Abstract
Background
Human immunodeficiency virus type-1 (HIV-1) infection leads to acquired immunodeficiency syndrome (AIDS), a severe viral infection that has claimed approximately 658,507 lives in the US between the years 2010–2014. Antiretroviral (ARV) therapy has proven to inhibit HIV-1, but unlike other viral illness, not cure the infection.
Objective
Among various Food and Drug Administration (FDA)-approved ARVs, nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) are most effective in limiting HIV-1 infection. This review focuses on NRTIs mechanism of action and metabolism.
Methods
A search of PubMed (1982–2016) was performed to capture relevant articles regarding NRTI pharmacology.
Results
The current classical NRTIs pharmacology for HIV-1 prevention and treatment are presented. Finally, various novel strategies are proposed to improve the efficacy of NRTIs, which will increase therapeutic efficiency of present-day HIV-1 prevention/treatment regimen.
Conclusion
Use of NRTIs will continue to be critical for successful treatment and prevention of HIV-1.
Keywords: Antiretroviral drugs, antiretroviral therapy, NRTIs, HIV-1, AIDS, nanomedicine
1. INTRODUCTION
According to the 2015 estimate made by Centers for Disease Control and Prevention (CDC), in the United States, approximately 1.2 million people are living with human immunodeficiency virus type 1 (HIV-1) infection and in recent years, the new HIV-1 infection incidence rate is relatively constant [1]. In the United States alone between years 2010–2014, about 678,509 people died with diagnosed stage 3, HIV/AIDS infection [2].
Since, the discovery of HIV-1 as the causative agent of AIDS in 1981 [3–5], it has been estimated that the HIV-1 pandemic main (M) group has spread the infection to over 60 million people globally within last 30 years, mainly by sexual, percutaneous and perinatal routes [6]. HIV-1 mainly attacks CD4+ cells, causing significant reductions in the CD4+ cell count leading to immunodeficiency, opportunistic infection(s), or opportunistic cancer. This in turn, gradually diminishes the ability of the body’s immune system to produce new T cells.
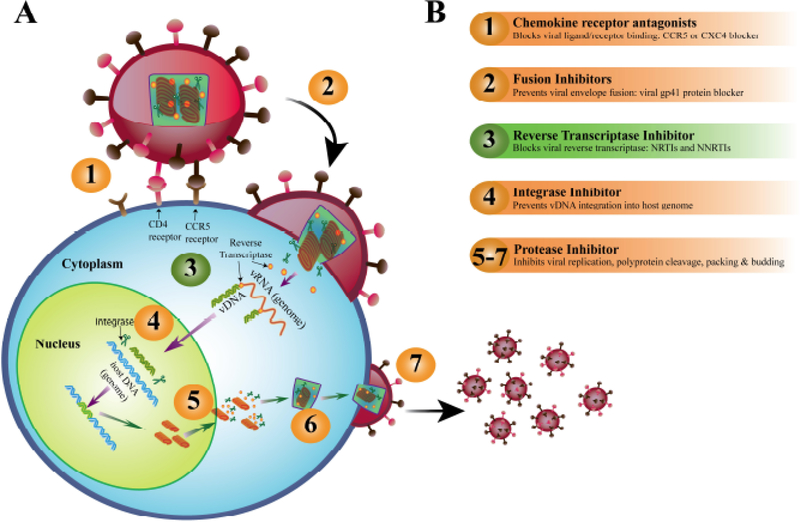
HIV-1 is a retrovirus; contains two copies of the RNA genome (Fig. 1). In addition, the HIV-1 virus also encapsidates several enzymes, such as reverse transcriptase (RT), integrase, and viral protease. These enzymes play an important role in making new copies of HIV and are the targets of antiretroviral drugs. The HIV viral particle, or virion, has a capsid which is cone-shaped and is enclosed in a lipid bilayer, or envelope [7]. This envelope contains viral glycoproteins that bind specifically to CD4 and CCR5 or CXCR4 cell receptors, enabling the virus to fuse with the host cell. While inside host cytoplasm, HIV-1 as all other retroviruses, transcribes the viral RNA (vRNA) genome into viral DNA (vDNA) with the help of RT enzymes. The vDNA is then shuttled to the nucleus and is incorporated into the host genome with the help of the integrase enzyme.
Fig. (1).
(A) HIV-1 infection pathway and (B) Antiretroviral drug (ARV) action site. The numbers in part (A), describes the ARVs target action site of part (B). vRNA, viral RNA genome; vDNA, complimentary viral DNA.
Based on its mechanism of action, antiretroviral drugs (ARVs) for HIV-1 treatment are categorized into the following different classes [8] (Fig. 1) i.e., i) viral entry blockers (the CCR5 receptors antagonists); ii) HIV-1 virus fusion inhibitors (mainly targeting the viral gp41 protein); iii) non-nucleoside and nucleoside RT inhibitors (NNRTIs and NRTIs, respectively); iv) integrase strand transfer inhibitors and v) protease inhibitors. Currently, the use of these ARVs as combination ARVs (cARVs) is the standard treatment strategy for HIV, termed antiretroviral therapy (ART). The present cARVs regimen involves the use of at least two different drug classes for HIV-1 protection regime.
In this review, the focus will be on NRTIs. The NRTIs are rather interesting because the associated viral mutations are very slow when compared to NNRTI [9]. Therefore, the detailed knowledge of NRTI action pathways can be a prime factor for designing effective HIV-1 therapeutic strategies. This review is focused on NRTIs mechanism of action, metabolism, and bottlenecks of NRTIs used for HIV-1 treatment. We will not discuss NRTI use for hepatitis B treatment. We will also comment on various strategies to improve the efficacy of NRTIs.
In last 30 years of ARV therapeutic targets, viral RT is a primary target for ARV drug design against HIV-1. As a matter of fact, RT was first identified using a viral reverse transcriptase inhibitor (vRTI) known as zidovudine (AZT). In 1987, AZT was approved for the treatment of HIV-1 infections [10]. Over 40 % of the present day ARVs approved by Food and Drug Administration (FDA) for the prevention/treatment of HIV-1 infection target the viral DNA (vDNA) polymerization, as RT plays a prime role in the HIV life-cycle [11]. Like DNA polymerases, RT requires both a primer and a template for vDNA synthesis. The HIV-1 RT uses host transfer RNA (tRNA) primer (i.e. tRNAlys3) and the vDNA polymerization reaction begins at the RT/(+)vRNA binding site, leading to a conformational change of RT which leads to the propagation of vDNA polymerization. The HIV-1 RT is a multifunctional enzyme with p66/p21 heterodimeric subunit. The p66 and p21 are two essential subunits of RT performing two distinct activities: the p66 subunit has the DNA polymerase property that actively propagates vDNA production either from vRNA or from complementary vDNA as a template; whereas the p21 subunit, the endonucleolytic ribonuclease H (RNase H) specifically degrades the RNA strand from the RNA:DNA duplexes [12].
There are two classes of vRTIs i.e. nucleoside and nucleotide RT inhibitors (NRTIs) and non-nucleoside RT inhibitors (NNRTIs). While intracellular, the NRTIs upon phosphorylation to their respective active di/triphosphate nucleoside/nucleotide base analogue compete with the natural nucleoside/nucleotide bases during vRNA to vDNA strand synthesis by the RT polymerase (Fig. 1a). The integration of the drug nucleoside/nucleotide analogue causes termination of vDNA synthesis, due to lack of 3’-hydroxyl group in NRTI active metabolite [13]. Therefore, NRTI leads to competitive inhibition whereas NNRTI exerts a non-competitive inhibition [10].
2. FDA APPROVED NRTIS AND THEIR MODE OF ACTION
NRTIs are prodrugs that require intracellular anabolic phosphorylation to be converted into their active form of phosphorylated NRTI metabolites; most of which have longer plasma half-lives than their parent compounds (Table 1 and 2) [14]. NRTIs are a class of drugs that inhibit the HIV-1 RT enzyme by competing with natural nucleosides (such as dTTP, dCTP, dGTP and dATP) and act by incorporation into viral DNA (Fig. 1).
Table 1.
NRTI cellular transport and half-lifes.
| NRTI Name | Diffusion/SLC Transporter [Ref.] | Active ddNTP & ddNDP analogue | Plasma Half-Life [Ref.] | Inn a cellular half-life [Ref.] |
|---|---|---|---|---|
| Lamivudine (3TC) | OCT1, OCT2, CNT1 [24, 25, 27] | 3TC-TP | 22 h [26] | 15 to 16 h [29] |
| Emtricitabine (FTC) | MATE1 [30] | FTC-TP | 37 h [31] | 39 h [31] |
| Tenofovir Disoproxil Funiarate (TDF) | Direct diffusion [32] | TFV-DP | ∼17h [33] | 164 h [31] |
| Tenofovir Alafenamide (TAF) | Direct diffusion [32] | TFV-DP | ∼125 h [33] | 164 h [31] |
| Abacavir (ABC) | Direct diffusion [18] | CBV-TP | ∼3.3 [34]h | ∼18 to 21 h [35] |
| Zidovudine (AZT) | OAT1-4, CNT1, CNT3, ENT2 | AZT-TP | ∼2 h [36] | ∼9 h [37] |
| Didanosine (ddI) | CNT2, CNT3, ENT1, ENT2 [23, 38] | ddI-TP | 2.3 h [39] | 24 h to 40 h [40] |
| Stavudine (d4T) | CNT1 [41] and. Direct diffusion [19] | d4T-TP | 7 h [42] | 4.5 h [43] |
CNT, concentrative nucleoside transporter; ENT, equilibrative nucleoside transporter; MATE, multidrug and toxin extrusion protein; OAT, organic anion transporter; OCT, organic cation transporter.
Table 2.
Efflux transporters involved in commercial NRTIs elimination.
| NRTI Drug | Brand Name, Company | FDA Approval Year | Type of Efflux Transporter | References |
|---|---|---|---|---|
| Lamivudine (3TC) | Epivir, GlaxoSmithKline | 1995 | BCRP | [65–67] |
| Emtricitabine (FTC) | Emtriva, Gilead Sciences | 2003 | MRP1 | [65, 66, 68] |
| Tenofovir disoproxil fumarate (TDF) | Viread, Gilead Sciences | 2001 | PgP | [32, 66, 69] |
| Tenofovir alafenamide (TAF) | Vemlidy, Gilead Sciences | 2015 | MRP4 | [70,71] |
| Abacavir (ABC) | Ziagen, GlaxoSmithKline | 1998 | Pgp, MRP4, BCRP | [67,72,73] |
| Zidovudine (AZT) | Retrovir, GlaxoSmithKline | 1987 | MRP4, BCRP | [67, 73–75] |
| Didanosine (ddl) | Videx EC (capsule), Bristol Myers-Squibb | 1991 | BCRP | [65, 67] |
| Stavudine (d4T) | Zerit, Bristol Myers-Squibb | 1994 | BCRP, MRP5 | [65,67,76] |
BCRP, breast cancer resistance protein transporter; MRP, multidrug resistance-associated protein transporter; Pgp, P-glycoprotein transporter.
2.1. NRTIs Mechanism of Action and their Metabolism
NRTIs are non-functional until the compound gets converted to its active di- or tri-phosphate metabolite form. However, transport and metabolism of NRTIs an highly dependent on the levels of activated NRTI derivatives and endogenous dNTP levels [15, 16]. In this section, we describe the mechanism of transportation, cellular metabolism, and catabolism of NRTIs.
2.2. Transport of NRTIs into Cells
NRTIs are transported into cells by simple diffusion or diffusion via nucleoside carrier-mediated transporter [17]. Lipophilic NRTIs, such as tenofovir disoproxil fumarate (TDF) as well as tenofovir alafenamide (TAF), AZT, abacavir (ABC), and stavudine (d4T), are known to diffuse passively through cellular membranes by non-facilitated diffusion mainly due to their hydrophobic characteristics [18–20]. However, NRTI can also diffuse across the cell membrane utilizing various cell surface transporters that induce facilitated diffusion through the cellular membrane. A series of transporter proteins on cellular surfaces regulates the cellular uptake of NRTIs. Several of these transporter proteins belong to the solute carrier transporter (SLC) families [21]. The SLC family members that are involved in NRTI transport include: organic cation transporters (OCTs), organic anion transporters (OATs), concentrative nucleoside transporters (CNTs), and equilibrative nucleoside transporters (ENTs) [22–24]. Depending on the type of organ involved, the type of SLC families involved in NRTI transport varies [24–28]. In the small intestine, the absorptive cells utilize OCT1, OCT2, CNT1–3, OAT2, ENT1, and ENT2 transporters for NRTI uptake. In lymphocytes, however, ENT1 and ENT2 regulate the uptake of some specific NRTIs such as AZT and ddI. Hepatocytes, on the other hand, use OAT2 and OCT1 transporters for NRTIs transportation. Whereas, the renal tubule epithelial cells directly transport NRTIs via CNT1, CNT2, OAT1–4, and OCT2 transporters. Table 1, represents the different transporters responsible for NRTIs cellular transportation [28].
2.3. NRTI Metabolism
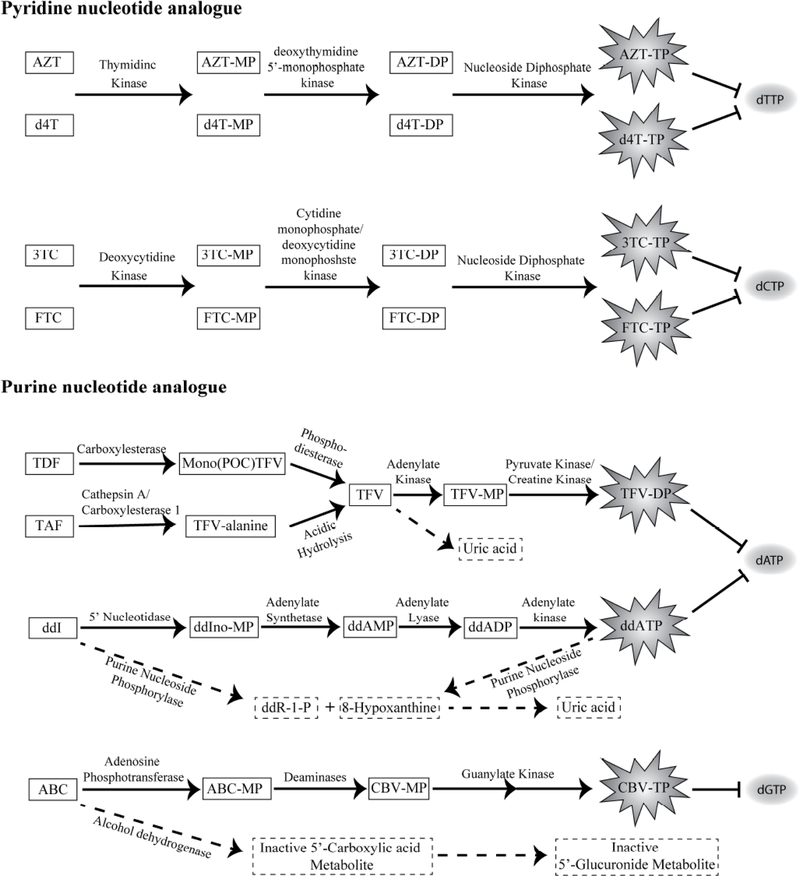
NRTIs are taken as prodrugs or as drugs. The NRTI prodrugs after cellular uptake, first get metabolized to their respective drug form. The drug form then gets phosphorylated to its active form i.e. diphosphate (DP) or triphosphate (TP) form. The active form of drug acts like functional nucleoside analogue, primarily blocking the enzymatic function of RT, in turn causing abrupt termination of vDNA synthesis (Fig. 2). In the cytoplasm, the NRTIs active drug-phosphate form accumulates to generate a cellular pool of analogue 2’, 3’-dideoxynucleoside 5’-triphosphates (ddNTPs) or 2’, 3’-dideoxynucleoside 5’-diphosphates (ddNDPs). The analogues, ddNTPs/ddNDPs, then compete with 2’-deoxynucleotide 5’-triphosphates (dNTPs) for substrate binding by RT enzyme. Once ddNTPs/ddNDPs analogues get incorporated, they cause premature termination of viral RNA transcription (Fig. 1).
Fig. (2).
NRTI metabolic pathways. The box represents the NRTIs and its metabolites. The activate metabolites of respective NRTI drugs are presented in the irregular star-shaped structure. In red, respective NRTI drug’s (ddNTPs), natural nucleotide analogues (dNTPs) are been presented. In case of Tenofovir (TFV), Abacavir (ABC) and Didanosine (ddI), the broken arrows and boxes below, represents respective catabolic pathway. NRTI, Nucleotide Reverse Transcriptase Inhibitors; ddNTPs, 2’, 3’-dideoxynucleoside 5’-triphosphates; ddNDPs, 2’, 3’-dideoxynucleoside 5’-diphosphate; ddR-1-P, 2′,3′-dideoxyribose-1-phosphate, ABC, Abacavir; CBV, Carbovir monophosphate, ddI, Didanosine; ddIno, dideoxyinosine; FTC, Emtricitabine; 3TC, Lamivudine; POC, isopropyloxymethyl carbonate; d4T, Stavudine; TFV, Tenofovir; TAF, Tenofovir alafenamide; TDF, Tenofovir disoproxil fumarate; AZT, Zidovudine; MP, Monophosphate; DP, Diphosphate; TP, Triphosphate.
The NRTI gets phosphorylated to its respective active analogue ddNTPs/ddNDPs in a stepwise fashion (Table 1). For phosphorylation, NRTIs utilize host endogenous nucleotide synthesis and nucleoside phosphorylation pathways. Since, different NRTIs are analogues of specific dNTP, each NRTI metabolism/phosphorylation utilizes different sets of enzymes and pathways to get converted to their respective di/triphosphate active form. For example, one of the highly studied NRTI drugs, TDF, a prodrug NRTI after converting to the drug form i.e. tenofovir (TFV), undergoes 2-step phosphorylation to its clinically active DP form i.e. from TFV-monophosphate (MP) to TFV-DP (Fig. 2). Whereas, intracellular AZT and d4T directly gets phosphorylated to their corresponding MPs [17, 44]. These MPs are then converted to their DPs and then to respective clinically active TP metabolite form [17] (Fig. 2).
The positive or negative feedback mechanism (≥1 enzyme) regulates the intracellular nucleoside analogue TPs concentration. In the phosphorylation pathways, there are more than one steps that act as rate limiting steps during the formation of active drug from NRTI prodrug. There are various cellular factors that regulate the RTs functionality of incorporating the ddNTPs in place of dNTP analogue into nascent proviral DNA. Those factors are: the presence of cellular kinases; the “error-prone” nature of RT; the ddNTP to endogenous dNTP ratio and the high affinity of ddNTP over dNTPs; all these factors contribute to promote premature proviral DNA chain termination [36]. Therefore, characterization of NRTIs intracellular metabolites (phosphorylated/ active form of NRTI i.e. ddNDPs or ddNTPs), instead of plasma concentrations of NRTIs, provides a better understanding of clinical efficacy of NRTIs in the HIV-infected patients [45]. In the next section, we illustrate some of the major metabolic pathways of NRTIs based on their respective analogues.
3. PYRIDINE NUCLEOTIDE ANALOGUE METABOLISM
Among various pyridine nucleotide analogues, AZT and d4T are the two most common thymidine analogue NRTI drugs [46]. Thymidine analogues are converted in their active TP form in three steps. The drug first converts to its MPs form by thymidine kinase 1 [47] (Fig. 2). In the second step, deoxythymidine 5’-monophosphate kinase (TMPK) phosphorylates the drug-MP to its DP form. Finally, the nucleoside diphosphate kinase converts the drug-DP to the respective clinically active drug-TP form. However, the phosphorylation kinetics of each drug significantly varies from the other. The rate-limiting step in case of AZT is the conversion of AZT-MP to AZT-DP intermediate due to poor reactivity of AZT-MP with TMPK enzyme [47]. This leads to accumulation of the AZT-MP overtime in the cell. Further, presence of excess AZT-MP intermediate competitively inhibits production of endogenous thymidine-MP, which in turn results in decreased intracellular thymidine-TP levels. Therefore, low indigenous intracellular thymidine-TP concentration increases the probability of utilizing AZT-TPs by DNA polymerase and can potentially cause toxicity by inhibiting normal DNA synthesis [48]. However, AZT-TP has 100 times higher affinity for HIV-1 RT than for host DNA polymerase, therefore low level of AZT-TP can show effective HIV-1 RT inhibition [36]. In case of non-mitotic cells of liver and heart that doesn’t expresses thymidine kinase 1, AZT inhibits mitochondrial thymine kinase 2 enzyme blocking production of AZT-MP as well as thymidine-MP production. This leads to mitochondrial toxicity as reduction of AZT-TP and thymidine-MP production causing slow mitochondrial DNA replication and thus leads to mitochondrial DNA deletion.
On the contrary, since d4T is a poor substrate for thymidine kinase, the very first step in phosphorylation i.e. production of d4T-MP derivative, is the rate limiting factor [49]. Even though d4T get easily accumulated in cells, due to slow phosphorylation to its active d4T-TP it shows delay in HIV blockage. Therefore, d4T drug is relatively less efficacious in viral load reduction.
The cytosine analogues such as 3TC and FTC are the 2 main analogue drugs that compete with endogenous 2’-deoxycytidine 5’-triphosphate (dCTP) during viral cDNA synthesis, causing pre-matured cDNA chain termination [50]. They also follow the same 3 steps of phosphorylation to get to their active drug-TP form. The deoxycytidine kinase catalyzes the respective drug-MPs conversion. Further, the cytidine monophosphate/deoxycytidine monophosphate kinase catalyzes the drug-MPs to the drug-DP formation. The final step of drug-TP production from DPs is carried out by the enzyme nucleoside diphosphate kinase (Fig. 2). The rate-limiting step for 3TC to 3TC-TPs is the conversion step from DP to TP form [29]. On the contrary, the rate rate-limiting step for FTC-TP is the FTC-TP utilization by RT during viral cDNA synthesis, which in turn depends upon the release of the elongated DNA product from RT [51]. Another rate limiting factor for cytosine analogues is the enantiomer effect. All the natural (+)D-isomers have higher RT binding affinity than their respective (−)L-isomers. The best substrate binding demonstrates strong binding leading to fast incorporation rate. Feng et. al. reported cytosine analogues following the incorporation efficiency order dCTP > ddCTP > (+)FTC-TP > (+)3TC-TP > (−)3TC-TP > (−)FTC-TP [51].
4. PURINE NUCLEOTIDE ANALOGUE METABOLISM
Purine nucleotide analogue NRTIs, are structural analogues of natural adenosine or guanosine. However, their phosphorylation pathways are very different. Certain purine nucleotide NRTIs analogues such as TDF and TAF, are ester prodrugs that first need to be converted into TFV before phosphorylation steps. The TDF prodrug has two bis (isopropyloxymethyl) carbonate (isopropyloxymethyl carbonate is abbreviated as POC) groups as phosphate protecting groups. After it diffuses across the cellular membrane, carboxylesterases mediate the conversion of TDF to its mono-ester form (i.e., mono(POC)TFV), that further gets converted into TFV by intracellular phosphodiesterases [52]. TAF, on the other hand, is a comparatively stable drug essentially in the biological fluid (i.e. in plasma) and follows a unique activation mechanism than that of TDF. In the PBMCs and liver, lysosomal serine protease cathepsin A (CatA) and carboxylesterase 1 (Ces1), catalyze selective hydrolysis of intracellular TAF to TFV with specific intermediate steps [53]. CatA first cleaves the carboxyester bond of the TAF prodrug moiety that generates a metastable metabolite, followed by the elimination of phenol group and hydrolysis leading to TFV-Alanine intermediate. The TFV-Alanine intermediate, however, spontaneously converts into TFV due to lysosomal acidic environment. Further, TFV follows the same fate as in case of TDF (Fig. 2). Furthermore, TFV, either converted from prodrug form (TDF or TAF) or directly internalized via OAT1/OAT3 channel, gets converted into TFV-MP by intracellular adenylate kinase isoform 2 (AK2) enzyme. The TFV-MP then gets converted into the TFV-DP form by various pyruvate/creatine kinases [52]. However, depending on the tissue type, transformation from TFV-MP to TFV-DP is carried out by various nucleotide kinase(s) such as pyruvate kinase in muscle (PKM); pyruvate kinase in the liver and red blood cell (PKLR); creatine kinase in muscle (CKM). Unlike other NRTIs, in case of TDF and TAF, diphosphate form i.e. TFV-DP is the clinically active analogue form of TFV that competes with endogenous 2′-deoxyadenosine 5’-triphosphate (dATP) to get incorporated into the viral cDNA by RT leading to premature chain termination [54]. Studies have shown that TAF has 1000- and 10-fold higher efficacy against HIV infection than TFV or TDF, as well as reduced off-target effects such as bone and kidney problem [55]. However, the rate limiting step for TFV is its conversion into TFV-DP form, probably because cellular kinases involved in TFV-DP conversion from TFV-MP, recognize phosphonate moiety of TFV as TFV-MP. Hence, it by-passes the initial phosphorylation step, a common rate limiting factor for nucleoside analogs [56].
Another adenosine analogue NRTI of the purine nucleoside group is didanosine (ddI). DdI is one of the first purine nucleoside analogues to be approved for HIV-1 treatment. The ddI follows very different pathway to get converted to its TP form. The cytosolic 5’ nucleotidase converts ddI to dideoxyinosine monophosphate (ddIno-MP) form [57]. Further, adenylate synthetase and adenylate lyase catalyze the conversion of ddIno-MP to dideoxyadenosine monophosphate (ddAMP) and to the diphosphate i.e. ddADP. Adenylate kinase further catalyzes ddADP to 2’, 3’-dideoxy- adenosine 5’-triphosphate (ddATP) conversion (Fig. 2).
Among purine guanine analogues, ABC is one of the potent NRTI drugs. ABC differs in the phosphorylation pathway from all other NRTIs. ABC is not a substrate for cellular nucleoside kinases; it first gets phosphorylated to ABC-MP by adenosine phosphotransferase [58]. Then cytosolic deaminases cause deamination of ABC-MP to carbovir monophosphate (CBV-MP). Further, guanylate kinase phosphorylates CBV-MP to CBV-DP and then to CBV-TP, which competes with endogenous 2’-deoxyguanosine 5’-triphosphate (dGTP) (Fig. 2). Interestingly, there is no rate-limiting step involved in the conversion of ABC to CBV-TP.
Based on the intracellular metabolite (mono-, di-, or triphosphate) domination, the conversion time of NRTIs into their active forms is affected. Thus, the half-life of activated NRTIs (either di- or triphosphate) varies significantly (Table 1), which in-turn limits their competition and inhibition efficiency, or even turnover to show RT inhibition.
5. NRTI CATABOLISM AND ELIMINATION
Mechanisms for NRTI degradation vary and are also dependent on cell type. Mostly NRTIs are degraded in the liver by enzymes that are involved in the purine or pyrimidine nucleoside salvage pathway. In general, ddNTPs get degraded to the oxidized ring compound, uric acid, in the case of purinergic bases, and to smaller compounds (such as β-amino acids), in the case of pyrimidine bases. However, these degraded small molecules then serve as precursors to induce de novo synthesis of purines and pyrimidines. One such enzyme, purine nucleoside phosphorylase (PNP), plays a role in the hydrolysis or phosphorolysis of guanosine and inosine nucleosides and is believed to be a mechanism for ddI clearance (Fig. 2) [59]. Similarly, ABC catabolism utilizes alcohol dehydrogenase to catalyze a reaction to form the 5’-carboxylic acid [60]. Further, glucuronyl transferase induces conversation of 5’-carboxylic acid to the 5’-glucuronide of ABC (Fig. 2).
Moreover, NRTIs that are not absorbed systemically, get eliminated through the kidney either by diffusion or via carrier-mediated transportation. ATP-binding cassette (abc) superfamily transporters are mainly involved in the drug efflux process for various drug classes, including NRTIs [61]. The prime members that mainly carry out NRTI efflux process are P-glycoprotein transporters (Pgp), breast cancer resistance protein transporters (BCRP), and multidrug resistance-associated protein transporters (MRPs) [62] (Table 2). The type of efflux transporter involved in efflux of NRTI differs based on the type of NRTI nucleoside analogue (Table 2). Moreover, many NRTIs also act as inhibitors, substrates, and/or inducers of abc-transporters [28] (Table 2). Unlike TDF, TAF easily gets absorbed and metabolized. Therefore, a minimal amount of NRTIs gets excreted via urine in unmodified form. TAF is predominantly eliminated as TFV or TFV metabolites, and uric acid via feces or urine. Renal elimination of some NRTIs (such as TFV) however, involves first active tubular secretion into proximal tubule by OAT1and OAT3 and followed by efflux by MRP4 [63, 64]. This is one of the causes of NRTI induced nephrotoxicity.
5.1. Setback for NRTIs as ARVs
Even though, NRTI as ARVs has improved the life expectancy of the HIV-1 infected patients, the daily high dosing of NRTIs results in various chronic complications. Presently, ARV toxicity is one of the major concerns of the HIV patient management [77]. Accumulation of several such factors resulted in disadvantages of the NRTIs as ARVs. Those factors include, the licensed NRTIs were marketed prior to their proper long-term safety evaluation. This was mainly to overcome the severity of the HIV epidemic. Secondly, as ARVs causes suppression of infection but not cure, therefore, daily dosage of ARVs intake by HIV-infected patient becomes a life-long affair to preserve the clinical benefits. Thirdly, ARV related toxicity was realized only after long-term ingestion. NRTI catabolism and elimination rate are other factors that hold back the ultimate success of the commercially available NRTIs (Table 2). Finally, the long-term ARVs treatment has shown the emergence of drug resistance [78]. The subsequent sections highlight the drawbacks of NRTIs that affect the potency of NRTIs as ARVs.
6. MITOCHONDRIAL TOXICITY
Several side-effects common to NRTI class of drugs can be linked to mitochondrial toxicity [79]. Mitochondria (known as power house of a cell) are present in every type of cells (except erythrocytes), their number ranges from 1 to 1000. They regulate cellular and tissue metabolism, by oxidative phosphorylation of adenosine triphosphate (ATP). The replication of mitochondrial DNA (mtDNA) is performed by the polymerase γ. Comparatively, polymerase γ results in relatively high error prone replication and has less efficient repair capacity. In-vitro studies have shown that the NRTIs also inhibit mtDNA synthesis by blocking polymerase γ resulting in insufficient energy production, which results in malfunctioning mitochondria of the tissues and organs. In addition, NRTIs also interfere with various other mitochondrial functions causing oxidative damage, mitochondrial enzymes inhibition, electron transport chain uncoupling affecting ATP synthesis, and ultimately inducing cellular apoptosis [80].
Indeed, continuous administration of ddC, ddI AZT, and d4T inhibits DNA polymerase γ by the active tri-phosphate metabolite in vitro [81]. This inhibition of DNA polymerase γ is hypothesized to be the reason for the bone marrow suppression of AZT, peripheral neuropathy of ddI and d4T. This group of compounds known as the “d group” of NRTIs that are relatively strong inhibitors of polymerase γ, has been linked to a time- and dose-dependent reduction in intracellular levels mtDNA [82]. These drugs target the liver, skeletal muscle, vessel endothelia, peripheral nerves, and potentially adipose tissue of lipoatrophic subjects [83]. Other NRTIs (i.e. tenofovir), taken up by renal tubular epithelia, could be responsible for the reduction in renal function common with TDF. The combinations of NRTIs reported to result in minimal toxicity in vitro include TFV and 3TC. The combination of TFV and FTC slightly has been observed to reduce cell proliferation without affecting mtDNA [84, 85]. Other combinations that include ddI, d4T, or ABC lead to toxic mitochondrial effects. Clinically, NRTIs induced mitochondrial dysfunction in patients has been the cause of severe side-effects such as proximal renal tubular dysfunction, hepatic steatosis, lactic acidosis, pancreatitis, cardiomyopathy, myopathy, and peripheral neuropathy.
However, mtDNA toxicity is not adequately monitored or able to be predicted, certain inhibitors (antioxidants, vitamin E, coenzyme Q10) have been reported to reduce or eliminate the mitochondrial toxicity from NRTIs [86–88]. These reports have been to date on mice/rats and thus, more research on humans is needed to make positive recommendations for these agents [89, 90].
6.1. Renal Toxicity
Renal accumulation of NRTIs (e.g. TFV) due to inefficient efflux functionality causes their accumulation at the proximal tubule of nephrons leading to nephrotoxicity [84]. Evidence of TDF-associated kidney dysfunction has been reviewed previously [91]. Risk factors for the development of TDF-associated nephrotoxicity include patients with increased age, low body weight, pre-existing reduction in kidney function and use of nephrotoxic agents [91]. Most of the TDF-associated kidney dysfunction is associated with high circulating plasma levels of TDF. TAF is a more efficient prodrug with higher intracellular active metabolite levels. The dose of TAF is significantly reduced compared to TDF [55, 92], reducing the risk of TAF-associated kidney dysfunction problems.
Unlike TDF and TAF, ddI is sensitive to low pH. Therefore, low stomach pH causes rapid conversion of the intermediate dideoxyadenosine (ddA) to free adenosine. The free adenosine gets metabolized to insoluble 2,8-dihydroxyadenine, which accumulates in the renal tubules leading to renal failure [57].
6.2. Hepatic Steatosis and Lactic Acidosis
Various cases of liver failure have been reported in HIV patients receiving NRTIs. Hepatic dysfunction is mainly associated with the mitochondrial toxicity, hampering fatty acid oxidation, causing triglycerides and fatty acids accumulation in microvesicules and macrovesicules. Inhibition of fatty acid oxidation, in turn, inhibits gluconeogenesis, preventing the lactic acid oxidation to pyruvate, thus, leading to high serum lactic acid levels and lactic acidosis (LA), a condition known as “fatty liver or hepatic steatosis disease”. Although the severe hepatic steatosis incidence reported in patients on NRTI was relatively low (1.7 to 25.2 cases per 1000 patient/years), the case fatality rate is very high among these patients ranging from 30% to 60% in different studies [93]. Presently, research is underway to overcome this serious LA problem. Thiamine supplement (e.g. Riboflavin) treatment has been shown to be effective in reversing thiamine deficiency and LA-induced complication of NRTI therapy [94].
However, some NRTIs such as ddI, in the gut environment cleave down to the respective base and sugar moiety, and further catabolism of the inosine base leads to increased uric acid [57]. This may be one of the leading causes of peripheral pancreatitis and neuropathy in HIV-1 patients.
6.3. Bone Toxicity
Low bone mineral density (BMD) is a very common long-term consequence of therapy in HIV patients. Moreover, highly active antiretroviral therapy (HAART) has shown to aggravate BMD problems in HIV patients resulting in disorders such as osteopenia and osteoporosis. Presently it has been estimated that 67% of HIV-1 patients exhibited low BMD, among which 15% have osteoporosis [95]. The low BMD side effect was not NRTI class specific. Randomized study has suggested that TFV has shown initial decline in the BMD compared to d4T. Similarly, the thymidine analogues d4T and AZT are the main attributes to lipoatrophy but other NRTIs such as, ABC do not demonstrate such effect [96].
The chronic acidosis induced by NRTIs leads to electrolyte imbalance. To restore electrolyte homeostasis, the calcium hydroxyapatite mobilization from bones takes place, especially from trabecular bones leading to bone erosion. Additionally, common NRTIs may also induce receptor nuclear factor-κB ligand (RANKL) receptor mediated osteoclastogenesis. The NRTI mediated nephrotoxicity also impairs phosphorus and vitamin D dynamics, which significantly deceases BMD, leading to bone fractures [97].
6.4. Resistance Development to NRTIs
NRTIs are the backbones of most first- and second-line antiretroviral therapy (ART) regimens of HIV-1 patients. Reportedly, long-term application of NRTIs on HIV-1 patients has been shown to develop resistance to one and multiple clinically used NRTIs [98, 99]. Historically, the use of two NRTIs combinations has become the backbone of combination ART, as this therapeutic approach delays the emergence of resistant viruses and prolongs viral suppression as compared to monotherapy with NRTI.
The failure of ART mostly is correlated to the NRTI resistance and cross-resistance development. The RT enzyme lacks 3” exonuclease activity and cannot proofread it’s own errors [99]. Errors occur at 1 base error per cycle [100]. Resistance to NRTIs results from base changes within the RT genome allowing amino acid substitutions in the transcribed enzyme, which then confer structural changes at the active site or functional areas. These adaptations allow the enzyme to function again with some level of efficiency in the presence of the NRTI drug. Table 3 shows the common NRTI mutations for the different NRTI compounds [98]. These mutations can accumulate and is the reason for the cross-resistance that can occur among different agents of the NRTI class.
Table 3.
| NRTI | Primary Mutations |
|---|---|
| Zidovudine (ZDV) | K70R, T215T/F, M41L, D76N, M184V, T215Y, K219N, T215F |
| Lamivudine (3TC) | M184V/I, E44D, V1181, M41L, D76N, M184V, T215Y, K219N, T215F, K70R, K219Q, L210W, A62V, L74V |
| Didanosine (ddl) | L74V, A62V, D67N, K70R, V75L, F116Y, Q151M, K219Q, M41L, M184V, L210W, T215Y, T215F |
| MDR1 | Q151M |
| MDR2 | T69S, thymidine analog mutations |
| Abacavir (ABC) | K65R, L74V, Y115F, M184V/I, M41L, D67N, T215Y, K219N/Q, A62V, K70R, V75I, F116Y, Q151M, L210W |
| Tenofovir (TFV) | K65R, M41L, L210W |
| Stavudine (d4T) | M41L, M184V, T215Y, A62V, D67N, K70R, V75I, F116Y, Q151M. K219Q |
NRTI, nucleoside reverse transcriptase inhibitors; MDR, multiple drug resistance.
At present as ART, NRTIs as backbone therapy are the ultimate way to control HIV infection progression for extended period. Improving patient adherence will reduce the development of drug resistance without affecting the ART potentiality.
7. CURRENT STRATEGIES TO IMPROVE NRTIS EFFICACY
The main drawbacks of NRTIs as ART, are lifelong requirement, daily dosing, pill fatigue, patient non-adherence and toxicities or resistance. Presently, the next-generation ART research focuses on developing strategies that ensure reduced dosage, longer acting, exerting fewer side effects and thus less toxicity. All the aforesaid strategies aim to ensure patient adherence and decrease the mutation rate to current regimens.
Certainly, investigational NRTIs are being developed to reduce long-term side-effects, improve resistance profile, and improve the conversion to active metabolites. A newly developed investigational NRTI, EFdA (4’-ethynyl-2-fluoro2’-deoxyadenosine) has impressive low-nanomolar or picomolar EC50 [88]. Additional advantage includes the oral long-acting potential [89].
Introduction of nano-drug delivery system has promising future that could improve the pharmacokinetics of NRTIs such as enhanced absorption, improved target tissue penetration, lower metabolic rate, and slower elimination rate. Some pre-clinical trials studies of nanoformulated NRTIs have already reported to prolong the maintenance of NRTI drug concentrations, and slow but sustained release properties with once biweekly application potential over conventional daily dosage regimen [101, 102]. Nano-encapsulation also reduces NRTIs toxicity by protecting drug off target effects. Additionally, nano-encapsulation prolongs the systemic as well as cellular availability of the NRTI drugs [91, 93].
Another strategy under investigation is the use of solid drug nanoparticles (SDNs) technology. Primarily, the SDNs are developed by nanomilling approaches or simply drug adherence approaches are applied. These approaches have been argued to show sustained-release NRTIs. As other novel formulation under development is subdermal implant delivering the potent prodrug tenofovir alafenamide (TAF) [103].
All the present strategies allow HIV-infected patients to follow one pill daily regime or receive a parenteral dosage monthly or bimonthly dosing. This would allow improved patient adherence to their drug regimen. Further details for medical follow-up including laboratory monitoring have yet to be fully explained. How patients will receive their long-acting parenteral dosage formulation may still require administration within a physician’s office, but potentially could be deployed by community health nurses similar to directly observed therapy for tuberculosis. Certainly, the future is optimistic for this class of antiretroviral drugs.
CONCLUSION AND FUTURE PROSPECTIVE
NRTIs are backbone drugs for both preferred and alternative antiretroviral therapy (ART) regimens for HIV-1 patients due to their efficient mechanism of blocking RT. However, present day-NRTIs have significant side-effects that require potential monitoring. Currently, the focus of major NRTI research is to develop strategies to over-come the potential drawbacks to improve ART adherence in HIV patients. The future prospective of the potential NRTIs will be combination of the conventional strategies with the advanced technologies.
ACKNOWLEDGEMENTS
S.M., A.H., and C.J.D. wrote manuscript. All authors were equally involved in editing and improving the manuscript.
CONFLICT OF INTEREST
Present work and publication has been funded by NIAID R01AI117740-01, 2015 (to C.J.D.). The content is solely the responsibility of the authors.
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
REFERENCES
- [1].CDC. HIV in the United States: At A Glance Atlanta, GA, USA: MMWR; 2012. [updated June 1, 2017 Available from: http://www.cdc.gov/hiv/statistics/overview/ataglance.html. [Google Scholar]
- [2].CDC. Diagnoses of HIV Infection in the United States and Dependent Areas, 2015. Atlanta, GA, USA: MMWR; 2015 [updated June 1, 2017 Available from: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2015-vol27.pdf. [Google Scholar]
- [3].Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). 1983. Rev Invest Clin. 2004; 56(2): 126–9. [PubMed] [Google Scholar]
- [4].Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983; 220(4599): 868–71. [DOI] [PubMed] [Google Scholar]
- [5].Popovic M, Sarngadharan MG, Read E, Gallo RC. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984; 224(4648): 497–500. [DOI] [PubMed] [Google Scholar]
- [6].Sharp PM, Hahn BH. Origins of HIV and the AIDS pandemic. Cold Spring Harbor perspectives in medicine. 2011; 1(1): a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhao G, Perilla JR, Yufenyuy EL, Meng X, Chen B, Ning J, et al. Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics. Nature. 2013; 497(7451): 643–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Diseases NIoAaI. Types of HIV/AIDS Antiretroviral Drugs [Available from: http://www.niaid.nih.gov/topics/HIVAIDS/Understanding/Treatment/pages/arvdrugclasses.aspx.
- [9].Kim D, Wheeler W, Ziebell R, Johnson J, Prejean J, Heneine W, et al. , editors. Prevalence of Transmitted Antiretroviral Drug Resistance among Newly-diagnosed HIV-1-infected Persons, US, 2007. 17th Conference on Retroviruses and Opportunistic Infections, 2010; 2010 February 16–19, 2010; San Francisco, USA. San Francisco, USA 2010. [Google Scholar]
- [10].Maga G, Radi M, Gerard MA, Botta M, Ennifar E. HIV-1 RT Inhibitors with a Novel Mechanism of Action: NNRTIs that Compete with the Nucleotide Substrate. Viruses. 2010; 2(4): 880–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].FDA. Antiretroviral drugs used in the treatment of HIV infection Silver Spring, MD: U.S. Department of Health and Human Services; 2016. [updated 08/09/2016 Available from: http://www.fda.gov/forpatients/illness/hivaids/ucm118915.htm. [Google Scholar]
- [12].Sluis-Cremer N, Temiz NA, Bahar I. Conformational Changes in HIV-1 Reverse Transcriptase Induced by Nonnucleoside Reverse Transcriptase Inhibitor Binding. Current HIV research. 2004; 2(4): 323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Squires KE. An introduction to nucleoside and nucleotide analogues. Antiviral therapy. 2001; 6 Suppl 3: 1–14. [PubMed] [Google Scholar]
- [14].Bazzoli C, Jullien V, Le Tiec C, Rey E, Mentre F, Taburet AM. Intracellular Pharmacokinetics of Antiretroviral Drugs in HIV-Infected Patients, and their Correlation with Drug Action. Clinical pharmacokinetics. 2010; 49(1): 17–45. [DOI] [PubMed] [Google Scholar]
- [15].Gao WY, Shirasaka T, Johns DG, Broder S, Mitsuya H. Differential phosphorylation of azidothymidine, dideoxycytidine, and dideoxyinosine in resting and activated peripheral blood mononuclear cells. The Journal of clinical investigation. 1993; 91(5): 2326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Robbins BL, Wilcox CK, Fridland A, Rodman JH. Metabolism of tenofovir and didanosine in quiescent or stimulated human peripheral blood mononuclear cells. Pharmacotherapy. 2003; 23(6): 695–701. [DOI] [PubMed] [Google Scholar]
- [17].Tan X, Chu CK, Boudinot FD. Development and optimization of anti-HIV nucleoside analogs and prodrugs: A review of their cellular pharmacology, structure-activity relationships and pharmacokinetics. Advanced drug delivery reviews. 1999; 39(1–3): 117–51. [DOI] [PubMed] [Google Scholar]
- [18].Mahony WB, Domin BA, Daluge SM, Zimmerman TP. Membrane permeation characteristics of abacavir in human erythrocytes and human T-lymphoblastoid CD4+ CEM cells: comparison with (−)-carbovir. Biochemical pharmacology. 2004; 68(9): 1797–805. [DOI] [PubMed] [Google Scholar]
- [19].August EM, Birks EM, Prusoff WH. 3’-Deoxythymidin-2’-ene permeation of human lymphocyte H9 cells by nonfacilitated diffusion. Molecular pharmacology. 1991; 39(2): 246–9. [PubMed] [Google Scholar]
- [20].Huttunen KM, Raunio H, Rautio J. Prodrugs--from serendipity to rational design. Pharmacological reviews. 2011; 63(3): 750–71. [DOI] [PubMed] [Google Scholar]
- [21].Han HK. Role of transporters in drug interactions. Archives of pharmacal research. 2011; 34(11): 1865–77. [DOI] [PubMed] [Google Scholar]
- [22].Takeda M, Khamdang S, Narikawa S, Kimura H, Kobayashi Y, Yamamoto T, et al. Human organic anion transporters and human organic cation transporters mediate renal antiviral transport. The Journal of pharmacology and experimental therapeutics. 2002; 300(3): 918–24. [DOI] [PubMed] [Google Scholar]
- [23].Yao SY, Ng AM, Sundaram M, Cass CE, Baldwin SA, Young JD. Transport of antiviral 3’-deoxy-nucleoside drugs by recombinant human and rat equilibrative, nitrobenzylthioinosine (NBMPR)-insensitive (ENT2) nucleoside transporter proteins produced in Xenopus oocytes. Molecular membrane biology. 2001; 18(2): 161–7. [DOI] [PubMed] [Google Scholar]
- [24].Jung N, Lehmann C, Rubbert A, Knispel M, Hartmann P, van Lunzen J, et al. Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug metabolism and disposition: the biological fate of chemicals. 2008; 36(8): 1616–23. [DOI] [PubMed] [Google Scholar]
- [25].Truong DM, Kaler G, Khandelwal A, Swaan PW, Nigam SK. Multi-level analysis of organic anion transporters 1, 3, and 6 reveals major differences in structural determinants of antiviral discrimination. The Journal of biological chemistry. 2008; 283(13): 8654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Anderson PL, Kiser JJ, Gardner EM, Rower JE, Meditz A, Grant RM. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. The Journal of antimicrobial chemotherapy. 2011; 66(2): 240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cano-Soldado P, Lorrayoz IM, Molina-Arcas M, Casado FJ, Martinez-Picado J, Lostao MP, et al. Interaction of nucleoside inhibitors of HIV-1 reverse transcriptase with the concentrative nucleoside transporter-1 (SLC28A1). Antiviral therapy. 2004; 9(6): 993–1002. [PubMed] [Google Scholar]
- [28].Kis O, Robillard K, Chan GN, Bendayan R. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends in pharmacological sciences. 2010; 31(1): 22–35. [DOI] [PubMed] [Google Scholar]
- [29].Else LJ, Jackson A, Puls R, Hill A, Fahey P, Lin E, et al. Pharmacokinetics of lamivudine and lamivudine-triphosphate after administration of 300 milligrams and 150 milligrams once daily to healthy volunteers: results of the ENCORE 2 study. Antimicrobial agents and chemotherapy. 2012; 56(3): 1427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Reznicek J, Ceckova M, Cerveny L, Muller F, Staud F. Emtricitabine is a substrate of MATE1 but not of OCT1, OCT2, P-gp, BCRP or MRP2 transporters. Xenobiotica; the fate of foreign compounds in biological systems. 2016: 1–9. [DOI] [PubMed] [Google Scholar]
- [31].Jackson A, Moyle G, Watson V, Tjia J, Ammara A, Back D, et al. Tenofovir, emtricitabine intracellular and plasma, and efavirenz plasma concentration decay following drug intake cessation: implications for HIV treatment and prevention. Journal of acquired immune deficiency syndromes (1999). 2013; 62(3): 275–81. [DOI] [PubMed] [Google Scholar]
- [32].Bam RA, Birkus G, Babusis D, Cihlar T, Yant SR. Metabolism and antiretroviral activity of tenofovir alafenamide in CD4+ T-cells and macrophages from demographically diverse donors. Antiviral therapy. 2014; 19(7): 669–77. [DOI] [PubMed] [Google Scholar]
- [33].Duwal S, Schutte C, von Kleist M. Pharmacokinetics and pharmacodynamics of the reverse transcriptase inhibitor tenofovir and prophylactic efficacy against HIV-1 infection. PloS one. 2012; 7(7): e40382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Daluge SM, Good SS, Faletto MB, Miller WH, St Clair MH, Boone LR, et al. 1592U89, a novel carbocyclic nucleoside analog with potent, selective anti-human immunodeficiency virus activity. Antimicrobial agents and chemotherapy. 1997; 41(5): 1082–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Moyle G, Boffito M, Fletcher C, Higgs C, Hay PE, Song IH, et al. Steady-state pharmacokinetics of abacavir in plasma and intracellular carbovir triphosphate following administration of abacavir at 600 milligrams once daily and 300 milligrams twice daily in human immunodeficiency virus-infected subjects. Antimicrobial agents and chemotherapy. 2009; 53(4): 1532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Piliero PJ. Pharmacokinetic properties of nucleoside/nucleotide reverse transcriptase inhibitors. Journal of acquired immune deficiency syndromes (1999). 2004; 37 Suppl 1: S2–S12. [DOI] [PubMed] [Google Scholar]
- [37].Flynn PM, Rodman J, Lindsey JC, Robbins B, Capparelli E, Knapp KM, et al. Intracellular pharmacokinetics of once versus twice daily zidovudine and lamivudine in adolescents. Antimicrobial agents and chemotherapy. 2007; 51(10): 3516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ritzel MW, Ng AM, Yao SY, Graham K, Loewen SK, Smith KM, et al. Molecular identification and characterization of novel human and mouse concentrative Na+-nucleoside cotransporter proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib). The Journal of biological chemistry. 2001; 276(4): 2914–27. [DOI] [PubMed] [Google Scholar]
- [39].Molina JM, Peytavin G, Perusat S, Lascoux-Combes C, Sereni D, Rozenbaum W, et al. Pharmacokinetics of emtricitabine, didanosine and efavirenz administered once-daily for the treatment of HIV-infected adults (pharmacokinetic substudy of the ANRS 091 trial). HIV medicine. 2004; 5(2): 99–104. [DOI] [PubMed] [Google Scholar]
- [40].Ritter J, Lewis L, Mant T, Ferro A. HIV and AIDS A Textbook of Clinical Pharmacology and Therapeutics. 5 ed. London: A Hodder Arnold Publication; 2008. p. 351–60. [Google Scholar]
- [41].Lostao MP, Mata JF, Larrayoz IM, Inzillo SM, Casado FJ, Pastor-Anglada M. Electrogenic uptake of nucleosides and nucleoside-derived drugs by the human nucleoside transporter 1 (hCNT1) expressed in Xenopus laevis oocytes. FEBS letters. 2000; 481(2): 137–40. [DOI] [PubMed] [Google Scholar]
- [42].Becher F, Landman R, Mboup S, Kane CN, Canestri A, Liegeois F, et al. Monitoring of didanosine and stavudine intracellular trisphosphorylated anabolite concentrations in HIV-infected patients. AIDS (London, England). 2004; 18(2): 181–7. [DOI] [PubMed] [Google Scholar]
- [43].Sy SK, Innes S, Derendorf H, Cotton MF, Rosenkranz B. Estimation of intracellular concentration of stavudine triphosphate in HIV-infected children given a reduced dose of 0.5 milligrams per kilogram twice daily. Antimicrobial agents and chemotherapy. 2014; 58(2): 1084–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Balzarini J, Herdewijn P, De Clercq E. Differential patterns of intracellular metabolism of 2’,3’-didehydro-2’,3’-dideoxythymidine and 3’-azido-2’,3’-dideoxythymidine, two potent anti-human immunodeficiency virus compounds. The Journal of biological chemistry. 1989; 264(11): 6127–33. [PubMed] [Google Scholar]
- [45].Piliero PJ. Pharmacokinetic Properties of Nucleoside/Nucleotide Reverse Transcriptase Inhibitors. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2004; 37: S2–S12. [DOI] [PubMed] [Google Scholar]
- [46].Stein DS, Moore KH. Phosphorylation of nucleoside analog antiretrovirals: a review for clinicians. Pharmacotherapy. 2001; 21(1): 11–34. [DOI] [PubMed] [Google Scholar]
- [47].Mu L, Zhou R, Tang F, Liu X, Li S, Xie F, et al. Intracellular pharmacokinetic study of zidovudine and its phosphorylated metabolites. Acta Pharmaceutica Sinica B. 2016; 6(2): 158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sales SD, Hoggard PG, Sunderland D, Khoo S, Hart CA, Back DJ. Zidovudine phosphorylation and mitochondrial toxicity in vitro. Toxicology and applied pharmacology. 2001; 177(1): 54–8. [DOI] [PubMed] [Google Scholar]
- [49].Hubscher U, Spadari S, Villani G, Maga G. Inhibitors of HIV-1 Reverse Transcriptase DNA Polymerases: Discovery, Characterization and Functions in Cellular DNA Transactions. Singapore: World Scientific Publishing Company; 2010. p. 301–6. [Google Scholar]
- [50].Scaglione F, Berrino L. Cytosine deoxyribonucleoside anti-HIV analogues: a small chemical substitution allows relevant activities. International journal of antimicrobial agents. 2012; 39(6): 458–63. [DOI] [PubMed] [Google Scholar]
- [51].Feng JY, Murakami E, Zorca SM, Johnson AA, Johnson KA, Schinazi RF, et al. Relationship between antiviral activity and host toxicity: comparison of the incorporation efficiencies of 2’,3’dideoxy-5-fluoro-3’-thiacytidine-triphosphate analogs by human immunodeficiency virus type 1 reverse transcriptase and human mitochondrial DNA polymerase. Antimicrobial agents and chemotherapy. 2004; 48(4): 1300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Taneva E, Crooker K, Park SH, Su JT, Ott A, Cheshenko N, et al. Differential Mechanisms of Tenofovir and Tenofovir Disoproxil Fumarate Cellular Transport and Implications for Topical Preexposure Prophylaxis. Antimicrobial agents and chemotherapy. 2016; 60(3): 1667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Birkus G, Bam RA, Willkom M, Frey CR, Tsai L, Stray KM, et al. Intracellular Activation of Tenofovir Alafenamide and the Effect of Viral and Host Protease Inhibitors. Antimicrobial agents and chemotherapy. 2016; 60(1): 316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Suo Z, Johnson KA. Selective inhibition of HIV-1 reverse transcriptase by an antiviral inhibitor, (R)-9-(2-Phosphonylmethoxypropyl)adenine. The Journal of biological chemistry. 1998; 273(42): 27250–8. [DOI] [PubMed] [Google Scholar]
- [55].Ray AS, Fordyce MW, Hitchcock MJ. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus. Antiviral research. 2016; 125: 63–70. [DOI] [PubMed] [Google Scholar]
- [56].Lee WA, He GX, Eisenberg E, Cihlar T, Swaminathan S, Mulato A, et al. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrobial agents and chemotherapy. 2005; 49(5): 1898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rosenthal GJ, Kowolenko M. Immunotoxicology And Immunopharmacology In: Jack HD, editor. Immunotoxicology And Immunopharmacology. TARGET ORGAN TOXICOLOGY SERIES. 2 ed. Immunotoxicologic Manifestations of AIDS Therapeutics: CRC Press; 1994. p. 761. [Google Scholar]
- [58].Ilina T, Parniak MA. Inhibitors of HIV-1 Reverse Transcriptase In: Murad F, Granner D, August JT, Jeang K-T, editors. HIV I: Molecular Biology and Pathogenesis: Clinical Applications. 2 ed. UK: Academic Press; 2008. p. 127–39. [Google Scholar]
- [59].Ray AS, Olson L, Fridland A. Role of purine nucleoside phosphorylase in interactions between 2’,3’-dideoxyinosine and allopurinol, ganciclovir, or tenofovir. Antimicrobial agents and chemotherapy. 2004; 48(4): 1089–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Healthcare V. Ziagen: Prescribing Information Interactions USA: ViiV Healthcare; 1996. [Available from: http://pi.lilly.com/us/zyprexa-pi.pdf. [Google Scholar]
- [61].Masereeuw R, Russel FG. Regulatory pathways for ATP-binding cassette transport proteins in kidney proximal tubules. The AAPS journal. 2012; 14(4): 883–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ho RH, Kim RB. Transporters and drug therapy: implications for drug disposition and disease. Clinical pharmacology and therapeutics. 2005; 78(3): 260–77. [DOI] [PubMed] [Google Scholar]
- [63].Cihlar T, Ho ES, Lin DC, Mulato AS. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides, nucleotides & nucleic acids. 2001; 20(4–7): 641–8. [DOI] [PubMed] [Google Scholar]
- [64].Ray AS, Cihlar T, Robinson KL, Tong L, Vela JE, Fuller MD, et al. [Google Scholar]
- [65].Weiss J, Weis N, Ketabi-Kiyanvash N, Storch CH, Haefeli WE. Comparison of the induction of P-glycoprotein activity by nucleotide, nucleoside, and non-nucleoside reverse transcriptase inhibitors. European journal of pharmacology. 2008; 579(1–3): 104–9. [DOI] [PubMed] [Google Scholar]
- [66].Weiss J, Theile D, Ketabi-Kiyanvash N, Lindenmaier H, Haefeli WE. Inhibition of MRP1/ABCC1, MRP2/ABCC2, and MRP3/ABCC3 by nucleoside, nucleotide, and non-nucleoside reverse transcriptase inhibitors. Drug metabolism and disposition: the biological fate of chemicals. 2007; 35(3): 340–4. [DOI] [PubMed] [Google Scholar]
- [67].Wang X, Furukawa T, Nitanda T, Okamoto M, Sugimoto Y, Akiyama S, et al. Breast cancer resistance protein (BCRP/ABCG2) induces cellular resistance to HIV-1 nucleoside reverse transcriptase inhibitors. Molecular pharmacology. 2003; 63(1): 65–72. [DOI] [PubMed] [Google Scholar]
- [68].Bousquet L, Pruvost A, Didier N, Farinotti R, Mabondzo A. Emtricitabine: Inhibitor and substrate of multidrug resistance associated protein. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2008; 35(4): 247–56. [DOI] [PubMed] [Google Scholar]
- [69].van Gelder J, Deferme S, Naesens L, De Clercq E, van den Mooter G, Kinget R, et al. Intestinal absorption enhancement of the ester prodrug tenofovir disoproxil fumarate through modulation of the biochemical barrier by defined ester mixtures. Drug metabolism and disposition: the biological fate of chemicals. 2002; 30(8): 924–30. [DOI] [PubMed] [Google Scholar]
- [70].Jin F, Fordyce M, Garner W, Ray A, Tanamly S, Lindow J, et al. , editors. Pharmacokinetics, metabolism and excretion of tenofovir alafenamide. 14th International Workshop on Clinical Pharmacology of HIV Therapy; 2013. April 22–24; Amsterdam, The Netherlands. [Google Scholar]
- [71].Ray AS, Cihlar T, Robinson KL, Tong L, Vela JE, Fuller MD, et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrobial agents and chemotherapy. 2006; 50(10): 3297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Storch CH, Theile D, Lindenmaier H, Haefeli WE, Weiss J. Comparison of the inhibitory activity of anti-HIV drugs on P-glycoprotein. Biochemical pharmacology. 2007; 73(10): 1573–81. [DOI] [PubMed] [Google Scholar]
- [73].Schuetz JD, Connelly MC, Sun D, Paibir SG, Flynn PM, Srinivas RV, et al. MRP4: A previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nature medicine. 1999; 5(9): 1048–51. [DOI] [PubMed] [Google Scholar]
- [74].Weiss J, Rose J, Storch CH, Ketabi-Kiyanvash N, Sauer A, Haefeli WE, et al. Modulation of human BCRP (ABCG2) activity by anti-HIV drugs. The Journal of antimicrobial chemotherapy. 2007; 59(2): 238–45. [DOI] [PubMed] [Google Scholar]
- [75].Pan G, Giri N, Elmquist WF. Abcg2/Bcrp1 mediates the polarized transport of antiretroviral nucleosides abacavir and zidovudine. Drug metabolism and disposition: the biological fate of chemicals. 2007; 35(7): 1165–73. [DOI] [PubMed] [Google Scholar]
- [76].Reid G, Wielinga P, Zelcer N, De Haas M, Van Deemter L, Wijnholds J, et al. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Molecular pharmacology. 2003; 63(5): 1094–103. [DOI] [PubMed] [Google Scholar]
- [77].Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet (London, England). 2000; 356(9239): 1423–30. [DOI] [PubMed] [Google Scholar]
- [78].WHO. HIV Drug Resistance Surveillance Network: WHO and International AIDS Society building; 2000. [Available from: http://www.who.int/drugresistance/hivaids/network/en/. [Google Scholar]
- [79].Chen CH, Vazquez-Padua M, Cheng YC. Effect of anti-human immunodeficiency virus nucleoside analogs on mitochondrial DNA and its implication for delayed toxicity. Molecular pharmacology. 1991; 39(5): 625–8. [PubMed] [Google Scholar]
- [80].Kohler JJ, Lewis W. A brief overview of mechanisms of mitochondrial toxicity from NRTIs. Environmental and molecular mutagenesis. 2007; 48(3–4): 166–72. [DOI] [PubMed] [Google Scholar]
- [81].Lewis W, Day BJ, Copeland WC. Mitochondrial toxicity of nrti antiviral drugs: an integrated cellular perspective. Nat Rev Drug Discov. 2003; 2(10): 812–22. [DOI] [PubMed] [Google Scholar]
- [82].Walker UA. Update on mitochondrial toxicity: where are we now? Journal of HIV therapy. 2003; 8(2): 32–5. [PubMed] [Google Scholar]
- [83].Carr A, Cooper DA. Adverse effects of antiretroviral therapy. The Lancet. 2000; 356(9239): 1423–30. [DOI] [PubMed] [Google Scholar]
- [84].Stray KM, Bam RA, Birkus G, Hao J, Lepist EI, Yant SR, et al. Evaluation of the effect of cobicistat on the in vitro renal transport and cytotoxicity potential of tenofovir. Antimicrobial agents and chemotherapy. 2013; 57(10): 4982–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Cihlar T, Birkus G, Greenwalt DE, Hitchcock MJ. Tenofovir exhibits low cytotoxicity in various human cell types: comparison with other nucleoside reverse transcriptase inhibitors. Antiviral Res. 2002; 54(1): 37–45. [DOI] [PubMed] [Google Scholar]
- [86].Glover M, Hebert VY, Nichols K, Xue SY, Thibeaux TM, Zavecz JA, et al. Overexpression of mitochondrial antioxidant manganese superoxide dismutase (MnSOD) provides protection against AZT-or 3TC-induced endothelial dysfunction. Antiviral Res. 2014; 111: 136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Oluwafeyisetan A, Olubunmi A, Peter O. Naringin Ameliorates HIV-1 Nucleoside Reverse Transcriptase Inhibitors- Induced Mitochondrial Toxicity. Curr HIV Res. 2016; 14(6): 506–16. [DOI] [PubMed] [Google Scholar]
- [88].Xue SY, Hebert VY, Hayes DM, Robinson CN, Glover M, Dugas TR. Nucleoside reverse transcriptase inhibitors induce a mitophagy-associated endothelial cytotoxicity that is reversed by coenzyme Q10 cotreatment. Toxicol Sci. 2013; 134(2): 323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Batterham M, Gold J, Naidoo D, Lux O, Sadler S, Bridle S, et al. A preliminary open label dose comparison using an antioxidant regimen to determine the effect on viral load and oxidative stress in men with HIV/AIDS. Eur J Clin Nutr. 2001; 55(2): 107–14. [DOI] [PubMed] [Google Scholar]
- [90].Rosenfeldt FL, Mijch A, McCrystal G, Sweeney C, Pepe S, Nicholls M, et al. Skeletal myopathy associated with nucleoside reverse transcriptase inhibitor therapy: potential benefit of coenzyme Q10 therapy. Int J STD AIDS. 2005; 16(12): 827–9. [DOI] [PubMed] [Google Scholar]
- [91].Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011; 57(5): 773–80. [DOI] [PubMed] [Google Scholar]
- [92].Mills A, Arribas JR, Andrade-Villanueva J, DiPerri G, Van Lunzen J, Koenig E, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016; 16(1): 43–52. [DOI] [PubMed] [Google Scholar]
- [93].Calza L, Manfredi R, Chiodo F. Hyperlactataemia and lactic acidosis in HIV-infected patients receiving antiretroviral therapy. Clinical nutrition (Edinburgh, Scotland). 2005; 24(1): 5–15. [DOI] [PubMed] [Google Scholar]
- [94].Kvq Lương, Nguyễn LTH. The role of thiamine in HIV infection. International Journal of Infectious Diseases. 2013; 17(4): e221–e7. [DOI] [PubMed] [Google Scholar]
- [95].Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS (London, England). 2006; 20(17): 2165–74. [DOI] [PubMed] [Google Scholar]
- [96].Hansen AB, Obel N, Nielsen H, Pedersen C, Gerstoft J. Bone mineral density changes in protease inhibitor-sparing vs. nucleoside reverse transcriptase inhibitor-sparing highly active antiretroviral therapy: data from a randomized trial*. HIV medicine. 2011; 12(3): 157–65. [DOI] [PubMed] [Google Scholar]
- [97].Arora S, Agrawal M, Sun L, Duffoo F, Zaidi M, Iqbal J. HIV and Bone Loss. Current Osteoporosis Reports. 2010; 8(4): 219–26. [DOI] [PubMed] [Google Scholar]
- [98].Loveday C International perspectives on antiretroviral resistance. Nucleoside reverse transcriptase inhibitor resistance. Journal of acquired immune deficiency syndromes (1999). 2001; 26 Suppl 1: S10–24. [DOI] [PubMed] [Google Scholar]
- [99].Wirden M, Simon A, Schneider L, Tubiana R, Paris L, Marcelin AG, et al. Interruption of nonnucleoside reverse transcriptase inhibitor (NNRTI) therapy for 2 months has no effect on levels of human immunodeficiency virus type 1 in plasma of patients harboring viruses with mutations associated with resistance to NNRTIs. Journal of clinical microbiology. 2003; 41(6): 2713–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Roberts JD, Preston BD, Johnston LA, Soni A, Loeb LA, Kunkel TA. Fidelity of two retroviral reverse transcriptases during DNA-dependent DNA synthesis in vitro. Molecular and cellular biology. 1989; 9(2): 469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Mandal S, Prathipati PK, Kang G, Zhou Y, Yuan Z, Fan W, et al. Tenofovir alafenamide and elvitegravir loaded nanoparticles for long-acting prevention of HIV-1 vaginal transmission. AIDS. 2017; 31(4): 469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Prathipati PK, Mandal S, Destache CJ. Simultaneous quantification of tenofovir, emtricitabine, rilpivirine, elvitegravir and dolutegravir in mouse biological matrices by LC-MS/MS and its application to a pharmacokinetic study. J Pharm Biomed Anal. 2016; 129: 473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Gunawardana M, Remedios-Chan M, Miller CS, Fanter R, Yang F, Marzinke MA, et al. Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrobial agents and chemotherapy. 2015; 59(7): 3913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]