Abstract
Straightforward synthesis of cholesterol functionalized aliphatic N-substituted 8-membered cyclic carbonate (Chol-8m) monomer is reported. Well-defined poly(ethylene glycol) (PEG) diblock copolymers were readily accessed via organo catalytic ring opening polymerization. These polymers show promise as building blocks for self-assembled nanostructures and steric stabilizers for liposomes.
Efficient and scalable synthesis of functional monomers is crucial for successful commercialization of degradable polymers for nanobiomedicine.1 Recently a few aliphatic carbonate platforms have enabled straightforward synthesis of functional monomers.2–5 Particularly, amino diol based precursors have emerged as an attractive starting material to access novel functional materials.3,4,6 An inherent difference in reactivity of amines and alcohols can be utilized to create functionalized cyclic carbonate monomers, in a chemo-selective and step-efficient manner. Amines in the presence of unprotected alcohols have been shown to react with a wide range of nucleophiles, including alkyl halides, chloroformates and acrylates. This first step results in a functionalized diol that can be further cyclized in an intra-molecular fashion to produce target monomers.3,4 With the relative ease in introduction of functionalities and no need for protection and deprotection of alcohols, potential large-scale production of a wide variety of functional monomers, and subsequently polymers, is feasible.
Serinol,3,6,7 ammediol,8 and diethanolamines4,9,10 have all been used to synthesize functionalized carbonate monomers. Depending upon the exact choice of amino diol, 6 or 8-mem-bered cyclic carbonates are produced. Among commercially available amino diols, diethanolamine (DEA) is appealing due its low cost. DEA allows for the synthesis of aliphatic N-substituted functional 8-membered cyclic carbonates.4,9,11 Moreover, several functionalized diethanolamines are readily available, rendering this an attractive platform. Herein, we report two-step access to Chol-8m from commercially available DEA and cholesteryl chloroformate. Subsequent organo-catalytic ring-opening polymerization of this monomer results in well-defined PEGylated amphiphilic diblock copolymers. The aqueous self-assembly behavior and potential utility of these block copolymers as an inexpensive steric stabilizer for liposomes are also discussed.
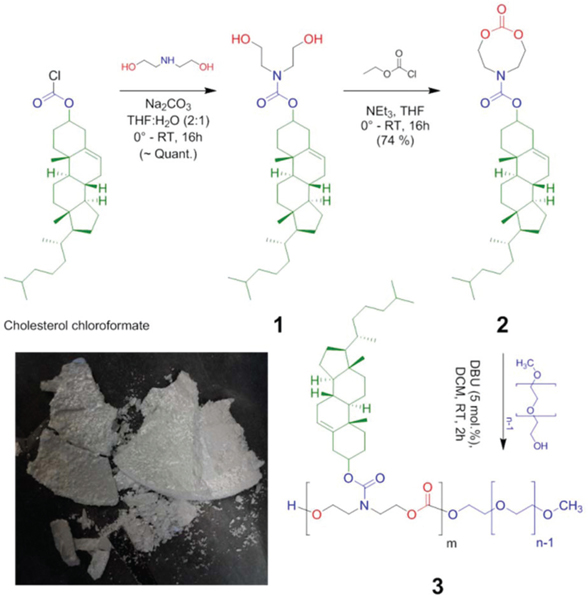
Cholesterol, a biologically relevant and hydrophobic moiety has been shown to direct self-assembly and also serve as an excellent handle to encapsulate hydrophobic drugs.12,13 Incorporation of cholesterol into biodegradable platforms allows for the development of materials well suited for biomedical applications.12,14 In this work, cholesterol-functionalized monomer was synthesized on multigram scale in a two-step process. First, DEA was allowed to react with commercially available cholesterol chloroformate in the presence of Na2CO3 in a tetrahydrofuran (THF)–water solvent mixture to result in cholesterol-functionalized diol 1 (Scheme 1). Functional diol was isolated in nearly quantitative yield through solvent–solvent extraction and was then subjected to intra-molecular cyclization with ethyl chloroformate as the cyclization reagent to yield target monomer 2 (Scheme 1). The crude product was purified by recrystallization from a dichloromethane (DCM) : diethyl ether mixture (1 : 1 by volume fraction), to result in polymerizable-grade monomer as shiny, flaky crystals and with an overall yield of ≈74%. In contrast to previous reports to synthesize cholesterol-functionalized cyclic carbonate monomers,3,5,15,16 the current approach with its straightforward purification (i.e., no need for flash-column chromatography) makes it more commercially practical.
Scheme 1.
Synthesis of PEGylated amphiphilic diblock copolymers (3) from Chol-8m (2). A photograph of monomer 2 crystals is included.
We explored organo-catalytic ring opening polymerization (ROP) of monomer 2, by using commercially available macroinitiator, polyethylene glycol monomethyl ether (mPEG-OH, 2.4 and 5.0 kDa). The ROP reaction was carried out at room temperature for about 2 h using 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, 5% by mol with respect to monomer) as the catalyst and DCM as the solvent. By varying the mPEG-OH and by changing the feed ratio of monomer versus initiator, a series of diblock copolymers, 3a–k (mP(EG)m-b-P(2)n), was readily synthesized (Table 1). In general the observed degree of polymerization (DPn) as determined by 1H nuclear magnetic resonance (NMR) spectroscopy (by comparing resonances due to signals from cholesteryl protons to that of signals from PEG protons), demonstrated very good agreement to the initial feed ratio (mPEG-OH : monomer 2). The series of synthesized block copolymers has amphiphilic balance tuned over a broad range (PEG mass fractions f ranging from 0.87 to 0.18). All polymers have a relatively low polydispersity (ĐM < 1.2), suggesting that the polymerization reaction proceeded with excellent control.
Table 1.
Characterization of amphiphilic diblock copolymers (3)
| S. no. | Code | mPEG-OH Mn a (kDa) | Targetb | Observedc | Mn c (kDa) | ĐMd | fe |
|---|---|---|---|---|---|---|---|
| 1 | 3a | 5.0 | 2.1 | 1.4 | 5.7 | 1.11 | 0.87 |
| 2 | 3b | 5.0 | 2.5 | 2.2 | 6.2 | 1.11 | 0.81 |
| 3 | 3c | 5.0 | 3.0 | 2.8 | 6.5 | 1.12 | 0.77 |
| 4 | 3d | 5.0 | 3.5 | 3.3 | 6.8 | 1.13 | 0.74 |
| 5 | 3e | 5.0 | 4.0 | 3.7 | 7.0 | 1.12 | 0.71 |
| 6 | 3f | 5.0 | 5.0 | 4.8 | 7.6 | 1.13 | 0.66 |
| 7 | 3g | 2.4 | 5.1 | 3.9 | 4.5 | 1.12 | 0.53 |
| 8 | 3h | 5.0 | 10.0 | 9.8 | 10.3 | 1.15 | 0.49 |
| 9 | 3i | 5.0 | 15.0 | 14.1 | 12.7 | 1.16 | 0.40 |
| 10 | 3j | 5.0 | 19.6 | 18.6 | 15.1 | 1.18 | 0.33 |
| 11 | 3k | 2.4 | 20.4 | 19.8 | 13.1 | 1.17 | 0.18 |
Relative number-average molecular mass as provided by the manufacturer.
Based on the initial molar feed ratio with respect to the mPEG-OH.
Based on 1H-NMR spectroscopy.
Dispersity index (ĐM) as determined by size-exclusion chromatography (SEC).
PEG mass fraction based upon: .
Nanostructures of various shape, obtained from block copolymers, find multitude of applications in medicine and consumer-care domains.17 Engineered block copolymer compositions, covering broad range of amphiphilic balance, allows for modulation of interfacial curvature and thereby accessing nanostructures of different shapes.18 Selected representative examples of block copolymers were investigated for their aqueous self-assembly via solvent exchange by dialysis (from THF to water) at room temperature (Fig. 1). As observed by conventional transmission electron micrographs (negatively stained with phosphotungstic acid), polymer 3a was found to form discrete disk-like micelles, whereas polymer 3e, was found to form a mixture of disk-like micelles and tape-like nanostructures. Depending upon orientation of nanostructures on the TEM grid, disks are observed in edge-on, face-one and intermediate orientations. Similar disk-like micelles and the transformation of disks-to-tapes have been observed for cholesterol-containing PEGylated systems.15,19 It is important to note that disk-like micelles are not commonly encountered and in most instances, they require sophisticated polymer design or processing conditions.20 It is remarkable that disk-like micelles can be accessed with these simple diblock copolymers. Two-dimensional sheet or membrane-like structures were formed by polymer 3k and these membranes were found to have internal stripped patterns, presumably due to the ordering of cholesteryl side-chains as noted earlier for other cholesterol functionalized block copolymers (Fig. S1†).16 Apart from exploring the self-assembly of these block copolymers, we are also interested in using them as an additive to impart stability to liposomes by the formation of hybrid stealth liposomes.
Fig. 1.
Aqueous self-assembly of amphiphilic diblock copolymers 3. (A) 3a, discrete disk-like micelles; (B) 3e, mixture of disk-like micelles and elongates tape-like structures and (C) 3k, membrane like structures.
Stealth liposomes are polymer coated lipid vesicles, wherein the water-soluble polymers anchor into vesicle bilayers through a covalently attached lipid (e.g., dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[mPEG-5000] (DPPE-PEG)).21–23 The polymer coating prevents liposome bilayer contact and consequently fusion, therefore sterically stabilizing liposomes in solution. Through their polymer coating, stealth liposomes dramatically outperform their unfunctionalized analogues in numerous attributes, including in vitro shelf-life and in vivo circulation times.22 Currently, these liposomes form a significant fraction of nanoformulations for a variety of therapeutics.23,24 However, the expensive nature of lipid-anchored polymers may restrict their wide-spread application.25 To address this limitation, we explored the use of block copolymer 3 as a liposome additive.
For these diblock copolymers to serve as a steric-stabilizing additive, their tendency to self-assemble into liposome bilayers (rather than reside in preformed micelles) is crucial.26 At lower , copolymer 3 will have a higher propensity to integrate into lipid membranes through insertion of the cholesteryl moieties. As a proof-of-concept study, liposomes were prepared from dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) by thinfilm rehydration and extrusion. Then copolymer 3a or DPPE-PEG was introduced at 5% by mol relative to the lipid. Hydrodynamic diameter of resultant stealth and hybrid stealth liposomes were monitored as a function of time by dynamic light scattering (DLS). From DLS data it is obvious that steric stabilizers are crucial for extended stability of liposomes (Fig. 2). Large aggregates that appear in a few days in the absence of steric stabilizers are not observed even up to 30 days with addition of 5% by mol of copolymer 3a or DPPE-PEG (Fig. 2D–F). These findings highlight the capability of the current block copolymers as a shelf-life extending additive for liposomes.27
Fig. 2.
Application of amphiphilic block copolymer 3a in the preparation of hybrid stealth liposomes. Schematic representations of a (A) standard liposome and (B) sterically-stabilized stealth liposomes; (C) chemical structure of liposome components, their corresponding schematic representations and bilayer configurations; and size distribution evolutions as a function of time for each type of liposome: (D) standard, (E) stealth, and (F) hybrid stealth. Formulations containing block copolymer 3a demonstrate temporal stability comparable to that of pre-existing stealth liposome technology.
In summary, Chol-8m (2), was synthesized through a commercially relevant synthetic approach. By using polyethylene glycol monomethyl ether as a macroinitiator, organo-catalytic ROP of monomer 2 was conducted to result in a series of PEGylated diblock copolymers, 3 (mPEG-b-P(2)) with tunable amphiphilic balance. These block copolymers were shown to undergo aqueous self-assembly to result in unique nano-structures, including disk-like micelles. It has also been demonstrated that synthesized diblock copolymers can serve as inexpensive steric stabilizers for liposomes. Owing to their biocompatible constituents and biodegradable hydrophobic block, these functional polymers are amenable for biomedical and consumer-care applications.
Supplementary Material
Acknowledgements
This work was funded by the Institute of Bioengineering and Nanotechnology (Biomedical Research Council, Agency for Science, Technology and Research, Singapore). K. P. M. acknowledges postdoctoral research support from the National Research Council Research Associateship Program.
Footnotes
Official contribution of the National Institute of Standards and Technology, not subject to copyright in the United States.
Electronic supplementary information (ESI) available: Details of experimental methods, additional TEM micrograph, NMR spectra and SEC chromatographs. See DOI: 10.1039/c8py00406d
Conflicts of interest
There are no conflicts of interest to declare.
Notes and references
- 1.Tian H, Tang Z, Zhuang X, Chen X and Jing X, Prog. Polym. Sci, 2012, 37, 237–280; [Google Scholar]; Feng J, Zhuo R-X and Zhang X-Z, Prog. Polym. Sci, 2012, 37, 211–236; [Google Scholar]; Tempelaar S, Mespouille L, Coulembier O, Dubois P and Dove AP, Chem. Soc. Rev, 2013, 42, 1312–1336. [DOI] [PubMed] [Google Scholar]
- 2.Pratt R, Nederberg F, Waymouth RM and Hedrick JL, Chem. Commun, 2008, 114–116; [DOI] [PubMed] [Google Scholar]; Sanders DP, Fukushima K, Coady DJ, Nelson A, Fujiwara M, Yasumoto M and Hedrick JL, J. Am. Chem. Soc, 2010, 132, 14724–14726; [DOI] [PubMed] [Google Scholar]; Mindemark J and Bowden T, Polym. Chem, 2012, 3, 1399–1401. [Google Scholar]
- 3.Venkataraman S, Veronica N, Voo ZX, Hedrick JL and Yang YY, Polym. Chem, 2013, 4, 2945–2948. [Google Scholar]
- 4.Venkataraman S, Ng VW, Coady DJ, Horn HW, Jones GO, Fung TS, Sardon H, Waymouth RM,Hedrick JL and Yang YY, J. Am. Chem. Soc, 2015, 137, 13851–13860. [DOI] [PubMed] [Google Scholar]
- 5.Olsson JV, Hult D, Cai Y, Garcia-Gallego S and Malkoch M, Polym. Chem, 2014, 5, 6651–6655. [Google Scholar]
- 6.Sanda F, Kamatani J and Endo T, Macromolecules, 2001, 34, 1564–1569. [Google Scholar]
- 7.Qiu F-Y, Song C-C, Zhang M, Du F-S and Li Z-C, ACS Macro Lett, 2015, 4, 1220–1224; [DOI] [PubMed] [Google Scholar]; Qiu F-Y, Zhang M, Du F-S and Li Z-C, Macromolecules, 2017, 50, 23–34; [Google Scholar]; Hu X, Chen X, Xie Z, Cheng H and Jing X, J. Polym. Sci., Part A: Polym. Chem, 2008, 46, 7022–7032. [Google Scholar]
- 8.Dong P, Sun H and Quan D, Polymer, 2016, 97, 614–622. [Google Scholar]
- 9.Tan JPK, Coady DJ, Sardon H, Yuen A, Gao S, Lim SW, Liang ZC, Tan EW, Venkataraman S, Engler AC, Fevre M, Ono R, Yang YY and Hedrick JL, Adv. Healthcare Mater, 2017, 6, 1601420; [DOI] [PubMed] [Google Scholar]; Venkataraman S, Tan JPK, Ng VWL, Tan EWP, Hedrick JL and Yang YY, Biomacromolecules, 2017, 18, 178–188; [DOI] [PubMed] [Google Scholar]; Qiu F-Y, Yu L, Du F-S and Li Z-C, Macromol. Rapid Commun, 2017, 38, 1700400. [DOI] [PubMed] [Google Scholar]
- 10.Chang YA, Rudenko AE and Waymouth RM, ACS Macro Lett, 2016, 5, 1162–1166. [DOI] [PubMed] [Google Scholar]
- 11.Pascual A, Tan JPK, Yuen A, Chan JMW, Coady DJ, Mecerreyes D, Hedrick JL, Yang YY and Sardon H, Biomacromolecules, 2015, 16, 1169–1178. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Briand VA, Sharma N, Ahn S. k. and Kasi RM, Materials, 2009, 2, 636–660. [Google Scholar]
- 13.Ercole F, Whittaker MR, Quinn JF and Davis TP, Biomacromolecules, 2015, 16, 1886–1914. [DOI] [PubMed] [Google Scholar]
- 14.Lee AL, Venkataraman S, Sirat SB, Gao S, Hedrick JL and Yang YY, Biomaterials, 2012, 33, 1921–1928; [DOI] [PubMed] [Google Scholar]; Coady DJ, Ong ZY, Lee PS, Venkataraman S, Chin W, Engler AC, Yang YY and Hedrick JL, Adv. Healthcare Mater, 2014, 3, 882–889; [DOI] [PubMed] [Google Scholar]; Lee ALZ, Venkataraman S, Fox CH, Coady DJ, Frank CW, Hedrick JL and Yang YY, J. Mater. Chem. B, 2015, 3, 6953–6963; [DOI] [PubMed] [Google Scholar]; Yang C, Liu SQ, Venkataraman S, Gao SJ, Ke X, Chia XT, Hedrick JL and Yang YY, J. Controlled Release, 2015, 208, 93–105. [DOI] [PubMed] [Google Scholar]
- 15.Venkataraman S, Lee AL, Maune HT, Hedrick JL, Prabhu VM and Yang YY, Macromolecules, 2013, 46, 4839–4846. [Google Scholar]
- 16.Zhou L, Zhang D, Hocine S, Pilone A, Trepout S, Marco S, Thomas C, Guo J and Li M-H, Polym. Chem, 2017, 8, 4776–4780. [Google Scholar]
- 17.Venkataraman S, Hedrick JL, Ong ZY, Yang C, Ee PLR, Hammond PT and Yang YY, Adv. Drug Delivery Rev, 2011, 63, 1228–1246. [DOI] [PubMed] [Google Scholar]
- 18.Mai Y and Eisenberg A, Chem. Soc. Rev, 2012, 41, 5969–5985; [DOI] [PubMed] [Google Scholar]; Epps TH III and O’Reilly RK, Chem. Sci, 2016, 7, 1674–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danino D, Abezgauz L, Portnaya I and Dan N, J. Phys. Chem. Lett, 2016, 7, 1434–1439. [DOI] [PubMed] [Google Scholar]
- 20.Holder SJ and Sommerdijk NAJM, Polym. Chem, 2011, 2, 1018–1028; [Google Scholar]; Li Z, Chen Z, Cui H, Hales K, Qi K, Wooley KL and Pochan DJ, Langmuir, 2005, 21, 7533– 7539; [DOI] [PubMed] [Google Scholar]; Li Z, Chen Z, Cui H, Hales K, Wooley KL and Pochan DJ, Langmuir, 2007, 23, 4689–4694; [DOI] [PubMed] [Google Scholar]; Lin Z, Sun J, Zhou Y, Wang Y, Xu H, Yang X, Su H, Cui H, Aida T, Zhang W and Cheng SZD, J. Am. Chem. Soc, 2017, 139, 5883–5889; [DOI] [PubMed] [Google Scholar]; Lodge TP, Hillmyer MA, Zhou Z and Talmon Y, Macromolecules, 2004, 37, 6680–6682; [Google Scholar]; Edmonds WF, Li Z, Hillmyer MA and Lodge TP, Macromolecules, 2006, 39, 4526–4530; [Google Scholar]; Zhu J, Zhang S, Zhang K, Wang X, Mays JW, Wooley KL and Pochan DJ, Nat. Commun, 2013, 4, 2297; [DOI] [PubMed] [Google Scholar]; Venkataraman S, Chowdhury ZA, Lee AL, Tong YW, Akiba I and Yang YY, Macromol. Rapid Commun, 2013, 34, 652–658. [DOI] [PubMed] [Google Scholar]
- 21.Lasic DD and Needham D, Chem. Rev, 1995, 95, 2601–2628. [Google Scholar]
- 22.Immordino ML, Dosio F and Cattel L, Int. J. Nanomed, 2006, 1, 297–315. [PMC free article] [PubMed] [Google Scholar]
- 23.Barenholz Y, J. Controlled Release, 2012, 160, 117–134. [DOI] [PubMed] [Google Scholar]
- 24.Allen TM and Cullis PR, Adv. Drug Delivery Rev, 2013, 65, 36–48. [DOI] [PubMed] [Google Scholar]
- 25.For example: 1,2-dipalmitoyl-sn-glycero-3-phosphoethano-lamine-N-[methoxy( polyethylene glycol)-5000] (ammonium salt), 880200P-1 g, $ 980 https://avantilipids.com/product/880200/, (accessed Jan 11, 2018).
- 26.Edwards K, Johnsson M, Karlsson G and Silvander M, Biophys. J, 1997, 73, 258–266; [DOI] [PMC free article] [PubMed] [Google Scholar]; Rovira-Bru M, Thompson DH and Szleifer I, Biophys. J, 2002, 83, 2419–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mineart KP, Venkataraman S, Yang YY, Hedrick JL and Prabhu VM, Macromolecules, 2018, DOI: 10.1021/acs.macromol.8b000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.