholar]
- Baggott, L.M. and Moore, H.D.M. (1990) Early embryonic development of the grey, short‐tailed opossum (Monodelphis domestica) in vivo and in vitro. Journal of Zoology, London, 222, 623–639. [Google Scholar]
- Barberini, F. , Sartori, S. and Motta, P. (1978) Changes in the surface morphology of the rabbit endometrium related to the estrous and progestational stages of the reproductive cycle. Cell and Tissue Research, 190, 207–222. 10.1007/BF00218170 [DOI] [PubMed] [Google Scholar]
- Bradshaw, F.J. and Bradshaw, D. (2011) Progesterone and reproduction in marsupials: a review. General and Comparative Endocrinology, 170(1), 18–40. 10.1016/j.ygcen.2010.07.015 [DOI] [PubMed] [Google Scholar]
- Carter, A.M. and Enders, A.C. (2016) Placentation in mammals: definitive placenta, yolk sac, and paraplacenta. Theriogenology, 86, 278–287. 10.1016/j.theriogenology.2016.04.041 [DOI] [PubMed] [Google Scholar]
- Carter, A.M. and Enders, A.C. (2013) The evolution of epitheliochorial placentation. Annual Review of Animal Biosciences, 1(1), 443–467. 10.1146/annurev-animal-031412-103653 [DOI] [PubMed] [Google Scholar]
- Carter, A.M. and Mess, A. (2007) Evolution of the placenta in eutherian mammals. Placenta, 28, 259–262. 10.1016/j.placenta.2006.04.010 [DOI] [PubMed] [Google Scholar]
- Crespi, B. and Semeniuk, C. (2004) Parent‐offspring conflict in the evolution of the vertebrate reproductive mode. American Naturalist, 163(5), 635–653. [DOI] [PubMed] [Google Scholar]
- Dudley, J.S. , Murphy, C.R. , Thompson, M.B. and McAllan, B.M. (2015) Desmoglein‐2 during pregnancy and its role in the evolution of viviparity in a marsupial (Sminthopsis crassicaudata; Dasyuridae). Journal of Morphology, 276, 261–272. [DOI] [PubMed] [Google Scholar]
- Dytham, C. (2011) Choosing and Using Statistics: A Biologist’s Guide, 3rd edition West Sussex, UK: Wiley Blackwell Publishing. [Google Scholar]
- Elliot, M.G. and Crespi, B.J. (2009) Phylogenetic evidence for early haemochorial placentation in Eutheria. Placenta, 30, 949–967. [DOI] [PubMed] [Google Scholar]
- Enders, A.C. and Schlafke, S. (1967) A morphological analysis of the early implantation stages in the rat. The American Journal of Anatomy, 120, 185–226. [Google Scholar]
- Enders, A.C. and Enders, R.K. (1969) The placenta of the four‐eyed opossum (Philander opossum). Anatomical Record, 165, 431–450. [DOI] [PubMed] [Google Scholar]
- Fadem, B.H. (1985) Evidence for the activation of female reproduction by males in a marsupial, the gray short‐tailed opossum (Monodelphis domestica). Biology of Reproduction, 33, 112–116. [DOI] [PubMed] [Google Scholar]
- Fadem, B.H. and Rayve, R. (1985) Characteristics of the oestrous cycle and influence of social factors in grey short‐tailed opossums (Monodelphis domestica). Journal of Reproduction and Fertility, 73, 337–342. 10.1530/jrf.0.0730337 [DOI] [PubMed] [Google Scholar]
- Ferner, K. and Mess, A. (2011) Evolution and development of fetal membranes and placentation in amniote vertebrates. Respiratory Physiology & Neurobiology, 178(1), 39–50. 10.1016/j.resp.2011.03.029 [DOI] [PubMed] [Google Scholar]
- Freyer, C. and Renfree, M.B. (2009) The mammalian yolk sac placenta. Journal of Experimental Zoology, 312B, 545–554. [DOI] [PubMed] [Google Scholar]
- Freyer, C. , Zeller, U. and Renfree, M.B. (2002) Ultrastructure of the placenta of the tammar wallaby, Macropus eugenii: comparison with the gray short‐tailed opossum, Monodelphis domestica . Journal of Anatomy, 20, 101–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer, C. , Zeller, U. and Renfree, M.B. (2003) The marsupial placenta: a phylogenetic analysis. Journal of Experimental Zoology, 299A, 59–77. [DOI] [PubMed] [Google Scholar]
- Freyer, C. , Zeller, U. and Renfree, M.B. (2007) Placental function in two distantly related marsupials. Placenta, 28, 249–257. 10.1016/j.placenta.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Griffith, O.W. , Chavan, A.R. , Protopapas, S. , Maziarz, J. , Romero, R. , and Wagner, G.P. (2017) Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proceedings of the National Academy of Sciences of the United States of America, 114(32), E6566–E6575. 10.1073/pnas.1701129114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith, O.W. , Chavan, A.R. , Pavlicev, M. , Protopapas, S. , Callahan, R. , Maziarz, J. , et al. (2019) Endometrial recognition of pregnancy occurs in the grey short‐tailed opossum (Monodelphis domestica). Proceedings of the Royal Society B, 286, 20190691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, V.L. , Schilkey, F.D. and Miller, R.D. (2016) Transcriptomic changes associated with pregnancy in a marsupial, the gray short‐tailed opossum Monodelphis domestica . PLoS ONE, 11, e0161608 10.1371/journal.pome.0161608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, V.L. , Faber, L.S. , Salehpoor, A.A. and Miller, R.D. (2017) A pronounced uterine pro‐inflammatory response at parturition is an ancient feature in marsupials. Proceedings. Biological Sciences, 284, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder, J.D. , Stonerook, M.J. and Pondy, J. (1993) Gestation and placentation in two new world opossums: Didelphis virginiana and Monodelphis domestica . Journal of Experimental Zoology, 266, 463–479. [DOI] [PubMed] [Google Scholar]
- Hayat, M.A. (1986) Basic Techniques for Transmission Electron Microscopy. Orlando, FL: Academic Press. [Google Scholar]
- Hinds, L.A. , Reader, M. , Wernberg‐Molller, S. and Saunders, N.R. (1992) Hormonal evidence for induced ovulation in Monodelphis domestica . Journal of Reproduction and Fertility, 95, 303–312. [DOI] [PubMed] [Google Scholar]
- Hosie, M.J. , Adams, S.M. , Thompson, M.B. and Murphy, C.R. (2003) Viviparous lizard, Eulamprus tympanum, shows changes in the uterine surface epithelium during early pregnancy that are similar to the plasma membrane transformation of mammals. Journal of Morphology, 258, 346–357. 10.1002/jmor.10163 [DOI] [PubMed] [Google Scholar]
- Hulstaert, C.E. , Kalicharan, D. and Hardonk, M.J. (1983) Cytochemical demonstration of phosphatases in the rat liver by a cerium‐ based method in combination with osmium tetroxide and potassium ferrocyanide postfixation. Histochemistry, 78, 71–79. 10.1007/BF00491113 [DOI] [PubMed] [Google Scholar]
- Kaneko, Y. , Lindsay, L.A. and Murphy, C.R. (2008) Focal adhesions disassemble during early pregnancy in rat uterine epithelial cells. Reproduction, Fertility and Development, 20(8), 892–899. 10.1071/RD08148 [DOI] [PubMed] [Google Scholar]
- Kazemian, A. , Hooshmandabbasi, R. , Schraner, E.M. , Boos, A. and Klisch, K. (2019) Evolutionary implications of fetal and maternal microvillous surfaces in epitheliochorial placentae. Journal of Morphology, 280, 615–622. 10.1002/jmor.20970 [DOI] [PubMed] [Google Scholar]
- Kin, K. , Maziarz, J. and Wagner, G.P. (2014) Immunohistological study of the endometrial stromal fibroblasts in the opossum, Monodelphis domestica: evidence for homology with eutherian stromal fibroblasts. Biology of Reproduction, 90(5), 1–12. [DOI] [PubMed] [Google Scholar]
- Laird, M.K. (2017) Uterine remodeling in marsupial pregnancy: implications for mammalian placental evolution. PhD thesis: University of Sydney. [Google Scholar]
- Laird, M.K. , Thompson, M.B. , Murphy, C.R. and McAllan, B.M. (2014) Uterine epithelial cell changes during pregnancy in a marsupial (Sminthopsis crassicaudata; Dasyuridae). Journal of Morphology, 275, 1081–1092. [DOI] [PubMed] [Google Scholar]
- Laird, M.K. , Turancova, M. , McAllan, B.M. , Murphy, C.R. and Thompson, M.B. (2017a) Uterine focal adhesion dynamics during pregnancy in a marsupial (Sminthopsis crassicaudata; Dasyuridae). Anatomical Record, 300, 1150–1159. [DOI] [PubMed] [Google Scholar]
- Laird, M.K. , McShea, H. , McAllan, B.M. , Murphy, C.R. and Thompson, M.B. (2017b) Uterine remodelling during pregnancy and pseudopregnancy in the brushtail possum (Trichosurus vulpecula; Phalangeridae). Journal of Anatomy, 231, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, M.K. , McShea, H. , Murphy, C.R. , McAllan, B.M. , Shaw, G. , Renfree, M.B. , et al. (2018) Non‐invasive placentation in the marsupials Macropus eugenii (Macropodidae) and Trichosurus vulpecula (Phalangeridae) involves redistribution of uterine Desmoglein‐2. Molecular Reproduction and Development, 85, 72–82. [DOI] [PubMed] [Google Scholar]
- Lillegraven, J.A. (1975) Biological considerations of the marsupial‐placental dichotomy. Evolution, 29, 707–722. [DOI] [PubMed] [Google Scholar]
- Ljungkvist, I. (1972) Attachment reaction of rat uterine luminal epithelium IV. The cellular changes in the attachment reaction and its hormonal regulation. Fertility and Sterility, 23, 847–865. [DOI] [PubMed] [Google Scholar]
- Mate, K.E. , Robinson, E.S. , Vandeberg, J.L. and Pedersen, R.A. (1994) Timetable of in vivo embryonic development in the grey short‐tailed opossum (Monodelphis domestica). Molecular Reproduction and Development, 39(4), 365–374. 10.1002/mrd.1080390404 [DOI] [PubMed] [Google Scholar]
- Mess, A. (2014) Placental evolution within the supraordinal clades of Eutheria with the perspective of alternative animal models for human placentation. Advances in Biology, 2014, 1–21. 10.1155/2014/639274 [DOI] [Google Scholar]
- Mess, A.M. and Ferner, K.J. (2010) Evolution and development of gas exchange structures in Mammalia: the placenta and the lung. Respiratory Physiology & Neurobiology, 173, S74–S82. [DOI] [PubMed] [Google Scholar]
- Mossman, H.W. (1987) Vertebrate Fetal Membranes. New Brunswick, NJ, USA: Rutgers University Press. [Google Scholar]
- Murphy, C.R. (1998) Commonality within diversity: the plasma membrane transformation of uterine epithelial cells during early placentation. Journal of Assisted Reproduction and Genetics, 15(4), 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, C.R. (2000) Understanding the apical surface markers of uterine receptivity: pinopods or uterodomes? Human Reproduction, 15, 2451–2454. [DOI] [PubMed] [Google Scholar]
- Murphy, C.R. (2004) Uterine receptivity and the plasma membrane transformation. Cell Research, 14(4), 259–267. [DOI] [PubMed] [Google Scholar]
- Murphy, C.R. , Hosie, M.J. and Thompson, M.B. (2000) The plasma membrane transformation facilitates pregnancy in both reptiles and mammals. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 127(4), 433–439. 10.1016/S1095-6433(00)00274-9. [DOI] [PubMed] [Google Scholar]
- Nikzad, H. , Kabir‐Salmani, M. , Shiokawa, S. , Akimoto, Y. and Iwashita, M. (2010) Co‐expression of galectin‐3 and ανβ3 integrin at pinopods of human endometrium. Iranian Journal of Reproductive Medicine, 8, 145–152. [Google Scholar]
- Nilsson, M.A. , Churakov, G. , Sommer, M. , Tran, N.V. , Zemann, A. , Brosius, J. , et al. (2010) Tracking marsupial evolution using archaic genomic retroposon insertions. PLoS Biology, 8, e1000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson, O. (1958) Ultrastructure of mouse uterine surface epithelium under different estrogenic influences. 1. Spayed animals and oestrous animals. Journal of Ultrastructure Research, 1, 375–396. [DOI] [PubMed] [Google Scholar]
- Pilton, P.E. and Sharman, G.B. (1962) Reproduction in the marsupial Trichosurus vulpecula . Journal of Endocrinology, 25(1), 119‐NP 10.1677/joe.0.0250119 [DOI] [PubMed] [Google Scholar]
- Potts, D.M. and Racey, P.A. (1971) A light and electron microscopic study of early development in the bat Pipistrellus pipistrellus . Micron, 2, 322–348. [Google Scholar]
- Preston, A.M. , Lindsay, L.A. and Murphy, C.R. (2004) Progesterone treatment and the progress of early pregnancy reduce desmoglein 1&2 staining along the lateral plasma membrane in rat uterine epithelial cells. Acta Histochemica, 106, 345–351. [DOI] [PubMed] [Google Scholar]
- Renfree, M.B. (2000) Maternal recognition of pregnancy in marsupials. Reviews of Reproduction, 5(1), 6–11. 10.1530/ror.0.0050006 [DOI] [PubMed] [Google Scholar]
- Renfree, M.B. , Suzuki, S. and Kaneko‐Ishino, T. (2013) The origin and evolution of genomic imprinting and viviparity in mammals. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1609), 1–11. 10.1098/rstb.2012.0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, C.T. and Breed, W.G. (1994) Embryonic‐maternal cell interactions at implantation in the fat‐tailed dunnart, a dasyurid marsupial. The Anatomical Record, 240(1), 59–76. 10.1002/ar.1092400107 [DOI] [PubMed] [Google Scholar]
- Rose, R.W. , Horak, J.A.A. , Shetewi, A.D. and Jones, S.M. (1999) Pregnancy in a marsupial, the Tasmanian pademelon (Thylogale billardierii). Reproduction, Fertility, and Development, 11, 175–182. [DOI] [PubMed] [Google Scholar]
- Schlafke, S. and Enders, A.C. (1975) Cellular basis of interaction between trophoblast and uterus at implantation. Biology of Reproduction, 12, 41–65. [DOI] [PubMed] [Google Scholar]
- Stavreus‐Evers, A. (2005) Characteristics and possible function of pinopods seen on the surface of the receptive human endometrium. Middle East Fertility Society Journal, 10, 22–28. [Google Scholar]
- Stroband, H.W.J. , Taverne, N. , Langenfeld, K. and Barends, P.M.G. (1986) The ultrastructure of the uterine epithelium of the pig during the estrous cycle and early pregnancy. Cell and Tissue Research, 246, 81–89. [DOI] [PubMed] [Google Scholar]
- Thompson, M.B. , Stewart, J.R. , Speake, B.K. , Hosie, M.J. and Murphy, C.R. (2002) Evolution of viviparity: What can Australian lizards tell us? Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 131(4), 631–643. 10.1016/S1096-4959(02)00013-1 [DOI] [PubMed] [Google Scholar]
- Tyndale‐Biscoe, C.H. and Renfree, M.B. (1987) Reproductive Physiology of Marsupials. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Vogel, P. (2005) The current molecular phylogeny of eutherian mammals challenges previous interpretations of placental evolution. Placenta, 26, 591–596. [DOI] [PubMed] [Google Scholar]
- Wagner, G.P. , Kin, K. , Muglia, L. and Pavličev, M. (2014) Evolution of mammalian pregnancy and the origin of the decidual stromal cell. International Journal of Developmental Biology, 58, 117–126. [DOI] [PubMed] [Google Scholar]
- Ward, S.J. and Renfree, M.B. (1988) Reproduction in females of the feathertail glider Acrobates pygmaeus (Marsupialia). Journal of Zoology, 216, 225–239. [Google Scholar]
- Wick, R. and Kress, A. (2002) Ultrastructural changes in the uterine luminal and glandular epithelium during the oestrous cycle of the marsupial Monodelphis domestica (grey short‐tailed opossum). Cells Tissues Organs, 170, 111–131. [DOI] [PubMed] [Google Scholar]
- Wildman, D.E. (2016) IFPA Award in placentology lecture: phylogenomic origins and evolution of the mammalian placenta. Placenta, 48, S31–S39. 10.1016/j.placenta.2016.04.004 [DOI] [PubMed] [Google Scholar]
- Winterhager, E. and Denker, H.‐W. (1990) Changes in lipid organization of uterine epithelial cell membranes at implantation in the rabbit. Trophoblast Invasion and Endometrial Receptivity, 4, 323–338. [Google Scholar]
- Zeller, U. and Freyer, C. (2001) Early ontogeny and placentation of the grey short‐ tailed opossum, Monodelphis domestica (Didelphidae: Marsupialia): contribution to the reconstruction of the marsupial morphotype. Journal of Zoological Systematics and Evolutionary Research, 39, 137–158. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


