Abstract
It is widely accepted that ornithodirans (bird lineage) and some pseudosuchians (crocodilian lineage) achieved fully erect limb posture in different ways. Ornithodirans have buttress‐erected hindlimbs, while some advanced pseudosuchians have pillar‐erected hindlimbs. Analysis of the musculoskeletal apparatus of the early dinosauriform Silesaurus opolensis challenges this view. This ornithodiran had pillar‐erected hindlimbs like some pseudosuchians. This condition could be autapomorphic or represents a transitional state between adductor‐controlled limb posture of early dinosauromorphs and the buttress‐erected hindlimbs of dinosaurs. This sequence of changes is supported by Triassic tracks left by animals of the dinosaurian lineage. It was associated with the strong development of knee flexors and extensors. Furthermore, the forelimbs of Silesaurus were fully erect, analogously to those of early sauropods. Members of both lineages reduced the muscles related to the protraction, retraction and bending of the limb. They used forelimbs more as a body support and less for propulsion. A similar scapula and humerus construction can be found in the Lagerpetidae and Lewisuchus, suggesting that long, slender, fully erected forelimbs are primitive for all Dinosauromorpha, not just Silesauridae. Early dinosaurs redeveloped several muscle attachments on the forelimb, probably in relation to bipedality.
Keywords: archosauria, dinosauriforms, forelimb, hindlimb, Late Triassic, myology, silesaurus
Short abstract
Silesaurus is the first known dinosauromorph that had pillar‐erected hindlimbs. The forelimbs were also erected but they were mainly adapted for body support like in sauropods. Such locomotor apparatus could be ancestral for dinosaurs.
1. INTRODUCTION
Dinosauromorpha have received considerable attention among researchers studying locomotor transitions in animal evolution (Fechner, 2009; Langer et al. 2013; Niedźwiedzki et al. 2013; Bittencourt et al. 2015; Tsai et al. 2018). This is because early dinosauromorphs achieved bipedalism, a rare mode of locomotion in other vertebrates. Dinosauromorpha are defined as archosaurs, more closely related to birds than to pterosaurs and crocodiles (Langer et al. 2013). The group encompasses small gracile Lagerpetidae (Müller et al. 2018), proto‐dinosaurs, such as Marasuchus (Sereno and Arcucci, 1994) and Lewisuchus (Bittencourt et al. 2015), beaked Silesauridae (Nesbitt et al. 2010), and dinosaurs. Lagerpetidae are the first known branch of dinosauromorphs. They remained primitive in the morphology of the pelvis and femur, but their foot was highly asymmetric. They are considered quadrupedal, with some ability to run bipedally (Fechner, 2009). Marasuchus was interpreted as a bipedal animal because of its short forelimbs (Sereno and Arcucci, 1994), but the forelimbs were later attributed to the sphenosuchian Hesperosuchus (Remes, 2008). As a consequence, its’ bipedalism remains questionable.
Silesauridae are considered as more advanced than Marasuchus, forming a sister group to dinosaurs or being early ornithischians (Dzik, 2003; Dzik and Sulej, 2007; Ferigolo & Langer, 2007; Fostowicz‐Frelik & Sulej, 2010; Nesbitt et al. 2010; Langer & Ferigolo, 2013). The best known silesaurid is Silesaurus opolensis from the late Carnian of Poland (Dzik, 2003; Figure 1). The most unusual aspect of its anatomy, apart from its beaked dentary, is the elongation and gracile appearance of its forelimbs. This morphology may be interpreted either as a stage in the transition from the plesiomorphic quadrupedality of its archosaurian ancestor or, conversely, as incipient secondary quadrupedality at the beginning of the dinosauromorph radiation. The relatively long trunk (Piechowski and Dzik, 2010) of Silesaurus (0.79 hindlimb/trunk length ratio), closed acetabulum, and untwisted femoral head (Dzik, 2003) can be used to argue for the first interpretation. The second interpretation is supported by the relatively narrow pelvis and functionally tridactyl foot. A detailed restoration of locomotory muscles will improve our understanding of the problem. The fossil bones of Silesaurus from Krasiejów (the age and sedimentological interpretation of the strata were reviewed by Dzik and Sulej, 2007 and recently by Szulc et al. 2015 and Dzik and Sulej, 2016) are preserved well enough to enable such research.
Figure 1.
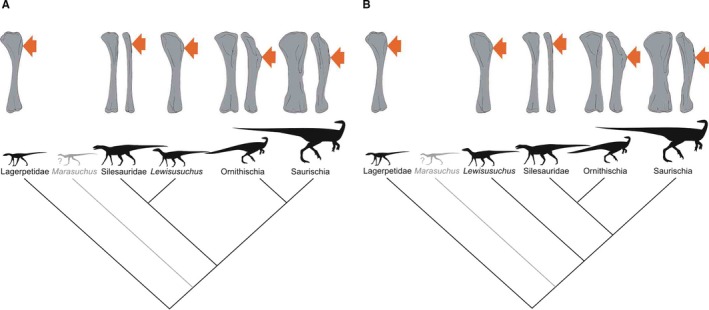
Phylogenetic framework of Dinosauromorpha used in this study with illustrations of humeri. (A) Phylogeny based on Nesbitt et al. (2017). (B) Phylogeny based on Cabreira et al. (2016)
Previous research by our team has resulted in reconstruction of the skeleton of S. opolensis (Dzik, 2003; Piechowski and Dzik, 2010), tracing its partial ontogeny, and recognition of probable sexual dimorphism (Piechowski et al. 2014). In the present paper, we discuss osteological features of the fore‐ and hindlimbs of Silesaurus that permit myological reconstructions. Biomechanical methods have already proved helpful in understanding dinosaurian locomotion (Gatesy, 1995; Carrano, 1998; Hutchinson and Gatesy, 2000; Hutchinson, 2002; Hutchinson and Garcia, 2002; Hutchinson et al. 2005; Sellers and Manning, 2007; Hutchinson et al. 2008; Otero et al. 2010; Maidment and Barrett, 2011; Otero, 2018), and their application to an early member of the dinosaur lineage is potentially valuable.
2. MATERIALS AND METHODS
The fossil material used in the present paper includes five partially articulated skeletons, ZPAL Ab/361 (holotype), ZPAL Ab/362, 363, 364 and 1930, as well as numerous isolated or semi‐articulated bones of the fore‐ and hindlimbs. All bones come from the Krasiejów locality (Dzik, 2003), and almost all come from one accumulation (upper bone bed). The exception is ZPAL Ab/1930, which was found in slightly older deposits (lower bone bed). Studies on skeletal variability found no evidence for the presence of more than one silesaurid taxon (Piechowski et al. 2014; Piechowski et al. 2019). The available material is generally well preserved, and shows clear muscle attachment features. Specimens were prepared with mechanical tools, cleaned with formic or acetic acids, and protected with cyanoacrylate adhesives.
Information about homology and myological arrangement in select extant phylogenetically relevant taxa was derived largely from the literature (see Section 3). For comparative purposes, bones and muscles of Caiman niger, Crocodylus niloticus, Alligator mississippiensis, Sphenodon punctatus, Struthio camelus, Rhea americana, Ciconia nigra, Anser anser, Gallus gallus, Tribolonotus novaeguineae and Neophron percnopteus from the collection of the Institute of Zoology, University of Warsaw were examined.
We adopted the Extant Phylogenetic Bracket method (Witmer, 1995) for the soft tissue inference. Witmer recognized three levels of phylogenetic inferences based on absence, presence or soft tissue in closely related extant taxa. Obtained levels of inference are provided in Tables 3, 4, 5, 6.
Table 3.
Synopsis of the pectoral and brachial musculature in Silesaurus opolensis, listing their names, origins, insertions and actions
| Muscle name | Origin | Insertion | Proposed function | Level of inference |
|---|---|---|---|---|
| M. serratus superficialis | Lateral surfaces probably of the 9th to 13th ribs | Posterior part of the ventral edge of the scapular blade | Retracts and depresses the scapula | I |
| M. serratus profundus | Lateral surfaces probably of the 10th to 12th ribs | Distal part of the ventral aspect of the scapular blade | Protracts the scapula | II |
| M. costocoracoideus | Anterior edge probably of the anteriormost dorsal ribs | Anteroventral portion of the lateral surface of the coracoid | Rotates, adducts and protracts the forelimb | III |
| M. rhomboideus | Neural spines probably of the anteriormost dorsal vertebrae | Distalmost end of the medial aspect of the scapular blade | Protracts the scapula | I |
| M. levator scapulae | Anterior cervical ribs | Dorsal edge of the scapular blade | Rotates the scapula, as well as lateral flexion of the neck | I |
| M. trapezius | Cervical and thoracodorsal fascia | Dorsal edge of the scapular blade | Rotates the scapular blade, likely assisting in protraction of the forelimb | I |
| M. latissimus dorsi | Neural spines or thoracodorsal fascia probably of the last cervical to the sixth or seventh dorsal vertebrae | Posterolateral side of the proximal humerus | Retracts the humerus | I |
| M. teres major | Posterior part of the lateral surface of the scapular blade? | Proximodorsal surface of the humerus? | Retracts the humerus | II |
| M. pectoralis | Gastral apparatus | Posterolateral surface of the deltopectoral crest of the humerus | Adducts and protracts the humerus | II |
| M. subscapularis | Medial side of the scapular blade | Medial tuberosity of the humerus | Retracts and rotates the humerus | II |
| M. subcoracoideus | Medial side of the coracoid | Medial tuberosity of the humerus | Adducts and laterally rotates the humerus | I |
| M. supracoracoideus | Subacromial depression of the scapula, and adjacent lateral surface of the coracoid | Lateral surface of the deltopectoral crest of the humerus | Protracts and abducts the humerus | II |
| M. supracoracoideus accessorius | Subacromial depression of the scapula | Proximal part of the deltopectoral crest of the humerus | Protracts and abducts the humerus | II |
| M. coracobrachialis brevis | Anteroventral portion of the lateral surface of the coracoid | Broad, subtriangular depression on the anterior surface of the humerus | Protracts the humerus | I |
| M. coracobrachialis longus | We refrained from reconstruction | II | ||
| M. scapulohumeralis caudalis | Medial side of the scapula, next to the glenoid and the ridge on the ventral margin of the scapular blade | Medial tuberosity of the humerus | Retracts the humerus | I |
| M. scapulohumeralis anterior | We refrained from reconstruction | I | ||
| M. deltoideus clavicularis | Acromion process of the scapula | Lateral surface of the deltopectoral crest of the humerus | Abducts and slightly protracts the humerus | II |
| M. deltoideus scapularis | Lateral blade of the scapula | Posterolateral surface of the proximal humerus | Abducts and retracts the humerus | II |
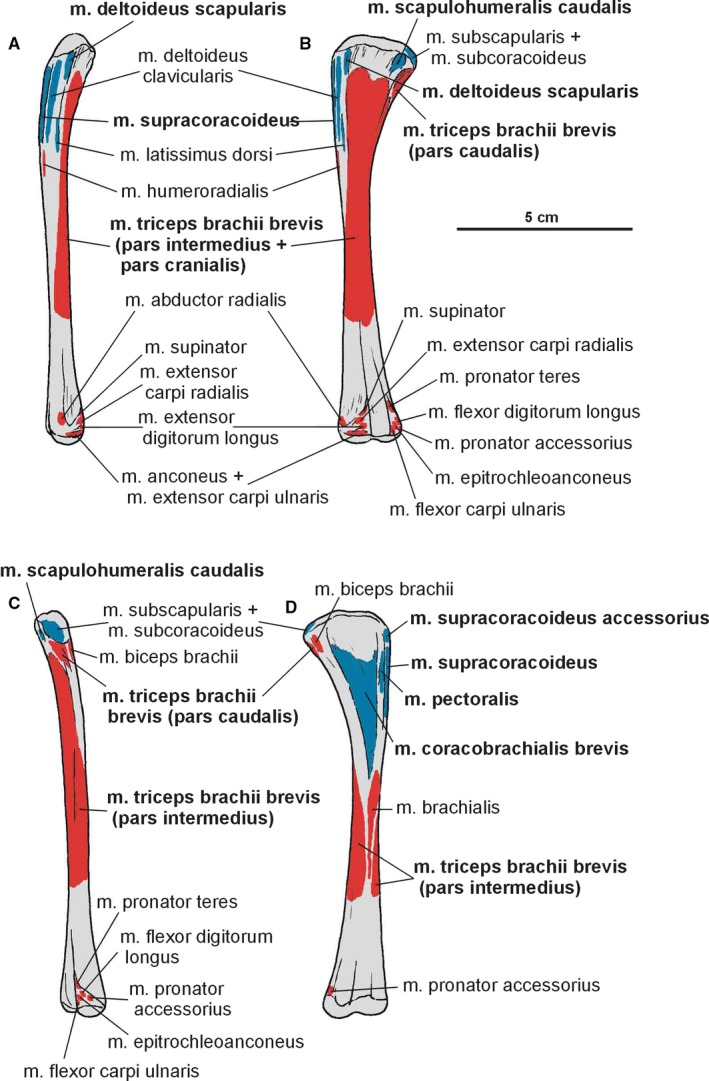
| M. triceps brachii longus and brevis | Lateroventral surface of the scapula just posterior to the glenoid (triceps branchii longus, pars lateralis); medial surface of coracoid just anterior to the glenoid and tentatively in the middle of the scapular blade ventrally (triceps branchii longus, pars caudalis); oval rugose surface just below the medial tuber of the humerus (triceps brachii brevis, pars caudalis); most of the posterior humeral shaft (triceps brachii brevis, pars intermedius) | Olecranon process of the ulna | Extends the antebrachium, as well as contributing to the extension of the humerus | I |
| M. biceps brachii | Anterior edge of the coracoid together with the biceps tubercle; anteromedial aspect of the proximal humerus? | Anterior sides of the proximal ulna and radius | Flexes the antebrachium | I |
| M. humeroradialis | Lateral side of the deltopectoral crest of the humerus | Anterolateral side of the proximal radius | Flexes the antebrachium | II |
| M. brachialis | Lateral humeral midshaft, distal to the deltopectoral crest | Anterior sides of the proximal ulna and radius | Flexes the forearm | I |
Muscle attachments in bold are those that have visible osteological correlates.
Table 4.
Summary table of the antebrachial musculature in Silesaurus opolensis, listing their names, origins, insertions and actions
| Muscle name | Origin | Insertion | Proposed function | Level of inference |
|---|---|---|---|---|
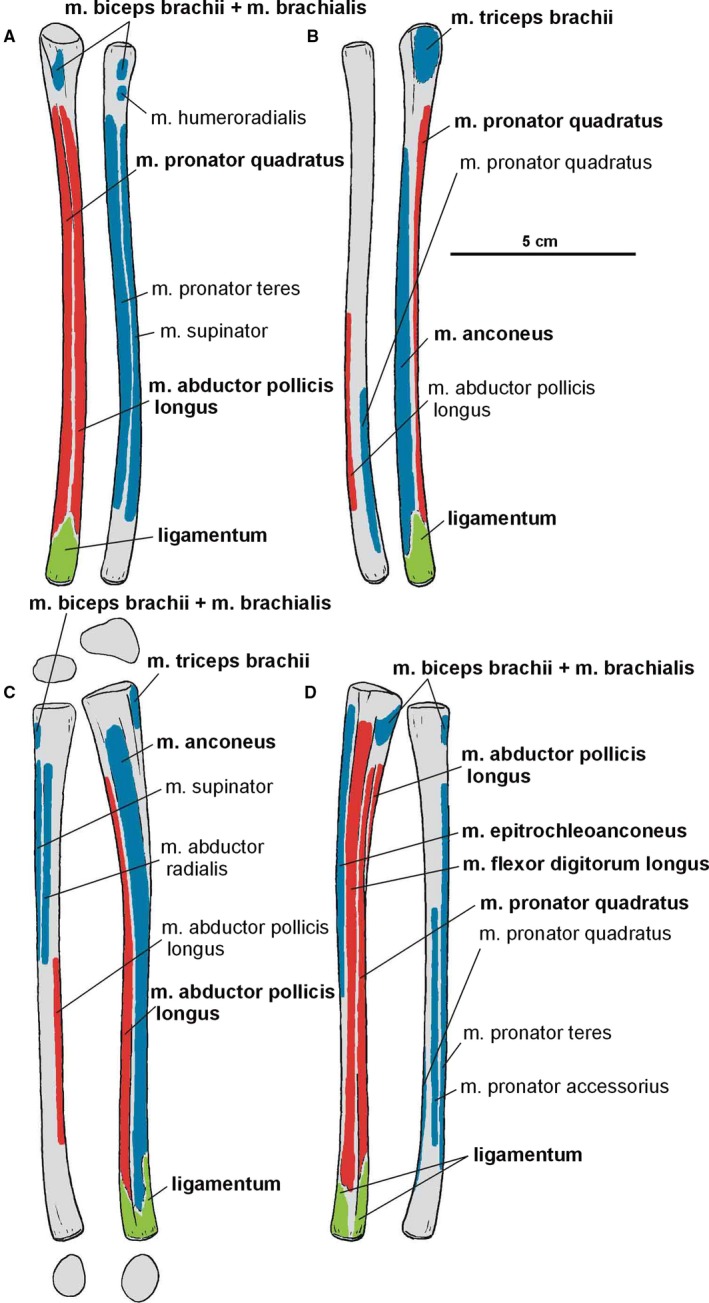
| M. anconeus | Ectepicondyle of the humerus | Lateral surface of the ulna | Flexes the forearm | I |
| M. extensor carpi ulnaris | Ectepicondyle of the humerus | Manus | Extends and abducts the wrist, along with extension of the forearm | III |
| M. supinator | Ectepicondyle of the humerus | Anterolateral surface of the radius | Flexes and supinates the forearm | I |
| M. extensor carpi radialis | Ectepicondyle of the humerus | Manus | Extends and adducts the wrist, as well as contributing to flexion of the forearm | I |
| M. abductor radialis | Ectepicondyle of the humerus | Proximal half of the lateral surface of the radius | Abducts and slightly flexes the forearm | II |
| M. abductor pollicis longus | Facing surfaces of the radius and ulna | Manus | Extends and abducts the wrist, as well as abduction of digit I | I |
| M. extensor digitorum longus | Ectepicondyle of the humerus | Manus | Extends the wrist | I |
| M. pronator teres | Entepicondyle of the humerus | Anteromedial shaft of the radius | Flexes the forearm and pronates the antebrachium | I |
| M. pronator accessorius | Entepicondyle of the humerus | Medial side of the distal radius | Flexes and pronates the antebrachium | II |
| M. pronator quadratus | Medial side of the proximal ulna | Posterior surface of the distal radius | Pronates the antebrachium and manus | I |
| M. epitrochleoanconeus | Entepicondyle of the humerus | Medioventral surface of the proximal ulna | Flexes the antebrachium | II |
| M. flexor carpi ulnaris | Entepicondyle of the humerus | Manus | Flexes and adducts the wrist | I |
| M. flexor digitorum longus | Entepicondyle of the humerus (flexor digitorum longus superficialis); medioventral surface of the ulna (flexor digitorum longus profundus) | Manus | Flexes the digits and the wrist | II |
Muscle attachments in bold are those that have visible osteological correlates.
Table 5.
Summary of the pelvic and leg musculature in Silesaurus opolensis, listing their names, origins, insertions and actions
| Muscle name | Origin | Insertion | Proposed function | Level of inference |
|---|---|---|---|---|
| M. iliotibialis | Dorsal border of the iliac blade | Cnemial crest of the tibia | Flexes, extends, and abducts the hip, as well as extending the knee | I |
| M. ambiens | Pubic tubercle | Cnemial crest of the tibia | Flexes the hip and extends the knee | I |
| M. femorotibialis | Femoral shaft | Cnemial crest of the tibia | Extends the knee | I |
| M. iliofibularis | Dorsolateral surface of the postacetabular process of the ilium | Spiral ridge of the fibula | Extends and abducts the hip, as well as flexes the knee | I |
| M. iliofemoralis | Lateral surface of the ilium | Anterior trochanter and the trochanteric shelf of the femur | Abducts the hip | I |
| M. puboischiofemoralis internus (pifi) | Anterior aspect of the ilium (pifi 1); lateroventral aspect of the anterior process of the ilium (pifi 2) | Femoral shaft, anterior to the fourth trochanter (pifi 1); anterolateral aspect of the femoral neck (pifi 2) | Flexes the hip | I |
| M. puboischiotibialis | Obturator plate of the ischium | Posteromedial aspect of the proximal tibia | Abducts and extends the hip, as well as flexes the knee | II |
| M. pubotibialis | Not reconstructed | |||
| M. flexor tibialis internus | Distalmost ischium ? (flexor tibialis internus 1); distinct, rugose ridge on the proximodorsal part of the ischium, posterior to the acetabulum (flexor tibialis internus 3) | Posteromedial aspect of the proximal tibia | Adducts and extends the hip, as well as flexes the knee | II |
| M. flexor tibialis externus | Postacetabular process of the ilium | Posteromedial aspect of the proximal tibia | Extends and adducts the hip, as well as flexes the knee | I |
| M. adductors | Ventral portion of the ischial body (adductor 1); dorsal margin of the posterior ischium (adductor 2) | Femoral shaft, between the medial and lateral condyle | Adducts the hip | I |
| M. puboischiofemoralis externus (pife) | Medial surface of the distal half of the pubic shaft (pife 1); lateral surface of the distal pubic shaft (pife 2); lateral ischial shaft (pife 3) | Dorsolateral ossification of the femur | Flexes and adducts the hip | II |
| M. ischiotrochantericus | Dorsomedial surface of the distal ischium | Dorsolateral trochanter of the femur | Lateral rotation (supination), and retraction of the hip | I |
| M. caudofemoralis brevis | Brevis fossa of the ilium | Femoral shaft, just posteriorly to the fourth trochanter | Extend and adduct the hip | I |
| M. caudofemoralis longus | Bodies of a varying number of caudal vertebrae and ventral surfaces of their transverse process | Oval concavity, anteromedial to the fourth trochanter of the femur; posterior aspect of the proximal fibula (secondary tendon) | Extends and adducts the hip | I |
Muscle attachments in bold are those that have visible osteological correlates.
Table 6.
Summary of the pes musculature in Silesaurus opolensis, listing the names, origins, insertions and actions
| Muscle name | Origin | Insertion | Proposed function | Level of inference |
|---|---|---|---|---|
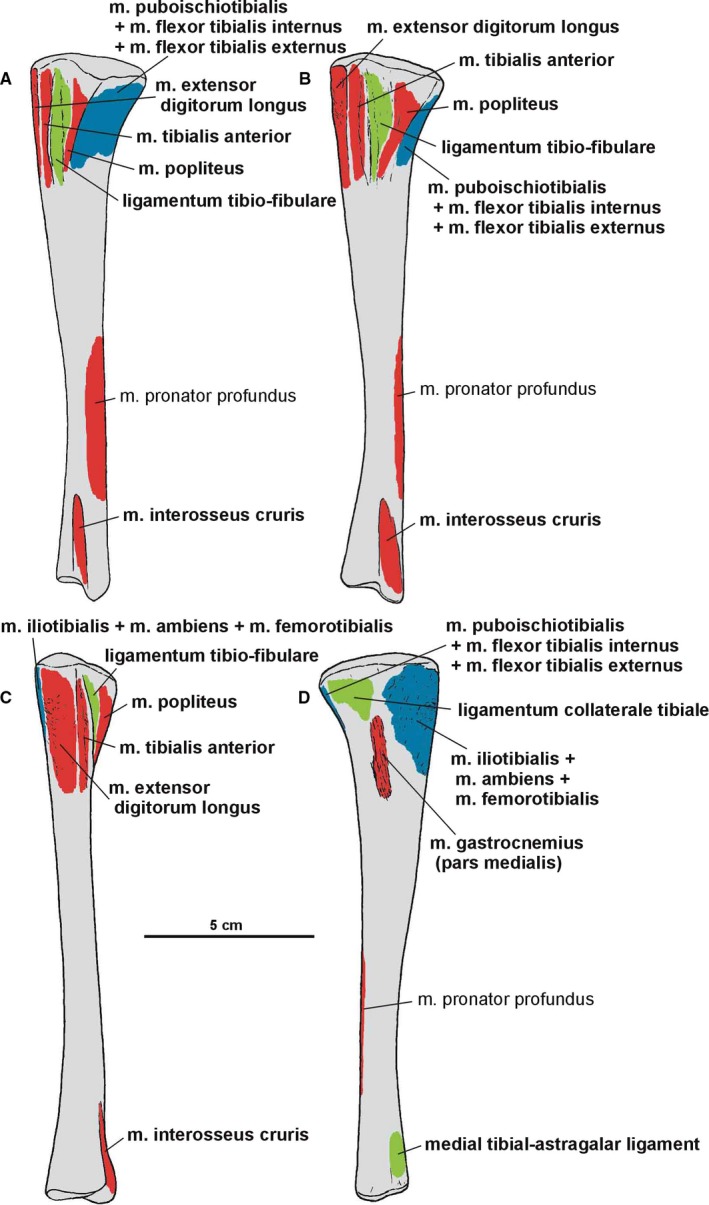
| M. gastrocnemius | Lateral femoral condyle (gastrocnemius pars lateralis); medial aspect of the proximal tibia (gastrocnemius pars medialis); posteromedial aspect of the femoral medial condyle? (gastrocnemius pars intermedius) | Ventral aspect of the metatarsals II–IV | Flexes the knee, and extends the ankle joint | I |
| M. tibialis anterior | Anterolateral side of the proximal tibia | Lateral surfaces of the proximal metatarsals II–IV | Flexes the ankle joint | I |
| M. popliteus | Posteromedial side of the proximal tibia | Facing side of the fibula | Rotates the fibula | I |
| M. interosseous cruris | Posteromedial aspect of the distal tibia | Facing side of the fibula | Flexes the ankle joint | II |
| M. pronator profundus | Posterior or posteromedial portion of the fibula and the lateral side of tibia | Ventromedial basis of the proximal metatarsal II | Flexes the ankle joint | II |
| M. fibularis longus and brevis | Lateral surface of the fibula | Ventral aspect of the calcaneum? (fibularis longus); ventral surface of the distal end of the metatarsal V (fibularis brevis) | Flexes the ankle joint | I |
| M. extensor digitorum longus and brevis | Cnemial crest of the tibia (extensor digitorum longus); dorsal aspect of the proximal tarsals? (extensor digitorum brevis) | Dorsal surface of the phalanges, and the dorsal aspects of the unguals | Flexes the ankle joint, and extend the pedal digits | I, II |
| M. flexor digitorum longus and brevis | Femoral lateral condyle, and posterolateral aspect of the proximal fibula (flexor digitorum longus); plantar aponeurosis? (flexor digitorum brevis) | Ventral surface of the unguals of digits II–IV (flexor digitorum longus); basis of the phalanges of digits II–IV? (flexor digitorum brevis) | Extends the ankle joint, and flexes the digits | I, II |
| M. extensor hallucis longus | Probably lost | |||
| M. flexor hallucis longus | Probably lost |
Muscle attachments in bold are those that have visible osteological correlates.
3. RESULTS
3.1. Bone orientation and limb proportions
We established limb proportions mostly from the holotype ZPAL Ab III/361, in which the scapulocoracoid, humerus, radius, ulna, pelvis, femur, tibia, fibula and most elements of the pes are nearly complete (Dzik, 2003). Additionally, we could observe most of these bones in two other specimens: ZPAL Ab III/364 and ZPAL Ab III/1930 (Table 1). Unfortunately, most of the manus is unknown in Silesaurus.
Table 1.
Length measurements of the most complete limb bones of Silesaurus opolensis
| Specimen | Scapulocoracoid length, mm | Humerus length, mm | Ulna length, mm | Radius length, mm | Pubis length, mm | Ischium length, mm | Femur length, mm | Tibia length, mm | Fibula length, mm | III metatarsal length, mm |
|---|---|---|---|---|---|---|---|---|---|---|
| Ab III/361 | 145.6 | 136 | 151.8 | 146.5 | 157 | 122 | 200 | 160 | 85 | |
| Ab III/362 | 137 | 160 | ||||||||
| Ab III/364 | 155.3 | 153.3 | 77 | |||||||
| Ab III/1930 | 119 | 160 | 142 |
The scapulocoracoid of Silesaurus was oriented subvertically in previous reconstructions (Dzik, 2003; Piechowski and Dzik, 2010). Our re‐examination suggests a less vertical position in lateral view (possibly about 45°) which was probably intermediate between that of birds (subhorizontal) and crocodilians (subvertical), although the scapulocoracoid apparently rotated moderately with the forelimb movement (Baier and Gatesy, 2013; Figure 2). The bone orientation is attributable to the geometry of the chest and the length of the anterior dorsal ribs. The presence of clear striations and a distinct ridge on the dorsal edge of the scapular blade provides evidence to reconstruct the musculus levator scapulae with the m. trapezius in Silesaurus (Figures 3A,C and 4A; Table 3). Because both muscles are hypothesized to have been lost due to the reorientation of the scapula into a subhorizontal position in birds (Jasinoski et al. 2006), they may be reconstructed in taxa lacking this scapular orientation (see also Burch, 2014).
Figure 2.
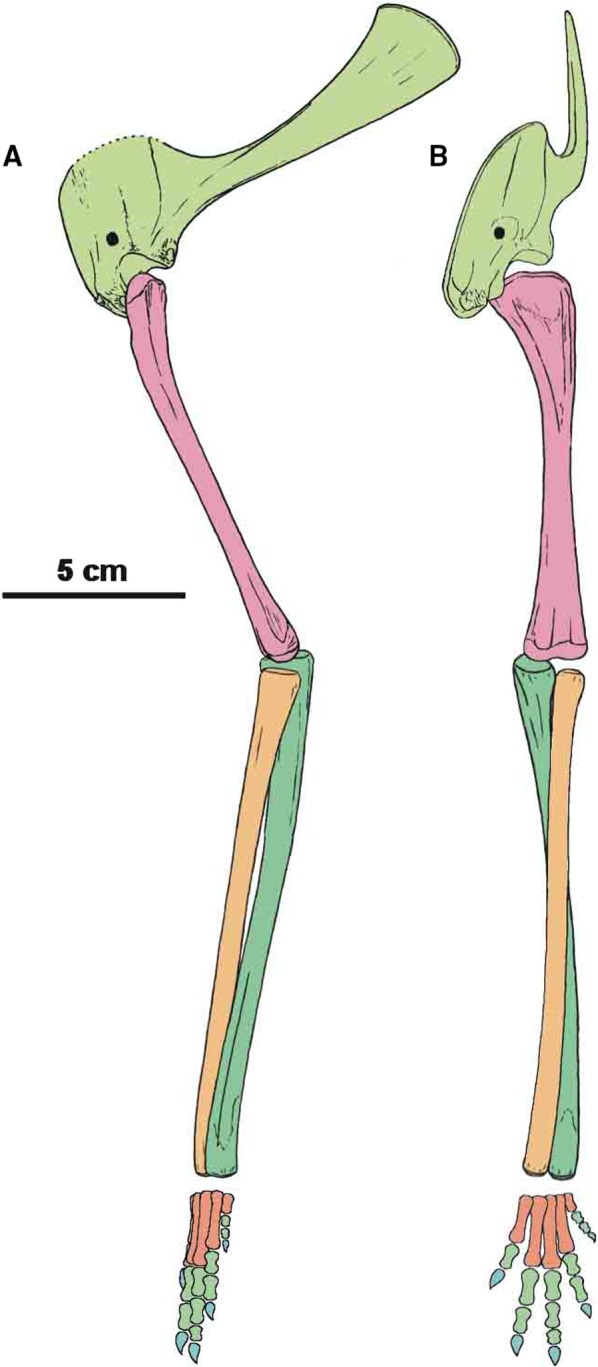
Restoration of forelimb skeleton of Silesaurus opolensis Dzik, 2003 (late Carnian, Krasiejów locality) in lateral (A) and anterior (B) views
Figure 3.
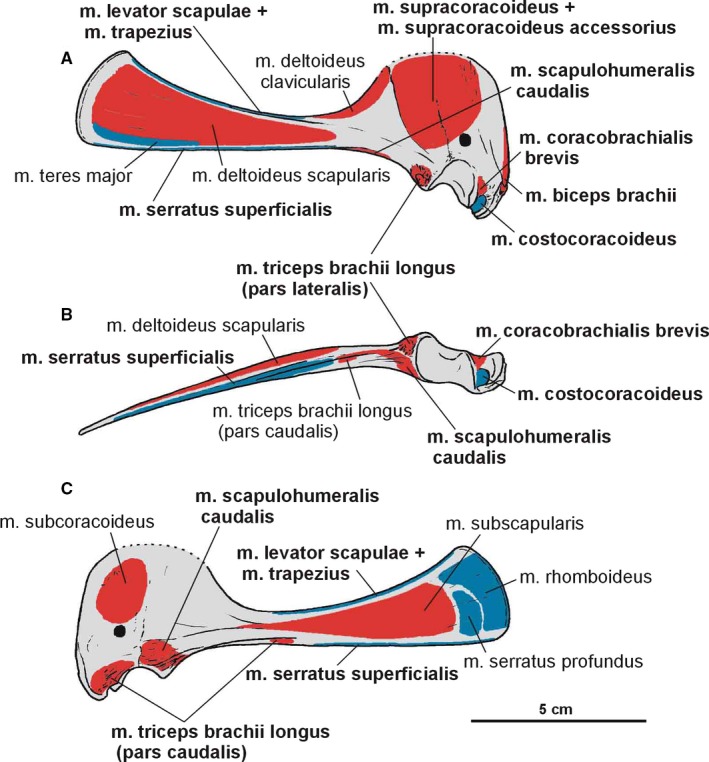
Attachments of muscles on the right scapulacoracoid of Silesaurus opolensis. Origins are in red, insertions are in blue. (A) Lateral view. (B) Ventral view. (C) Medial view. Muscle attachments in bold are those that have visible osteological correlates
Figure 4.
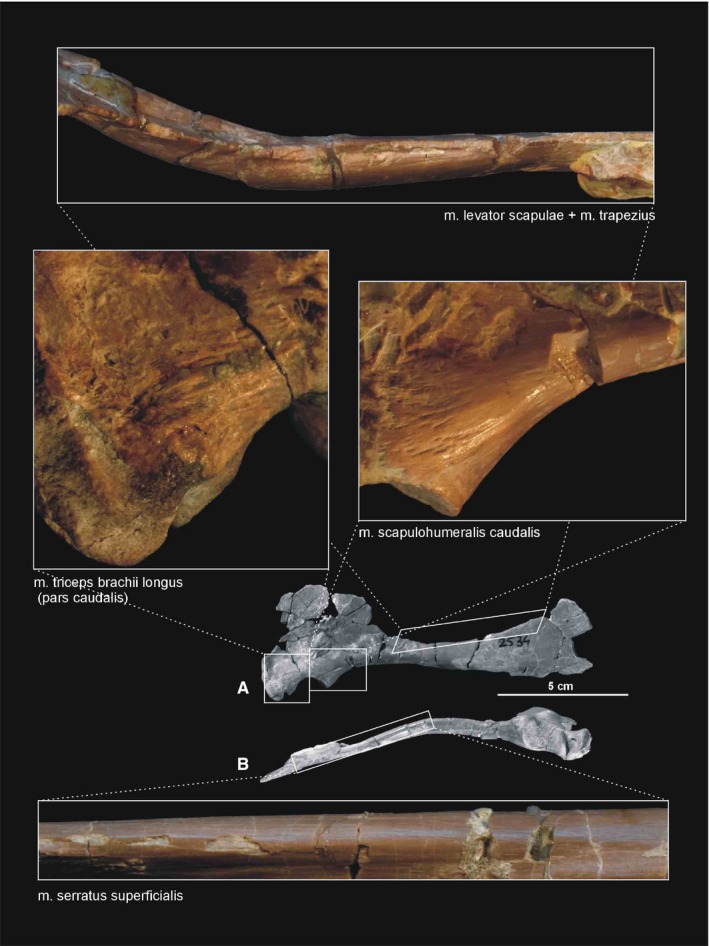
Muscle scars visible on the medial, dorsal and ventral aspects of the scapulocoracoid of Silesaurus opolensis. All photographs of ZPAL AbIII/2530 except the lower one (ZPAL AbIII/404/8). Photograph of m. levator scapulae and m. trapezius rotated 180°. (A) Right scapulocoracoid in medial view. (B) Right scapulocoracoid in ventral view
Both bones (scapula and coracoids) are firmly fused together. They form a pair lateromedially flattened elements, which follow the contour of the rib cage (Figure 2).
The scapula is approximately equal in length to the humerus (Table 1). It forms a slender blade which became thicker anteriorly. The spatulate blade is narrow, with a very thin posterodorsal projection. This is why its margin is broken in most specimens. The projection is wide and flares anterodorsaly. Its anterodorsal corner is sharp whereas the posteroventral one is rounded and obtuse (Figures 2A and 3A,C). Between them, the posterodorsal margin is convex. The area is more porous and rough, suggesting cartilaginous extension (Langer et al. 2007). The scapular blade bears subtle longitudinal striations. The thinnest part of the scapula is the anterodorsally expanded scapular prominence (‘acromial process’; Nicholls & Russell, 1985). Its concave lateral surface forms the ‘preglenoid fossa’ (Madsen and Welles, 2000; Welles, 1984) or ‘subacromial depression’ (Currie & Zhao 1993). A gentle ridge (‘preglenoid ridge’; Madsen and Welles, 2000) extends ventrally, posterodorsal to that. The most massive part of the bone locates at the basal portion (caput scapulae Baumel et al. 1993), near the glenoid. Ventrally, this thickened portion forms a subtriangular connection with the coracoid in the cross‐section. The glenoid surface can be divided into two planes. Thereby, the glenoid articular surface orients mainly ventrally, but this part is also directed somewhat laterally.
The degree of fusion between the scapula and coracoid is similar in all more complete specimens. The suture is clear in its ventral part, near the glenoid, where the coracoid seems to slightly overlap the scapula laterally. A marked tubercle is located on the basis of the scapula, just posteriorly to the glenoid. A similar structure also occurs in many dinosaurs (Walker, 1961; Ostrom, 1974; Butler, 2005; Figures 2A, 3A,B and 5).
Figure 5.
Muscle scars visible on the lateral aspect of the right scapulocoracoid of Silesaurus opolensis. All photographs of ZPAL AbIII/2530
The coracoid has subrhomboidal outline in the lateral view, with a greatly expanded and rounded anterodorsal area. The bone is thin and plate‐like anteriorly, in accordance with the development of the scapular acromion. The plate is enhanced by slight thickening which is parallel to the preglenoid ridge of the scapula. The coracoid thickens ventrally, where it contributes to the glenoid fossa. Thus, the scapulocoracoid is relatively massive only around the glenoid. The coracoidal portion of the glenoid has a tongue‐like appearance in the posteroventral view. The glenoid articular surface is subcircular in lateral view. A distinct coracoid foramen appears dorsal to the glenoid anterior to the coracoid‐scapula suture on the lateral, slightly convex surface of the bone. A complex structure is located anteriorly to the glenoid (Figures 3A,B and 5). It resembles strongly Saturnalia but the bone was described by Langer et al. (2007) in different orientation. The structure faces laterally and projects ventrally as a pointed, deflected process. From this point, it extends as a thickened embankment (‘elongated tuber’ Langer et al. 2007; ‘biceps tubercle’ (Nesbitt, 2011) dorsally on the anterior margin of the bone. It has a subtriangular outline from this view. A distinct semilunar groove separates the structure from the tongue‐like lateroventral margin of the glenoid.
A distinct attachment for the clavicle is visible medially on the anteroventral edge of the coracoid (ZPAL Ab III 2534; Figures 3C and 4A); however, no ossified clavicles are in the material. They were probably cartilaginous or are not preserved.
The orientation of the articular surfaces of the humerus and scapulacoracoid suggests that the humerus could move slightly anteriorly and much further posteriorly. The orientation of the scapulacoracoid implies subvertical orientation of the humerus when the animal was standing still. The torsion of humeral heads was much weaker than in the first reconstruction (Dzik, 2003).
The humerus (Figures 2 and 6) is a very slender and slightly curved bone. The bone is hollow but has fairly thick walls like the other long bones. Its moderately convex head (‘caput articulare humeri’ Baumel et al. 1993) occupies most of the proximal end. In proximal view, the head is kidney‐shaped, with the slightly concave border facing anteromedially. A slightly swollen medial tuberosity (tuberculum ventrale Baumel et al. 1993) forms the medial margin of the proximal humerus. The tuberosity projects dorsally, and its medial surface is convex. Originally, it was probably capped by a thick cartilage (Dzik, 2003). Two ridges run longitudinally from the proximal head of the humerus, surrounding a shallow midline concavity on the anterior surface. The ridges are subvertical and low, and they diminish before the midshaft. The deltopectoral crest is the smaller one located on the anterolateral side of bone (Figures 2, 6C,D and 7B). The shaft of the bone is almost straight in the anterior view. The shaft is nearly circular in cross‐section in mid‐length, but increases its width lateromedially towards the heads. The distal expansion is about two‐thirds of the width of the proximal one. The distal end is sinuous in profile and is divided into two rounded convexities. The lateral (radial) condyle being slightly larger than the medial (ulnar) condyle. The condyles are separated from each other by a shallow trochlea. The radial condyle is trapezoidal in distal view. It is strongly convex anteroposteriorly and gently concave medially. The radial condyle projects directly distally. The articular surface of the ulnar condyle, in contrast, is oval (rounded) in distal view and reaches further distally. Epicondylar rugosities are well developed on both the lateral and medial sides of the distal end of the humerus. The entepicondyle is present on the medial side of the bone just above the ulnar condyle. The ectepicondyle is localized above the radius condyle on the lateral side of the distal end. They are more widely separated, and expand towards the midshaft of the bone in larger specimens.
Figure 6.
Attachments of muscles on the left humerus of Silesaurus opolensis. Origins are in red, insertions are in blue. (A) Lateral view. (B) Posterior view. (C) Medial view. (D) Anterior view. Muscle attachments in bold are those that have visible osteological correlates
Figure 7.
Muscle scars visible on the anterior and medial aspects of the humerus of Silesaurus opolensis. All photographs of ZPAL AbIII/452 except the one on the right (ZPAL AbIII/1930). (A) Left humerus in lateral view. (B) Left humerus in posterior view
The forearm bones have articular surfaces directed proximally (Figure 2). This means that they were located exactly below the humerus, allowing a nearly vertical orientation. This was aided by the almost complete reduction of the olecranon process of the ulna. The ulna has a subtriangular outline, while the radius is semi‐oval in dorsal view. Both radius and ulna display a slight curvature, so they were not completely parallel to each other. This feature probably enabled some rotation of the forearm.
Despite some controversy (Hutson and Hutson, 2015, 2017), it is possible that some active pronation of the manus may have been possible in Silesaurus as it occurs in lepidosaurs and crocodiles (Landsmeer, 1983; Baier and Gatesy, 2013). Silesaurus anatomy could allow semipronation by rearranging of the whole antebrachium via long‐axis rotation at the elbow joint. This is suggested by the articular surfaces on the radius and ulna that indicate how these bones fit together (ZPAL Ab III 361 and 453; Figures 2, 8C and 9A,C). Additionally, in quadrupedal forms, muscle action is highly influenced by limb posture, more so than morphology (Otero et al. 2017).
Figure 8.
Attachments of muscles and ligaments on the left antebrachium of Silesaurus opolensis. Origins are in red, insertions are in blue, ligaments are in green. (A) Anterior view. (B) Posterior view. (C) Lateral view. (D) Medial view. Muscle and ligament attachments in bold are those that have visible osteological correlates
Figure 9.
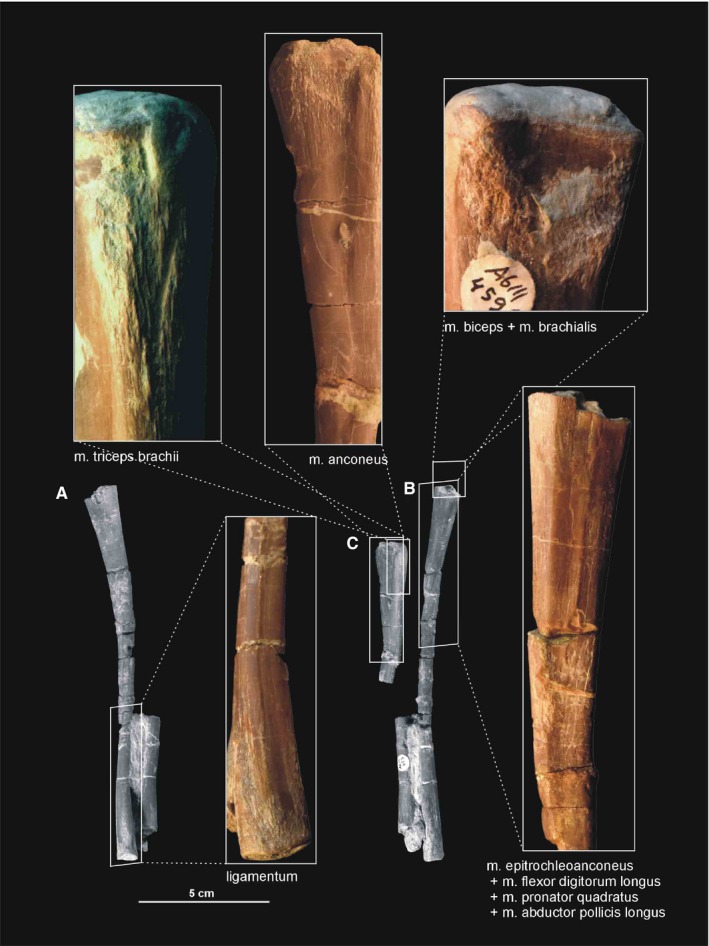
Muscle and ligament scars visible on the ulna and radius of Silesaurus opolensis. Upper left and right photographs from ZPAL AbIII/453/3, middle upper photograph and C from ZPAL AbIII/407/3, the rest are from ZPAL AbIII/453. (A) Partial left antebrachium in lateral view. (B) Partial left antebrachium in medial view. (C) Partial left ulna in lateral view
The ulna (Figures 2, 8 and 9) is unusually slender and longer than the humerus. Its subtriangular proximal surface has a shallow depression. On the lateral side of the proximal end, a slightly concave facet for articulation with the radius is present. Although the proximal end with its lateral portion is expanded, the distinct olecranon process is not visible. Anterior and posterior margins of the proximal end show indistinct ligament scars and ridges that pass along the length of the shaft.
The ulnar shaft is subtriangular proximally, but from the midshaft it becomes semicircular in cross‐section (Figure 8C). The shaft curves slightly medially in its distal part. The ends of the bone are expanded and flattened, the proximal much more so than the distal end. The convex distal lateral facet contacted a slight depression on the distal medial wall of the radius. The articular surface is semicircular in the distal view. The distinct ligament scars and a longitudinal ridge are visible just above them. This suggests that strong ligaments may have bounded the distal ends of the ulna and radius.
The radius (Figures 2 and 8) is an extremely slender bone, only slightly shorter than the ulna. Its proximal end is slightly concave and subtriangular in cross‐section, but the bone becomes semicircular distally. The radius is a relatively simple bone, with slightly expanded proximal and distal ends. The distal end is slightly depressed at its contact with the ulna.
The manus of Silesaurus has never been described before. Dzik (2003) mentioned the existence of two possible carpals but we could not detect them in the collection. However, we found four other bones that are probably from the manus of one individual (ZPAL AbIII/455). In this material (Figure 10), the only complete bone is a distal phalanx. It is a small, short element with a slightly asymmetrical proximal articular facet. The shaft is wider mediolaterally than dorsoventrally, and resembles a wedge in lateral view. The lateral and medial sides of the distal expansion bear a deep ligament pit that is bordered by rounded ridges. The sides are not of equal size. The next specimen represents the proximal half of a possible metacarpal IV. Its planar proximal end has a subtriangular, transversely elongated form. The shaft is slender and trapezoidal in cross‐section. The last two incomplete specimens may represent asymmetric distal ends of metacarpals or phalanges. Both the lateral and medial sides of the distal expansion bear a ligament pit that is bordered by rounded ridges.
Figure 10.
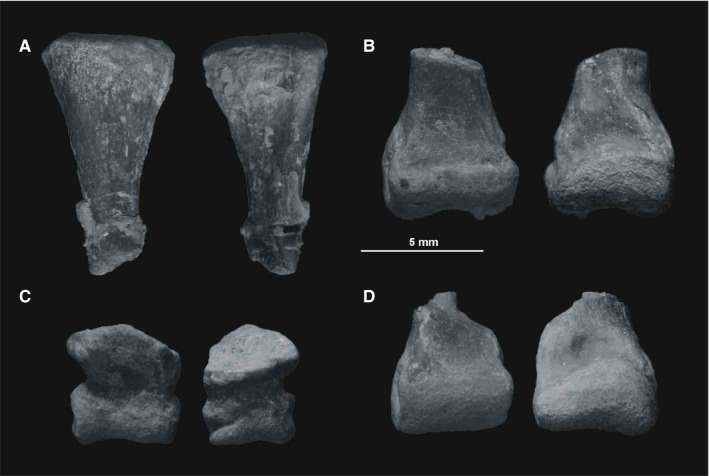
Preserved elements of the manus of Silesaurus opolensis. (A) Proximal half of a metacarpal (probably third) in medial and lateral views. (B) Distal half of a metacarpal in ventral and dorsal views. (C) Distal phalanx in ventral and dorsal views. (D) Distal half of a metacarpal or phalanx in ventral and dorsal views
The ilium of Silesaurus is as long as the four sacral vertebrae in ZPAL Ab III/362 (that is longer than that restored by Dzik, 2003; Figure 11A,B). The bone was inclined at ~30° to the vertical plane, more than in the original reconstruction (Figure 11B,D). The acetabulum faced more ventrally than laterally. The articulation surfaces for the pubis and ischium were not in the same line. The latter was parasagittal, while the former was inclined laterally (Figure 11D,E).
Figure 11.
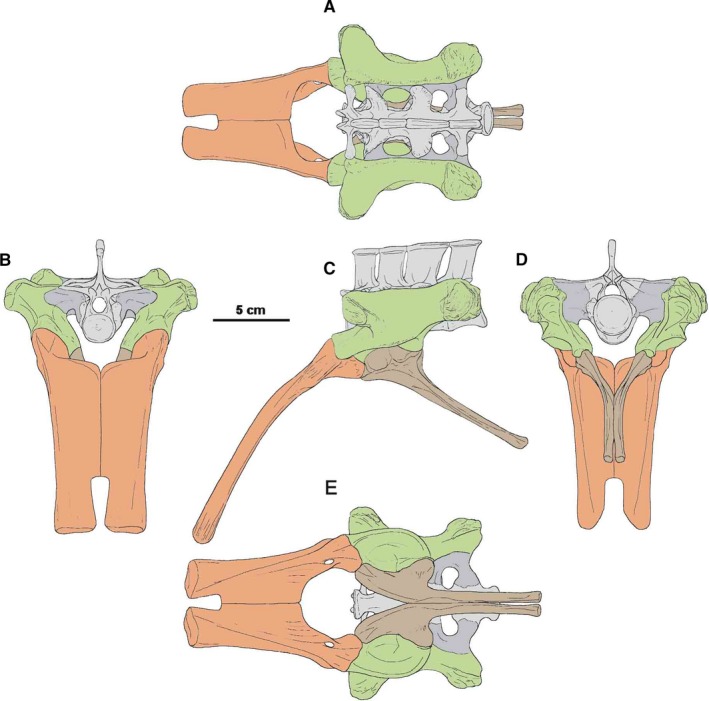
Restoration of pelvis and sacrum of Silesaurus opolensis based mostly on ZPAL AbIII/362, 925, 404/2, 404/5, 411/1. (A) Dorsal view. (B) Anterior view. (C) Lateral view. (D) Posterior view. (E) Ventral view
The best preserved ilia of Silesaurus, ZPAL Ab III/361, 362, 363 and 404/2, show an extremely thin, almost vertical (contra Dzik, 2003) iliac blade, inclined towards wing‐like apophyses of the sacral vertebrae (Dzik, 2003). Specimens ZPAL Ab III361 and 362 show how the ilium articulated the sacrum. Unfortunately, this specimen is crooked and accurate geometry of the pelvis is difficult to determine (Figures 12 and 13A,B). The blade (Figures 11A,C, 12 and 14B,C) formed a saddle‐like structure between the anterior and postacetabular processes of the ilium, and seems to have been originally in contact with apophyses of the sacrals (Dzik, 2003). The medial surface of the ilium bears facets for three sacral ribs.
Figure 12.

Muscle scars visible on the lateral aspect of the ilium of Silesaurus opolensis. All photographs of ZPAL AbIII/362 (mirrored). Pelvis in lateral view
Figure 13.
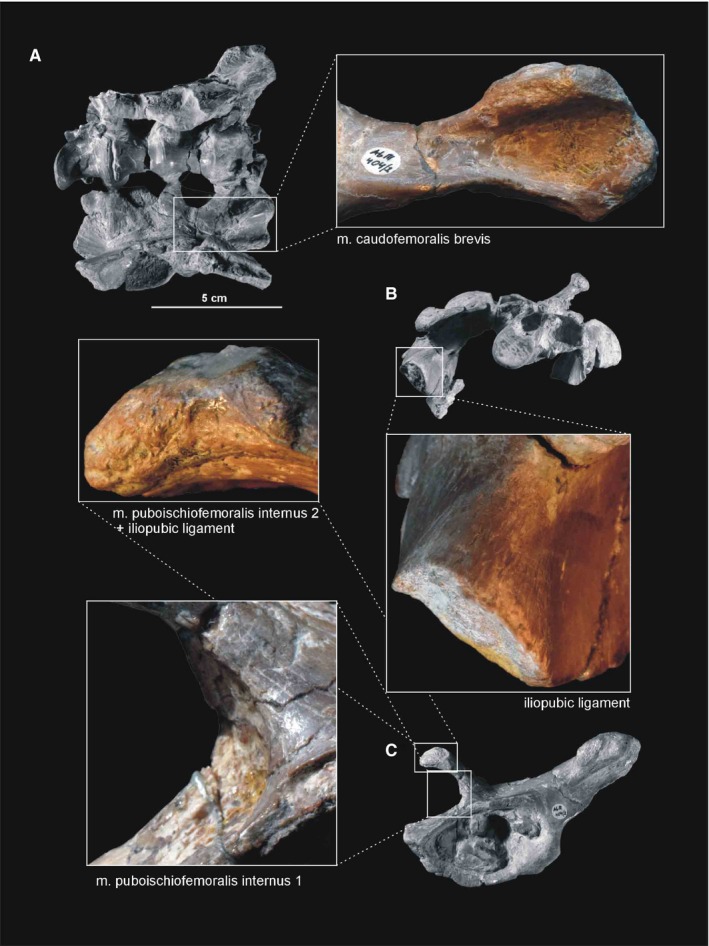
Muscle and ligament scars visible on the ventral and anterior aspects of the ilium of Silesaurus opolensis. A, B, and iliopubic ligament of ZPAL AbIII/362, the others from ZPAL AbIII/404/2. (A) Crushed pelvis in ventral view. (B) Crushed pelvis in anterior view. (C) Left ilium in ventrolateral view
Figure 14.
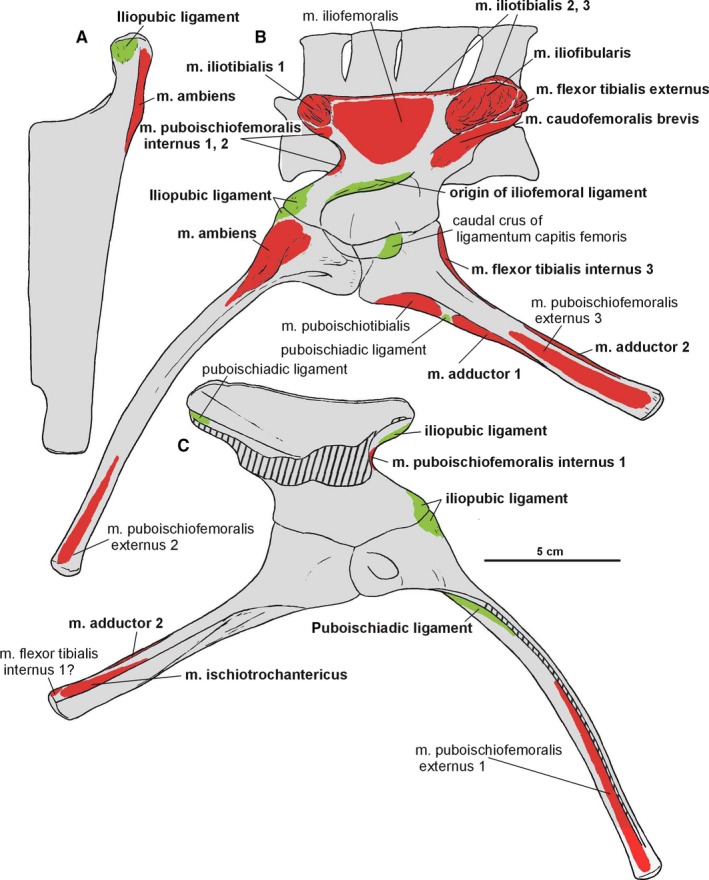
Attachments of muscles and ligaments on the left pelvis of Silesaurus opolensis based mostly on the holotype. Origins are in red, ligaments are in green. (A) Anterodorsal view of pubis. (B) Ventrolateral view of pelvis. (C) Dorsomedial view of pelvis. Muscle and ligament attachments in bold are those that have visible osteological correlates
The relatively short anterior process projects anterodorsally and curves laterally (Dzik, 2003). Its distal surface was covered originally by cartilage. This structure has a very variable outline in population from Krasiejów (Piechowski et al. 2014). A distinct tear‐shaped scar is marked on the ventrolateral side of the anterior process (Figure 13C).
The postacetabular process of the iliac blade is the strongest and most prominent part of the ilium, giving its posterior margin a semicircular curvature (Dzik, 2003). The apical surface of the process is mostly roughened analogously to the anterior one, which is better expressed in adult specimens. A prominent, posteroventrally oriented ridge (brevis shelf) separates two longitudinal areas for muscle attachments on the postacetabular process.
The acetabulum (Figure 11C,E) is large relative to the head of the femur. A strong semicircular supra‐acetabular crest overhangs the acetabulum, obscuring the dorsal part of the fossa. The most prominent section of the crest is located in the middle. The ilium contributes to the two‐thirds of the acetabular wall. The acetabulum is not opened and the iliac wall shows an extensive ventral contact with other pelvic bones. The surface of the antitrochanter rises relative to the anterior portion of the acetabulum.
The pubis occupies a broader space than the ischium (Figure 11B,D,E). The obturator plate flares medially, thus the obturator foramen was visible in anterior view. The pubic bones have an almost straight shaft in anterior view (not slightly bent as proposed by Dzik, 2003). The shafts contact each other by a thin medial blade that is broader proximally than distally because the shafts are oriented ventromedially.
The pubis is preserved in articulation with the ilium in ZPAL Ab III/361 (Dzik, 2003). Unfortunately, the extremely thin medial blade is incomplete in all isolated specimens (Dzik, 2003). The best preserved of these is ZPAL Ab III 404/5, however, the actual extent of the blade is traceable on the basis of the partially articulated specimen ZPAL Ab III/363 (Dzik, 2003). The pubis of Silesaurus is very long, only slightly shorter than the femur and considerably longer than the ilium (Figure 15B). The pubis is curved and expands anteroventrally in the lateral view. The two pubes are joined for much of their length by a strong plate‐like structure, with comma‐shaped (Dzik, 2003) cross‐section. Their transverse width decreases slightly from the proximal to distal ends in the anterior view. The proximal end of each pubis shows two robust articulations. The pubes diverge from each other at about one‐third of their length dorsally, and each bone extends upward and slightly laterally to articulation with the ilium. As a consequence, there is an anterior opening in the pelvis. The articulation with the ischium is oriented directly posteriorly. The medium obturator foramen appears close to this articulation. The pubes are separated distally for a short distance down to their tips. The distal ends of the pubis are slightly rounded, covered originally by cartilage.
Figure 15.
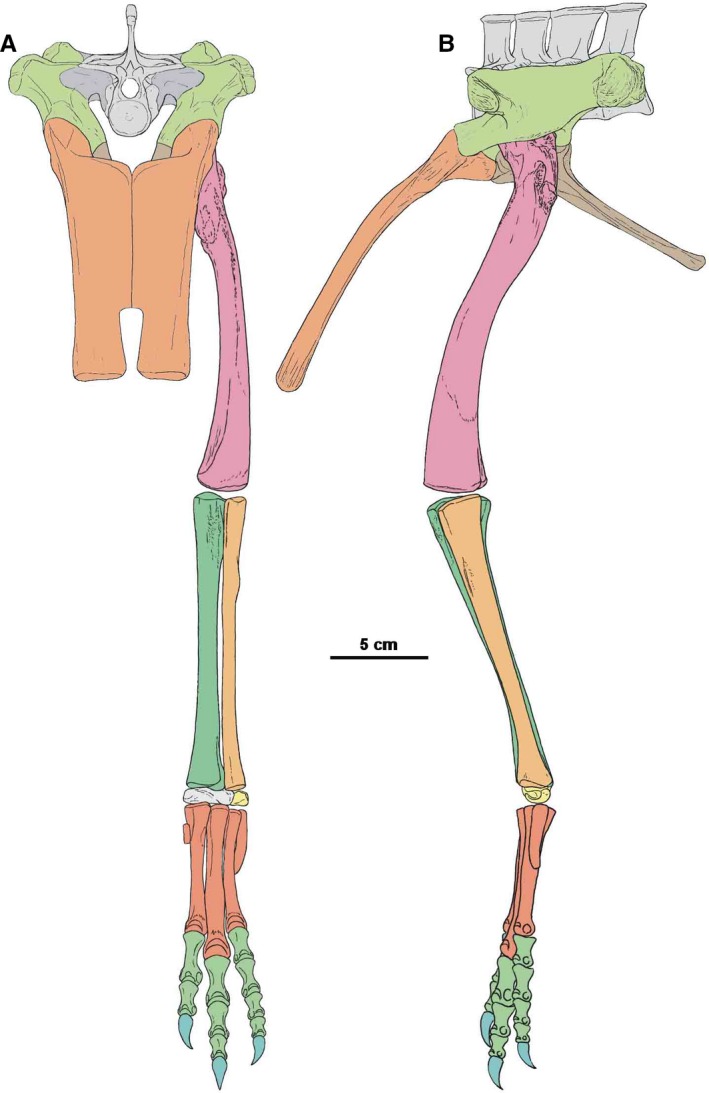
Restoration of pelvis, sacrum and hindlimb of Silesaurus opolensis in anterior view (A) and lateral view (B)
Dzik (2003) reconstructed the pelvis of Silesaurus, with ischia meeting each other only at their distalmost end. This was because the pubis is lateromedially broad, while the ischia have only a slight curvature at their proximal ends, requiring a narrow space between them to be able to meet. However, Nesbitt (2011) noticed that isolated ischia of Silesaurus bear a symphysis throughout most of the anteromedial margins. Our observations confirm this (Figure 11D,E). The ilium was inclined medially, so the proximal parts of the ischia were close to each other. The symphysis between the ischia appears just below the contact with the ilium, and continues along the shaft to the distal end.
Almost all ischia are more or less disarticulated. This bone is also elongated, being about two‐thirds of the length of the femur. Proximally, the ischium branches dorsally to meet the ischial peduncle and anteriorly to articulate with the pubis. Distally, the ischiatic shaft is laterally compressed, with slightly expanded end, originally covered by cartilage. Isolated specimens ZPAL Ab III/404/1, 404/7 and 925 show a ‘symphysis’ throughout the anteromedial margins. The ischia connected each other probably by ligament, which remained as a rough, flat, symphyseal‐like, medial surface.
The femur is the longest hindlimb bone. It is proportionally longer in larger specimens (Piechowski et al. 2014; Figure 15; Table 1). The proximal head is not rotated medially as in typical dinosaurs. However, as seen in anterior view, the bone is slightly curved medially in its proximal half. As a result, the proximal articular surface is not parallel to the distal one. Furthermore, the distal half of the bone was oriented at right angles to the ground, while the proximal half was inclined to meet the acetabulum.
The femur (Figures 15, 16, 17, 18, 19) is semitriangular in the proximal view, with the broader margin facing the acetabulum. A straight groove passes through most of the articular surface on the proximal head. In some cases, the proximal articular surface forms a gentle overhang posteriorly (Piechowski et al. 2014). The proximal head is poorly defined without recognizable neck between the femoral head and shaft.
Figure 16.
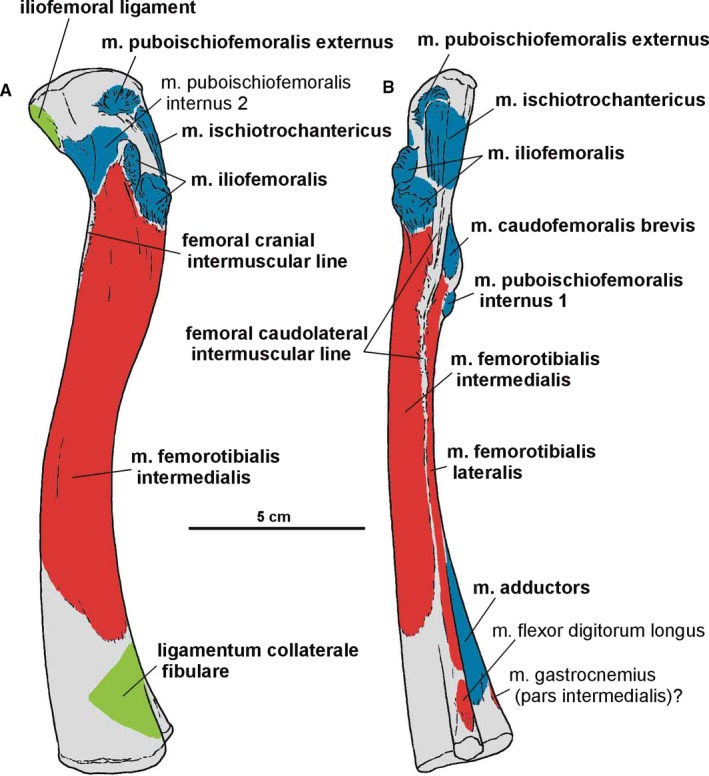
Attachments of muscles and ligaments on the left femur of Silesaurus opolensis. Origins are in red, insertions are in blue, ligaments are in green. (A) Lateral view. (B) Posterior view. Muscle, intermuscular lines and ligament attachments in bold are those that have visible osteological correlates
Figure 17.
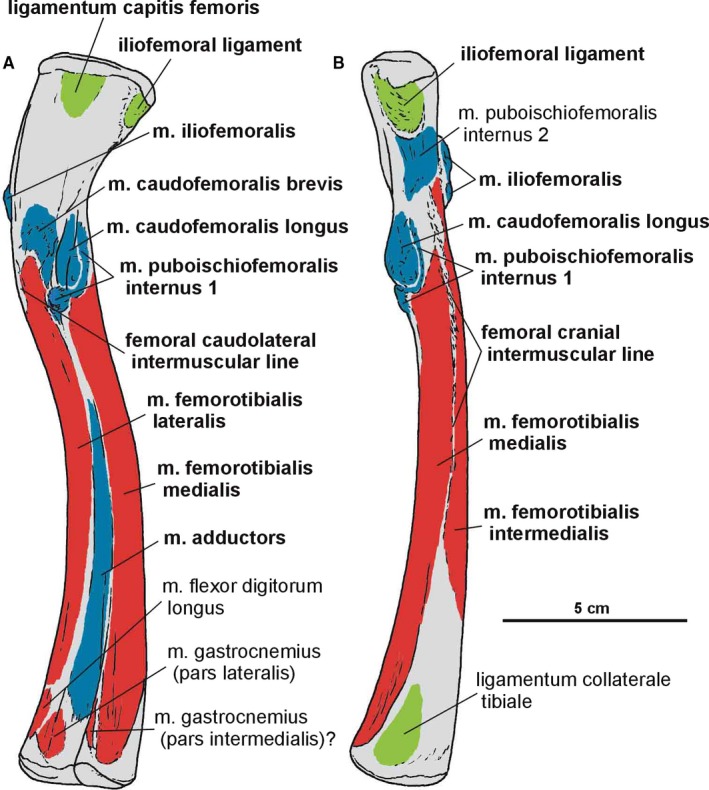
Attachments of muscles and ligaments on the left femur of Silesaurus opolensis. Origins are in red, insertions are in blue, ligaments are in green. (A) Medial view. (B) Anterior view. Muscle, intermuscular lines and ligament attachments in bold are those that have visible osteological correlates
Figure 18.
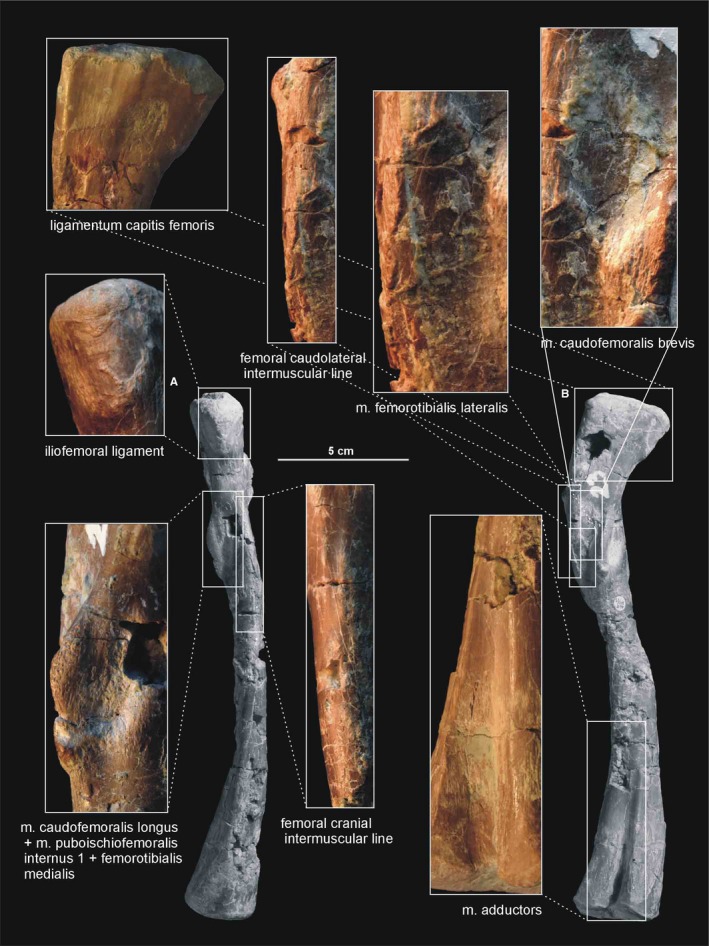
Muscle and ligament scars visible on the anterior and medial aspect of the femur of Silesaurus opolensis. Upper photograph of ligament from ZPAL AbIII/457, the surrounding two photographs are from ZPAL AbIII/361/21, the remainder from ZPAL AbIII/361/23. (A) Left femur in anterior view. (B) Left femur in medial view
Figure 19.
Muscle and ligament scars visible on the lateral aspect of the femur of Silesaurus opolensis. Muscle scars in ZPAL AbIII/361/21, ligament scar in ZPAL AbIII/405, complete bone in ZPAL AbIII/361/23. (A) Left femur in posterior view. (B) Left femur in lateral view
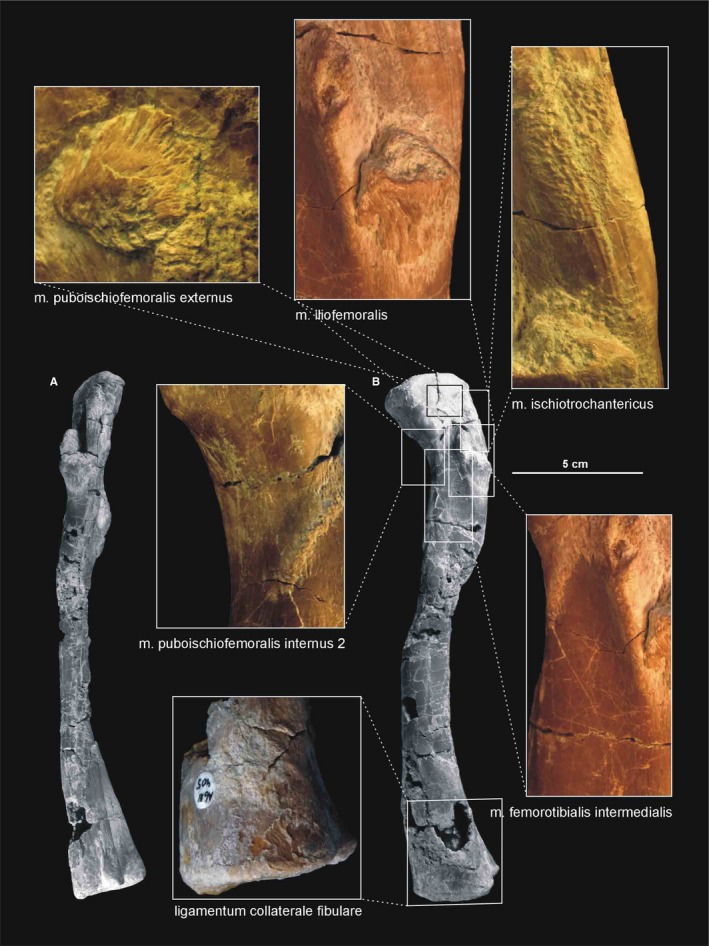
The greater trochanter is marked by an indistinct ridge (Dzik, 2003) on the posterolateral side of the head. In Silesaurus, the anterior (lesser) trochanter is very prominent, as a longitudinal ridge on the anterolateral surface, below the head. This ridge is stronger and more pointed in proximal aspect. The trochanteric shelf (the lateral ossification Piechowski et al. 2014) extends posteriorly along the entire posterolateral surface of the bone from the base of anterior trochanter, but only in some specimens. An additional tuberosity, the dorsolateral ossification (Piechowski et al. 2014) is present above the anterior trochanter, on the head in some specimens. Posteriorly to them, a longitudinal ridge, the dorsolateral trochanter continues down to the trochanteric shelf level.
The fourth trochanter forms an elongated ridge on the medial surface of the bone. It is located at nearly one‐third of the length of the femur from its proximal end. Its curvature is different from the proximal curvature of the femoral shaft. This ridge is occupied by a small ossification, but only in some specimens (Piechowski et al. 2014). The proximal and distal margins of the fourth trochanter run at nearly equal, low angles to the femoral shaft. A round indistinct depression is present next to the anterior border of the trochanter. In the femur of Silesaurus, the dorsolateral ossification always coexists with the lateral ossification, ‘overhang calcification’ and ossification of the fourth trochanter (Piechowski et al. 2014).
A clear femoral cranial intermuscular line (Figures 16A, 17B and 18A) appears on the anterodorsal surface of the bone just behind the neck. A prominent femoral caudolateral intermuscular line (Figures 16B, 17A and 18B) extends distally between the lateral ossification and fourth trochanter on the posterior surface of the shaft.
The distal end of the femur is oriented posteriorly and its articular surface bears two conjoined condyles: a larger one for articulation with the tibia (the lateral condyle) and the smaller one with the fibula (the fibular condyle). A third distinct condyle (the medial condyle) is located in opposition to them. Posteriorly, the articular surface bears a depression, which divides the distal head into medial and lateral areas.
Both epipodials are represented as articulated in the specimens ZPAL Ab III/361/8, 364, 1930 and 362. The tibia (Figures 15 and 20, 21, 22) is a robust, straight bone that is shorter in length than the femur. The proximal end of the tibia is subtriangular, with an anteroposterior elongation. It is much stronger than its distal end. The proximal articulation surface shows well‐developed internal and fibular condyles, on the posteromedial and posterior side, respectively. A straight cnemial crest appears on the anterior side. A low fibular flange (Dzik, 2003) occurs proximally on the lateral surface of the tibia. The shaft of the tibia is robust.
Figure 20.
Attachments of muscles and ligaments on the left tibia of Silesaurus opolensis. Origins are in red, insertions are in blue, ligaments are in green. (A) Posterolateral view. (B) Lateral view. (C) Anterior view. (D) Medial view. Muscle and ligament attachments in bold are those that have visible osteological correlates
Figure 21.
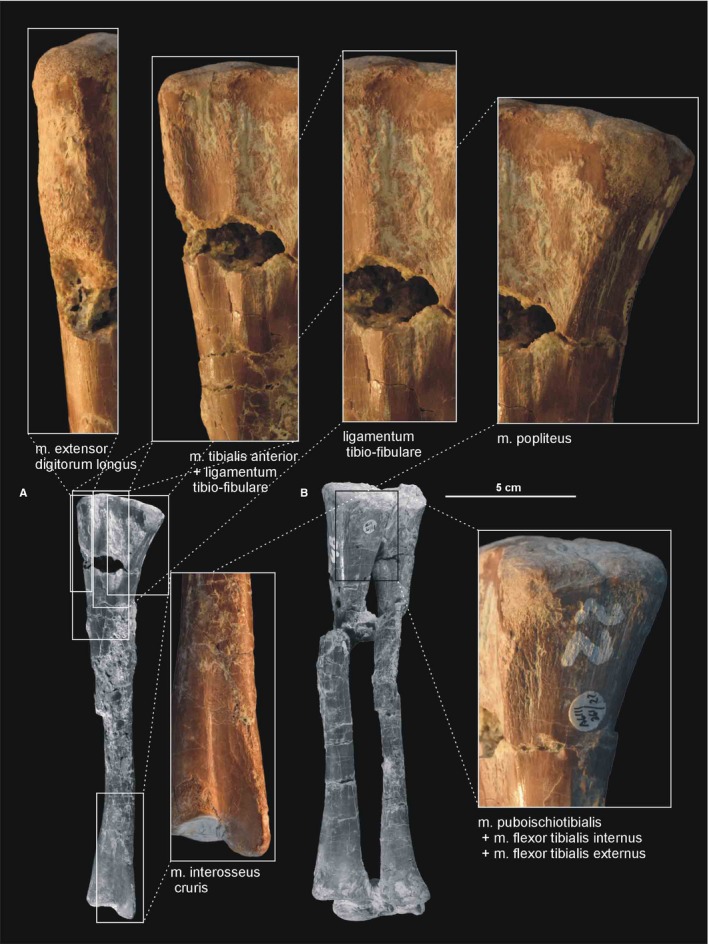
Muscle and ligament scars visible on the lateral aspect of the tibia of Silesaurus opolensis. All scars are from ZPAL AbIII/361/22. (A) Left tibia in lateral view (ZPAL AbIII/361/22). (B) Right shank and astragalocalcaneum in posterior view (ZPAL AbIII/361/48)
Figure 22.
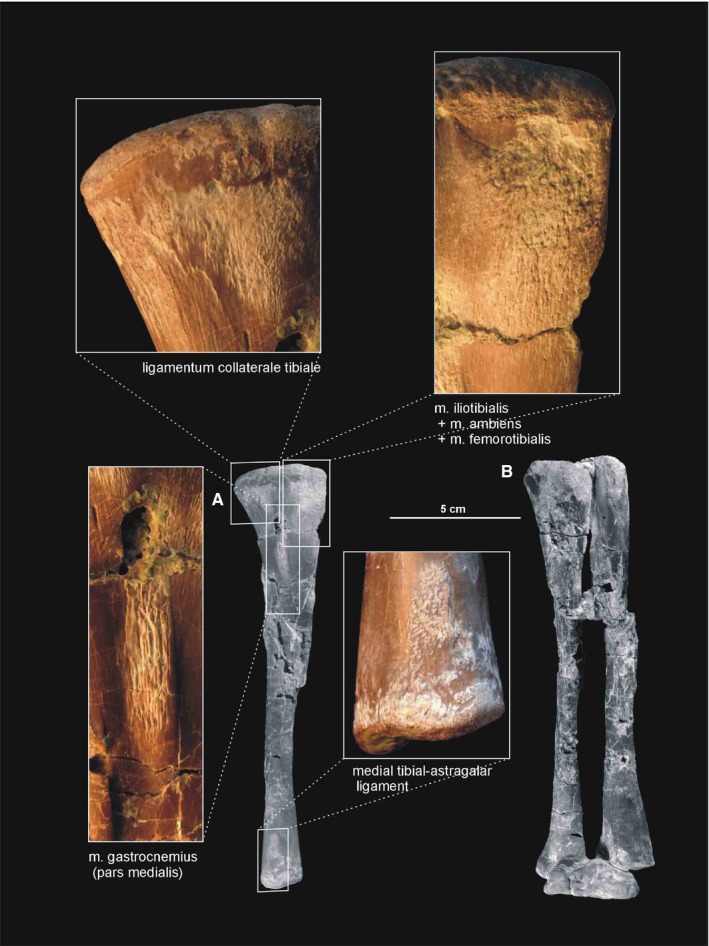
Muscle and ligament scars visible on the medial aspect of the tibia of Silesaurus opolensis. All scars are on ZPAL AbIII/361/22. (A) Left tibia in medial view (ZPAL AbIII/361/22). (B) Right shank and astragalocalcaneum in anterior view (ZPAL AbIII/361/48)
The distal end of the tibia is slightly broader than longer anteroposteriorly because its distal articular surface is oriented in a transverse plane. Its articular surface has a rounded anteromedial corner with a prominent astragalar overhang. The distal lateral end of the tibia forms a wall‐like descending process (Figure 21A). It overlaps the posterior surface of the astragalar ascending process. A gentle vertical groove on the lateral surface of the tibia separates its descending process from the articular surface for the ascending process of the astragalus. The groove terminates distally as a shallow notch in the distal articular surface, which is large and broader than the descending process.
The fibula (Figures 15, 21B, 22B, 23 and 24) is more slender than the tibia. The fibula is closely attached to the tibia proximally and distally, but separated throughout the rest of its length. As a result, there is a narrow gap between them. The proximal end of the fibula is anteroposteriorly expanded, and its central portion articulates with the fibular condyle of the tibia. The fibular shaft is straight. The spiral ridge (Dzik, 2003) is developed as a low crest on the anterior margin of its proximal part.
Figure 23.
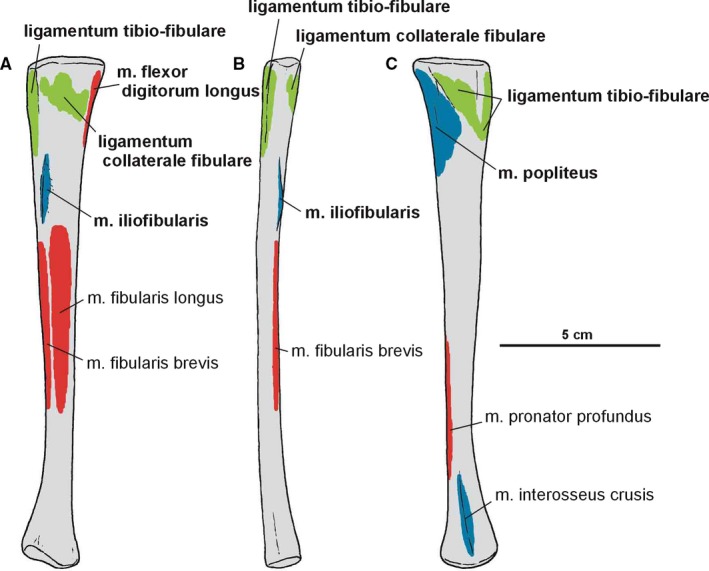
Attachments of muscles and ligaments on the left fibula of Silesaurus opolensis. Origins are in red, insertions are in blue, ligaments are in green. (A) Lateral view. (B) Anterior view. (C) Medial view. Muscle and ligament attachments in bold are those that have visible osteological correlates
Figure 24.
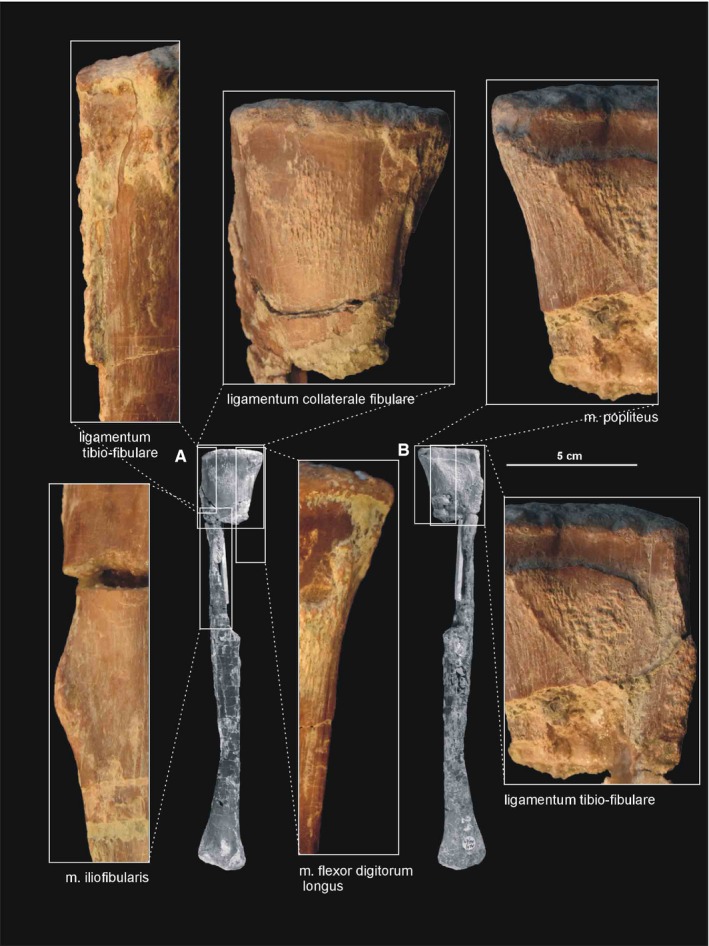
Muscle and ligament scars visible on the lateral and medial aspects of the fibula of Silesaurus opolensis. All scars are on ZPAL AbIII/361/24 except that of m. iliofibularis (ZPAL AbIII/416). (A) Left fibula in lateral view. (B) Left fibula in medial view (both views from ZPAL AbIII/361/48)
The fibula continues distally slightly more than the tibia. Its articular surface is elliptical in the distal view, with oblique medioanterior to lateroposterior orientation. The lateral part of the articular surface meets the calcaneum, while its medioanterior and medial edges articulate distally with the astragalus.
In Silesaurus, like in dinosaurs, the midtarsal joint is well developed. Two conjoined bones, astragalus and calcaneum, connected the epipodials with the rest of the pes. In all retained specimens, the astragalus and calcaneum are tightly connected (Figures 15, 25A and 26D), with the oblique straight suture between them (Dzik, 2003).
Figure 25.
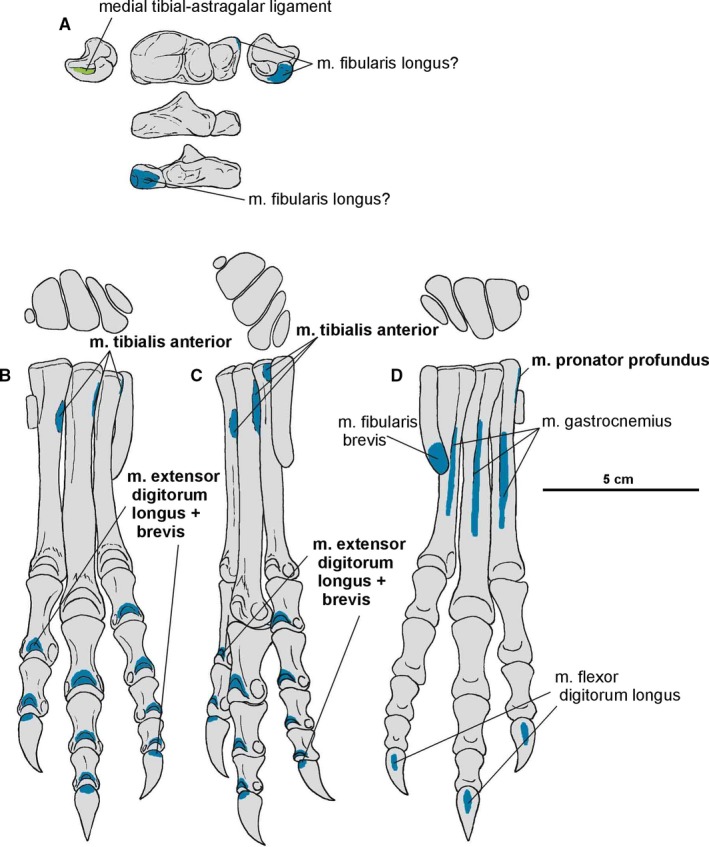
Attachments of muscles and ligaments on the left pes of Silesaurus opolensis. Insertions are in blue, ligaments are in green. (A) Astragalocalcaneum in medial, dorsal, lateral, anterior and posterior views. (B) Metatarsals and digits in dorsal and anterior view. (C) Metatarsals and digits in dorsal and lateral view. (D) Metatarsals and digits in dorsal and medial view. Muscle attachments in bold are those that have visible osteological correlates
Figure 26.
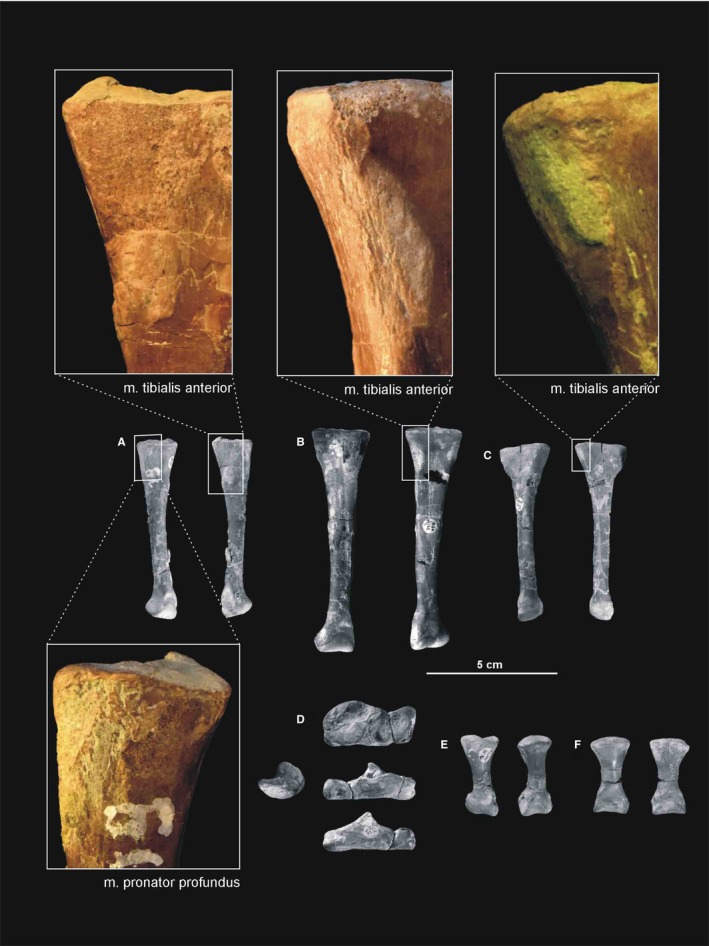
Muscle scars visible on the pes of Silesaurus opolensis. All detailed photographs are from illustrated bones (see below). (A) Left metatarsal II in medial and lateral views (ZPAL AbIII/361/19). (B) Right metatarsal III in dorsal and ventral view (ZPAL AbIII/361/14). (C) Left metatarsal IV in dorsal and ventral view (ZPAL AbIII/361/2). (D) Left astragalocalcaneum in medial, dorsal, anterior and posterior views (ZPAL AbIII/361/20). (E) First phalanx of the digit II from the right pes in ventral and dorsal views (ZPAL AbIII/361/13). (F) Proximal phalanx of digit III in ventral and dorsal views (ZPAL AbIII/1930)
The astragalus is a strong, transversely elongated bone. A vertical, non‐articular surface separates the dorsal and ventral articular facets in the anterior view. A shallow depression occurs on the anterior surface of the astragalus. The posterior side of the astragalus has a similar, but gently convex non‐articular surface. A roughly horizontal groove is visible on the medial side.
The astragalus is almost trapezoidal in the dorsal view, with anteriorly expanded medial part. The uneven tibial facet is separated from the fibular one by a pyramidal crest of the ascending process. Three broad concavities extend through its surface. The anterior margin of the ascending process continues on the rest of the surface. Posteriorly, the ascending process borders with the dorsal basin, which articulates with the descending process of the tibia. The ascending process bears a posteromedial ridge, which demarcates the dorsal basin from the medial articular surface of the astragalus. The lower posterior part of the ascending process articulates with a notch on the distal end of the tibia. Lateral to the ascending process, the bone is low in dorsoventral aspects and shows an oblique straight suture to border the tight articulation with the calcaneum. The concave lateral surface exposes the fibular facet of the astragalus. The fibula articulates with this articular surface, as well as with the lateral surface of the ascending process. The ventral articular surface of the astragalus articulates with the proximal ends of the first to third metatarsals. Although this facet shows a slight mediolaterally concave curvature, the articular surface is anteroposteriorly convex.
The calcaneum is a relatively small subtrapezoidal bone, with a lateroproximal expanded rim. As a result, the calcaneal tuberosity projects lateroposteriorly. The dorsal articular surface for the fibula meets medially with the articular surface of the astragalus. In ventral view, the calcaneum shows a convex elliptical surface for articulation with the fourth metatarsus. Laterally, a distinct notch extends anteriorly on a short distance. The astragalus and calcaneum belong functionally to the epipodials, while the bones of proximal tarsus constitute a functional part of the pes.
Articulated metatarsals are known from specimen ZPAL Ab III/364. Dzik (2003) reconstructed them as contacting each other parallel to the long axis of the leg. However, our inspection of the specimen revealed that they overlap each other, as in modern crocodiles and many other taxa. As a result, their proximal heads were rotated medially in relation to the rest of the bone (Figure 25B–D).
Metatarsals show much variability in the shape of their proximal ends (Dzik, 2003). The proximal ends of the second and third metatarsals are in almost horizontal alignment with the proximal end of the fourth metatarsal. Their articular surfaces are slightly concave to accommodate the distal surface of the astragalus and calcaneum. In the dorsal view, the second metatarsal is trapezoidal, third metatarsal is usually parallelogram, and fourth metatarsal shows a comma‐like surface, which fits the oval fifth metatarsal. The shafts of the second to fourth metatarsal are straight and closely appressed throughout most of their lengths. The third metatarsal is the most robust and longest in the series. The second and fourth are somewhat shorter than the third, but are equal to each other in length. Although the specimens are usually twisted by deformations, the central parts of the metatarsals show variability corresponding to their proximal ends. Metatarsals II–IV have well‐developed distal articular surfaces that contacted the proximal phalanges. The distal ends of the metatarsals have dorsal extensor depressions for intercondylar processes of their respective proximal phalanges. Pits for the collateral ligaments are also present in the metatarsals. In addition, scars for the insertion of the collateral ligaments are present on the proximal end of bones. The fifth digit is represented only by the metatarsal, which angles mediodistally across the posterior side of the metatarsus. The possible first digit is a narrow rib‐like bone attached to the right metatarsal second in the specimen ZPAL Ab III/364 (Dzik, 2003).
Description of the pedal phalanges (Figures 25B25, 26D and 25, 26E,F; Table 2) is based mostly on the articulated specimen ZPAL Ab III/364. Individual morphology of particular phalanges is supported by the isolated specimens ZPAL Ab III/361/13, 32 and 1930. The pedal phalangeal formula of Silesaurus is 0 – 3 – 4 – 5 – 0. The phalanges have a distally rounded articular surface, which corresponds to concave surfaces on the proximal ends of the succeeding phalanges. This proximal surface presents a dorsoproximal prong. The distal articular surface of most nonungual phalanges bears well‐developed pits for the extensor ligaments. Distinct pits for the collateral ligaments are present on all nonungual phalanges. They are approximately of the same depth on both sides of the bones.
Table 2.
Length measurements of the pes bones of Silesaurus opolensis ZPAL AbIII/364
| I, mm | II, mm | III, mm | IV, mm | V, mm | |
|---|---|---|---|---|---|
| Metatarsals | ? | 64 | 77 | 63 | 33 |
| Phalanx 1 | 23 | 25 | 19 | ||
| Phalanx 2 | 16 | 19 | ~14 | ||
| Phalanx 3 | 14 | ~10 | |||
| Phalanx 4 | ~9 | ||||
| Unguals | 16 | ? | 10 |
Knowledge of digit I is uncertain and limited to a single piece of bone.
The unguals (Figure 25B–D; Table 2) are subtriangular in cross‐section and curved, each with a convex dorsal and concave ventral edge. Their proximal articular surfaces are similar to proximal ones of preceding phalanges. The dorsal surface of unguals bears scars for the extensor attachment. The unguals are elongated and they possess a sharp point.
3.2. Pectoral and brachial musculature
3.2.1. Musculus serratus superficialis
The origin of the m. serratus superficialis is tentatively reconstructed in Silesaurus based on other studies (Meers, 2003; Jasinoski et al. 2006; Remes, 2008; Burch, 2014) as arising from the lateral surfaces of several anteriormost dorsal ribs. The muscle inserts on the posterior part of the ventral edge of the scapular blade (compare with Fürbringer, 1900; Miner, 1925; Figure 3; Table 3). In our material, the insertion area can be recognized in specimens ZPAL Ab III/2534, 404/8 and 406/7. The condition proposed here for Silesaurus resembles that in crocodilians (Meers, 2003) and lepidosaurs (Russell and Bauer, 2008) in having a single elongated insertion along the ventral edge of the scapula, as marked by longitudinal striations (Figure 4B). The m. serratus superficialis retracts and depresses the scapula (see Meers, 2003; Table 3).
3.2.2. Musculus serratus profundus
In Silesaurus, the origin of the m. serratus profundus is similar to that in Tawa (Burch, 2014), where it arose from several anteriormost dorsal ribs (compare also with Fürbringer, 1900; Jasinoski et al. 2006; Remes, 2008). The insertion is not osteologically distinguishable on the scapula of Silesaurus, but probably lay behind the distal insertion of the m. subscapularis (compare with Jasinoski et al. 2006; Remes, 2008; Burch, 2014; Figure 3C; Table 3). The m. serratus profundus acted as a protractor of the scapula (see Burch, 2014; Table 3).
3.2.3. Musculus costocoracoideus
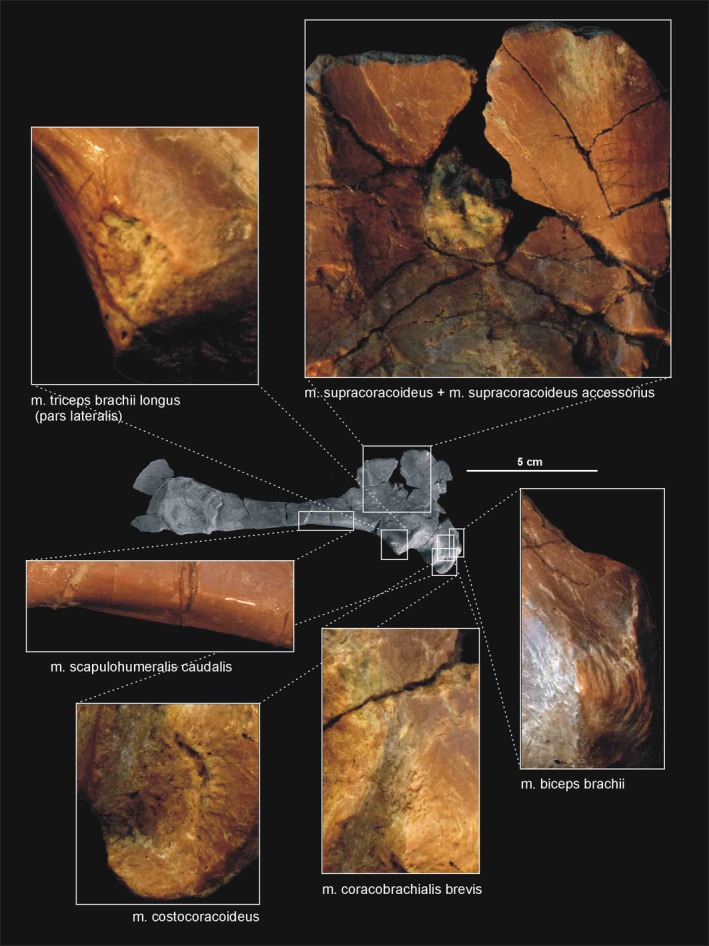
Phylogenetic bracketing suggests the presence of this muscle in Silesaurus. Because a large keeled sternum is a bird apomorphy, the origin of the m. costocoracoideus in Silesaurus was presumably located on the ribs, as in crocodiles (compare Jasinoski et al. 2006; Remes, 2008). The insertion was probably located on the anteroventral portion of the lateral surface of the coracoid (compare Meers, 2003; Remes, 2008; Figure 3A,B; Table 3), posteroventral to the origin of m. biceps brachii. The ventral (posteroventral; Burch, 2014) process of the coracoid of Silesaurus (ZPAL Ab III/2534 and 1203) possesses a distinct rugose subglenoid fossa that is the likely insertion point (Figure 5). A similar fossa is visible in many dinosaurs (Santa‐Luca, 1980; Jasinoski et al. 2006; Langer et al. 2007). The action of the m. costocoracoideus is to rotate, adduct and protract the forelimb (Table 3).
3.2.4. Musculus rhomboideus
Based on the scapula orientation in Silesaurus, which was probably intermediate between that of birds (subhorizontal) and crocodilians (subvertical), it is possible that the m. rhomboideus was transitional in its origin, arising from fascia and several anterior dorsal neural spines (compare with Fürbringer, 1876; Fürbringer, 1888; Fürbringer, 1902; Fitzgerald, 1969; Jasinoski et al. 2006; Remes, 2008; Burch, 2014). The muscle is reconstructed as inserting on the distalmost end of the medial scapular blade as in Tawa (Burch, 2014; compare with Cong et al. 1998; Fürbringer, 1900; Meers, 2003; Remes, 2008; Figure 3C; Table 3), although the reconstruction of this muscle is tentative. What is clear is that the widening of the scapular blade provides a more extensive surface for the muscle. The m. rhomboideus acted as protractor of the scapula (see Burch, 2014; Table 3).
3.2.5. Musculus levator scapulae
The m. levator scapulae is located on the lateral side of the neck, medial to the m. trapezius (Jasinoski et al. 2006; Remes, 2008). In Silesaurus, the anterior cervical ribs are parallel to the neck and extend backward for a few vertebral lengths (Piechowski and Dzik, 2010); therefore, they could serve as a muscle attachment. The presence of clear striations and a distinct ridge (the latter only in the smaller specimen ZPAL AbIII/2534) on the dorsal edge of the scapular blade provides evidence to reconstruct the m. levator scapulae with the m. trapezius in Silesaurus (compare with Fürbringer, 1876; Fürbringer, 1900; Romer, 1922; Meers, 2003; Remes, 2008; Burch, 2014; Figure 3A,C; Table 3). The insertion area can also be recognized in specimens ZPAL Ab III/404/8, 406/7 and 411/12 (compare with Meers, 2003; Jasinoski et al. 2006; Burch, 2014; Figure 4A). The m. levator scapulae acted as a rotator of the scapular blade, as well as a lateral flexor of the neck (see Burch, 2014; Table 3).
3.2.6. Musculus trapezius
In Silesaurus, as a result of sudden change in morphology of the ribs at the cervical to dorsal boundary, the first 10 to 11 dorsal ribs are especially strong and long (Piechowski and Dzik, 2010); therefore, the scapula of Silesaurus could not keep completely horizontal position. Given osteological evidence for the presence of the m. levator scapulae (see above), we include the m. trapezius in the reconstruction of musculature of Silesaurus (compare with George and Berger, 1966; Meers, 2003; Russell and Bauer, 2008; Burch, 2014; Fearon and Varricchio, 2016; Figures 3A,B and 4A; Table 3). This superficial muscle acted as a rotator of the scapular blade, likely assisting in protraction of the forelimb (see Burch, 2014; Table 3).
3.2.7. Musculus latissimus dorsi
The m. latissimus dorsi is reconstructed here as a single muscle that originates on the neural spines or thoracodorsal fascia, probably in the region from the last cervical to the sixth or seventh dorsal vertebrae (compare with Romer, 1922, 1944; Meers, 2003; Russell and Bauer, 2008; Burch, 2014; Figure 6A,B; Table 3). The insertion of the muscle is tentatively reconstructed on the proximal posterolateral side of the humerus (compare with Sullivan, 1962; George and Berger, 1966). The m. latissimus dorsi acted as a retractor of the humerus (see Burch, 2014; Table 3).
3.2.8. Musculus teres major
Because no osteological correlates are present in Silesaurus, the m. teres major is shown tentatively in the reconstruction (Figure 3A; Table 3). Being a specialized part of the m. latissimus dorsi (Remes, 2008), the m. teres major retracted the humerus (see Butler, 2010; Table 3).
3.2.9. Musculus pectoralis
Because of the lack of ossified and preserved sternum elements of Silesaurus, it is difficult to determine the origin of the m. pectoralis (see Padian, 2004; Remes, 2008; Burch, 2014; Fearon and Varricchio, 2016). Nevertheless, Fürbringer (1900) assumed that the well‐developed gastral apparatus found in many fossil amniotes might have served as an anchor for the m. pectoralis. Such a well‐developed gastral apparatus is present in the skeleton of Silesaurus (Piechowski and Dzik, 2010). The insertion of the m. pectoralis is located on the posterolateral surface of the low deltopectoral crest preserved in specimens ZPAL Ab III/1930 and 411/11 (Figures 6D and 7B; Table 3). The m. pectoralis would have adducted and protracted the humerus (see Burch, 2014; Table 3).
3.2.10. Musculus subscapularis
Phylogenetic inference suggests an origin of the m. subscapularis from the medial surface of the scapular blade in Silesaurus, as in crocodilians (compare with Romer, 1944; Sullivan, 1962; Meers, 2003; Maidment and Barrett, 2011; Figure 3C; Table 3). The insertion of the m. subscapularis is equivocally located on the medial tuberosity of the humerus (compare with Meers, 2003; Maidment and Barrett, 2011; Figure 6B–D; Table 3), sharing an insertion with the m. subcoracoideus. The m. subscapularis would have retracted and rotated the humerus (see Burch, 2014; Table 3).
3.2.11. Musculus subcoracoideus
Phylogenetic inference suggests the m. subcoracoideus originated on the medial side of the coracoid in Silesaurus (compare with Romer, 1944; Sullivan, 1962; Meers, 2003; Jasinoski et al. 2006; Maidment and Barrett, 2011; Burch, 2014; Fearon and Varricchio, 2016; Figure 3C; Table 3). The m. subcoracoideus equivocally shares a tendon insertion on the medial tuberosity of the humerus with the m. subscapularis (Figure 6B–D; Table 3). The m. subcoracoideus adducted and laterally rotated the humerus (see Burch, 2014; Table 3).
3.2.12. Musculus supracoracoideus
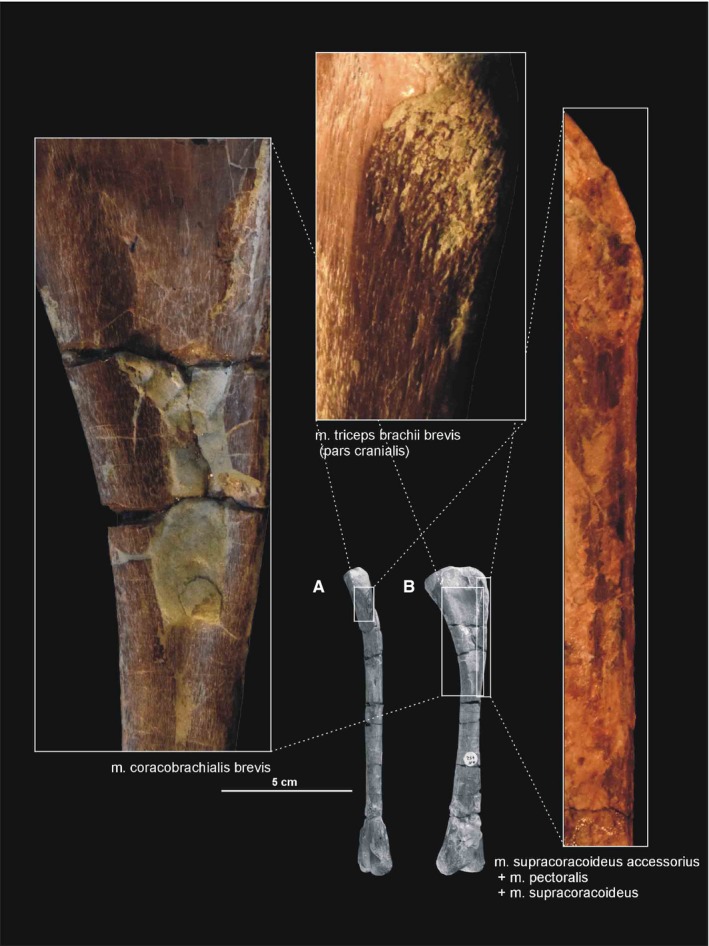
Because we cannot distinguish separate attachments on the surface of the coracoid, we reconstruct the m. supracoracoideus of Silesaurus as a muscle complex without distinguishing multiple heads (compare with Romer, 1944; Sullivan, 1962; Meers, 2003; Remes, 2008; Maidment and Barrett, 2011). The muscle originates on the subacromial depression of the scapula and extends on to the adjacent lateral surface of the coracoid, providing a clear broad, flat area on both bones (Figures 3A and 5; Table 3). The posteroventral extent of the m. supracoracoideus is delimited by a distinct bowed scar, which is clearly visible on specimens ZPAL AbIII/404/8 and 2634. Unfortunately, the dorsal range of the muscle attachment of this region is difficult to determine because of poor preservation in all specimens. The m. supracoracoideus is inserted on the deltopectoral crest of the humerus. A small longitudinal depression located on the lateral surface of the deltopectoral crest in specimens ZPAL Ab III/1930, 452, 411/11 is consistent with this site of insertion and indicates the lateral extent of the insertion (Figures 6A6, 7D and 6, 7B; Table 3). The m. supracoracoideus acted as a protractor and abductor of the humerus (see Burch, 2014; Table 3).
3.2.13. Musculus supracoracoideus accessorius
We tentatively reconstruct the origin of the m. supracoracoideus accessorius on the subacromial depression of the scapula, together with area for the m. supracoracoideus (compare with Burch, 2014; Figures 3A and 5; Table 3). The m. supracoracoideus accessorius inserted on the proximal part of the deltopectoral crest (anterior side) of Silesaurus and may be marked by a distinct semi‐oval depression in ZPAL AbIII/1930 (Figures 6D and 7B; Table 3). The role of the muscle is the same as the previous one.
3.2.14. Musculus coracobrachialis brevis
The origin of the m. coracobrachialis brevis is unequivocally reconstructed here, based on the origin of the crocodilian and ornithischian pars ventralis (compare with Romer, 1944; Sullivan, 1962; Meers, 2003; Maidment and Barrett, 2011; Burch, 2014). According to this, the muscle arises from the lateral aspect of the coracoids (Figure 3A,B; Table 3). A distinct fossa appears between the glenoid and the ventral process (ZPAL Ab III/2534 and 1203). The fossa is rugose and subdivided into two basins by an anteroposterior constriction. The ventral basin served for insertion of the m. costacoracoideus (see above). The dorsal basin belongs to the origin of the m. coracobrachialis brevis. Rugosities observed above this structure probably represent the extension of this origin (Figure 5). The insertion of this muscle is also phylogenetically unequivocal, situated on the broad, subtriangular depression that covers most of the anterior surface of the humerus (compare with Meers, 2003; Maidment and Barrett, 2011; Figure 6D; Table 3). In Silesaurus, this area is clearly visible on specimens ZPAL Ab III/452 and 411/11. Consistent with this morphology, the primary action of the m. coracobrachialis brevis would be protraction of the humerus (see Burch, 2014; Table 3).
3.2.15. Musculus coracobrachialis longus
Crocodilians lack the m. coracobrachialis longus, making it phylogenetically equivocal (Burch, 2014). We opted not to reconstruct it in Silesaurus because of the lack of osteological correlates (Table 3).
3.2.16. Musculus scapulohumeralis caudalis
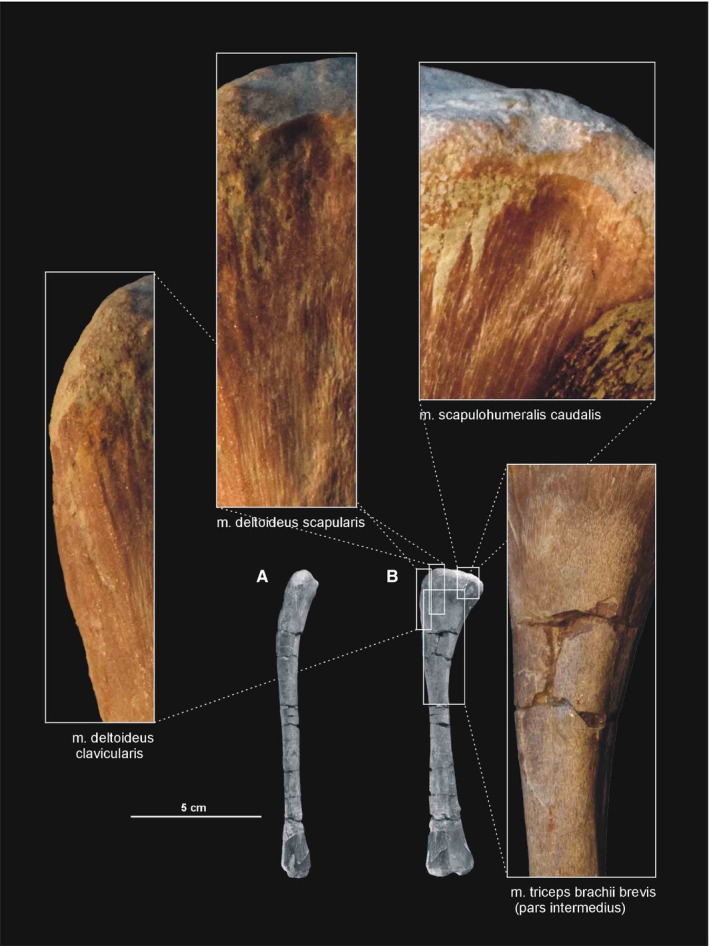
There is a distinct muscle scar next to glenoid on the medial side of the scapula in ZPAL AbIII/2534, 404/8 and 406/7. This rugose area is tear‐shaped. There is also a distinct ridge on the ventral surface of the scapular blade just posteriorly to the scar. We identify these areas as the origin of the m. scapulohumeralis caudalis because it is in a location similar to that of crocodiles (compare with Romer, 1944; Meers, 2003; Burch, 2014; Figures 3, 4A and 5; Table 3). The insertion of the m. scapulohumeralis caudalis is located on the medial tuberosity of the humerus. Similar to that of some dromaeosaurids and Tawa (Burch, 2014; compare with George and Berger, 1966; Meers, 2003; Maidment and Barrett, 2011), the humerus of Silesaurus (ZPAL AbIII/452, 411/11 and 1930) has an oval depression on the posterior surface of the medial tuberosity that probably corresponds to the insertion site of this muscle (Figures 6B,C and 27B; Table 3). The m. scapulohumeralis caudalis acted as a retractor of the humerus (see Burch, 2014; Table 3).
Figure 27.
Muscle scars visible on the posterior aspect of the humerus of Silesaurus opolensis. All photographs of ZPAL AbIII/452 except the scar for m. latissimus dorsi (ZPAL AbIII/1930). (A) Left humerus in lateral view. (B) Left humerus in posterior view
3.2.17. Musculus scapulohumeralis anterior
We failed to trace any insertion for the m. scapulohumeralis anterior in Silesaurus. The m. scapulohumeralis anterior is reconstructed in non‐avian theropods by homology with birds and lepidosaurs. These dinosaurs bear a scar or a weak fossa on the posterior portion of the scapular blade, which marks the origin of the muscle. In contrast to birds, theropods have no trace of this muscle insertion on the humerus. The muscle is absent in crocodiles (see Burch, 2014; Table 3).
3.2.18. Musculus deltoideus clavicularis
The origin of the m. deltoideus clavicularis is tentatively reconstructed here as a semilunar area restricted to the anterodorsal part of the lateral surface of the acromion process of the scapula (compare with Romer, 1944; Meers, 2003; Remes, 2008; Figure 3A; Table 3). The prominent acromial process of Silesaurus is similar in its development to that of ornithischians and crocodiles (Coombs, 1978; Norman, 1986; Johnson and Ostrom, 1995; Dilkes, 2000; Meers, 2003). The insertion of the m. deltoideus clavicularis is visible as a distinct longitudinal area on the lateral surface of the deltopectoral crest (compare with Sullivan, 1962; George and Berger, 1966; Dilkes, 2000; Meers, 2003; Burch, 2014; Figure 4). The m. deltoideus clavicularis would have abducted and slightly protracted the humerus (see Burch, 2014; Table 3).
3.2.19. Musculus deltoideus scapularis
In Silesaurus, the m. deltoideus scapularis probably originated on the lateral scapular blade which provides a large area of attachment (compare with Fürbringer, 1876; Romer, 1944, 1956; Sullivan, 1962; Meers, 2003; Remes, 2008; Maidment and Barrett, 2011; Figure 3A,B; Table 3). The muscle inserted on the posterolateral surface of the proximal humerus (compare with George and Berger, 1966; McGowan, 1982; Meers, 2003; Figure 6A,B; Table 3). There are subtle striations in this location (ZPAL AbIII/452) that probably represent a scar for this muscle (Figure 27B). The m. deltoideus scapularis would have abducted and retracted the humerus (see Burch, 2014; Table 3).
3.2.20. Musculus triceps brachii longus and brevis
In Silesaurus, clear striations appear on the lateroventral surface of the scapula just posterior to the scapular glenoid fossa, and form a distinct rugose tubercle. This can be easily homologized with the m. triceps branchii longus lateralis origin as it has the same location in crocodiles and birds (compare with Romer, 1944; George and Berger, 1966; Meers, 2003; Remes, 2008; Maidment and Barrett, 2011; Figures 3A,B and 5; Table 3). The tubercle is present in a similar location in the basal ornithischians Heterodontosaurus (Santa‐Luca et al. 1976; Santa‐Luca, 1980) and Eocursor (Butler, 2010). A rugosity in this area is variably developed across theropods (Burch, 2014). The m. triceps branchii longus caudalis origin is visible on the medial surface of the coracoid just anterior to the glenoid fossa, where the origin forms a distinct rugose concavity (Figures 3C and 4A; Table 3). Numerous authors recognized only one origin of the m. triceps on this bone in various early dinosaurs (Langer et al. 2007; Maidment and Barrett, 2011; Burch, 2014) as the second origin is equivocal and has no osteological correlates. However, Delcourt and Azevedo (2012) found a shallow pit on the medial portion of the scapular blade in Saturnalia. It has very similar form and position to the scapular attachment of m. triceps brachii longus in Caiman brevirostris. Based on that, we have tentatively reconstructed this attachment in Silesaurus (Figure 3; Table 3). The origins of the m. triceps brachii are also clearly visible on the humerus of Silesaurus (ZPAL AbIII/452 and 411/11). In Silesaurus, the m. triceps brachii brevis caudalis occupied a distinct oval rugose surface. It is located in Silesaurus on the posterior side of the bone, just below the medial tuber (Figures 6B6, 7D and 6, 7A; Table 3). The m. triceps brachii brevis intermedius originated just distal to the pars caudalis and continued distally along the humeral shaft. The origin has heart‐like outline on the posterior side of the bone where it is expanded and bifurcated proximally (Figures 6A6, 27C and 6, 27B; Table 3). If present, the m. triceps brevis cranialis continued along the lateral border of the pars intermedius as a narrow strip. The common insertion of the m. triceps brachii is on the olecranon process of the ulna (compare with Meers, 2003; Remes, 2008; Maidment and Barrett, 2011; Burch, 2014; Figure 8B,C; Table 3). Although the Silesaurus olecranon is vestigial against many other Triassic Dinosauromorpha, it bears clear striations for this muscle in ZPAL AbIII/431/1, 459/3 and 407/3 (Figure 9C). The primary action of the m. triceps branchii would be in extending the antebrachium, as well as contributing to extension of the humerus (see Burch, 2014; Table 3).
3.2.21. Musculus biceps brachii
The origin of the m. biceps brachii is reconstructed here along the anterior edge of the coracoids (compare with Romer, 1944; Goslow et al. 1989; Meers, 2003; Remes, 2008; Maidment and Barrett, 2011; Burch, 2014; Fearon and Varricchio, 2016; Figure 3A; Table 3). Its ventral border is marked by a distinct biceps brachii tubercle (ZPAL AbIII/2534 and 1203; Figure 5). The tubercle appears anterior to the glenoid and dorsal to the ventral process of the coracoid. It is wider than high, and directed anterolaterally. Our reconstruction of the m. biceps brachii is in contrast to those proposed by some authors (Langer et al. 2007; Burch, 2014), which locate its origin on the ‘elongated tuber’ of the coracoid. However, we note that, in extant archosaurs, the origin of this muscle is on the acromial part of the coracoids (Meers, 2003), far from the glenoid area. Our interpretation is congruent with many others (i.e. Borsuk‐Bialynicka, 1977; Maidment and Barrett, 2011; Fearon and Varricchio, 2016). The humeral head of the m. biceps brachii is present only in birds among modern archosaurs (Vanden Berge and Zweers, 1993; Jasinoski et al. 2006; Remes, 2008) and is not reconstructed in non‐theropod dinosaurs (i.e. Langer et al. 2007; Maidment and Barrett, 2011; Fearon and Varricchio, 2016). However, it is reconstructed even in the Triassic non‐avian theropods (Burch, 2014). We observe an indistinct rugose surface in Silesaurus on the anteromedial aspect of the medial tuber that perhaps represents the biceps brachii humeral origin (Figure 6C,D). It is preserved only in ZPAL AbIII/411/11. As for the insertion, there is a distinct muscle scar on the anterior side of the ulna in Silesaurus (ZPAL AbIII/2538, 407/3, 407/12, 459/3 and 431/1) that corresponds with this attachment in extant taxa (Figures 8A8, 9D and 8, 9B; Table 3). It is located just distal to the articular surface and has a subtriangular outline that expands posterodistally. The radius (ZPAL AbIII/407/12) bears only delicate rugosities on its surface in an analogous area. The primary action of the m. biceps brachii would be flexion of the antebrachium (see Burch, 2014; Table 3).
3.2.22. Musculus humeroradialis
Because both origin and insertion are indistinguishable in Silesaurus, the presence of m. humeroradialis is inferred based on some theropods and crocodiles (compare with Fürbringer, 1876; Romer, 1944; Sullivan, 1962; Meers, 2003; Diogo and Abdala, 2010; Burch, 2014; Figures 6A,B and 8A; Table 3). The muscle lacks osteological correlates in any tetrapod group other than these two (Remes, 2008; Burch, 2014). The m. humeroradialis would have flexed the antebrachium (see Burch, 2014; Table 3).
3.2.23. Musculus brachialis
The origin of the m. brachialis of Silesaurus is located on the lateral humeral midshaft, distal to the deltopectoral crest (compare with Walker, 1973; Meers, 2003; Russell and Bauer, 2008; Maidment and Barrett, 2011; Figure 6D; Table 3), where an indistinct flat longitudinal surface is present. The surface is oriented proximodistally along the humeral shaft. The separate insertion of the m. brachialis is reconstructed together with the origin of the m. biceps brachii on the proximal ends of the radius and ulna (compare with Remes, 2008; Figures 8A,D and 9B; Table 3). In our material, the insertion area can be recognized in specimens ZPAL Ab III/2538, 459/3 and 407/3,12. A similar condition is present in crocodiles and lepidosaurs. In birds, it is restricted to the proximal ulna (Baumel et al. 1993). The m. brachialis would have flexed the forearm (see Burch, 2014; Table 3).
3.3. Antebrachial musculature
3.3.1. Musculus anconeus
The origin of the m. anconeus in Silesaurus is tentatively reconstructed here on the ectepicondyle of the humerus, where it should share a tendon with the m. extensor carpi ulnaris (compare with Miner, 1925; Haines, 1939; Walker, 1973; Vanden Berge and Zweers, 1993; Meers, 2003; Russell and Bauer, 2008; Burch, 2014; Figure 6A,B; Table 4). The muscle insertion is reconstructed unequivocally on the lateral surface of the ulna, just behind the proximal articular surface of the bone and extending for most of its length (compare with Haines, 1939; Sullivan, 1962; Burch, 2014; Figure 8B,C; Table 4). It is marked by a relatively broad, longitudinal concavity on the lateral ulnar shaft (ZPAL Ab III/2538, 459/3,4, 4073 and 407/12; Figure 9C). In Silesaurus, a prominent ridge begins at the ulnar midshaft and extends towards the distal end providing a distinct surface for the distal part of the m. anconeus and separating its insertion from the origin of the m. abductor pollicis longus. A similar condition in present in Tawa (Burch, 2014). The m. anconeus would have flexed the forearm (see Burch, 2014; Table 4).
3.3.2. Musculus extensor carpi ulnaris
The muscle is relatively conservative in non‐archosaurian reptiles and birds (Burch, 2014); therefore, this muscle was probably present in Silesaurus. The origin of the m. extensor carpi ulnaris is tentatively reconstructed here on the ectepicondyle of the humerus, in the same place as the anconeus (Figure 6A,B; Table 4). Because the manus of Silesaurus is poorly known, the insertion of the m. extensor carpi ulnari cannot be reconstructed. M. extensor carpi ulnaris would have extended and abducted the wrist, along with extension of the forearm (see Burch, 2014; Table 4).
3.3.3. Musculus supinator
Here, we tentatively reconstruct the origin of the m. supinator as the most proximal muscle attachment on the ectepicondyle in Silesaurus, just above origin of the m. extensor carpi radialis and close to that of the m. abductor radialis (compare with Haines, 1939; Remes, 2008; Russell and Bauer, 2008; Figure 6A,B; Table 4). The insertion of the m. supinator is located on the anterolateral surface of the radius behind the proximal articular surface and extending for most of its length (compare with Vanden Berge and Zweers, 1993; Vazques, 1994; Remes, 2008; Burch, 2014; Figure 8A,C; Table 4). The precise location of the insertion on the radial shaft is unclear in Silesaurus. The m. supinator would have flexed and supinated the forearm (see Burch, 2014; Table 4).
3.3.4. Musculus extensor carpi radialis
The origin of the m. extensor carpi radialis in Silesaurus is reconstructed on the ectepicondyle (compare with Haines, 1939; Baumel et al. 1993; Meers, 2003; Remes, 2008; Russell and Bauer, 2008; Figure 6A,B; Table 4) in a location similar to that of crocodiles, lepidosaurs and turtles. The insertion (see Meers, 2003; Burch, 2014) cannot be reconstructed due to the incomplete manus. The m. extensor carpi radialis would have extended and adducted the wrist, as well as contributing to flexion of the forearm (see Burch, 2014; Table 4).
3.3.5. Musculus abductor radialis
The m. abductor radialis is reconstructed here as originating on the ectepicondyle, just proximal to the origin of both the m. anconeus and the m. extensor carpi ulnaris (compare with Haines, 1939; Remes, 2008; Russell and Bauer, 2008; Burch, 2014; Figure 6A,B; Table 4), as in crocodiles. We reconstruct its insertion on the proximal half of the lateral radial surface (compare with Meers, 2003; Remes, 2008; Burch, 2014; Figure 8C; Table 4). According to this interpretation, the m. abductor radialis would have abducted and slightly flexed the forearm (see Burch, 2014; Table 4).
3.3.6. Musculus abductor pollicis longus
The m. abductor pollicis longus originated unequivocally from the internal (interosseous) surfaces of the radius and ulna in Silesaurus (compare with Haines, 1939; George and Berger, 1966; Remes, 2008; Russell and Bauer, 2008; Burch, 2014; Figure 8A,C,D; Table 4). On the ulna, a distinct scar marks its distal extent (ZPAL Ab III/453 and 459/3,4; Figure 9B), with a faint ridge separating its proximal limit medially from the m. pronator quadratus on the same bone. It cannot be reconstructed because the adequate elements of the hand are not preserved. The m. abductor pollicis longus would have extended and abducted the wrist, as well as abducted digit I (see Burch, 2014; Table 4).
3.3.7. Musculus extensor digitorum longus
The m. extensor digitorum longus is reconstructed here as originating from approximately the middle of the ectepicondyle, between the origins of the m. extensor carpi ulnaris and the m. extensor carpi radialis or the m. supinator (compare with Burch, 2014; Figure 6A,B; Table 4), as in most living taxa. The insertion cannot be reconstructed. M. extensor digitorum longus would have extended the wrist (see Burch, 2014; Table 4).
3.3.8. Musculus pronator teres
Although the entepicondyle of Silesaurus lacks a distinct ridge or anterior projection, its proximal extension probably corresponds to the origin of the m. pronator teres (compare with Livezey, 1990; Remes, 2008; Figure 6B,C; Table 4). Based on Tawa (Burch, 2014), the muscle is reconstructed as inserting in a line along the anteromedial shaft of the radius for more than half of its overall length (compare with Straus, 1942; Haines, 1950; Remes, 2008; Russell and Bauer, 2008; Burch, 2014; Figure 8A,D; Table 4). M. pronator teres would have flexed the forearm and pronated the antebrachium (see Burch, 2014; Table 4).
3.3.9. Musculus pronator accessorius
After Burch (2014), the origin of the m. pronator accessorius of Silesaurus is reconstructed as being more distal than the origin of the m. pronator teres, at the distal end of the entepicondyle near to the origin of the m. flexor digitorum longus (Figure 6B–D; Table 4). We tentatively reconstructed the insertion of the m. pronator accessorius as running on the medial side of the distal radius of Silesaurus for slightly over half of its length (compare with Haines, 1950; George and Berger, 1966; Russell and Bauer, 2008; Burch, 2014; Figure 8D; Table 4). The m. pronator teres would have flexed and pronated the antebrachium (see Burch, 2014; Table 4).
3.3.10. Musculus pronator quadratus
Two faint ridges on the medial side of the proximal ulna are interpreted as delimiting the origin of the m. pronator quadratus in Silesaurus (ZPAL Ab III/453, 407/3, 459/3, 131/1), thus the muscle origin is reconstructed as covering most of the length of the bone (compare with Ribbing, 1907; Straus, 1942; Haines, 1950; Sullivan, 1962; George and Berger, 1966; Walker, 1973; Meers, 2003; Remes, 2008; Russell and Bauer, 2008; Figures 8A8, 9C and 8, 9B; Table 4), as in Tawa (Burch, 2014). The radial insertion of the m. pronator quadratus in Silesaurus is demarcated on the posterior surface of the AbIII 431/4 by a light narrow distal scar, suggesting a much shorter (Figure 8B,D; Table 4) insertion than that reconstructed for Tawa (Burch, 2014). The carpal attachment remains unknown. The primary action of the m. pronator quadratus would be pronation of the antebrachium and manus (see Burch, 2014; Table 4).
3.3.11. Musculus epitrochleoanconeus
The m. epitrochleoanoconeus is tentatively reconstructed in Silesaurus as originating between the origins of the m. flexor carpi ulnaris and the m. pronator accesorius on the entepicondyle (compare with George and Berger, 1966; Remes, 2008; Burch, 2014; Figure 6B,C; Table 4). Its insertion is defined by a distinct ridge on the ventromedial surface of the proximal ulna (compare with Miner, 1925; Remes, 2008; Burch, 2014; ZPAL Ab III/453, 407/3, 459/3 and 431/1), just behind the proximal articular surface and covering the medioventral surface of the bone, but restricted to its proximal half (Figures 8D and 9B; Table 4). The m. epitrochleoanoconeus would have flexed the antebrachium (see Burch, 2014; Table 4).
3.3.12. Musculus flexor carpi ulnaris
The m. flexor carpi ulnaris is reconstructed in Silesaurus as originating from a single tendon on the posterodistal aspect of the entepicondyle as in other diapsids (compare with Miner, 1925; Remes, 2008; Burch, 2014), just above the distal articular surface (Figure 6B,C; Table 4). The insertion cannot be reconstructed. The m. flexor carpi ulnaris would have flexed and adducted the wrist (see Burch, 2014; Table 4).
3.3.13. Musculus flexor digitorum longus
The origin of the m. flexor digitorum longus superficialis is reconstructed in Silesaurus in the same location on the humerus as in most modern taxa (Figure 6B; Table 4), and on the medioventral surface of the ulna, as is seen in crocodiles and lepidosaurs (compare with Straus, 1942; Fisher and Goodman, 1955; George and Berger, 1966; Fitzgerald, 1969; Cong et al. 1998; Meers, 2003; Russell and Bauer, 2008; Burch, 2014; Figures 8D and 9B; Table 4). The area is marked by two ridges (ZPAL Ab III/453, 407/3, 459/3 and 431/1), which separate it from the attachment site of the m. pronator quadratus anteriorly and the m. epitrochleoanoconeus posteriorly. The insertion cannot be reconstructed. The function of the m. flexor digitorum longus is to flex the digits and the wrist (see Burch, 2014; Table 4).
3.4. Myology of the Pelvic Girdle and Hindlimb
3.4.1. Musculus triceps femoris
The term ‘triceps femoris’ is used in a wider context, subsuming three distinct muscles: the m. iliotibialis, the m. ambiens, and the m. femorotibialis. In all extant taxa, these three divisions coalesce into a common femoropatellar tendon that inserts on the cnemial crest of the tibia. The primary action of the m. triceps femoris would be in flexing the hip and extending the knee (after Schachner et al. 2011; Table 5).
3.4.2. Musculus iliotibialis
In Silesaurus ZPAL Ab III/361, 362, 404/1,2, the origin of the m. iliotibialis is marked by a distinct longitudinal narrow rugosity along the dorsal border of the iliac blade (compare with Vanden Berge and Zweers, 1993; Carrano and Hutchinson, 2002; Fechner, 2009; Schachner et al. 2011; Liparini and Schultz, 2013; Figures 12 and 14B; Table 5). The rugosity expands onto the anterior process, covering its anterolateral surface. It is very broad there and has a rounded lateroventral margin. The part situated on the iliac blade expands posteriorly to cover the dorsalmost part of the postacetabular process. It is difficult to determine the exact boundaries between the different parts of the m. iliotibialis. The presence of the anteriorly straight cnemial crest indicates that the m. iliotibialis of Silesaurus attached to the anteromedial aspect of the crest (compare with Romer, 1923b; Maidment and Barrett, 2011; Figures 20C,D and 22A; Table 5). In our material, the insertion area can be recognized in specimens ZPAL Ab III/361, 403, 414, 2539, 1245, 1246, 1930 and 404/10. There is a large, distinct scar, marking the position of the common insertion for three muscles (m. iliotibialis, m. ambiens and m. femorotibialis).
3.4.3. Musculus ambiens
The different origination patterns of the m. ambiens in modern diapsids make reconstructions in extinct taxa difficult. Phylogenetic bracketing indicates that the double‐headed m. ambiens is a derived feature of crocodiles (Maidment and Barrett, 2011). However, it is possible that the second head evolved earlier and was secondarily lost in advanced dinosaurs (Langer, 2003). We reconstructed the origin of the m. ambiens as lying laterally on the pubic tubercle in Silesaurus (Figure 14A,B; Table 5), as in lepidosaurs and basal archosaurs (compare with Romer, 1923b, 1927b; George and Berger, 1966; Vanden Berge and Zweers, 1993; Fechner, 2009; Schachner et al. 2011; Liparini and Schultz, 2013). This position is supported by a large scar that covers the whole anterior surface of the tubercle and extends craniodorsally to the dorsal rim of the pubis and also ventrally (ZPAL Ab III/361, 363, 404/5, 1930, 3339 and 3340; Figure 28B). In most dinosaurs, the dorsal rim of the pubis is somewhat protruding, while the pubic tubercle is absent (Langer, 2003). This led various authors to locate the ambiens origin dorsally near proximal end of the pubis, as in crocodiles (Romer, 1923a; Romer, 1927b; Borsuk‐Bialynicka, 1977; Dilkes, 2000; Langer, 2003). The m. ambiens had a common insertion with the m. iliotibialis and the m. femorotibialis (compare with Liparini and Schultz, 2013; Figures 20C,D and 22A; Table 5). It is impossible to determine whether a secondary tendon crossed the knee extensor tendon and inserted on the m. gastrocnemius lateralis as in extant archosaurs.
Figure 28.
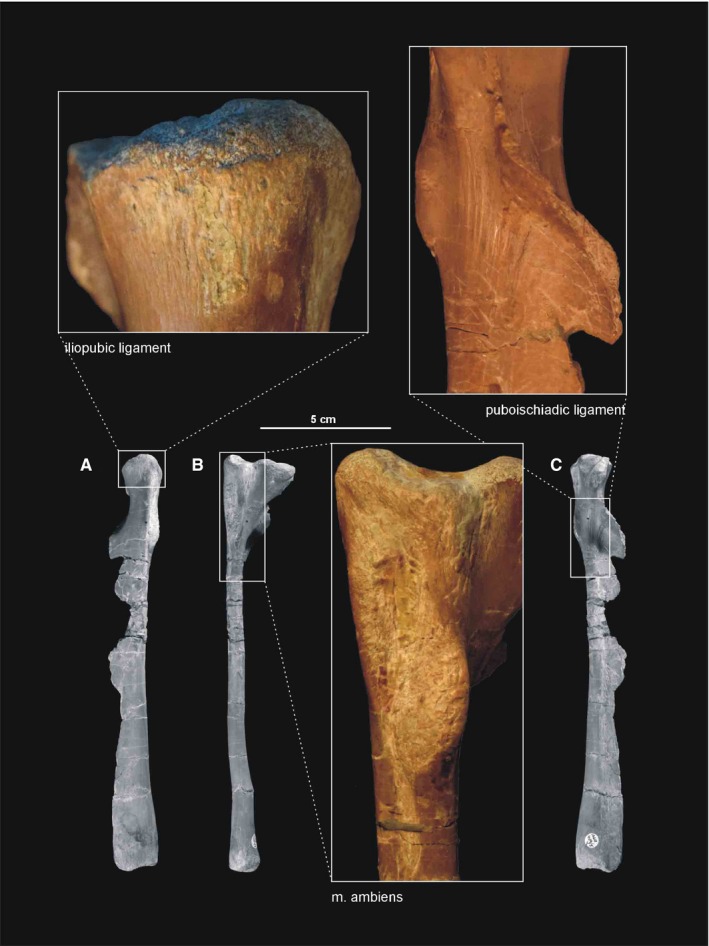
Muscle and ligament scars visible on the pubis of Silesaurus opolensis. All photographs of ZPAL AbIII/404/5. (A) Left pubis in anterodorsal view. (B) Left pubis in lateral view. (C) Left pubis in posteroventral view
3.4.4. Musculus femorotibialis
We reconstructed three parts for the m. femorotibialis in Silesaurus, as in birds and non‐avian dinosaurs (Langer, 2003; Fechner, 2009); contra Carrano and Hutchinson (2002) and Maidment and Barrett (2011). On the femur, a proximodistally oriented cranial intermuscular line (Figures 16A, 17B and 21A) clearly separates the origins of the m. intermedialis and medialis, whereas a caudolateral intermuscular line (Figures 16B, 17A and 18B) separates the origins of the m. intermedialis and lateralis. As a result, the intermedialis part covers most of the anterolateral surface of the femoral shaft (Figure 16; Table 5). A distinct scar at approximately the mid‐level of the anterior trochanter marks the proximal limit of the origin (Figure 19B). Distally, the scar is very faint but it is possible to recognize its posterolateral elevation. The origin of the m. femorotibialis medialis surrounds the depression marking the caudofemoralis longus origin proximally (Figures 17 and 18A; Table 5), whereas the distal limit is marked by a clear rounded scar. The proximal scar for the m. femorotibialis lateralis origin is pointed and located posterior to the fourth trochanter (Figures 16B, 17A and 18B; Table 5). The distal limit of this origin is marked by a rounded scar on the lateral condyle. In our material, the area of origin is visible in specimens ZPAL Ab III/361, 405 and 1263. The m. femorotibialis inserts in the same place as the previous two muscles.
3.4.5. Musculus iliofibularis
Despite the relatively smooth surface of the dinosaurian ilium, the postacetabular process of Silesaurus has a raised and rugose dorsolateral surface that probably marks the origin of m. iliofibularis (compare with Romer, 1923b; Gangl et al. 2004; Maidment and Barrett, 2011; Schachner et al. 2011). It is the largest rugose surface on the ilium (Figure 14B; Table 5) and, in our material, is visible in specimens ZPAL Ab III/361, 362 and 404/1,2 (Figure 12). The insertion is marked by a spiral ridge (Dzik, 2003) less than one‐third of the way down the anterolateral surface of the fibular shaft (compare with Fechner, 2009; Schachner et al. 2011; Figure 23A,B; Table 5). The ridge is visible in specimens ZPAL Ab III/361, 416 and 3342 (Figure 24A). The primary action of the m. iliofibularis would be to extend and abduct the hip, as well as flexing the knee (see Schachner et al. 2011; Table 5).
3.4.6. Musculus iliofemoralis
The lateral surface of the ilium of Silesaurus presents a marked, smooth concavity above the acetabulum that is probably related to the origin of the m. iliofemoralis (Figure 14B; Table 5), based on comparison with crocodilians (compare with Romer, 1923b; Rowe, 1986; Hutchinson, 2002; Fechner, 2009; Maidment and Barrett, 2011; Schachner et al. 2011). However, Silesaurus lacks a ridge on the iliac blade, making it difficult to judge if the m. iliofemoralis was divided into the m. iliotrochantericus caudalis and the m. iliofemoralis externus as in birds (compare with Osborn and Mook, 1916; Romer, 1927b; George and Berger, 1966; Osmólska et al. 1972; Bonaparte, 1986; Rowe, 1986; Barsbold and Maryańska, 1990; Hutchinson, 2001b; Carrano and Hutchinson, 2002; Langer, 2003; Maidment and Barrett, 2011; Schachner et al. 2011). However, the anterior trochanter and the trochanteric shelf (lateral ossification Piechowski et al. 2014) of Silesaurus might correspond respectively to the insertion of those two muscles (Figures 16 and 17; Table 5). In our material, the insertion area is visible in ZPAL Ab III/361, 405, 457, 1930, 460/1 and 411/4 (compare with Fechner, 2009; Schachner et al. 2011; Figure 19B). The m. iliofemoralis abducted the hip (see Schachner et al. 2011; Table 5).
3.4.7. Musculus puboischiofemoralis internus
The m. puboischiofemoralis internus 1 is reconstructed here as arising from a distinct fossa on the anterior aspect of the ilium (ZPAL Ab III/361, 362, 404/1,2), located exactly between the pubic peduncle and the anterior process (compare with Romer, 1927b; Fechner, 2009; Maidment and Barrett, 2011; Schachner et al. 2011; Figures 13C and 14B,C; Table 5). The fossa has an elliptical outline. The area of origin of the m. puboischiofemoralis internus 2 in Silesaurus could be on the lateroventral aspect of the anterior process similar to the position in birds (compare with Romer, 1927b; Rowe, 1986; Carrano and Hutchinson, 2002; Fechner, 2009; Schachner et al. 2011; Figures 13C and 14B; Table 5). There is a small tubercle next to the lateral extremity of the m. iliotibialis 1 that continues ventrally as a small ridge anterodorsal to the m. puboischiofemoralis internus 1. The insertion of the m. puboischiofemoralis internus 1 in Silesaurus apparently retained the crocodilian condition (compare with Romer, 1923b; George and Berger, 1966; Hutchinson, 2001b; Fechner, 2009; Maidment and Barrett, 2011; Figures 16B and 17; Table 5). It forms an arch along a low ridge that surrounds the semi‐oval depression for the m. caudofemoralis longus mediodistally (Figure 18A). The insertion site extends on to the distalmost part of the fourth trochanter, the ossification of which reflects this attachment (Piechowski et al. 2014). The m. puboischiofemoralis internus 2 insertion occupies the anterolateral aspect of the femoral neck (compare with Fechner, 2009; Maidment and Barrett, 2011; Figures 16A and 17B). A distinct scar bounds the attachment area anteriorly, extended over the anterior trochanter (ZPAL Ab III/361; Figure 19B), but its medial outline is difficult to determine. This muscle would have flexed the hip (see Schachner et al. 2011; Table 5).
3.4.8. Musculus puboischiotibialis
The reconstruction of the m. puboischiotibialis in Silesaurus is equivocal (see Walker, 1973; Fechner, 2009; Liparini and Schultz, 2013). The muscle usually originates from the obturator plate of the ischium (Fechner, 2009; Schachner et al. 2011). A lateral depression on the proximoventral ischium, slightly separated from the reconstructed origin of the adductor muscles, was interpreted by some authors as a site of origin of the m. puboischiotibialis (Schachner et al. 2011; Liparini and Schultz, 2013). If present, the m. puboischiotibialis of Silesaurus may have arisen from a similar position (Figure 14B; Table 5) and inserted on the posteromedial surface of the proximal tibia (Figure 20A,B,D; Table 5). The rugose, common insertion area for the pubotibialis, flexor tibialis internus and externus is visible in specimens ZPAL Ab III/361, 403, 414, 1246, 1930, 460/3, 411/2 and 1239 (compare with Schachner et al. 2011; Figure 21B). The m. puboischiotibialis would have abducted and extended the hip, as well as flexed the knee (see Schachner et al. 2011; Table 5).
3.4.9. Musculus pubotibialis
We were not able to identify any osteological correlates for the m. pubotibialis in Silesaurus and did not attempt to reconstruct it (Table 5).
3.4.10. Musculus flexor tibialis internus
This muscle is variously reconstructed in fossil taxa (compare with Romer, 1923b; George and Berger, 1966; Borsuk‐Bialynicka, 1977; Dilkes, 2000; Hutchinson, 2001a; Langer, 2003; Gangl et al. 2004; Fechner, 2009; Maidment and Barrett, 2011; Schachner et al. 2011; Liparini and Schultz, 2013) due to the differences between modern birds and crocodiles. We provisionally followed the crocodilian arrangement because Silesaurus has similar distribution of rugosities on the ischium. However, we only reconstructed two parts, one of which is uncertain. A distinct rugose ridge on the proximodorsal part of the ischium posterior to the acetabulum is attributed to the origin of m. flexor tibialis internus 3 (Figure 14B; Table 5). This ridge is proximodistally elongated and runs parallel to the bone axis. It has convex surface facing laterally and tapers distally along approximately one‐third of the bone (ZPAL Ab III/361, 362, 925, 1228, 3226 and 404/1; Figure 29A). The m. flexor tibialis internus 1 may have originated from the dorsal part of the distalmost ischium (Figure 14C; Table 5), where an indistinct surface can be seen on some specimens (i.e. ZPAL AbIII/3288; Figure 29D). The insertion was probably on the posteromedial surface of the proximal tibia, together with m. puboischiotibialis and m. flexor tibialis externus (Figure 20A,B,D; Table 5). In our material, the insertion area is visible in ZPAL Ab III/361, 403, 414, 1246, 1930, 460/3, 411/2 and 1239 (compare with Fechner, 2009; Schachner et al. 2011; Figure 21B). The action of the m. flexor tibialis internus would have been adduction and extension of the hip, as well as flexion of the knee (see Schachner et al. 2011; Table 5).
Figure 29.

Muscle scars visible on the ischium of Silesaurus opolensis. The uppermost photograph is of ZPAL AbIII/3226, the first below ZPAL AbIII/404/7, the others from ZPAL AbIII/925. (A) Left ischium in lateral view. (B) Left ischium in medial view. (C) Left ischium in ventral view. (D) Left ischium in dorsal view
3.4.11. Musculus flexor tibialis externus
In Silesaurus, the m. flexor tibialis externus originates from the posterior iliac crest (compare with Romer, 1923b; George and Berger, 1966; Gangl et al. 2004; Schachner et al. 2011). A rugose tuberosity on the postacetabular process is divided into two distinct portions. The largest marks the origin of the m. iliofibularis, while the smaller marks the origin of the m. flexor tibialis externus. The latter is located more posteroventrally (Figures 12 and 14B; Table 5). The insertion of the m. flexor tibialis externus is reconstructed here on the posteromedial surface of the proximal tibia (ZPAL Ab III/361, 403, 414, 1246, 1930, 460/3, 411/2 and 1239), together with the insertions of m. flexor tibialis internus and m. puboischiotibialis (compare with Schachner et al. 2011; Figures 21B and 20A,B,D; Table 5). The m. flexor tibialis externus would have extended and adducted the hip as well as flexed the knee (see Schachner et al. 2011; Table 5).
3.4.12. Musculus adductors
Silesaurus is interpreted as having two parts to the m. adductors, both originating from the lateral side of the ischium (ZPAL Ab III/361, 925 and 404/7), as evidenced by the presence of two clear longitudinal scars. The first scar runs along the dorsal margin of the posterior ischium, proximolateral to the m. ischiotrochantericus origin. The second scar is on the ventral portion of the ischial body, distal to the origin of the m. puboischiotibialis (compare with Romer, 1923b; Hutchinson, 2001b; Fechner, 2009; Schachner et al. 2011; Figure 14B,C; Table 5). The insertion of the m. adductors is clearly visible (ZPAL Ab III/361, 1914 and 460/1) as a distinct scar between the medial and lateral femoral condyles (compare with Romer, 1923b; Fechner, 2009; Maidment and Barrett, 2011; Figures 16B, 17A and 18B; Table 5). The scar is rounded and marks the distalmost extremity of this muscle attachment. Its proximal limit is difficult to trace. The action of the m. adductors would be adduction and extension of the hip (see Schachner et al. 2011; Table 5).
3.4.13. Musculus puboischiofemoralis externus
The orientation of the pubis and ischium remains plesiomorphic in Silesaurus. For that reason, the m. puboischiofemoralis externus is reconstructed in three parts (compare with Hutchinson, 2001b; Fechner, 2009; Figure 14B,C; Table 5). It was the main muscle attached to the pubic shaft. M. puboischiofemoralis externus 1 probably originated from the medial surface of the distal half of the shaft, but our reconstruction should be treated as tentative because this area is not well preserved in available specimens. M. puboischiofemoralis externus 2 is reconstructed as originating on the lateral surface of the distal pubic shaft, with an indistinct scar marking its distal limit (compare with Hutchinson and Gatesy, 2000; Hutchinson, 2001a; Fechner, 2009). The m. puboischiofemoralis externus 3 probably occupied the lateral ischial shaft distally between the sites of origin of the m. adductors, as seen in crocodiles (compare with Gadow, 1882; Romer, 1923b; Fechner, 2009). Some longitudinal striations close to the distal end of the bone may indicate its presence. All parts of the muscle inserted on a dorsolateral ossification (‘anterolateral scar’ Griffin and Nesbitt, 2016; e.g. ZPAL Ab III/361, 405 and 411/4; Figure 19B) or rugose scar (e.g. ZPAL Ab III/457, 460/1, 1930, 2063 and 563/7) on the femoral head (compare with Gadow, 1882; Romer, 1923a; George and Berger, 1966; Hutchinson, 2001b; Fechner, 2009; Maidment and Barrett, 2011; Figure 16; Table 5). The m. puboischiofemoralis externus would have flexed and adducted the hip (see Schachner et al. 2011; Table 5).
3.4.14. Musculus ischiotrochantericus
The m. ischiotrochantericus is reconstructed here as originating from the dorsomedial surface of the distal ischium (compare with Romer, 1923b; Gangl et al. 2004; Fechner, 2009; Maidment and Barrett, 2011; Liparini and Schultz, 2013; Figure 14C; Table 5), marked by a proximodistally elongated rugose scar (ZPAL Ab III/3223, 925 and 404/7; Figure 29B). Its site of insertion is located on the dorsolateral trochanter (‘greater trochanter’ Griffin and Nesbitt, 2016), as indicated by a prominent scar in some specimens (Figures 16 and 19B; Table 5). Proximally, this insertion lies next to that of the m. puboischiofemoralis externus as in modern archosaurs (Hutchinson, 2001b; Fechner, 2009; Maidment and Barrett, 2011). In our material, the insertional area is visible in specimens ZPAL Ab III/361, 405, 457, 1930, 460/1 and 411/4. The m. ischiotrochantericus would have laterally rotated (supination) and retracted the hip (see Schachner et al. 2011; Table 5).
3.4.15. Musculus caudofemoralis brevis
In Silesaurus, a large ventrally concave surface on the anteroventral portion of the postacetabular ala (Figure 14B; Table 5), the brevis fossa (Novas, 1996), marks the origin of the m. caudofemoralis brevis. It is visible in ZPAL Ab III/361, 362 and 404/1,2 (compare with Romer, 1923b; Gauthier, 1986; Fechner, 2009; Schachner et al. 2011; Figure 13A). The fourth trochanter of Silesaurus bears an extensive scarring on its posteromedial surface, marking the insertion of the muscle (Figures 16B and 17A; Table 5). The insertional area is relatively wide in the middle, but tapers proximally and distally. It is visible in ZPAL Ab III/361 and 1914 (compare with George and Berger, 1966; Gangl et al. 2004; Maidment and Barrett, 2011; Figure 18B). The m. caudofemoralis brevis would have extended and adducted the hip (see Schachner et al. 2011; Table 5).
3.4.16. Musculus caudofemoralis longus
Silesaurus had a long, strong tail (Piechowski and Dzik, 2010) so it is likely that the origin of the m. caudofemoralis longus resembled that of crocodiles (compare with Romer, 1923a; Fechner, 2009; Schachner et al. 2011, contra Gatesy, 1990; Gangl et al. 2004; Maidment and Barrett, 2011), with an insertion on the femur marked by an oval concavity anteromedial to the fourth trochanter (Langer, 2003; Figure 17; Table 5). The insertion area can be seen in ZPAL Ab III/361, 1930, 1914, 2063 and 411/4 (compare with Fechner, 2009; Maidment and Barrett, 2011; Schachner et al. 2011; Figure 18A; Table 5). There may have been a second insertion behind the knee (see Liparini and Schultz, 2013). The m. caudofemoralis longus would have extended and adducted the hip (see Schachner et al. 2011; Table 5).
3.5. Muscles to the Pes
3.5.1. Musculus gastrocnemius
Based on the phylogenetic bracket, Silesaurus probably had at least two parts of the m. gastrocnemius (compare with Hutchinson, 2002; Fechner, 2009; Schachner et al. 2011). As reconstructed, its pars lateralis arose from a rugose area on the lateral femoral condyle, just distal to the insertion of the m. adductors (Figure 17A; Table 6), whereas the pars medialis originated from the medial aspect of the proximal tibia (Figure 20D; Table 6). The area displays distinct longitudinal scarring (ZPAL Ab III/361, 403, 414, 1245, 1246, 2539; Figure 22A). It is difficult to judge whether Silesaurus had the avian pars intermedius. If it was present, it could have arisen from the posteromedial aspect of the femoral medial condyle between the m. adductors and the m. femorotibialis pars medialis. Langer (2003), after Romer (1927b), suggested that the m. gastrocnemius originated from the distal inflection of the fourth trochanter of Saturnalia; however, this is not supported by the anatomy of extant archosaurs (Fechner, 2009; Schachner et al. 2011). In Silesaurus, all divisions of the m. gastrocnemius probably merged into common tendon that ran distal to the calcaneum and inserted along the ventral aspect of metatarsals II–IV (compare with Gadow, 1882; Hudson et al. 1959; McGowan, 1979; Nickel et al. 2003; Gangl et al. 2004; Fechner, 2009; Schachner et al. 2011; Figure 25D; Table 6). The m. gastrocnemius would have flexed the knee and extended the ankle joint (see Schachner et al. 2011; Table 6).
3.5.2. Musculus tibialis anterior
In Silesaurus, the m. tibialis anterior clearly originated from the lateral side of the tibial cnemial crest (Langer, 2003), as evidenced by scars for muscle attachment (compare with Gadow, 1882; Dilkes, 2000; Fechner, 2009; Schachner et al. 2011; ZPAL Ab III/361, 403, 414, 1930 and 2539; Figure 20A–C; 21A; Table 6). The presence of a second head in Silesaurus is uncertain, as there is no obvious attachment surface on the femur (compare with Hudson et al. 1959; McGowan, 1979; Nickel et al. 2003; Gangl et al. 2004; Fechner, 2009; Schachner et al. 2011). Distinct muscle insertions are visible on the proximolateral surfaces of metatarsals II–IV in ZPAL Ab III/361 and 439/2 (compare with Hudson et al. 1959; McGowan, 1979; Dilkes, 2000; Nickel et al. 2003; Gangl et al. 2004; Fechner, 2009; Schachner et al. 2011; Figures 25B,C and 26E,F,G; Table 6). The insertion on metatarsal II is located further distally than others and is relatively short, whereas that on metatarsal III is slightly more proximal and twice as long and that on metatarsal IV lies at the proximal end of the bone, and is short and wide. The m. tibialis anterior would have flexed the ankle joint (see Schachner et al. 2011; Table 6).
3.5.3. Musculus popliteus
The reconstruction of the m. popliteus in Silesaurus is unequivocal, with an attachment to the facing (interosseal) surfaces of the proximal fibula and tibia where both bones display clear longitudinal concavities (compare with Osawa, 1898; Romer, 1922; Hudson et al. 1959; McGowan, 1979; Nickel et al. 2003; Gangl et al. 2004; Fechner, 2009; Schachner et al. 2011; Figures 20A20, 25C and 20, 25C; Table 6). In our material, the area of origin area is visible in specimens ZPAL Ab III/361, 403, 414, 1245 and 1930 (Figures 21A and 24B), and the insertion site is visible on ZPAL Ab III/361 and 416. The m. popliteus would have rotated the fibula (see Schachner et al. 2011; Table 6).
3.5.4. Musculus interosseous cruris
Reconstruction of the m. interosseous cruris in Silesaurus is unequivocal as the distal fibula is not reduced, and therefore, the muscle would have attached to the facing surfaces of the tibia and fibula (compare with Gadow, 1882; Kriegler, 1961; Vanden Berge and Zweers, 1993; Carrano and Hutchinson, 2002; Hutchinson and Garcia, 2002; Fechner, 2009; Figure 23C; Table 6). The tibia bears a distinct groove that may mark the attachment (ZPAL Ab III/ 361, 403/3, 411/2, 1225, 1930 and 1248; Figure 21A) whereas the distal fibula has a small ridge in that area (ZPAL Ab III/361). The m. interosseous cruris would have flexed the ankle joint (Table 6).
3.5.5. Musculus pronator profundus
The fibula of Silesaurus is not reduced, so we reconstructed the m. pronator profundus like that of a crocodile. It originated unequivocally on the posterior or posteromedial portion of the fibula and the lateral side of the tibia (compare with Gadow, 1882; Tarsitano, 1981; Hutchinson and Garcia, 2002; Fechner, 2009; Figures 20A,B,D and 23C; Table 6), and inserted on the ventromedial surface of the base of metatarsal II, due to reduction of metatarsal I (compare with Gadow, 1882; Kriegler, 1961; Fechner, 2009; Figure 25D; Table 6). The bone has a clearly visible scar in that area (Figure 26A). The m. pronator profundus would have flexed the ankle joint (Table 6).
3.5.6. Musculus fibularis longus and brevis
Silesaurus lacks any osteological correlates for the m. fibularis; however, it probably retained a fibular origin for both muscle heads because its fibula is not reduced and the tibia lacks cristae like those that serve for the m. fibularis longus attachment in birds (compare with Fechner, 2009; Schachner et al. 2011; Figure 23A,B; Table 6). The calcaneum of Silesaurus lacks a calcaneal tuber (Nesbitt, 2011; Figure 25A; Table 6), so the m. fibularis longus could have inserted elsewhere, as in birds. The m. fibularis brevis probably inserted on the distoventral surface of metatarsal V (compare with Hudson et al. 1959; McGowan, 1979; Nickel et al. 2003; Gangl et al. 2004; Fechner, 2009; Schachner et al. 2011; Figure 25D; Table 6). The m. fibularis would have flexed the ankle joint (see Schachner et al. 2011; Table 6).
3.5.7. Musculus extensor digitorum longus and brevis
Reconstruction of the m. extensor digitorum longus in Silesaurus was difficult. We correlated a shift in its origin from femur to tibia with the appearance of a cnemial crest (ZPAL Ab III/361, 2539, 1930 and 403/3,4); thus, the muscle may have originated from the anterior face of the cnemial crest as in birds (compare with Gadow, 1882; Hudson et al. 1959; Kriegler, 1961; McGowan, 1979; Tarsitano, 1981; Dilkes, 2000; Nickel et al. 2003; Gangl et al. 2004; Fechner, 2009; Schachner et al. 2011; Figures 20A20, 21C and 20, 21A; Table 6). One specimen (ZPAL AbIII/361/22) bears a distinct oval tuberosity distal to the origin. It could be interpreted as a distal extension of the origin but is probably some kind of pathology as it is absent in other specimens. The insertion site is equivocal due to the differences in extant archosaurs (see above). As Silesaurus was digitigrade with partially integrated metatarsals, it may have approached the avian in which the m. extensor digitorum longus and brevis could already be fused (Hutchinson and Garcia, 2002), with a single insertion on the dorsal surface of the phalanges and on the proximodorsal aspects of the unguals (compare with Gadow, 1882; Hudson et al. 1959; McGowan, 1979; Tarsitano, 1981; Vanden Berge and Zweers, 1993; Carrano and Hutchinson, 2002; Nickel et al. 2003; Fechner, 2009; Schachner et al. 2011; Figure 25B,C; Table 6) as seen in ZPAL Ab III/361. Both the m. extensor digitorum longus and brevis would have flexed the ankle joint and extended the pedal digits (see Schachner et al. 2011; Table 6).
3.5.8. Musculus flexor digitorum longus and brevis
In Silesaurus the origin of the m. flexor digitorum longus is marked by a clear tear‐shaped region on the femoral lateral condyle, distal to the origin of the m. femorotibialis lateralis (compare with Dilkes, 2000; Fechner, 2009; Schachner et al. 2011; Figures 16B and 17A; Table 6). A slightly rugose surface on the posterolateral aspect of the proximal fibula is interpreted as an origin site of the second head (compare with Hudson et al. 1959; McGowan, 1979; Nickel et al. 2003; Gangl et al. 2004; Fechner, 2009; Schachner et al. 2011; Figures 23A and 24A; Table 6). The muscle would have been inserted on the ventral surface of the unguals of digits II–IV (compare with Hudson et al. 1959; Kriegler, 1961; McGowan, 1979; Tarsitano, 1981; Dilkes, 2000; Nickel et al. 2003; Gangl et al. 2004; Fechner, 2009; Schachner et al. 2011; Figure 25D; Table 6).
The m. flexor digitorum brevis is not reconstructed for Silesaurus as its presence is uncertain, given its absence in birds (see Gadow, 1882; Kriegler, 1961; Vanden Berge and Zweers, 1993; Dilkes, 2000; Fechner, 2009). If present it would have originated from the plantar aponeurosis and inserted on the bases of the phalanges of digits II–IV. Both the m. flexor digitorum longus and brevis would have extended the ankle joint and flexed the digits (see Schachner et al. 2011; Table 6).
3.5.9. Musculus extensor hallucis longus
In Silesaurus, in which we observed a reduction of digit I, the m. extensor hallucis longus was probably lost together as in some modern ratites (McGowan, 1979; compare with Gadow, 1882; McGowan, 1979; Carrano and Hutchinson, 2002; Nickel et al. 2003; Fechner, 2009; Schachner et al. 2011; Table 6).
3.5.10. Musculus flexor hallucis longus
The muscle was probably lost in Silesaurus, together with digit I (compare with Carrano and Hutchinson, 2002; Fechner, 2009; Schachner et al. 2011; Table 6).
3.6. Ligaments
In this section we describe ligaments that left a trace on the appendicular skeleton of Silesaurus. This description is far from exhaustive as we focused only on ligament attachments that could be confused with muscle attachments. At least two ligaments left distinct scars on the anterior and the posterior aspects of the distalmost ulna (Figures 8 and 9A). Their surface is more rugose than muscle scars preserved in the same specimens.
The pelvis of lepidosaurs and crocodiles is associated with a series of ligaments that act as structural supports and attachment sites for the pelvic muscles (Schachner et al. 2011). Hutchinson (2001a) argued that the primary semicircular ilio‐ and ischiopubic ligaments of birds are probably homologous to those of extant crocodilians.
3.6.1. Iliopubic ligament
In the above taxa, this ligament or its homologues (Hutchinson, 2001a) generally runs from the preacetabular ilium to the pubic tubercle, and serves as a site of origin for some of the hypaxial musculature (Schachner et al. 2011). The attachment areas of the iliopubic ligament in Silesaurus resemble those in Poposaurus (Schachner et al. 2011). The ligament arose from the ventral surface of the preacetabular process of the ilium (Figures 13C and 14C), as indicated by a longitudinal groove just medial to the origin of the m. puboischiofemoralis internus 2. The insertion is clearly visible as a rugose area on the medial side of the iliopubic articulation (Figures 13b, 14 and 28A). The attachments of the iliopubic ligament are visible in ZPAL Ab III/2517, 404/1,2,5 and 462.
3.6.2. Puboischiadic ligament
In extant archosaurs, the puboischiadic ligament originates on the caudoproximal aspect of the pubis and inserts on the proximal ischium (Hutchinson, 2001a; Schachner et al. 2011). Distinct striations on the ventral pubis of Silesaurus (ZPAL Ab III/404/5) may represent an origin site for this ligament (Figures 14C and 28C), whereas the ischial insertion may have been between the origins of the m. puboischiotibialis and the m. adductor 1, at the ventral margin of the bone (Figure 14B).
3.6.3. Ilioischiadic ligament
The ilioischiadic membrane of Neornithes and the dense fascia in crocodiles are probably homologues of the ilioischiadic ligament of lepidosaurs (Hutchinson, 2001a; Schachner et al. 2011). We followed the reconstruction of Poposaurus (Schachner et al. 2011), where the ligament was reconstructing as arising from a distal pit on the ventral surface of the postacetabular process. The ilium of Silesaurus has a clear rugosity in the same position in ZPAL Ab III/362 and 404/1,2 (Figure 14C). As in Poposaurus, the ischial attachment site is unclear.
3.6.4. Ligamentum capitis femoris
In crocodiles, this ligament has two crura that originate from the pelvis and merge into a single attachment on the femur. The caudal crus originates from the acetabular part of the pubic peduncle of the ischium, and the condition in Silesaurus was probably similar as the ischium bears a rugose attachment area (Figure 14B). The rostral crus in crocodiles is a continuation of the deep meniscus (Tsai and Holliday, 2015). The two crura join and insert on the surface of the posteromedial tuber (Figure 17A), which is variably developed in Silesaurus (Piechowski et al. 2014). The attachment site of the ligamentum capitis femoris is visible in ZPAL Ab III/361, 405 and 411/4 (Figure 18B).
3.6.5. Iliofemoral ligament
In crocodiles, the iliofemoral ligament originates dorsally on the acetabular labrum of the acetabular crest (Tsai and Holliday, 2015). The area is greatly expanded in Silesaurus, forming a distinct supra‐acetabular crest that creates a roof for the acetabulum and bears clear rugosities along its margin (Figures 12 and 14B). The insertion site of the iliofemoral ligament in Silesaurus is identified as a large, rounded area on the anteromedial aspect of the femoral head, limited distally by a semilunate scar (Figures 16A and 17). The attachments of the iliofemoral ligament are visible in ZPAL Ab III/361, 362, 404/2 and 407/6 (Figure 18A).
3.6.6. Ligamentum collaterale tibiale
The ligament covered the medial side of the knee joint and connected the distal femur with proximal tibia (Haines, 1942). Silesaurus has a weak scar for this ligament on the medial surface of the medial femoral condyle (Figure 17B), and the proximal head of the tibia bears a clear irregular scar posteromedially (Figure 20D). The attachments of the ligamentum collateral tibiale are visible in ZPAL Ab III/361, 403, 416 and 2539 (Figure 22A).
3.6.7. Ligamentum collaterale fibulare
This ligament connects the distal femur with the proximal fibula, on the lateral side of the knee joint (Haines, 1942). Silesaurus bears large scars for this ligament. The lateral condyle of the femur bears semitriangular rugose area on its lateral surface (Figure 16A). It is visible on ZPAL AbIII/361, 363, 1263, 1914 and 403/5 (Figure 19B). The insertion covers most of the lateral surface of the proximal fibular head (Figure 23A,B), as evidenced by extensive but irregular scarring, especially visible on ZPAL AbIII/361/24 (Figure 24A).
3.6.8. Ligamentum tibio‐fibulare
This ligament connects the tibia and fibula anteriorly, below the knee joint in crocodiles (Haines, 1942). Intensive scarring on the anterior surface of the proximal fibula of Silesaurus marks the presence of this ligament (Figure 23A,B), as may further intensive scarring on the medial concavity of the fibular head and on the fibular articular facet on the lateral aspect of the tibia (Figures 20A20, 23C and 20, 23C). The attachments of the ligamentum tibio‐fibulare are visible in ZPAL Ab III/361 and 416 (Figures 21A and 24).
3.6.9. Medial tibial‐astragalar ligament
This is one of the ligaments connecting the tibia and astragalus of crocodiles (Brinkman, 1980). It limits rotation between the two bones (Brinkman, 1980). In Silesaurus (ZPAL Ab III/361, 403/1, 411/2 and 1247: Figure 22A), the distal tibia bears a tongue‐shaped scar on its medial aspect (Figure 20D). The ligament probably inserted on the medial aspect of the astragalus (Figure 25A) that bears a distinct semihorizontal groove in that area.
4. DISCUSSION
4.1. Musculature of Silesaurus compared with that of extant archosaurs
The derived condition of extant crocodiles and birds makes interpretation of primitive archosaurian musculature difficult. Silesaurus is one of the earliest members of the dinosaur‐line archosaurs. Given its geological age and phylogenetic position, it may help us to understand the polarity of archosaurian characters. The pectoral musculature of Silesaurus (3and30, 31, 32, 33, 34, 35) was obviously more crocodile‐like than bird‐like because birds have highly modified forelimbs for flight. The scapular blade of Silesaurus and crocodiles has a machete‐like shape (Figures 2 and 3A,C), whereas in birds it is narrow and sabre‐like. A prominent acromial process contributes to a large area for attachment of the supracoracoideus, which protracts, retracts and abducts the humerus in crocodiles and thus also Silesaurus (Figures 2, 3A,C and 30, 31, 32, 33, 34). Birds reduced this process in association with changes in the musculature. Birds and crocodiles convergently expanded the coracoid toward the sternum. Triassic archosaurs including Silesaurus restrict this part of the coracoid to a small tuber (Figures 2 and 3). The complex architecture of this tuber in Silesaurus probably reflects attachments of various muscles (m. costocoracoideus, m. triceps and m. coracobrachialis) grouped on a small area (Figures 3 and 30, 31, 32, 33, 34), and may represent the plesiomorphic archosaurian condition. The anatomy of the forelimb is simplified in Silesaurus by comparison to Euparkeria and Osmolskina (see below), so the typical archosaurian condition should not be expected in its brachial musculature.
Figure 30.
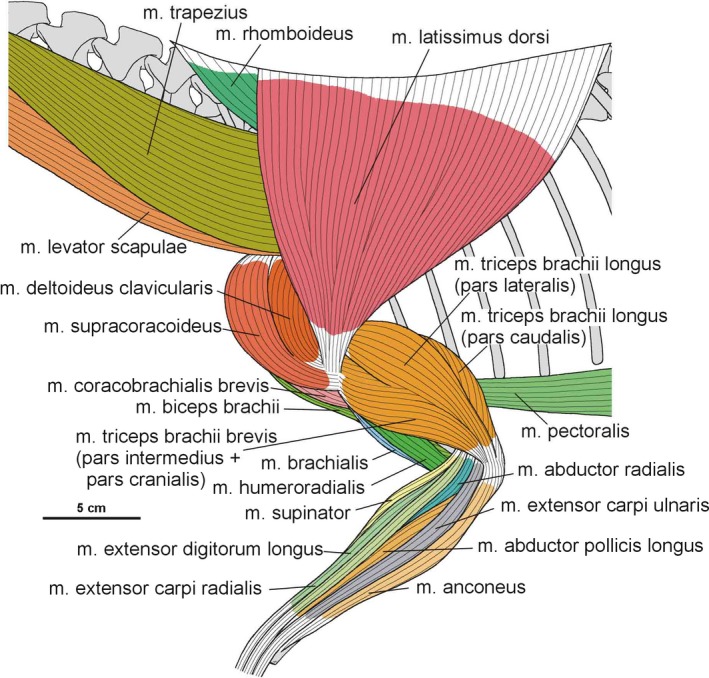
Muscle disposition on the forelimb of Silesaurus opolensis in lateral view
Figure 31.
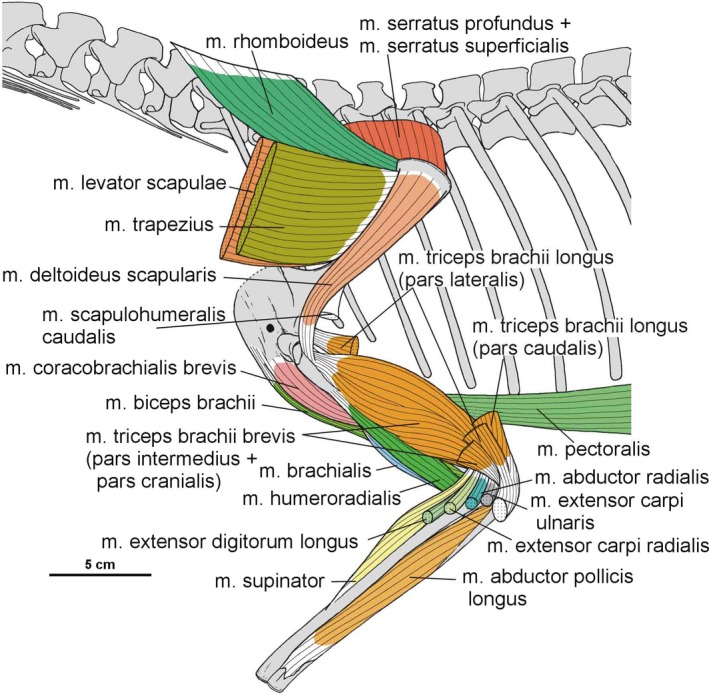
Muscle disposition on the forelimb of Silesaurus opolensis in lateral view. Some muscles are removed
Figure 32.
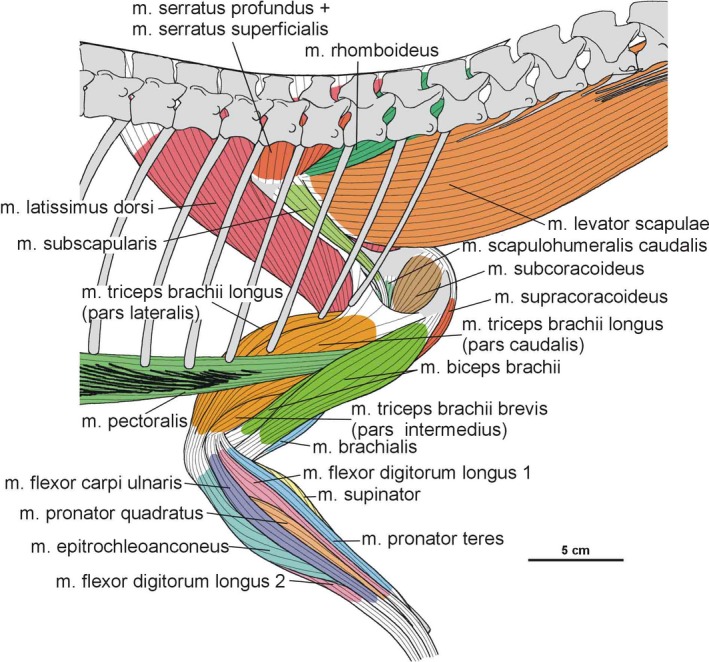
Muscle disposition on the forelimb of Silesaurus opolensis in medial view
Figure 33.
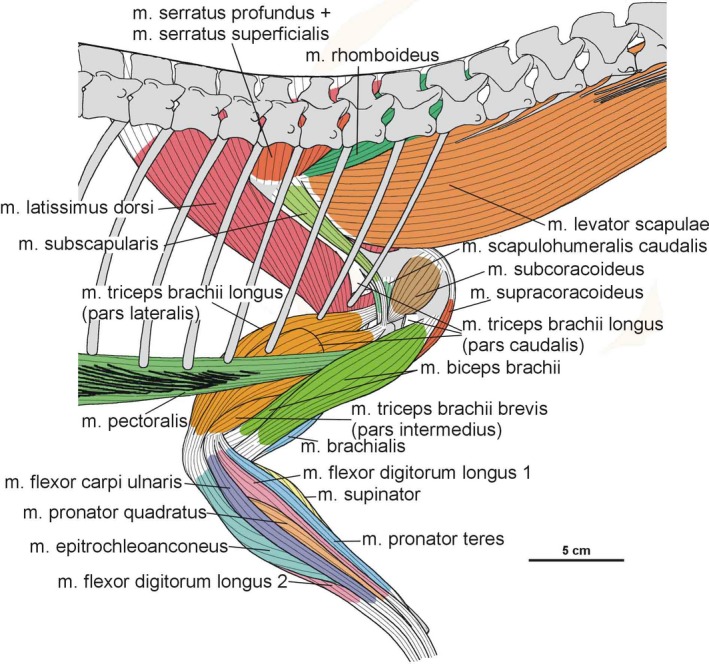
Muscle disposition on the forelimb of Silesaurus opolensis in medial view. Some muscles are removed
Figure 34.
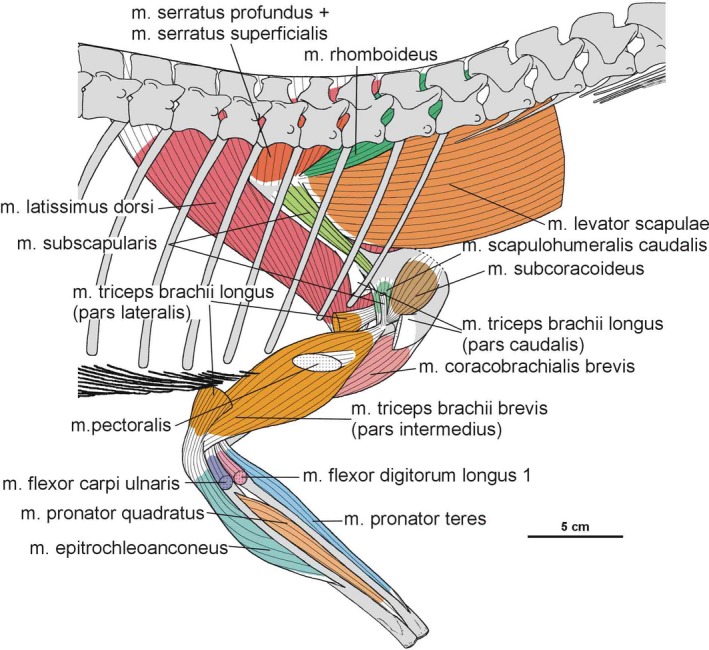
Muscle disposition on the forelimb of Silesaurus opolensis in medial view. Some muscles are removed
Figure 35.
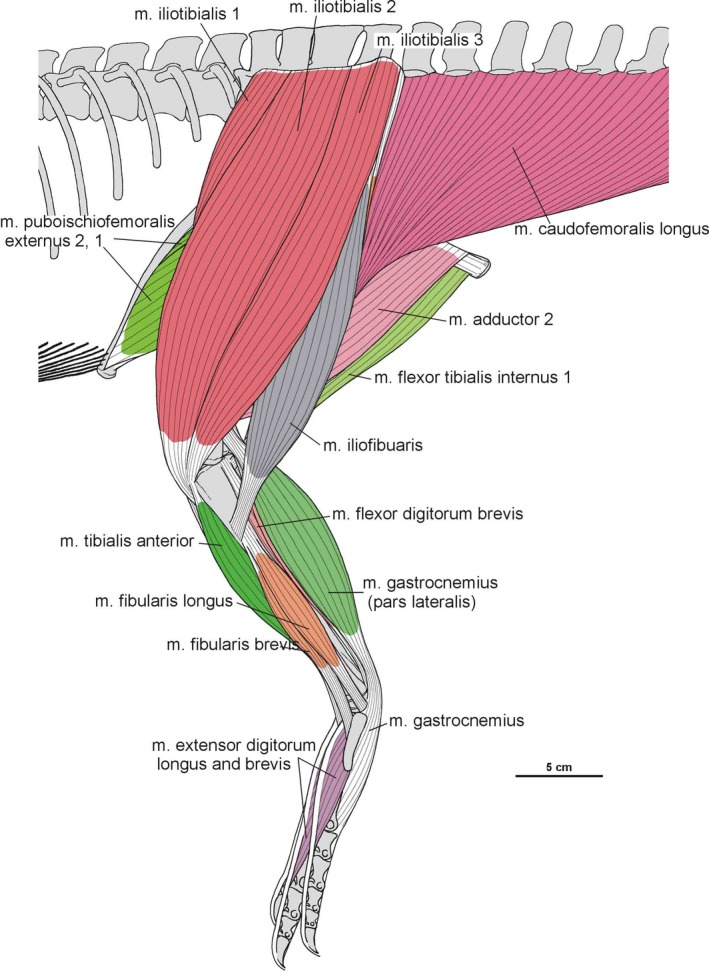
Muscle disposition on the hindlimb of Silesaurus opolensis in lateral view
Intermuscular lines on the femur of Silesaurus indicate that the m. femorotibialis had three distinct parts, as in birds, although crocodiles have only two (Figures 16, 17 and 35, 36, 37, 38, 39, 40). It remains unknown whether the ancestor of the dinosaur‐bird clade gained the third part or the common ancestors of archosaurs already had it. In the latter scenario, crocodiles secondarily lost the third part. As in crocodiles, osteological correlates are present for only two heads of the m. puboischiofemoralis internus in Silesaurus (Figures 14B,C and 36, 37, 38, 39, 40). The insertion sites for this muscle on the femur of Silesaurus are similar to those in crocodiles. If our identification is correct, the muscle originated from the well‐developed anterior process of the ilium, which would approach the bird condition (Hutchinson and Gatesy, 2000). Because more derived archosaurs on the lineage to birds expanded the process further anteriorly, this change would be homologous with the condition found in Aves. The obturator plate is reduced in Silesaurus, so the m. puboischiotibialis was also reduced in size (Figures 14B,C, 20A,B,D and 38, 39, 40; it is absent in birds). The ischium of Silesaurus has a scar in the same place as the crocodilian pit for the m. flexor tibialis internus 3, suggesting this is the primitive condition (Figures 14B,C and 36, 37, 38, 39, 40). The ischium of Silesaurus has more posterior orientation than that of crocodiles but the position of the m. adductors origin on that bone is homologous (Figures 14B,C and 36, 37, 38, 39, 40). The development of the cnemial crest is probably correlated with the more proximal position of origins of the m. tibialis anterior and the m. extensor digitorum longus (Figures 20A20, 36, 37, 38, 39, 40C and 20, 36, 37, 38, 39, 40), but insertions of the m. tibialis anterior are primitive (crocodilian‐like) due to the separated metatarsals. Insertions of the m. extensor digitorum longus are difficult to determine (Figure 25B,C). The well‐developed distal part of fibula and the scaring on the distal tibia and fibula suggest a primitive condition for the m. interosseous cruris (Figure 20A–C), and also for the m. pronator profundus, the m. fibularis longus and brevis, and the m. popliteus (Hutchinson, 2002; Figures 20, 23 and 35, 36, 37, 38). The femur of Silesaurus retained a tear‐shaped scar for the m. flexor digitorum longus (Figures 16B and 17A), while in birds the origin had shifted to the tibia.
Figure 36.
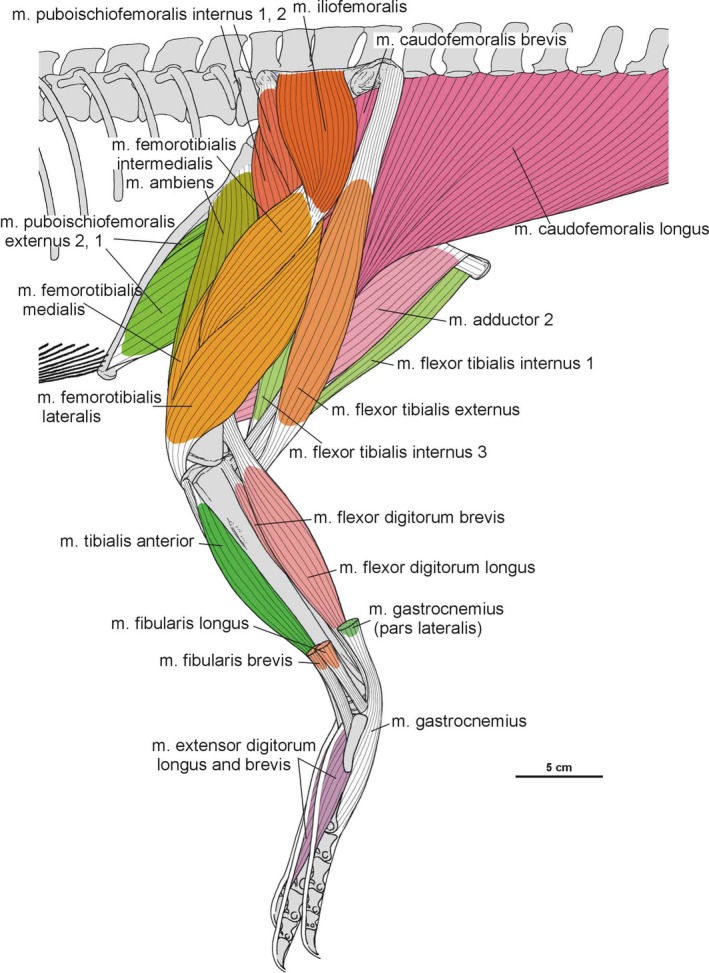
Muscle disposition on the hind limb of Silesaurus opolensis in lateral view. Some muscles are removed
Figure 37.
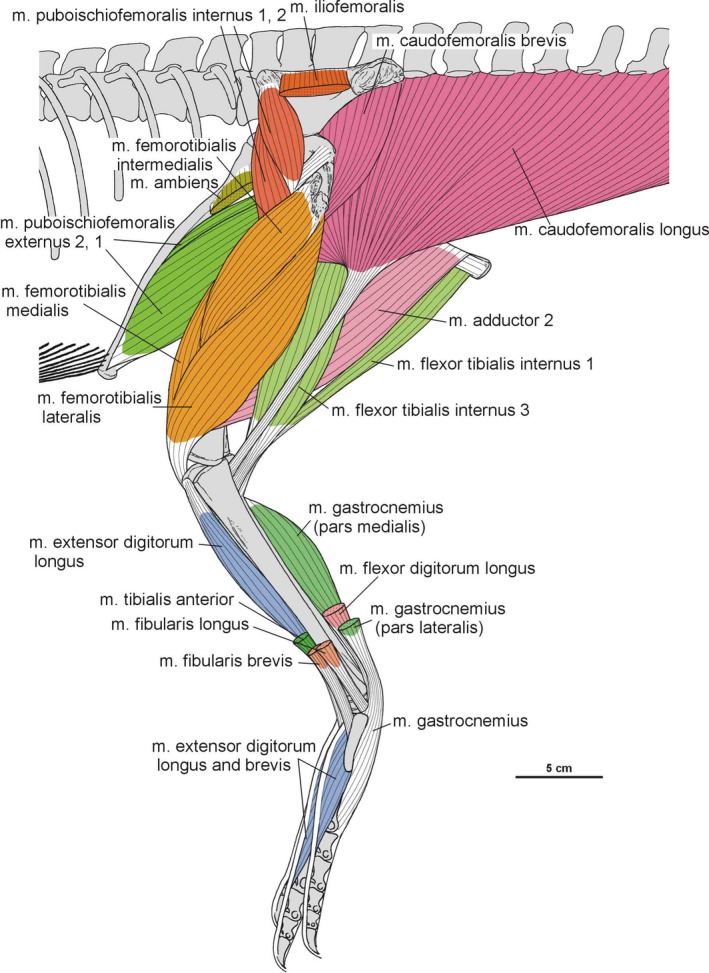
Muscle disposition on the hind limb of Silesaurus opolensis in lateral view. Some muscles are removed
Figure 38.
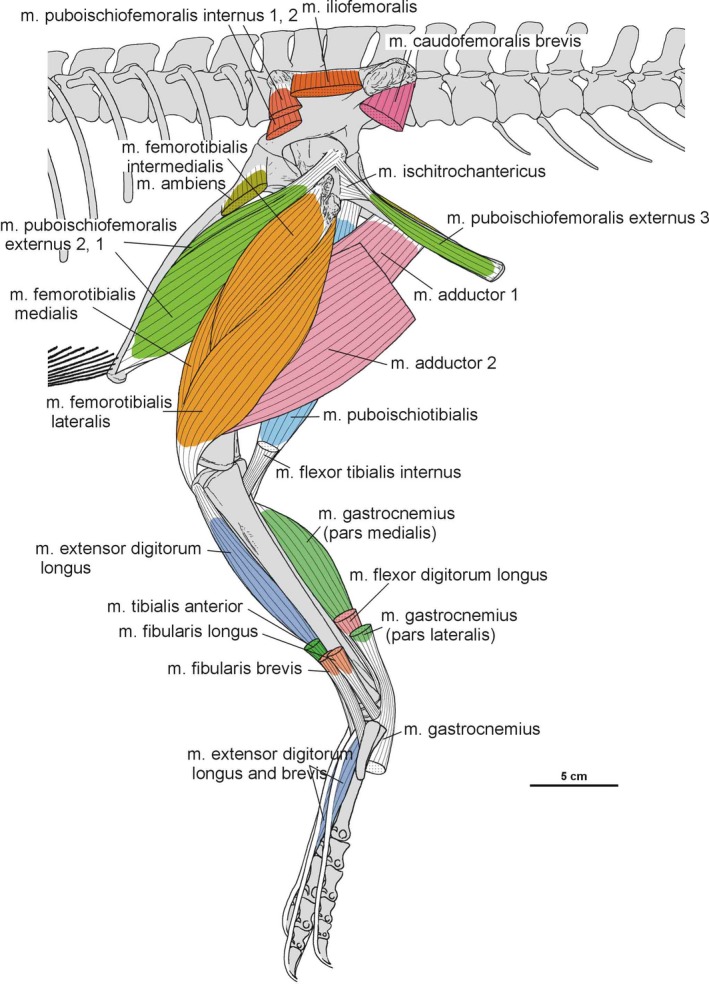
Muscle disposition on the hind limb of Silesaurus opolensis in lateral view. Some muscles are removed
Figure 39.
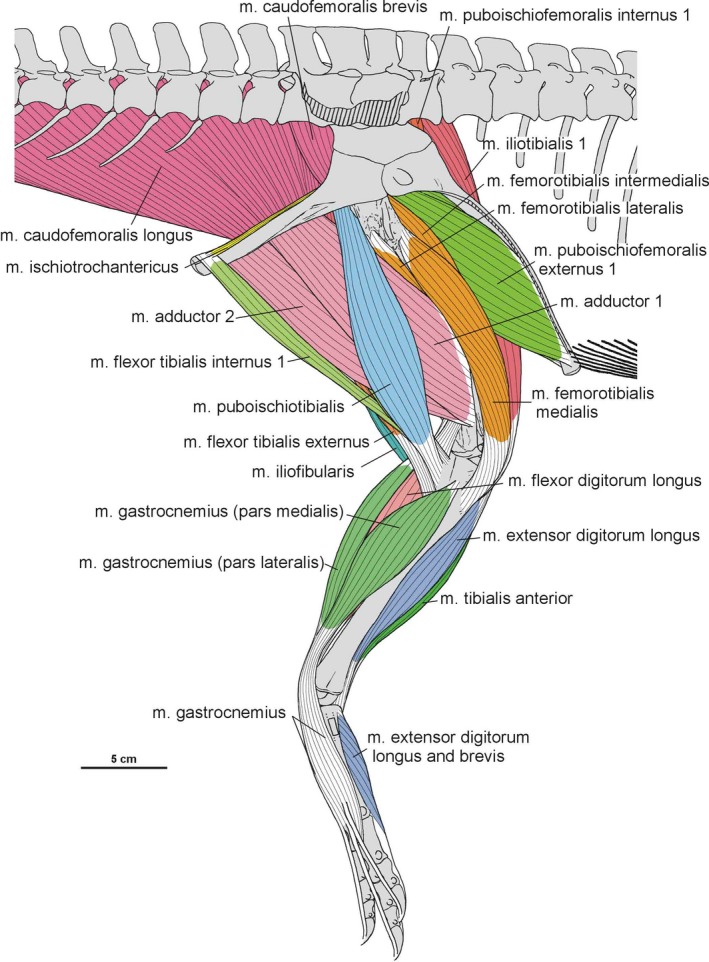
Muscle disposition on the hind limb of Silesaurus opolensis in medial view
Figure 40.
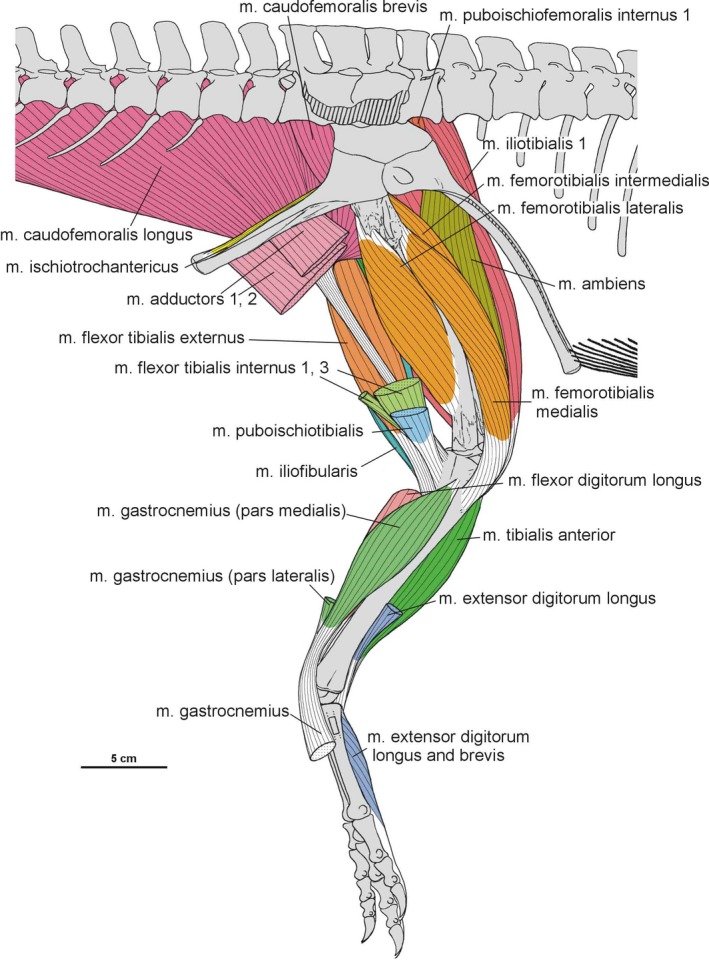
Muscle disposition on the hind limb of Silesaurus opolensis in medial view. Some muscles are cut off
4.2. Locomotion of Silesaurus opolensis
The atypical limb proportions of Silesaurus have previously elicited discussion among researchers about locomotion of this animal. Dzik (2003) reconstructed Silesaurus in a quadrupedal stance, and Fechner (2009), as well as Kubo and Kubo (2012) concluded that Silesaurus was a slow, obligate quadruped based on its body proportions. Later, Piechowski and Dzik (2010) also agreed that Silesaurus evolved toward quadrupedality (Figure 41). However, they suggested that the gracile forelimbs and large counterbalancing tail might indicate an ability to run bipedally at speed (Figure 42). Otero et al. (2019) point out that relative development of the tail and neck plays a more important role in supporting bipedal locomotion than influence of hindlimb/forelimb lengths.
Figure 41.
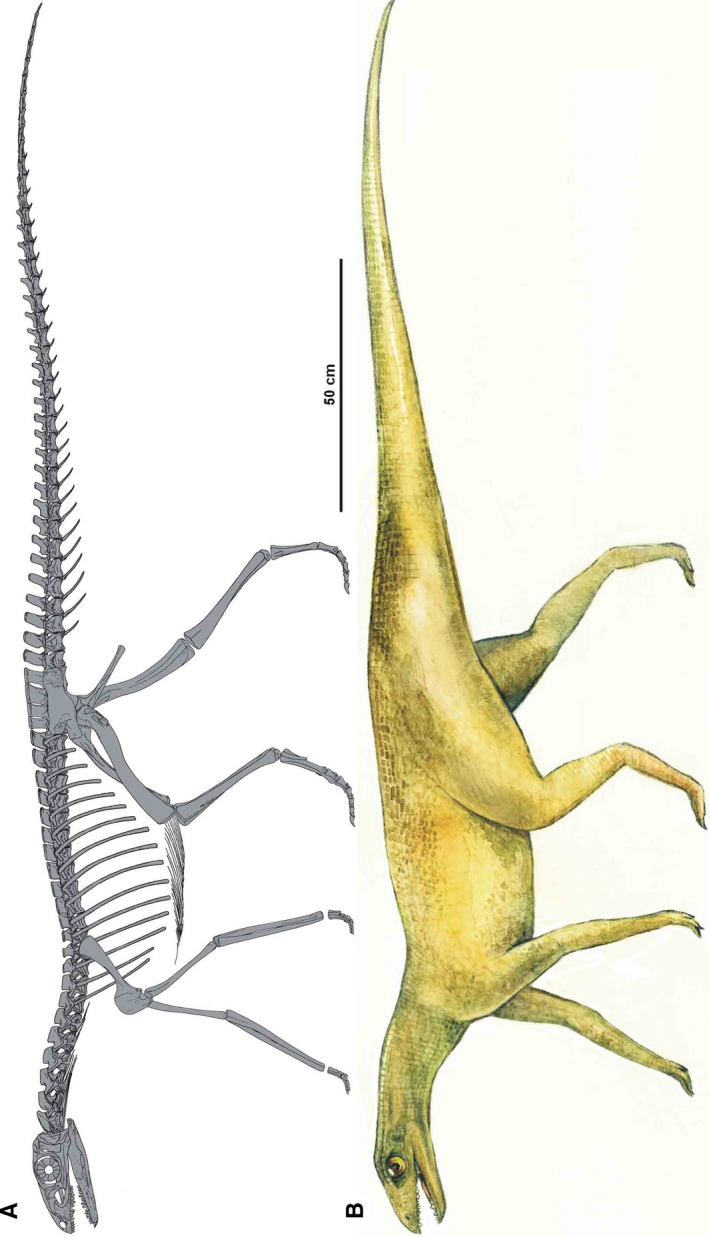
Restoration of Silesaurus opolensis in a quadrupedal pose. (A) Skeletal reconstruction in lateral view (by Rafał Piechowski). (B) Body reconstruction in lateral view (drawing by Małgorzata Czaja based on reconstruction by Rafał Piechowski)
Figure 42.
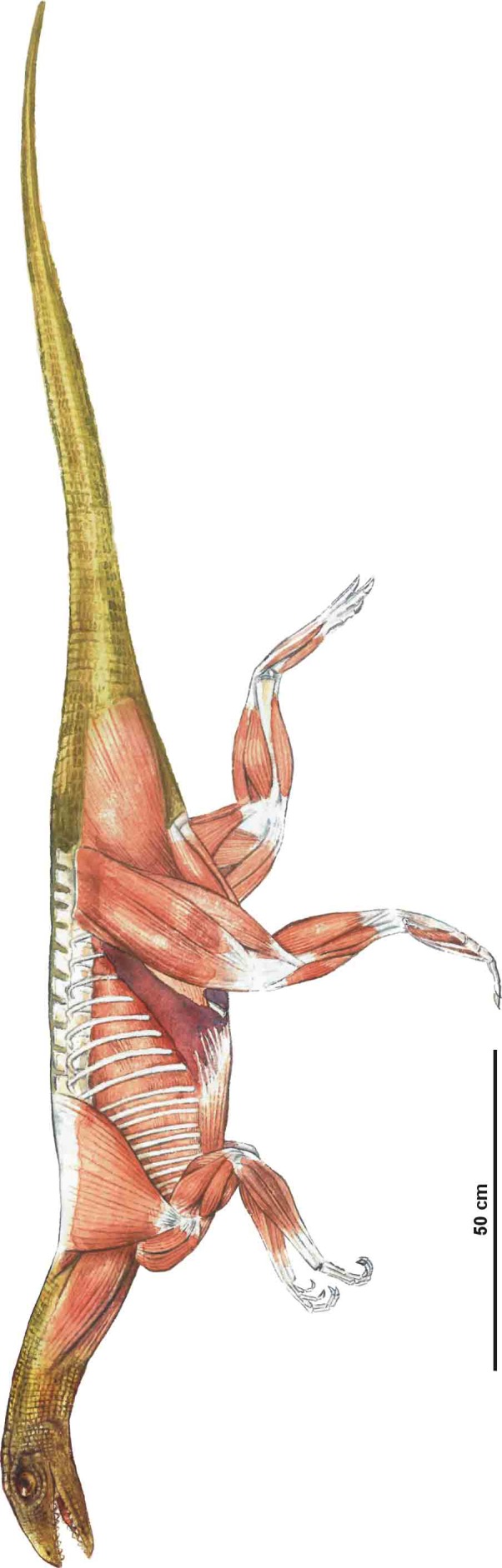
Restoration of muscles in Silesaurus opolensis in a facultative bipedal running pose. Note that muscles are slightly separated from each other for greater visibility. Drawing by Małgorzata Czaja based on reconstruction by Rafał Piechowski
The hindlimb to trunk length ratio of Silesaurus is 0.79, which is similar to that of obligate quadrupeds (Remes, 2008). The antebrachium is similar in length to the humerus in Silesaurus (1.1), a very high value compared to that of basal dinosaurs and Euparkeria in which the antebrachium ranges from 0.62 to 0.84 of humeral length (Remes, 2008). It means that elongation of the forelimb of Silesaurus was achieved mainly by prolonging the antebrachium. In sum, short hindlimbs (relative to the trunk) and elongated forelimbs support previous hypotheses that Silesaurus was an obligate quadruped (Figure 41).
It is interesting in this context that we found many similarities between the forelimbs of Silesaurus and those of non‐neosauropod non‐mamenchisaurid sauropods like Patagosaurus and Cetiosaurus. These are: (1) weakly expanded distal end of scapula; (2) long, slender and straight scapular blade; (3) convex anterior (dorsal) margin of scapula; (4) deep scapular head occupied by large oval fossa; (5) slightly expanded humeral heads relative to the shaft; (6) subtriangular proximal half of humerus in anterior view; (7) indistinct torsion of humeral heads; (8) reduced deltopectoral crest; (9) distal humeral head narrower than the proximal one; (10) relatively slender radius and ulna; and (11) reduced olecranon process.
The obvious difference between Silesaurus and early sauropods is the robustness of the forelimb elements, which is a consequence of size and weight in these animals. The common characters of the scapula are not restricted to Silesaurus and sauropods. They rather represent the primitive condition and function. However, the anatomy of the humerus, ulna and radius is derived in early sauropods compared to prosauropods. The same situation applies to Silesaurus compared to Teleocrater, Osmolskina or Euparkeria. Reduction of deltopectoral crest marks a decreasing role of m. pectoralis and m. deltoideus clavicularis (Figures 6D and 30, 31, 32, 33, 34). Remes (2008) correlates this with the vertical orientation of the humerus in sauropods. The same may apply to Silesaurus (Figures 2 and 43), which also had reduced humeral protractors. Protraction of humerus was limited by the position of the coracoids (Remes, 2008). The tightly spaced distal condyles of the humerus indicate reduced rotation capabilities in this joint (Remes, 2008; Figures 2 and 6). The olecranon process works as a lever for m. triceps brachii. Reduction of this structure leads to less effective extension movements of the forelimb in Silesaurus and early sauropods (Figures 2 and 43). It also indicates columnar alignment of the elbow joint (Wilson, 2005). Furthermore, these reptiles reduced rotation of humerus, while the ulna is rotated laterally and the radius anteriorly, modifications that enabled effective and permanent pronation of the manus.
Figure 43.
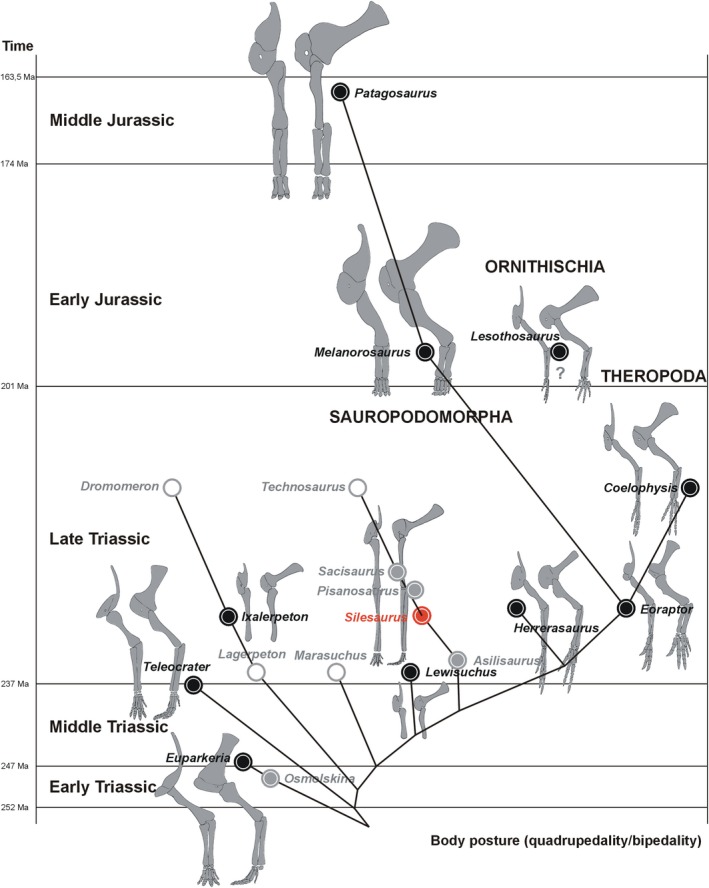
Evolution of forelimb skeleton and its posture in selected early dinosaurs and their predecessors. Black dots represent illustrated taxa. Grey dots are unillustrated taxa having some forelimb bones known. White dots are unillustrated taxa with no forelimb material known. Silesaurus has a red dot. The topology of the tree follows Müller et al. (2018) with the addition of Osmolskina, Melanorosaurus and Patagosaurus. Closely related taxa from different time horizons are illustrated as one lineage for clarity of the figure
In conclusion, Silesaurus and early sauropods achieved a fully quadrupedal stance through analogous joint and muscle modifications. However, they faced the problem of limited forelimb pronation (Bonnan and Yates, 2007). The erect humerus is blocked anteriorly by the coracoidal part of the glenoid (Remes, 2008). The forelimb can make only short steps, prohibiting fast locomotion (Remes, 2008). Large herbivores like sauropods were slow animals. In the case of Silesaurus, Langer et al. (2013) concluded that the simple rounded proximal articular surfaces of the ulna and radius enabled pronation (Figure 2). Thus, the unusually elongated antebrachium of Silesaurus could have been an adaptation to allow greater range of protraction, extending the step length and improving locomotion speed.
In contrast, the hindlimb seems to have been capable of greater speed. Fechner (2009) and Tsai et al. (2018) pointed out several features indicating erect hindlimb posture in Silesaurus. It lacks only a perforated acetabulum (Figure 14B,C). However, Tsai et al. (2018) concluded that Silesaurus had a greater range of hindlimb abduction and axial rotation than was inferred by previous studies. They argued that the femoral epiphysis articulated dorsally with the supraacetabular labrum during parasagittal limb movement. This was based on an incorrect orientation (Dzik, 2003) of the pelvis. The acetabular wall was inclined 30° dorsoventral to the sagittal plane, as in the ilium as a whole. The supra‐acetabular crest of Silesaurus was lateroventrally, not laterally, oriented, (Figures 11B11, 15D and 11, 15). As a consequence, it could not articulate with a dorsomedially oriented femoral head. Instead, it probably restricted femoral abduction and rotation (Hutchinson and Gatesy, 2000; Bates and Schachner, 2012; Tsai et al. 2018). The femoral epiphysis entered the deep acetabulum and articulated with the ilium at the junction of the supra‐acetabular crest and the acetabular wall. This means that Silesaurus obtained a pillar‐erect hindlimb posture similar to that of some pseudosuchians (Benton and Clark, 1988; Bates and Schachner, 2012; Figure 15A). This is a novel insight as ornithodirans were considered to have only buttress‐erect limb posture (Sullivan, 2015).
Reduced muscular abduction–adduction of the femur is congruent with our reconstruction of Silesaurus. The iliofemoralis (abductor) is altered in comparison with more primitive archosaurs. Its origin (Figure 14B,C) is not discernable on the iliac blade and the bone is very thin and delicate in that area. The insertion is marked by well‐developed anterior trochanter and trochanteric shelf (Figures 16 and 17), but this insertion is located much further proximally than in crocodiles and Lagerpetidae (Fechner, 2009; Figure 44), closer to the bird condition (Hutchinson and Garcia, 2002). This might reflect a change of activity of this muscle, related to increasing bipedal abilities. This is reflected in a shift of the muscle activity from swing phase to stance phase abduction and gave rise to an iliofemoralis capable of medial femoral rotation (Hutchinson and Gatesy, 2000). The reduced obturator plate and delicate ischium (Figure 14B,C) without distinct scarring, indicate a decreased role for the hip adductors compared to non‐dinosauriform archosaurs. This suggests that adductor‐controlled postural support was no longer required by Silesaurus. In contrast to the adductors, the muscles involved in flexing and extending the knee have large tuberosities or scars marking their attachment areas (mm. iliotibialis, ambiens, femorotibialis, iliofibularis, flexor tibialis internus and externus; Figures 14, 16, 17, 20 and 23A,B; Table 5). Silesaurus had narrow ischia connected through most of their length (Figure 11A,D,E), resulting in a decreased interacetabular distance, a condition necessary to reduce the lever arm of the ground reaction force, an adaptation observed in obligate bipedal dinosaurs (Fechner, 2009).
Figure 44.
Comparison of the adductor and abductor musculature of extant and extinct archosaurs. (A) Gallus (based on Fechner, 2009). (B) Silesaurus (this study). (C) Lagerpeton (based on Fechner, 2009). (D) Alligator (based on Fechner, 2009)
To summarize, Silesaurus had simplified, fully erect forelimbs capable of parasagittal movements and permanent pronation of the manus. This position resembles that of early sauropods, suggesting that both groups used the forelimb in a similar manner, mainly to support the anterior part of the body during slow quadrupedal locomotion (Figure 43). However, Silesaurus had a shorter humerus and a more elongated antebrachium (Figure 2), probably to increase the range of pronation. As a consequence, Silesaurus could make longer steps and gain greater speed than early sauropods.
The hindlimbs of Silesaurus were also fully erect but, in contrast to sauropods and other dinosaurs, the acetabulum was directed ventrolaterally, not laterally (Figure 11B11, 15D and 11, 15). This pillar‐erected hip joint was previously known only in some pseudosuchians. Reduction of adductors, modification of abductor m. iliofemoralis, strong flexors and extensors of the knee, supraacetabular crest limiting femoral abduction and rotation, and mesaxonic pes all suggest a narrow parasagittal gait. Some modern lizards are able to run bipedally to avoid danger (Persons and Currie, 2017). Fossil evidence suggests that lagerpetids could also run bipedally at higher speeds (Fechner, 2009). Silesaurus has a much more efficient locomotor apparatus than these two groups because its joints were aligned closer to the vector of the ground reaction force; therefore, less muscle energy was involved in limb posture control. This locomotor morphology could be explained as an adaptation to greater body mass (Fechner, 2009), as some bones belonged to individuals of at least three meters in length (R.P. and M.T., personal observations). Alternatively, it could be related to a greater capacity for facultative bipedal running (Piechowski and Dzik, 2010; Figure 42), as this animal had no clear adaptations to avoid predators other than running. Of course, the two hypotheses are not mutually exclusive.
Our reconstruction of locomotion and posture of Silesaurus is congruent with the ichnological record. The Middle‐Late Triassic dinosauriform tracks called Atreipus match those of Silesauridae anatomically and stratigraphically (Olsen and Baird, 1986; Haubold and Klein, 2000; Safran and Rainforth, 2004; Porchetti et al. 2008). The two co‐occur at the Woźniki locality (Sulej et al. 2011). These tracks were left by a medium‐sized quadrupedal animal. The manus imprints are small, digitigrade, and consist of three to four digits. They are oriented parallel to the walk direction and close to the trackway axis. This suggests that the track‐maker had fully erected forelimbs that acted in a parasagittal plane just like the forelimbs of Silesaurus (Figures 2 and 10). The pes of Atreipus is mesaxonic, tulip‐shaped, and has three functional digits as in Silesaurus (Porchetti et al. 2008). The hallux is not preserved even in very deep tracks (Olsen and Baird, 1986). The phalangeal formula reconstructed by Olsen and Baird (1986) matches that of Silesaurus. The gait of Atreipus is narrow and the pedes are parasagittaly oriented. Again, this reflects our reconstruction of Silesaurus. The trackway YPM 9962 attributed to Atreipus milfordensis (Olsen and Baird, 1986) shows an ability for facultative bipedality.
4.3. Evolution of limb postures
The common ancestors of pseudosuchians and dinosaurs had already semi‐erect limbs, which could be pulled beneath the body, resulting in a narrower gait. However, they had a primitive ‘crocodile‐normal’ ankle joint and adductor‐based postural support (Hutchinson, 2006; Fechner, 2009). Most authors agree that Euparkeria and Osmolskina are anatomically similar to the ancestral form (Figures 43 and 45). A large, shallow acetabulum permitted a wide range of femoral abduction and rotation. They were quadrupedal animals with poor, if any, bipedal capabilities. Euparkeria had a pectoral girdle that was as wide as it was high, with a vertically oriented scapular blade and a horizontal coracoid. As a result, the humerus was oriented laterally, generating a sprawling posture (Remes, 2008). The well‐developed deltopectoral crest in Euparkeria and Osmolskina (Borsuk‐Białynicka and Sennikov, 2009) indicates strong humeral retractors (Remes, 2008), whereas the olecranon process (triceps brachii insertion) was moderately developed. These reptiles probably still used a sprawled forelimb in propulsion.
Figure 45.
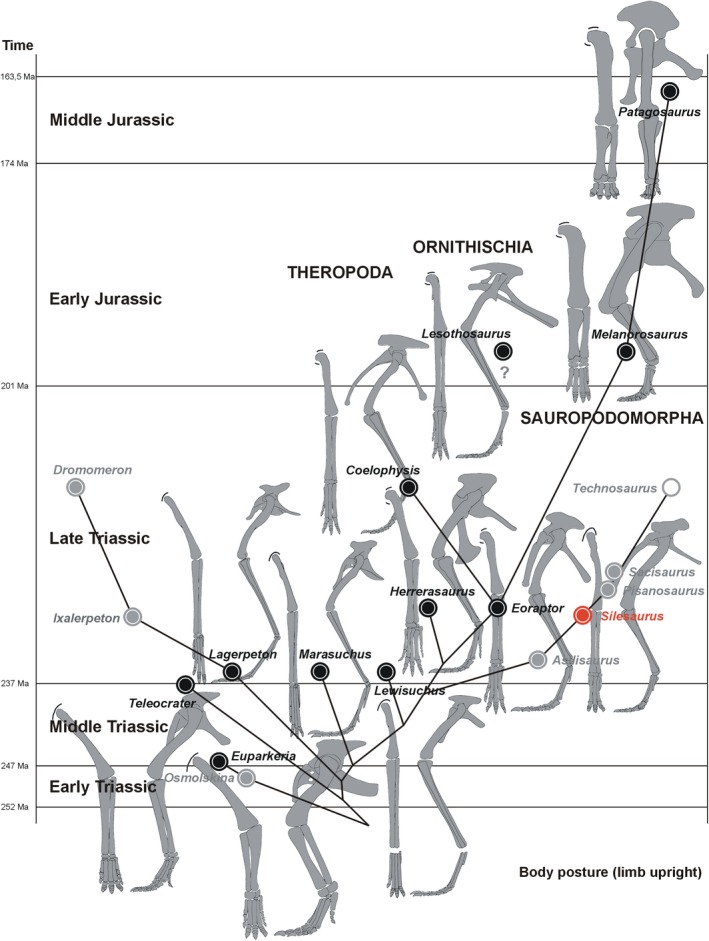
Evolution of hindlimb skeleton and its posture in selected early dinosaurs and their predecessors. Black dots represent illustrated taxa. Grey dots are unillustrated taxa having some forelimb bones known. White dots are unillustrated taxa with no forelimb material known. Silesaurus has a red dot. The topology of the tree follows Müller et al. (2018) with addition of Osmolskina, Melanorosaurus and Patagosaurus. Closely related taxa from different time horizons are illustrated as one lineage for clarity of the figure
These primitive features were largely inherited by Teleocrater from the earliest Carnian (Nesbitt et al. 2017; Figures 43 and 45). Its larger size is probably an independent advancement as lagerpetids Marasuchus and, most importantly, Early‐Mid Triassic dinosauromorph tracks are much smaller (Fechner, 2009; Brusatte et al. 2011; Niedźwiedzki et al. 2013). The dinosauromorph lineage achieved digitigrady (Hutchinson, 2006) early and reorganized their digits, as shown by Early and Mid‐Triassic tracks (Brusatte et al. 2011; Niedźwiedzki et al. 2013). An advanced mesotarsal joint and a more parasagittal gait probably evolved during that time.
Lagerpetids (Figure 45) possess a well‐developed anterior process of the ilium and more cursorial hindlimb proportions (Sereno and Arcucci, 1994; Hutchinson, 2006; Fechner, 2009). They were predominantly quadrupedal but were capable of bipedal running at higher speeds (Fechner, 2009). Despite these advancements, they retain a large obturator plate, a large, shallow acetabulum, and an asymmetric pes. These features imply a high degree of hindlimb rotation and abduction, and a primitive adductor‐controlled postural support (Fechner, 2009) resulting in a semi‐erect posture. Until recently, our knowledge about their forelimb was restricted only to manus imprints. Based on those, we know that the forelimb had a narrower gait than the hindlimb; it moved parasagittally, and produced relatively short steps (Fechner, 2009). Because of the latter, it was inferred that lagerpetids had short forelimbs (Fechner, 2009; Brusatte et al. 2011), but recently the humerus and scapula of a late Carnian lagerpetid were discovered (Cabreira et al. 2016; Müller et al. 2018). They show surprising similarities to the corresponding elements of the early dinosauriforms Asilisaurus (Nesbitt et al. 2010), Lewisuchus (Remes, 2008) and Silesaurus (Dzik, 2003) in having a long, slender scapular blade, low humeral deltopectoral crest, a weakly expanded distal humeral head, and low torsion of the humeral shaft. This suggests that the forelimb locomotor characteristics of Silesaurus and their resemblance to those of early sauropods were not restricted to this taxon but represent the primitive condition of all dinosauromorphs. This group fully erected their forelimbs resulting in a narrow and parasagittal gait (Figure 43). The forelimbs predominantly supported the body while propulsion was generated mainly by the hindlimbs. If our hypothesis is correct, then the short step of lagerpetids was a result of restricted humeral pronation, not short forelimbs. We predict that forelimb proportions were similar in lagerpetids, silesaurids and Lewisuchus (Figure 43).
Fully erect hindlimbs evolved later. Marasuchus from the early Carnian represents this transition (Sereno and Arcucci, 1994; Figure 45). The obturator plate is smaller, the acetabulum is deeper with a more developed supraacetabular crest, and the pes is more symmetrical than that of lagerpetids. These features imply a lesser role for the adductors in limb posture, and more erect hindlimbs that moved almost parasagittally with only three functional digits.
The process was complete when a deep acetabulum fully encompassed the femoral head. This resulted in the pillar‐erected hindlimb of Silesaurus. This pseudosuchian‐like construction was possible because the femoral head was not rotated medially, whereas the acetabulum and supraacetabular crest were oriented ventrolaterally, not laterally as in previous reconstructions. It is unclear when this orientation of the acetabulum appeared in the evolution of Dinosauromorpha. There are two possibilities. The condition could be an autapomorphy of Silesaurus, or a pillar‐erected hindlimb was a necessary step before the femoral head rotated medially and the acetabulum became open in the buttress‐erected limb posture of dinosaurs. If the second hypothesis is correct then the condition in Silesaurus represents a transition toward the improved locomotion of typical dinosaurs.
Primitive dinosaurs still retain a closed acetabulum but their femoral head is rotated medially. The ilium is anteroposteriorly short in Herrerasaurus and moderately elongated in Saturnalia. More advanced dinosaurs have a fully opened acetabulum and an anteroposteriorly expanded ilium for an enlarged iliofemoralis, a key muscle in an abductor‐controlled limb. There is growing evidence that this process of pelvic modification occurred in parallel in different dinosaur lineages (Tsai et al. 2018).
The same probably applies to the forelimb of early dinosaurs. According to our results, dinosaurs redeveloped a large deltopectoral crest on the humerus and some convergently to each other acquired a large olecranon process on the ulna. These changes show an increasing role of humeral protractors and forelimb extension. It is not easy to determine which early dinosaurs were fully bipedal and which were not (Remes, 2008; Fechner, 2009), but with increasing bipedal abilities, the forelimb was shortened and could be engaged in new functions.
In conclusion, our musculoskeletal reconstruction of S. opolensis provides a good fit for the Middle‐Late Triassic tracks called Atreipus. This animal was mainly quadrupedal. It used forelimbs mainly for support, whereas propulsion was generated mainly by the hindlimbs. The forelimbs were fully erect and moved mainly in a parasagittal plane. This resulted in reduction of several muscle attachment sites related to sprawling posture. That is why Silesaurus shows surprising similarities to primitive sauropods in its forelimbs.
The ilium of Silesaurus was inclined about 30° dorsomedially. As a consequence the acetabulum and supraacetabular crest fully encompassed the femoral head resulting in pillar‐erected hindlimbs like some pseudosuchians. The ischia were in contact through most of their length. As a result, the pelvis of Silesaurus was wide dorsally and narrow ventrally and had a short interacetabular distance. This improvement probably enabled a more efficient bipedal run.
At the beginning of their evolution, Dinosauromorpha acquired fully erect, elongated and simplified forelimbs like Silesaurus while retaining a primitive adductor‐controlled hindlimb posture. Early dinosauriforms fully erected their hindlimbs by deepening the acetabulum and developing a supraacetabular crest above the femur. This pillar‐erected limb posture was probably necessary before the femur of early dinosaurs rotated medially to meet the laterally directed acetabulum. After obtaining full bipedality, the forelimb of early dinosaurs redeveloped attachment sites for retractors, flexors and extensors to meet functions other than support of the body. Our results agree with those of Persons and Currie (2017) that obligatory bipedality was a response to increasing cursorial abilities, while the change in forelimb function was secondary.
CONFLICT OF INTEREST
None declared.
Supporting information
ACKNOWLEDGEMENTS
We thank Jerzy Dzik (Institute of Paleobiology PAS and University of Warsaw, Poland) and Tomasz Sulej (Institute of Paleobiology PAS, Poland) for access to and/or loan of specimens in their care. We would like to thank Susan Evans (University College London) for improving the language of the manuscript. We thank Marian Dziewiński (Institute of Paleobiology PAS, Poland) and Małgorzata Czaja (Academy of Fine Arts in Kraków, Poland) for technical support during preparation of photographs. Remarks provided by John Hutchinson, Corwin Sullivan and Henry Tsai helped improve the manuscript. We thank Dawid Dróżdż (Institute of Paleobiology PAS, Poland) for technical support during three‐dimensional reconstruction of the pelvis. The authors are also grateful to Małgorzata Czaja, Dawid Dróżdż, Marian Dziewiński, Olga Lisiewicz, Tomasz Piechowski, Paweł Raźny, Justyna Słowiak, Przemysław Świś, Tomasz Sulej and Tomasz Szczygielski for participating in excavations in Woźniki, Poręba and Zawiercie.
Piechowski R, Tałanda M. The locomotor musculature and posture of the early dinosauriform Silesaurus opolensis provides a new look into the evolution of Dinosauromorpha. J. Anat. 2020;236:1044–1100. 10.1111/joa.13155
REFERENCES
- Baier, D.B. and Gatesy, S.M. (2013) Three dimensional skeletal kinematics of the shoulder girdle and forelimb in walking Alligator. Journal of Anatomy, 223, 462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, K.T. and Schachner, E.R. (2012) Disparity and convergence in bipedal archosaur locomotion. Journal of the Royal Society, Interface, 9(71), 1339–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsbold, R. and Maryańska, T. (1990) Segnosauria In: Weishampel D.B., Dodson P. and Osmólska H. (Eds.) The Dinosauria. Berkeley, CA: University of California Press, pp. 408–415. [Google Scholar]
- Baumel, J.J. , King, A.S. , Breazile, J.E. , Evans, H.E. and Vanden Berge, J.C. (1993) Handbook of Avian Anatomy: Nomina Anatomica Avium, 2nd edition. Cambridge: Nuttall Ornithological Club, p. 779. [Google Scholar]
- Benton, M.J. and Clark, J.M. (1988) Archosaur phylogeny and the relationships of the Crocodylia. The Phylogeny and Classification of the Tetrapods, 1, 295–338. [Google Scholar]
- Bittencourt, J.S. , Arcucci, A.B. , Marsicano, C.A. and Langer, M.C. (2015) Osteology of the Middle Triassic archosaur Lewisuchus admixtus Romer (Chañares Formation, Argentina), its inclusivity, and relationships amongst early dinosauromorphs. Journal of Systematic Palaeontology, 13, 189–219. [Google Scholar]
- Bonaparte, J.F. (1986) Les Dinosaures (Carnosaures, Allosauridés, Sauropodes, Cétiosauridés) du Jurassique moyen de Cerro Cóndor (Chubut, Argentine). Annales de Paleontologie, 72(247–289), 326–386. [Google Scholar]
- Bonnan, M.F. and Yates, A.M. (2007) A new description of the forelimb of the basal sauropodomorph Melanorosaurus: implication for the evolution of pronation, manus shape and quadrupedalism in sauropod dinosaurs. Special Papers in Palaeontology, 77, 139–155. [Google Scholar]
- Borsuk‐Bialynicka, M. (1977) A new camarasaurid sauropod Opisthocoelicaudia skarzynskii gen. n., sp. n. from the Upper Cretaceous of Mongolia. Palaeontologica Polonica, 37(5), 5–64. [Google Scholar]
- Borsuk‐Białynicka, M. and Sennikov, A.G. (2009) Archosauriform postcranial remains from the Early Triassic karst deposits of southern Poland. Palaeontologica Polonica, 65, 283–328. [Google Scholar]
- Brinkman, D. (1980) The hind limb step cycle of Caiman sclerops and the mechanics of the crocodile tarsus and metatarsus. Canadian Journal of Zoology, 58(12), 2187–2200. [Google Scholar]
- Brusatte, S.L. , Niedźwiedzki, G. and Butler, R.J. (2011) Footprints pull origin and diversification of dinosaur stem lineage deep into Early Triassic. Proceedings of the Royal Society of London. Series B, Biological Sciences, 278(1708), 1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, R.J. (2005) The ‘fabrosaurid’ ornithischian dinosaur of the upper Elliot Formation (Lower Jurrasic) of South Africa and Lesotho. Zoological Journal of the Linnean Society, 145, 175–218. [Google Scholar]
- Butler, R.J. (2010) The anatomy of the basal ornithischian dinosaur Eocursor parvus from the lower Elliot Formation (Late Triassic) of South Africa. Zoological Journal of the Linnean Society, 160, 648–684. [Google Scholar]
- Burch, S.H. (2014) Complete forelimb mycology of the basal theropod dinosaur Tawa hallae on a novel robust muscle reconstruction method. Journal of Anatomy, 225, 271–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreira, S.F. , Kellner, A.W.A. , Dias‐da‐Silva, S. , Roberto da Silva, L. , Bronzati, M. , Marsola, J.C.A. ,,, … et al. (2016) A unique Late Triassic dinosauromorph assemblage reveals dinosaur ancestral anatomy and diet. Current Biology, 26, 1–6. [DOI] [PubMed] [Google Scholar]
- Carrano, M.T. (1998) Locomotion in non‐avian dinosaurs: integrating data from hindlimb kinematics, in vivo strains and bone morphology. Paleobiology, 24, 450–469. [Google Scholar]
- Carrano, M.T. and Hutchinson, J.R. (2002) Pelvic and hindlimb musculature of Tyranosaurus rex (Dinosauria: Theropoda). Journal of Morphology, 253, 207–228. [DOI] [PubMed] [Google Scholar]
- Cong, L.‐Y. , Hou, L.‐H. , Wu, X.‐C. and Hou, J.‐F. (1998) The Gross Anatomy of Alligator Sinensis Fauvel. Beijing, China: Science Press. [Google Scholar]
- Coombs, W.P. (1978) Forelimb muscles of the Ankylosauria (Reptilia, Ornithischia). Journal of Paleontology, 52, 642–657. [Google Scholar]
- Currie, P.J. and Zhao, X.-J. (1993) A new carnosaur (Dinosaura, Theropoda) from the Jurassic of Xinjiang, People's Republic of China. Canadian Journal of Earth Sciences, 30, 2037–2081. [Google Scholar]
- Delcourt, R. and Azevedo, S.A.K. (2012) New information on the scapular musculature of Saturnalia tupiniquim (Dinosauria, Saurischia). Gaea – Journal of Geoscience, 8(1), 1–5. [Google Scholar]
- Dilkes, D.W. (2000) Appendicular mycology of the hadrosaurian dinosaurs Maiasauria peeblesorum from the Late Cretaceous (Campanian) of Montana. Transactions of the Royal Society of Edinburgh, Earth Science, 90, 87–125. [Google Scholar]
- Diogo, R. and Abdala, V. (2010) Muscles of Vertebrates: Comparative Anatomy, Evolution, Homologies, and Development. Enfield, NH: Science Publishers. [Google Scholar]
- Dzik, J. (2003) A beaked herbivorous archosaur with dinosaur affinities from the early Late Triassic of Poland. Journal of Vertebrate Paleontology, 23, 556–574. [Google Scholar]
- Dzik, J. and Sulej, T. (2007) A review of the early Late Triassic Krasiejów biota from Silesia, Poland. Palaeontologica Polonica, 64, 1–27. [Google Scholar]
- Dzik, J. and Sulej, T. (2016) An early Late Triassic long‐necked reptile with a bony pectoral shield and gracile appendages. Palaeontologica Polonica, 61(4), 805–823. [Google Scholar]
- Fearon, J.L. and Varricchio, D.J. (2016) Reconstruction of the forelimb musculature of the Cretaceous ornithopod dinosaur Oryctodromeus cubicularis: implications for digging. Journal of Vertebrate Paleontology, 36(2), e1078341. [Google Scholar]
- Fechner, R. (2009) Morphofunctional evolution of the pelvic girdle and hindlimb of Dinosauromorpha on the lineage to Sauropoda. PhD Dissertation, Fakultät für Geowissenschaften, Ludwig‐Maximilians‐Universität, Munich. [Google Scholar]
- Ferigolo, J. and Langer, M.C. (2007) A Late Triassic dinosauriform from south Brazil and the origin of the ornithischian predentary bone. Historical Biology, 19(1), 23–33. [Google Scholar]
- Fisher, H.I. and Goodman, D.C. (1955) The mycology of the Whooping Crane, Grus americana . Illinois Biology Monographs, 24, 1–127. [Google Scholar]
- Fitzgerald, T.C. (1969) The Conturnix Quail: Anatomy and Histology. Des Moines, IA: The Lowa State University Press. [Google Scholar]
- Fostowicz-Frelik, Ł. and Sulej, T. (2010) Bone histology of Silesaurus opolensis Dzik, 2003 from the Late Triassic of Poland. Lethaia, 43(2), 137–148. [Google Scholar]
- Fürbringer, M. (1876) Zur vergleichenden Anatomie des Schultermuskeln – 3. Teil: Capitel IV: Saurier und Crocodile. Gegenbaurs Morphologisches Jahrbuch, 1, 636–816. [Google Scholar]
- Fürbringer, M. (1888) Untersuchungen zur Morphologie und Systematik der Vögel, zugleich ein Beitrag zur Anatomie der Stütz‐ und Bewegungsorgane. Amsterdam, The Netherlands: T. van Jolkema, p. 40. [Google Scholar]
- Fürbringer, M. (1900) Zur vergleichenden Anatomie des Brustschulterapparates und der Schultermuskeln, IV. Teil: Reptilien. Jenaische Zeitschrift für Naturwissenschaft, 36, 215–712. [Google Scholar]
- Fürbringer, M. (1902) Zur vergleichenden Anatomie des Brustschulterapparates und der Schultermuskeln, V. Teil: Vögel. Jenaische Zeitschrift für Naturwissenschaft, 36, 289–736. [Google Scholar]
- Gadow, H. (1882) Beiträge zur Myologie der hinteren Extremität der Reptilien. Morphologisches Jahrbuch, 7, 329–466. [Google Scholar]
- Gangl, D. , Weissengruber, G.E. , Egerbacher, M. and Forstenpointner, G. (2004) Anatomical description of the muscles of the pelvis limb in the Ostrich (Struthio camelus). Anatomy, Histology, Embryology, 33, 100–114. [DOI] [PubMed] [Google Scholar]
- Gatesy, S.M. (1990) Caudofemoral musculature and the evolution of theropod locomotion. Paleobiology, 16, 170–186. [Google Scholar]
- Gatesy, S.M. (1995) Functional evolution of the hindlimb and tail from basal theropods to birds In: Thomason J.J. (Ed.) Functional Morphology in Vertebrate Palaeontology. Cambridge: Cambridge University Press, pp. 219–234. [Google Scholar]
- Gauthier, J. (1986) Saurischian monophyly and the origin of birds. Memoirs of the California Academy of Sciences, 8, 1–55. [Google Scholar]
- George, J.C. and Berger, A.J. (1966) Avian Myology. New York, NY: Academic Press, p. 500. [Google Scholar]
- Goslow, G.E. , Dial, K.P. and Jenkins, F.A. (1989) The avian shoulder: an experimental approach. American Zoologist, 29(1), 287–301. [Google Scholar]
- Griffin, C.T. and Nesbitt, S.J. (2016) The femoral ontogeny and long bone histology of the Middle Triassic (? late Anisian) dinosauriform Asilisaurus kongwe and implications for the growth of early dinosaurs. Journal of Vertebrate Paleontology, 36(3), e1111224. [Google Scholar]
- Haines, R.W. (1939) A revision of the extensor muscles of the forearm in tetrapods. Journal of Anatomy, 73, 211–233. [PMC free article] [PubMed] [Google Scholar]
- Haines, R.W. (1942) The tetrapod knee joint. Journal of Anatomy, 76, 270. [PMC free article] [PubMed] [Google Scholar]
- Haines, R.W. (1950) The flexor muscles of the forearm and hand in lizard and mammals. Journal of Anatomy, 84, 12–29. [PMC free article] [PubMed] [Google Scholar]
- Haubold, H. and Klein, H. (2000) Die dinosauroiden Fährten Parachirotherium–Atreipus–Grallator aus dem unteren Mittelkeuper (Obere Trias: Ladin, Karn,? Nor) in Franken. Hallesches Jahrbuch für Geowissenschaften B, 22, 59–85. [Google Scholar]
- Hudson, G.E. , Hoff, K.M. , Berge, J.V. and Trivette, E.C. (1959) A numerical study of the wing and leg muscles of Lari and Alcae. Ibis, 111, 459–524. [Google Scholar]
- Hutchinson, J.R. (2001a) The evolution of hindlimb anatomy and function in theropod dinosaurs. Unpublished Ph.D. diss., University of California, Berkeley. [Google Scholar]
- Hutchinson, J.R. (2001b) The evolution of the femoral osteology and soft tissues on the line to extant birds (Neornithes). Zoological Journal of the Linnean Society, 131, 169–197. [Google Scholar]
- Hutchinson, J.R. (2002) The evolution of hindlimb tendons and muscles on the line to crown‐group birds. Comparative Biochemistry and Physiology Part A, 133, 1051–1086. [DOI] [PubMed] [Google Scholar]
- Hutchinson, J.R. (2006) The evolution of locomotion in archosaurs. Comptes Rendus Palevol, 5(3–4), 519–530. [Google Scholar]
- Hutchinson, J.R. and Garcia, M. (2002) Tyrannosaurus was not a fast runner. Nature, 415, 1018–1021. [DOI] [PubMed] [Google Scholar]
- Hutchinson, J.R. and Gatesy, S.M. (2000) Adductors, abductors, and the evolution of archosaur locomotion. Paleobiology, 26(4), 734–751. [Google Scholar]
- Hutchinson, J.R. , Anderson, F.C. , Blemker, S.S. and Delp, S.L. (2005) Analysis hindlimb muscle moment arms in Tyrannosaurus rex using a three‐dimensional musculoskeletal computer model: implications for stance, gait, and speed. Paleobiology, 31, 676–701. [Google Scholar]
- Hutchinson, J.R. , Miller, C. , Fritsch, G. and Hildebrandt, T. (2008) The anatomical foundation for multidisciplinary studies of animal limb function: examples from dinosaur and elephant limb imaging studies In: Endo H. and Frey R. (Eds.) Anatomical Imaging Techniques: Towards a New Morphology. Berlin, Germany: Springer‐Verlag, pp. 23–38. [Google Scholar]
- Hutson, J.D. and Hutson, K.N. (2015) An examination of forearm bone mobility in Alligator mississipiensis (Daulin, 1802) and Struthio camelus Linnaeus, 1758 reveals that Archaeopteryx and dromaeosaurus shared an adaptation for gilding and/or flapping. Geodiversitas, 37(3), 325–344. [Google Scholar]
- Hutson, J.D. and Hutson, K.N. (2017) An investigation of the locomotor function of therian forearm pronation provides renewed support for an arboreal, chameleon‐like evolutionary stage. Journal of Mammalian Evolution, 24(2), 10.1007/s10914-016-9341-1 [DOI] [Google Scholar]
- Jasinoski, S.C. , Russel, A.P. and Currie, P.J. (2006) An integrative phylogenetic and extrapolatory approach to the reconstruction of dromaeosaur (Theropoda: Eumaniraptora) shoulder musculature. Zoological Journal of the Linnean Society, 146, 301–344. [Google Scholar]
- Johnson, R.E. and Ostrom, J.H. (1995) The forelimb of Torosaurus and an analysis of the posture and gait of ceratopsian dinosaurs In: Thomason J.J. (Ed.) Functional Morphology in Vertebrate Paleontology. Cambridge: Cambridge University Press, pp. 205–218. [Google Scholar]
- Kriegler, W. (1961) Zur Myologie des Beckens und der Hinterextremität der Reptilien. Morphologisches Jahrbuch, 101, 541–625. [Google Scholar]
- Kubo, T. and Kubo, M.O. (2012) Associated evolution of bipedality and cursoriality among Triassic archosaurs: a phylogenetically controlled evaluation. Paleobiology, 38(3), 474–485. [Google Scholar]
- Landsmeer, J.M.F. (1983) The mechanism of forearm rotation in Varanus exanthematicus. Journal of Morphology, 175(2), 119–130. [DOI] [PubMed] [Google Scholar]
- Langer, M.C. (2003) The pelvic and hind limb anatomy of the stem‐sauropodomorph Saturnalia tupiniquim (Late Triassic, Brazil). PaleoBios, 23, 1–40. [Google Scholar]
- Langer, M.C. and Ferigolo, J. (2013) The Late Triassic dinosauromorph Sacisaurus agudoensis (Caturrita Formation; Rio Grande do Sul, Brazil): anatomy and affinities. Geological Society, London, Special Publications, 379(1), 353–392. [Google Scholar]
- Langer, M.C. , França, M.A.G. and Gabriel, S. (2007) The pectoral girdle and forelimb anatomy of the stem‐sauropodomorph Saturnalia tupiniquim (Upper Triassic, Brazil). Special Papers in Palaeontology, 77, 113–137. [Google Scholar]
- Langer, M.C. , Nesbitt, S.J. , Bittencourt, J.S. and Irmis, R.B. (2013) Non‐dinosaurian Dinosauromorpha In: Nesbitt S.J., Desojo J.B. and Irmis R.B. (Eds.) Anatomy, Phylogeny and Palaeobiology of Early Archosaurs and their Kin, vol. 379 London, UK: Geological Society, Special Publications, pp. 157–186. [Google Scholar]
- Liparini, A. and Schultz, C.L. (2013) A reconstruction of the thigh musculature of the extinct pseudosuchian Prestosuchus chiniquensis from the Dinodontosaurus Assemblage Zone (Middle Triassic Epoch), Santa Maria 1 Sequence, southern Brasil. Geological Society, London, Special Publications, 379(1), 441–468. [Google Scholar]
- Livezey, B.C. (1990) Evolutionary morphology of flightlessness in the Auckland Islands Teal. The Condor, 92, 639–673. [Google Scholar]
- Madsen, J.H.J. and Welles, S.P. (2000) Ceratosaurus (Dinosauria, Theropoda). A revised osteology. Miscellaneous Publication, Utah Geological Survey 00–2:1–80.
- Maidment, S.C.R. and Barrett, P.M. (2011) The locomotor musculature of basal ornithischian dinosaurs. Journal of Vertebrate Paleontology, 31, 1265–1291. [Google Scholar]
- McGowan, C. (1979) The hind limb musculature of the Brown Kiwi, Apteryx australis mantelli . Journal of Morphology, 160, 33–74. [DOI] [PubMed] [Google Scholar]
- McGowan, C. (1982) The wing musculature of the Brown Kiwi, Apteryx australis mantelli and its bearing on ratite affinities. Journal of Zoology, 197, 173–219. [Google Scholar]
- Meers, M.B. (2003) Crocodilian forelimb musculature and its relevance to Archosauria. The Anatomical Record. Part A, Discoveries in Molecular, Cellular, and Evolutionary Biology, 274, 891–916. [DOI] [PubMed] [Google Scholar]
- Miner, R.W. (1925) The pectoral limb of Eryops and other primitive tetrapods. Bulletin of the American Museum of Natural History, 51, 145–312. [Google Scholar]
- Müller, R.T. , Langer, M.C. and Dias‐Da‐Silva, S. (2018) Ingroup relationships of Lagerpetidae (Avemetatarsalia: Dinosauromorpha): a further phylogenetic investigation on the understanding of dinosaur relatives. Zootaxa, 4392(1), 149–158. [DOI] [PubMed] [Google Scholar]
- Nesbitt, S.J. (2011) The early evolution of archosaurs: relationships and the origin of major clades. Bulletin of the American Museum of Natural History, 352, 1–292. [Google Scholar]
- Nesbitt, S.J. , Butler, R.J. , Ezcurra, M.D. , Charig, A.J. and Barrett, P.M. (2017) The anatomy of Teleocrater rhadinus, an early avemetatarsalian from the lower portion of the Lifua Member of the Manda Beds (Middle Triassic). Journal of Vertebrate Palaentology, 37(sup1), 142–177. [Google Scholar]
- Nesbitt, S.J. , Sidor, C.A. , Irmis, R.B. , Angielczyk, K.D. , Smith, R.M.H. and Tsuji, L.A. (2010) Ecologically distinct dinosaurian sister group shows early diversification of Ornithodira. Nature, 464, 95–98. [DOI] [PubMed] [Google Scholar]
- Nicholls, E.L. and Russell, A.P. (1985) Structure and function of the pectoral girdle and forelimb of Struthiomimus altus (Theropoda, Ornithomimidae). Palaentology, 28, 643–677. [Google Scholar]
- Nickel, R. , Schummer, A. , Seiferle, E. and Habermehl, K.‐H. (2003) Lehrbuch der Anatomie der Haustiere I: Bewegungsapparat: Bd I. Stuttgart: Parey Verlag. [Google Scholar]
- Niedźwiedzki, G. , Brusatte, S.L. and Butler, R.J. (2013) Prorotodactylus and Rotodactylus tracks: an ichnological record of dinosauromorphs from the Early‐Middle Triassic of Poland. Geological Society, London, Special Publications, 379(1), 319–351. [Google Scholar]
- Norman, D.B. (1986) On the anatomy of Iguanodon atherfieldensis (Ornithischia, Ornithopoda). Bulletin de L`Institut Royal des Sciences Naturelle de Belgique: Sciences de la Terre, 56, 281–372. [Google Scholar]
- Novas, F.E. (1996) Dinosaur monophyly. Journal of Vertebrate Paleontology, 16, 723–741. [Google Scholar]
- Olsen, P.E. and Baird, D. (1986) The ichnogenus Atreipus and its significance for Triassic biostratigraphy In: Padian K. (Ed.) The Beginning of the Age of Dinosaurs: Faunal Change Across the Triassic‐Jurassic Boundary. New York, NY: Cambridge University Press, pp. 61–87. [Google Scholar]
- Osawa, C. (1898) Beiträge zur Anatomie von Hatteria punctata . Archiv für mikroskopische Anatomie und Entwicklungsgeschichte, 51, 548–675. [Google Scholar]
- Osborn, H.F. and Mook, C.C. (1916) Skeletal adaptations of Ornitholestes, Struthiomimus, Tyrannosaurus . Bulletin of the American Museum of Natural History, 35, 733–771. [Google Scholar]
- Osmólska, H. , Maryanska, T. and Barsbold, R. (1972) A new dinosaur, Gallimimus bullatus n. gen., n. sp. (Ornithomimidae) from the Upper Cretaceous of Mongoli. Palaeontologica Polonica, 27, 103–143. [Google Scholar]
- Ostrom, J.H. (1974) The pectoral girdle and forelimb function of Deinonychus (Reptilia: Saurischia): a correction. Postilla, 165, 1–11. [Google Scholar]
- Otero, A. , Gallina, P.A. and Herrera, Y. (2010) Pelvic musculature and function of Caiman latirostis . The Herpetological Journal, 20, 173–184. [Google Scholar]
- Otero, A. , Allen, V. , Pol, D. and Hutchinson, J.R. (2017) Forelimb muscle and joint actions in Archosauria: insights from Crocodylus johnstoni (Pseudosuchia) and Mussaurus patagonicus (Sauropodomorpha). PeerJ, 5, e3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero, A. (2018) Forelimb musculature and osteological correlates in Sauropodomorpha (Dinosauria, Saurischia). PLoS ONE, 13(7), e0198988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero, A. , Cuff, A.R. , Allen, V. , Sumner‐Rooney, L. , Pol, D. and Hutchinson, J.R. (2019) Ontogenetic changes in the body plan of the sauropodomorph dinosaur Mussaurus patagonicus reveal shifts of locomotor stance during growth. Scientific Reports, 9, 7614 10.1038/s41598-019-44037-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padian, K. (2004) Basal Avialae In: Weishampel D.B., Dodson P. and Osmolska H. (Eds.) The Dinosauria, 2nd edition. Berkeley, CA: University of California Press, pp. 210–231. [Google Scholar]
- Persons, W.S. and Currie, P.J. (2017) The functional origin of dinosaur bipedalism: cumulative evidence from bipedally inclined reptiles and disinclined mammals. Journal of Theoretical Biology, 420, 1–7. [DOI] [PubMed] [Google Scholar]
- Piechowski, R. and Dzik, J. (2010) The axial skeleton of Silesaurus opolensis . Journal of Vertebrate Paleontology, 30, 1127–1141. [Google Scholar]
- Piechowski, R. , Niedźwiedzki, G. and Tałanda, M. (2019) Unexpected bird-like features and high intraspecific variation in the braincase of the Triassic relative of dinosaurs. Historical Biology, 31(8), 1065–1081. [Google Scholar]
- Piechowski, R. , Tałanda, M. and Dzik, J. (2014) Skeletal variation and onkogeny of the Late Triassic dinosauriform Silesaurus opolensis . Journal of Vertebrate Paleontology, 34(6), 1383–1393. [Google Scholar]
- Porchetti, S.D. , Nicosia, U. , Mietto, P. , Petti, F.M. and Avanzini, M. (2008) Atreipus‐like footprints and their co‐occurrence with Evazoum from the upper Carnian (Tuvalian) of Trentino‐Alto Adige. Studi Trentini di Scienze Naturali. Acta Geologica, 83, 277–287. [Google Scholar]
- Remes, K. (2008) Evolution of the pectoral girdle and forelimb in sauropodomorpha (Dinosauria, Saurischia): osteology, mycology, and function. Ph. D. Dissertation, Ludwig‐Maximilians‐Uniwersität, Munich. [Google Scholar]
- Ribbing, L. (1907) Die distale armmuskulatur der Amphibien, Reptilien und Säugetiere. Zoologische Jahrbücher. Abteilung für Anatomie und Ontogenie der Tiere, 23, 587–682. [Google Scholar]
- Romer, A.S. (1922) The locomotor apparatus of certain primitive and mammal‐like reptiles. Bulletin of the American Museum of Natural History, 46, 517–606. [Google Scholar]
- Romer, A.S. (1923a) crocodilian pelvic muscles and their avian and reptilian homologues. Bulletin of the American Museum of Natural History, 48, 533–552. [Google Scholar]
- Romer, A.S. (1923b) The ilium in dinosaurs and birds. Bulletin of the American Museum of Natural History, 48, 141–145. [Google Scholar]
- Romer, A.S. (1927b) The pelvic musculature of the ornithischian dinosaurs. Acta Zoologica, 8, 225–275. [Google Scholar]
- Romer, A.S. (1944) The development of tetrapod limb musculature–the shoulder region of Lacerta. Journal of Morphology, 74, 1–41. [Google Scholar]
- Romer, A.S. (1956) The Osteology of the Reptiles. Chicago, IL: The University of Chicago Press. [Google Scholar]
- Rowe, T. (1986) Homology and evolution of the deep dorsal thigh musculature in birds and other Reptilia. Journal of Morphology, 189, 327–346. [DOI] [PubMed] [Google Scholar]
- Russell, A.P. and Bauer, A.M. (2008) The appendicular locomotor apparatus of Sphenodon and normal‐limbed squamates In: Gans C., Gaunt A.S. and Adler K. (Eds.) Biology of the Reptilia 24, Morphology 1. Ithaca, NY: Society for the Study of Amphibians and Reptiles, pp. 1–466. [Google Scholar]
- Safran, J. and Rainforth, E.C. (2004) Distinguishing the tridactyl dinosaurian ichnogenera Atreipus and Grallator: where are the latest Triassic Ornithischia in the Newark Supergroup. Geological Society of America, Abstracts with Program, 36(2), 96. [Google Scholar]
- Santa‐Luca, A.P. , Crompton, A.W. and Charig, A.J. (1976) A complete skeleton of the Late Triassic ornithischian Heterodontosaurus tucki . Nature, 264, 324–328. [Google Scholar]
- Santa‐Luca, A.P. (1980) The postcranial skeleton of Heterodontosaurus tucki (Reptilia, Ornithischia) from the Stromberg of South Africa. Annals of the South African Museum, 79, 159–211. [Google Scholar]
- Schachner, E.R. , Manning, P.L. and Dodson, P. (2011) Pelvic and hindlimb myology of the basal Archosaur Poposaurus gracilis (Archosauria: Poposauroidea). Journal of Morphology, 272, 1464–1491. [DOI] [PubMed] [Google Scholar]
- Sellers, W.L. and Manning, P.L. (2007) Estimating dinosaur maximum running speeds using evolutionary robotics. Proceedings of the Royal Society of London, Series B, 274, 2711–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno, P.C. and Arcucci, A.B. (1994) Dinosaurian precursors from the Middle Triassic of Argentina: Marasuchus lilloensis, gen. nov. Journal of Vertebrate Paleontology, 14, 53–73. [Google Scholar]
- Shah, R.V. and Patel, V.B. (1964) Myology of the chelonian pectoral appendage. The Journal of Animal Morphology and Physiology, 11, 58–84. [Google Scholar]
- Straus, W.L. (1942) The homologies of the forearm flexors: urodeles, lizards, mammals. The American Journal of Anatomy, 70, 281–316. [Google Scholar]
- Sulej, T. , Bronowicz, R. , Tałanda, M. and Niedźwiedzki, G. (2011) A new dicynodont–archosaur assemblage from the Late Triassic (Carnian) of Poland. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 101(3–4), 261–269. [Google Scholar]
- Sullivan, G.E. (1962) Anatomy and embryology of the wing musculature of the domestic flow (Gallus). Australian Journal of Zoology, 10, 458–518. [Google Scholar]
- Sullivan, C. (2015) Evolution of hind limb posture in Triassic archosauriforms Great Transformations in Vertebrate Evolution. Chicago, IL: University of Chicago Press, pp. 107–124. [Google Scholar]
- Szulc, J. , Racki, G. and Jewuła, K. (2015) Key aspects of the biostratigraphy of the Upper Silesian middle Keuper, southern Poland. Annales Societatis Geologorum Poloniae, 85, 557–686. [Google Scholar]
- Tarsitano, S.F. (1981) Pelvic and hindlimb musculature of archosaurian reptiles. PH.D., City University, New York. [Google Scholar]
- Tsai, H.P. and Holliday, C.M. (2015) Articular soft tissue anatomy of the archosaur hip joint: structural homology and functional implications. Journal of Morphology, 276(6), 601–630. [DOI] [PubMed] [Google Scholar]
- Tsai, H.P. , Middleton, K.M. , Hutchinson, J.R. and Holliday, C.M. (2018) Hip joint articular soft tissues of non‐dinosaurian Dinosauromorpha and early Dinosauria: evolutionary and biomechanical implications for Saurischia. Journal of Vertebrate Paleontology, 38, e1427593. [Google Scholar]
- Vanden Berge, J.C. and Zweers, G.A. (1993) Myologia In: Baumel J.J., King A.S., Breazile J.C., Evans H.E. and Vanden Berge J.C. (Eds.) Handbook of Avian Anatomy: Nomina Anatomica Avium. Cambridge: Nuttall Ornithological Club, pp. 189–247. [Google Scholar]
- Vazques, R.J. (1994) The automating skeletal and muscular mechanism of the avian wing (Aves). Zoomorphology, 114, 59–71. [Google Scholar]
- Walker, A.D. (1961) Triassic reptiles from the Elgin area: Stagonolepis, Dasygnathus and their allies. Philosophical Transactions of the Royal Society of London, Series B, 244, 103–204. [Google Scholar]
- Walker, J.W.F. (1973) The locomotor apparatus of testudines In: Gans C. and Parsons T.S. (Eds.) Biology of the Reptilia 4. New York, NY: Academic Press, pp. 1–100. [Google Scholar]
- Welles, S.P. (1984) Dilophosaurus wetherilli (Dinosauria, Theropoda). Osteology and Comparisons. Palaeontologica A, 185, 85–180. [Google Scholar]
- Wilson, J.A. (2005) Integrating ichnofossil and body fossil records to estimate locomotor posture and spatiotemporal distribution of early sauropod dinosaurs: a stratocladistic approach. Paleobiology, 31(3), 511–518. [Google Scholar]
- Witmer, L.M. (1995) The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils In: Thomason J.J. (Ed.) Functional Morphology in Vertebrate Paleontology. Cambridge: Cambridge University Press, pp. 19–33. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


