Abstract
Clarifying individual differences that predict resilience or vulnerability to emotional distress is crucial for identifying etiological factors contributing to affective disturbances, and to promoting emotional well-being. Despite recent progress identifying specific brain regions and personality traits, it remains unclear whether there are common factors underlying the structural aspects of the brain and the personality traits that, in turn, protect against symptoms of emotional distress. In the present study, an integrative structural equation model was developed to examine the associations among (1) a latent construct of Control, representing the volumes of a system of prefrontal cortical (PFC) regions including middle, inferior, and orbital frontal cortices; (2) a latent construct of Resilience personality traits including cognitive reappraisal, positive affectivity, and optimism; and (3) Anxiety and Depression symptoms, in a sample of 85 healthy young adults. Results showed that the latent construct of PFC volumes positively predicted the latent construct of Resilience, which in turn negatively predicted Anxiety. Mediation analysis confirmed that greater latent PFC volume is indirectly associated with lower Anxiety symptoms through greater latent trait Resilience. The model did not show a significant mediation for Depression. These results support the idea that there are common volumetric and personality factors that help protect against symptoms of emotional distress. These findings provide strong evidence that such brain-personality-symptom approaches can provide novel insights with valuable implications for understanding the interaction of these factors in healthy and clinically diagnosed individuals.
Key words: emotion, individual differences, neuroimaging, personality, affective disorders
Anxiety and depression are among the most common mental disorders in the United States (Anxiety and Depression Association of America, 2016; National Institute of Mental Health, 2016), and among the most prevalent causes of disability worldwide (World Health Organization, 2017). Hence, it is critical to improve current understanding of the neurobehavioral mechanisms associated with functions that are altered in these conditions. Despite recent progress regarding specific brain regions and personality traits (e.g., DeYoung et al., 2010; Giuliani, Drabant, Bhatnagar, & Gross, 2011), it remains unclear whether there are common latent brain and personality factors that might predict resilience or vulnerability to emotional distress.
Volumetric alterations in the brain and individual differences in personality traits that support resilience to emotional distress have been consistently associated with anxiety and depression (Chang et al., 2011; Gross & John, 2003; Martin & Dahlen, 2005; Talati, Pantazatos, Schneier, Weissman, & Hirsch, 2013; van Tol et al., 2010; Watson, Clark, & Carey, 1988; Wu et al., 2013). However, it remains unclear how these factors are interrelated, and whether there are common brain and personality factors underlying these associations. Examining this issue using integrative models combining neural correlates, personality traits, and measures indexing symptoms of distress in healthy populations provides the opportunity to identify common individual difference factors associated with different types of distress that may index resilience to, or risk/vulnerabilities for, psychopathologies (Cuthbert & Insel, 2013). Hence, in this proof-of-concept study, we adopted a brain-personality-distress symptom framework, using an integrative structural equation modeling approach, to examine associations among latent constructs of brain region volumes and personality traits, and the presence of anxiety and depression symptoms in a sample of healthy young adults.
A growing body of work from brain imaging research supports the idea that the brain can be conceptualized as a collection of interrelated systems or networks, and that brain regions that are involved in similar processes appear to be interrelated at both structural and functional levels (Alexander-Bloch, Giedd, & Bullmore, 2013; Bullmore & Sporns, 2009; Dosenbach et al., 2007; Dosenbach et al., 2006; Mechelli, Friston, Frackowiak, & Price, 2005; Power et al., 2011; Power & Petersen, 2013; Yeo, Krienen, Chee, & Buckner, 2014; Yeo et al., 2011). Although the exact delineation and dynamics of such networks continues to be an area of debate (e.g., Dosenbach et al., 2006; Power et al., 2011; Power & Petersen, 2013; Yeo et al., 2011), multiple networks appear to play key roles in top-down processing, cognitive control, and integration of emotional information (Dosenbach et al., 2006; Power et al., 2011; Power & Petersen, 2013; Seeley et al., 2007; Yeo et al., 2011). A shared feature of these networks is that key nodes exist within the prefrontal cortex (PFC), which has long been identified as a sector of the brain important for cognitive control and executive function (Gilbert & Burgess, 2008; Miller, 2000; Ochsner & Gross, 2005). Although the relative unity and diversity of cognitive control functions, and of the PFC, continues to be an ongoing area of research (Collette et al., 2005; Duncan, Johnson, Swales, & Freer, 1997; Friedman & Miyake, 2017; Miller, 2000; Miyake et al., 2000; Teuber, 1972), available evidence converges on the shared role of PFC regions in functions that contribute to the ability to cope with emotional challenges, such as cognitive reappraisal (Buhle et al., 2013; Goldin, McRae, Ramel, & Gross, 2008), positive affect (Ashby, Isen, & Turken, 1999; Davidson & Irwin, 1999), and optimism (Dolcos, Hu, Iordan, Moore, & Dolcos, 2016; Kringelbach, 2005; Sharot, Riccardi, Raio, & Phelps, 2007). We therefore introduce these associations below.
Within the PFC, the middle frontal cortex (MFC), inferior frontal cortex (IFC), and orbital frontal cortex (OFC) have each been linked to integration and control of emotion. Greater engagement of these regions has been found consistently in association with cognitive reappraisal (Buhle et al., 2013; Goldin et al., 2008; Kalisch, 2009), an emotion regulation strategy that involves construing a particular situation in a way that changes its emotional impact (Gross & John, 2003; Lazarus & Alfert, 1964). Individual differences in habitual engagement of reappraisal have also been associated with changes in brain response to emotional stimuli in the PFC (Drabant, McRae, Manuck, Hariri, & Gross, 2009). This suggests that the ways in which individuals typically control their emotions impacts neural processing and might, over the course of development, alter the structure of the underlying brain regions. Interestingly, engagement of reappraisal for longer durations seems to shift activity from left to right lateral PFC (Kalisch, 2009), and habitual engagement of reappraisal is positively associated with the volume of the right MFC (Moore et al., 2016). The right MFC has also been shown to be negatively associated with symptoms of emotional distress, such as depression (Bora, Fornito, Pantelis, & Yucel, 2012; Chang et al., 2011). Together, these results suggest that within the MFC, the volume of the right hemisphere is particularly associated with individual differences in cognitive control of emotion and protection against symptoms of emotional distress.
The PFC has also been identified as playing an important role supporting positive affect (Davidson & Irwin, 1999). In particular, convergent evidence from lesions, electroencephalography, and neuroimaging studies suggests that the left PFC is part of a system facilitating approach toward positive affective stimuli (Canli, Desmond, Zhao, Glover, & Gabrieli, 1998; Davidson & Irwin, 1999; Dolcos, LaBar, & Cabeza, 2004; Eddington, Dolcos, Cabeza, Krishnan, & Strauman, 2007; Harmon-Jones, 2003). The left PFC has also been linked to trait optimism (Dolcos et al., 2016), which refers to the dispositional tendency for people to hold generalized favorable expectancies about their future (Carver, Scheier, & Segerstrom, 2010). For example, disruption of the left PFC using transcranial magnetic stimulation has been shown to enhance the ability to incorporate negative information into beliefs (Sharot et al., 2012), suggesting that the left PFC facilitates the optimistic bias. Consistent with this idea, the left IFC and OFC have been shown to be negatively associated with symptoms of anxiety (Hu & Dolcos, 2017; Shang et al., 2014; Talati et al., 2013) and depression (Bremner et al., 2002; Lai, Payne, Byrum, Steffens, & Krishnan, 2000; Shah, Ebmeier, Glabus, & Goodwin, 1998). This suggests that within the IFC and OFC, the volume of the left hemisphere is particularly important in supporting cognitive control of emotion and protection against symptoms of emotional distress.
Consistent with the idea that the dispositional traits of cognitive reappraisal, positive affectivity, and optimism protect against symptoms of distress, these factors have been shown to be positively associated with one another (Chang, Maydeu-Olivares, & D’Zurilla, 1997; Gross & John, 2003), and to negatively predict anxiety and depression (Gross & John, 2003; Martin & Dahlen, 2005; Scheier, Carver, & Bridges, 1994; Watson, Clark, & Tellegen, 1988), suggesting that each of these traits might be an indicator of a common factor indexing well-being. There is also evidence suggesting that each of these constructs is related to cognitive control. For example, reappraisal has been shown to be linked to cognitive control abilities, such as working memory capacity (McRae, Jacobs, Ray, John, & Gross, 2012), positive affect has been shown to be linked to increased cognitive flexibility and reduced perseveration (Dreisbach & Goschke, 2004), and optimism has been shown to be associated with self-report indices of executive function such as organization (Kruger, 2011). Together, the available evidence suggests that cognitive reappraisal, positive affectivity, and optimism share a common association involving adaptive control of emotion that protects against negative emotional outcomes and emotional distress.
To summarize, greater engagement within a system of PFC regions has been associated with more adaptive responses to emotional challenges (Buhle et al., 2013; Davidson & Irwin, 1999; Goldin et al., 2008; Harmon-Jones, 2003; Kalisch, 2009), suggesting that a similar pattern may exist at the level of brain structure (Davidson & Irwin, 1999; Dolcos et al., 2016; Hu & Dolcos, 2017; Moore et al., 2016). The dispositional traits of cognitive reappraisal, positive affectivity, and optimism are personality dimensions that help to protect against symptoms of emotional distress (Carver, Scheier, & Segerstrom, 2010; Gross & John, 2003; Martin & Dahlen, 2005; Scheier, Carver, & Bridges, 1994; Watson, Clark, & Tellegen, 1988). Finally, indices of emotional distress, including anxiety and depression, have been linked to reduced volume in PFC structures (Bora et al., 2012; Bremner et al., 2002; Chang et al., 2011; Hu & Dolcos, 2017; Lai et al., 2000; Shah et al., 1998; Shang et al., 2014; Talati et al., 2013), and reduced indices of the resilience-related personality traits (Carver, Scheier, & Segerstrom, 2010; Gross & John, 2003; Martin & Dahlen, 2005; Scheier, Carver, & Bridges, 1994; Watson, Clark, & Tellegen, 1988).
However, what remains unclear is whether the suggested pattern of common factors in brain structure and in personality predicts lower symptoms of distress. To clarify this issue, the current study employed structural equation modeling using a brain-personality-distress symptom framework, and explored a possible mediating role of resilience-related personality traits in the link between PFC volume and measures of distress (i.e., anxiety and depression), in a sample of healthy young adults. The overall concept for the present report was informed by the existing literature (Colibazzi et al., 2008; Kim, Zhu, Chang, Bentler, & Ernst, 2007; Marsh et al., 2010; Yeh et al., 2010), and builds on previous findings coming from our work that targeted specific brain regions and factors (Dolcos et al., 2016; Hu & Dolcos, 2017; Moore et al., 2016) with a goal of testing for an integrated and comprehensive model. By incorporating factors that reflect individual differences in PFC volume, personality traits associated with enhanced positive affectivity, and measures of distress, the current study integrates control- and resilience-related constructs in a comprehensive brain-personality-symptom framework. Such an approach has the potential to advance our understanding of resilience and vulnerability to emotional distress and its mechanisms. The current study tested the following hypotheses: (a) A latent construct of PFC volume, including the MFC, IFC, and OFC, would be positively associated with latent trait Resilience; (b) a latent construct of trait Resilience would be negatively associated with Anxiety and Depression; and (c) the latent PFC volume would negatively predict symptoms of Distress through greater latent trait Resilience.
1. Methods
1.1. Participants
Data were collected from a sample of 85 healthy young participants (18–34 years old, 48 females), who had undergone magnetic resonance imaging (MRI) scanning. Some individual differences measures were not completed by all participants (see Analytic Overview subsection and Supplementary Materials Table 1 for details of final sample sizes, as well as details of statistical outlier assessment and removal that preceded all reported results). No participants had previously been diagnosed with any neurological, psychiatric, or personality disorders. Potential outlier cases were assessed and excluded from final analyses, based on procedures described below. The neuropsychological testing and structural brain imaging procedures were part of a common protocol across multiple individual functional brain imaging studies that also involved completion of behavioral tasks in the scanner. Participants completed questionnaires in one or more sessions at a computer terminal in the lab, which typically occurred within a few weeks around the MRI scanning session, depending on the specific functional studies. The present sample overlaps with samples previously reported elsewhere (Dolcos et al., 2016; Hu & Dolcos, 2017; Moore et al., 2016). The experimental protocol was approved for ethical treatment of human participants by the institutional Health Research Ethics Board, and participants provided written consent and were compensated with either course credit or money. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
1.2. Structural MRI data acquisition and preprocessing
Anatomical images (3D MPRAGE, repetition time=1,600 ms; echo time=3.82 ms; field of view=256×256 mm2; volume size=112 slices; voxel size=1×1×1 mm3) were obtained using a 1.5 Tesla Siemens Sonata scanner. To examine volumetric associations, brain imaging data were processed using a surface-based morphometric procedure. Surface-based cortical reconstruction and volumetric segmentation were performed with the FreeSurfer image analysis suite (FreeSurfer Version 5.3) (Fischl, 2012), which is freely available to download online (https://surfer.nmr.mgh.harvard.edu/). Specifically, raw DICOM images were imported directly into FreeSurfer (Fischl, 2012), where a semiautomatic workflow was adopted to ensure quality control at the stages of Talairach registration, skull stripping, white matter surface reconstruction, and pial surface reconstruction. Output from each of these stages was visually examined for quality assurance, and major errors were corrected using standard adjustment parameters or manual intervention before rerunning the necessary processing steps again until results were of good quality.
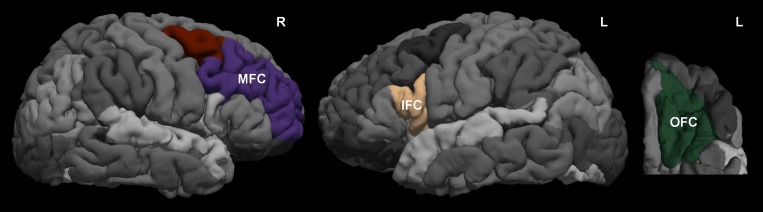
Volume measures from regions of interest (ROIs) were extracted using the parcellation from Desikan et al. (2006). Specifically, the right MFC (combined caudal and rostral MFC) ROI identified the region bordered by the superior frontal sulcus, the inferior frontal sulcus, and the precentral sulcus. For the left IFC (pars opercularis) ROI, the whole IFC was identified as the area delineated anteriorly by the rostral extent of the inferior frontal sulcus, posteriorly by the precentral gyrus, laterally by the lateral bank of the inferior frontal sulcus, and medially by the medial bank of the lateral orbital sulcus and/or the circular insular sulcus. The subdivision pars opercularis was defined on this IFC ROI as the first gyrus from the precentral gyrus. Finally, the left OFC (lateral OFC) ROI identified the region lateral to the medial orbital sulcus, within the rostral and caudal extent of the lateral orbital gyrus, bordering the lateral bank of the lateral orbital sulcus and/or the circular insular sulcus at the lateral aspect. Figure 1 shows an example of the ROI delineations. To account for overall brain size differences, the brain region volumes were scaled. Specifically, the MFC, IFC, and OFC volumes were divided by total intracranial volume, then multiplied by a constant (i.e., 1,000), to bring the scaled values to a variance range similar to the other variables in the structural equation model.
Figure 1.
The regions of interest selected for prefrontal cortex volumes. The Desikan-Killiany atlas was used to extract volumes for the MFC, IFC, and OFC for each participant. L = Left; R = Right; MFC = middle frontal cortex; IFC = inferior frontal cortex; OFC = orbital frontal cortex.
1.3. Individual differences measures
Personality and symptom measures included the Emotion Regulation Questionnaire (ERQ) (Gross & John, 2003), Positive and Negative Affect Schedule (PANAS) (Watson, Clark, & Tellegen, 1988), Life Orientation Test-Revised (LOT-R) (Scheier, Carver, & Bridges, 1994), State-Trait Anxiety Inventory (STAI) (Spielberger, Gorsuch, & Lushene, 1970), and the Beck Depression Inventory (BDI) (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961; Sanz & Garcia-Vera, 2007).
The ERQ assesses the habitual engagement of two emotion regulation strategies, reappraisal and suppression, using a 7-point Likert scale that ranges from 1=“strongly disagree,” to 7=“strongly agree.” Examples of statements from the reappraisal dimension include “I control my emotions by changing the way I think about the situation I’m in,” and statements from the suppression dimension include “I keep my emotions to myself” (Gross & John, 2003). In this sample, the Cronbach’s α was .73 for reappraisal, and .79 for suppression (n=80).
The PANAS is a widely used measure of current/trait affect (Watson, Clark, & Tellegen, 1988). It includes a list of 20 adjective descriptors of 10 positive (e.g., “interested,” “enthusiastic”) and 10 negative (e.g., “irritable,” “upset”) affects. Items are rated on a 5-point scale from 1=“very slightly or not at all,” to 5=“extremely” according “to what extent [the person] feels this way right now” or during a longer period of time (i.e., “in general”). In the current study, the trait measure for positive affect (Cronbach’s α=.92, n=78) over a longer period of time was used (negative affect Cronbach’s α=.79, n=78).
The LOT-R consists of 10 statements (e.g., “I’m always optimistic about my future,” “I rarely count on good things happening to me”), which measure the degree of optimism or pessimism (Scheier, Carver, & Bridges, 1994). Each statement is rated on a 5-point scale from 0=“strongly disagree,” to 4=“strongly agree.” Cronbach’s α was .76 in this sample (n=58).
The STAI provides measures of the temporary condition of “state anxiety” and the more general and long-standing quality of “trait anxiety” in adults (Spielberger, Gorsuch, & Lushene, 1970). The STAI consists of two scales containing 20 items each, with the trait anxiety measure evaluating how the participant feels “generally.” It uses “I feel/I am” statements that are rated on 4-point scale from 1=“not at all,” to 4=“very much so.” In the current study, the total trait measure of how a participant feels generally was used (Cronbach’s α=.88, n=81).
Finally, the BDI is a commonly used measure of depression (Beck et al., 1961; Sanz & Garcia-Vera, 2007). It consists of 21 items, each of them having four possible options to select from, ranging in intensity from 0 to 3 (e.g., 0=“I do not feel sad;” 1=“I feel sad;” 2=“I am sad all the time and I can’t snap out of it;” 3=“I am so sad or unhappy that I can’t stand it”). A value of 0–3 is assigned to each item and the total score determines the depression severity, the higher the score the more severe the depression (Cronbach’s α=.80, n=79).
1.4. Analytic overview
Structural MRI data were analyzed in conjunction with the individual difference measures introduced above, to examine associations among brain structure, personality, and distress symptoms. Analyses were carried out for testing statistical models involving brain region volumes, personality measures, and symptom measures, using R 3.4.3 with RStudio 1.1.423 and statistical package lavaan (Rosseel, 2012). The models that were tested were informed by the available anatomical literature, as well as theory regarding factors of resilience and emotional distress. Data were first assessed for potential outlier cases at a univariate level using a criterion of 3 SDs (Osborne & Overbay, 2004), for brain, trait, and symptom measures. Four participants were excluded from final analyses. Two of the participants were excluded because of outlier scaled ROI volumes, one participant was excluded because of outlier scores on reappraisal, anxiety, and depression measures, and one participant was excluded because of outlier score on optimism. In addition, some trait and symptom measures were not completed by all participants, thus handling of missing data is described below and final sample sizes are noted in the Supplementary Materials. Supplementary Materials Figure 1 shows scatterplots of the questionnaire data after outlier removal.
Path analyses were conducted using R package lavaan (Rosseel, 2012). Analyses completed in lavaan used settings that parallel other software packages such as AMOS standard settings, including Wishart estimation, maximum likelihood estimation for handling missing data, and the use of expected information for estimating standard error variance (Arbuckle, 2016; Rosseel, 2012). Within the hypothetical model, a latent factor was constructed for volumes of a PFC brain system of Control, another latent factor was constructed for a Resilience personality variable, and Distress was represented as manifest variables for total Anxiety and total Depression. Variables of sex and age were included in the regressions on the mediator and symptom variables, to control for the influence of these demographics. Given that a reversal of the proposed direction of effects is also statistically plausible, we tested two alternative models to examine the possibility that Resilience mediates the link from Distress to Control, or that Control mediates the link from Resilience to Distress. To determine the model fit, we examined the χ2/df ratio, comparative fit index (CFI), and root mean square error of approximation (RMSEA). A good model fit is reflected by χ2/df ratios <3 (Kline, 1998), fit indices above .90 (Bentler, 1990; Kline, 1998), and RMSEA values ≤.08.
2. Results
Analyses were conducted on brain, personality, and distress measures using the hypothetical structural equation model. The structural equation model included confirmatory factor analysis of the manifest brain and personality variables into latent variable constructs, which then were tested for predicted associations among each other and anxiety and depression measures using regression and mediation analyses. Supplementary Materials Table 1 provides descriptive information and intercorrelations for the targeted variables.
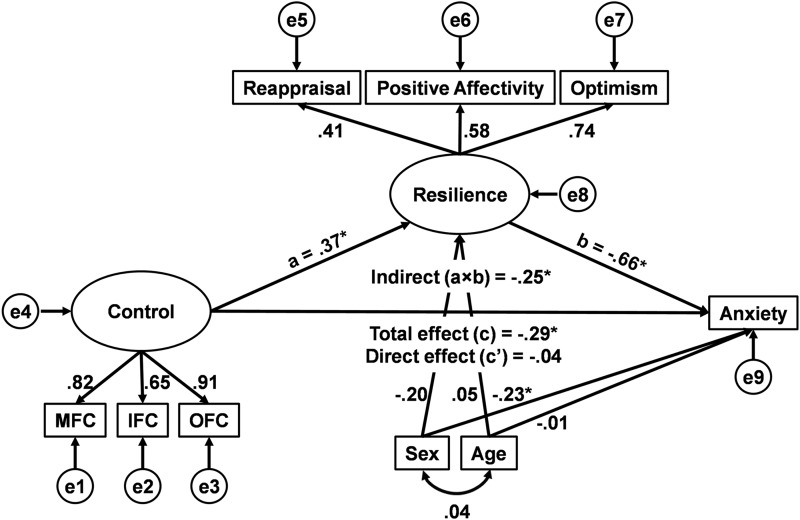
Figure 2 displays the latent variable mediation model for statistically predicting Anxiety with standardized path coefficients. The model predicting Anxiety showed a strong fit to the data, χ2(22)=21.16, ns, χ2/df=.96, CFI=1.00, RMSEA=.00. As expected, the scaled PFC volumes contributed significantly to the latent construct of Control (ps<.001), and the personality traits of reappraisal, positive affectivity, and optimism contributed significantly to the latent construct of Resilience (ps<.01). Consistent with the idea that brain regions engaged in cognitive control are associated with protection against emotional distress, the latent construct of PFC system volume positively predicted the latent construct of trait Resilience, and latent trait Resilience negatively predicted Anxiety. Furthermore, mediation analysis confirmed that greater latent PFC volume is indirectly associated with lower Anxiety symptoms through greater latent trait Resilience (see Figure 2).
Figure 2.
Structural equation model of latent prefrontal cortical (PFC) volume, latent trait Resilience, and Anxiety. Results from the proposed model confirm that latent PFC volume is associated with lower Anxiety, through greater latent trait Resilience. Standardized coefficients are shown for each path. MFC=middle frontal cortex (right side); IFC=inferior frontal cortex (left side); OFC=orbital frontal cortex (left side); e1–9=error terms. *Indicates p<.05 for the mediation analysis.
Consistent with the idea that anxiety and depression are often comorbid but also have nonoverlapping aspects (Bishop & Forster, 2013; Fava et al., 2000; Ormel et al., 2013; Pollack, 2005), the hypothesized model did not work as well for statistically predicting Depression. Specifically, fitting the same model that was used for predicting Anxiety to predict Depression showed an overall good fit to the data, χ2(22)=21.32, ns, χ2/df=.97, CFI=1.00, RMSEA=.00, but mediation analysis revealed only a trend level of significance for an indirect effect (a=.32, p=.074; b=−.56, p=.018; c=−.12, p=.299; c′=.06, p=.626; ab=−.18, p=.058). This possibly suggests that a similar model could be helpful for examining Depression, but that the current model might be better suited for understanding and predicting Anxiety in particular.
As expected, although the alternative model testing whether Resilience mediates the link from Anxiety to Control provided a good fit to the data, χ2(20)=21.06, ns, χ2/df=1.05, CFI=.99, RMSEA=.03, paths from Anxiety and Resilience to Control were not significant and a significant indirect effect was not found (ps>.19). The alternative model testing whether Control mediates the link from Resilience to Anxiety also provided a good fit, χ2(22)=23.06, ns, χ2/df=1.05, CFI=.99, RMSEA=.02, but the path from Control to Anxiety was not significant, and a significant indirect effect was also not found (ps>.68). For Depression, the alternative model testing whether Resilience mediates the link from Depression to Control provided a good fit, χ2(20)=21.25, ns, χ2/df=1.06, CFI=.99, RMSEA=.03, but paths from Depression and Resilience to Control were not significant and a significant indirect effect was not found (ps>.08). The alternative model testing whether Control mediates the link from Resilience to Depression also provided a good fit, χ2(22)=25.61, ns, χ2/df=1.16, CFI=.97, RMSEA=.05, but the path from Control to Depression was not significant, and a significant indirect effect was not found (ps>.56). In addition, results from supplementary analyses supported the idea that the associations identified among the targeted brain regions, personality traits, and symptoms are specific. Tests of alternative or additional brain, personality, and symptom measures did not appear to work better than the featured model (see Supplementary Materials).
3. Discussion
The present study demonstrated the successful implementation of a structural equation modeling approach to a brain-personality-distress symptom framework. Results showed that within an integrative structural equation model, latent factors of PFC brain volume and trait Resilience could be constructed and examined in association with symptoms of Anxiety and Depression. As expected, results showed that the latent construct of PFC volumes positively predicted the latent construct of Resilience, which in turn negatively predicted Anxiety. Furthermore, mediation analysis confirmed that greater latent PFC volume is indirectly associated with lower Anxiety symptoms through greater latent trait Resilience. Interestingly, the model fit well for Anxiety but did not show a significant mediation for Depression, which tentatively suggests that the associations with Anxiety are clearer, and more research will be needed to clarify the associations with Depression.
The latent construct of PFC region volumes is consistent with recent work suggesting an underlying framework of structural covariance in the brain (Alexander-Bloch, Giedd, & Bullmore, 2013; Baskin-Sommers, Neumann, Cope, & Kiehl, 2016; Bullmore & Sporns, 2009; Colibazzi et al., 2008; Mechelli et al., 2005; Yeh et al., 2010), and extends this idea to a system of PFC regions associated with the integration and control of emotion. The volumetric association between regions of the brain that are functionally related is consistent with the idea of use-dependent plasticity (Bütefisch et al., 2000; Nudo, Milliken, Jenkins, & Merzenich, 1996; for reviews of relevant studies in humans, see Draganski & May, 2008; May, 2011), which suggests that repeated patterns of neuronal firing may lead to increased synaptic connectivity (Hebb, 1949) and increases in gray matter volume (Draganski et al., 2006). Furthermore, the present results suggest that latent variable analysis is a feasible way of assessing the structural associations of functionally related brain regions, which complements other structural and functional approaches commonly used in the field to assess brain systems and networks (Bullmore & Sporns, 2009).
The contribution of the right MFC to the latent factor of Control is consistent with evidence that the right PFC is associated with engagement of reappraisal to decrease negative emotional response (Ochsner, Silvers, & Buhle, 2012), and with a system facilitating avoidance of aversive stimuli (Canli et al., 1998; Davidson & Irwin, 1999; Dolcos, LaBar & Cabeza, 2004; Eddington et al., 2007; Spielberg, Stewart, Levin, Miller, & Heller, 2008). In addition, the MFC has been emphasized in the integration of emotion and cognition (Gray, Braver, & Raichle, 2002), and in executive processes such as working memory (Curtis & D’Esposito, 2003), suggesting that there are multiple possibly interrelated processes that engage the MFC. Consistent with this idea, in the present study, MFC volume tended to be positively associated with Resilience-related traits and negatively associated with Anxiety. Taken together, these findings suggest that the MFC plays a key role in the integration and control of emotion, and that this role might involve or emerge from common functions that engage this brain region along with the IFC and OFC.
The left IFC and OFC contribution to the latent PFC construct is consistent with evidence that the left PFC is involved in a system facilitating approach toward appetitive stimuli (Davidson & Irwin, 1999), protecting against anxiety (Hu & Dolcos, 2017; Shang et al., 2014; Talati et al., 2013), and supporting optimism (Dolcos et al., 2016). The present findings suggest that larger volume of left PFC protects against symptoms of Anxiety, and that the OFC and MFC share positive associations with optimism (see Supplementary Materials Table 1). This is in line with the notion that optimism is a higher level trait that, beyond reflecting reward-related processing, also reflects individual differences in self-regulation and goal-directed behavior (Carver, Scheier, & Segerstrom, 2010; Nes & Segerstrom, 2006).
The latent construct of trait Resilience is consistent with the idea that cognitive reappraisal, positive affectivity, and optimism are associated traits (Chang, Maydeu-Olivares, & D’Zurilla, 1997; Gross & John, 2003), that each tap into a common factor that protects against emotional challenges. These traits also appear to be associated with individual differences in cognitive control and executive functions (Dreisbach & Goschke, 2004; Kruger, 2011; McRae et al., 2012), which supports the idea of examining latent constructs that might pull out common variance from across the observed personality traits. For example, it is interesting that positive affectivity was not directly correlated with any of the PFC volumes. However, positive affectivity contributed to the latent Resilience trait that was associated with PFC system volume, which suggests a more complex relation between a very general emotional trait, such as positive affectivity, and PFC volume. It is possible that general emotional traits such as positive affectivity have a diffuse association with brain volume, which is more readily captured with latent construct analyses as opposed to manifest variable assessments.
The strong fit of the structural equation model and significant prediction of Anxiety is consistent with previous evidence showing negative associations between PFC volume and anxiety (Dolcos et al., 2016; Hu & Dolcos, 2017; Shang et al., 2014; Talati et al., 2013). It is also consistent with previous evidence showing that cognitive reappraisal, positive affect, and optimism negatively predict anxiety (Gross & John, 2003; Martin & Dahlen, 2005). Together with the significant mediation, these findings suggest that, while there are common factors at the levels of PFC system volume and trait Resilience, the current model primarily describes interrelations that are associated with Anxiety. Interestingly, the model did not work as well for Depression, which was also not directly correlated with any of the scaled PFC volumes in this sample. This possibly suggests that, although the regions included here have been shown to be associated with depression in other research (Bora et al., 2012; Bremner et al., 2002; Chang et al., 2011; Lai et al., 2000; Shah et al., 1998), perhaps other regions that appear to be affected by depression would fit better in a latent factor analysis targeting depression specifically.
It is also possible that the results were somewhat influenced by aspects of the anxiety and depression measures themselves. The present study used a specific version of the STAI measure that targets trait anxiety, whereas the BDI typically assesses symptoms in a time range that includes the day of assessment. To further clarify these aspects, it would be important to investigate other brain regions that are also implicated in emotional dysregulation (Mayberg, 1997, 2006), and perhaps further explore other measures that are associated with emotional distress (e.g., neuroticism, Costa & McCrae, 1992). At any rate, the present mediation results are more interpretable in the prediction of anxiety symptoms, and future research is needed with regard to depression.
Overall, the present mediation findings are important because they suggest that, by modifying brain- and/or personality-level factors, it might be possible to change behavioral-level outcomes reflected in symptoms of anxiety, even within the spectrum of healthy functioning. The volume of PFC regions has been shown to change in response to experience and training (May, 2011), and interventions designed to train cognitive control of emotion hold promise in alleviating symptoms of emotional distress and affective disturbances (Fava et al., 2000; Siegle, Ghinassi, & Thase, 2007). The plasticity of brain structures and trait-level resilience factors reflects the dynamic interaction between the brain and behavior, and points to the possibility that resilience and well-being can be enhanced through training (Davidson & McEwen, 2012). Hence, by identifying concrete brain and personality factors that protect against symptoms of emotional distress, the present investigation highlights possible targets and related training areas (e.g., executive function, emotion control) for future interventions. To further investigate this possibility, future research could target tasks related to cognitive/executive control and emotion processing (e.g., Affective Go/No-Go task; Hu & Dolcos, 2017).
Since a common function of the presently identified PFC system appears to be the cognitive control of emotion, future work should test the association of such a PFC system with other indicators of cognitive control, to tease apart the different roles that the system might play compared to other systems or networks. For example, externalizing and substance abuse are important factors often examined in clinical research, which might be linked to these brain regions, but might also be linked to other systems such as fronto-striatal circuits (Limbrick-Oldfield, van Holst, & Clark, 2013; Shannon, Sauder, Beauchaine, & Gatzke-Kopp, 2009). Regions such as the anterior cingulate cortex (ACC) also emerge in the literature related to cognitive control of emotion, resilience, anxiety, and depression, but the commonality of these associations is less clear. More specifically, it has long been posited that within the ACC the dorsal anterior portion might be relatively more involved in “cognitive” processes and the ventral anterior portion might be relatively more involved in “affective” processes (Bush, Luu, & Posner, 2000). This made the inclusion of the ACC a challenge for the current study, which aimed at making initial steps in identifying regions involved in the integration and control of emotion to test for a common latent factor. On the one hand, the available literature has shown that while the volume of dorsal ACC is associated with habitual reappraisal, the volume of the ventral ACC is not (Giuliani, Drabant, & Gross, 2011). On the other hand, response in the ventral ACC has been shown to be positively associated with optimism (Sharot et al., 2007). These mixed results suggest that although the ACC plays a key role in the processes targeted in the present study, it might be a heterogeneous and complex role, and hence it should be targeted in future research building upon these initial findings. Other regions such as the amygdala are also important to consider given their interaction with the PFC during cognitive control of emotion (e.g., Buhle et al., 2013; Denkova, Dolcos, & Dolcos, 2015; Dolcos, Kragel, Wang, & McCarthy, 2006; Goldin et al., 2008), and should be examined in future investigations building on the presented model. We opted to not target regions such as the amygdala in the current analysis, because our previous work has indicated that automated tools such as the one used here for extracting cortical parcellations are not as ideal as manual tracing for extracting region volume in the medial temporal lobe (Hu et al., 2018; Moore et al., 2014). Overall, the present study provides insights that can guide future research targeting latent constructs of brain structure and function to further elucidate these relations and interactions.
Caveats. First, mediation models of cross-sectional data are limited in the extent to which they can explain dynamic relations among the variables being examined, and thus further empirical studies are needed to verify the directionality of these relations by manipulating and assessing changes at different levels in a longitudinal design. With this caveat in mind, based on the current results, it appears more likely that changes at the brain level, such as trainings that would target PFC-related cognitive control functions, may help to strengthen favorable effects of greater trait Resilience and reduced symptoms of Distress. These results point to promising possible future avenues for intervention studies in healthy populations, and provide novel insights with valuable implications for understanding how these mechanisms might be altered in clinical groups, which should also be tested in future studies. Second, it would also be ideal to have a larger sample size with statistical power that allows for the inclusion of more variables and the use of more conservative statistical criteria, including correction for multiple comparisons, which were not applied here. Third, in the present report, we tested for sex differences at the level of between-group main effects for each variable, and then included sex as a variable of no interest in the structural equation models. This is consistent with common practice in the literature and with previous findings that did not identify sex differences for some of the variables targeted here (Llewellyn, Dolcos, Iordan, Rudolph, & Dolcos, 2013). However, future research would ideally expand on the current study with larger sample sizes and multigroup analyses to further tease apart possible sex differences with appropriate statistical power.
Fourth, when targeting regions of the brain for analysis, it is important to consider the delineation used to define the ROIs. In the present analysis, a standard anatomical atlas was used to define and extract ROI volumes (see Figure 1). However, there are many possible anatomically or functionally informed atlases that are commonly used (e.g., Destrieux, Fischl, Dale, & Halgren, 2010), and the use of different atlases may contribute to variability in reported findings in the literature. Future work should further examine the brain structural correlates of emotional integration and control using multiple methods. Finally, further testing for specificity (e.g., to anxiety vs. depression) is also important. Consistent with the primary analyses, the results of the main model when tested for Anxiety with univariate statistical outliers included showed the same pattern of associations. Interestingly, when tested for Depression with outliers included, the mediation for Depression appeared to go from marginal to significant. However, we are cautious in interpreting this result as it appears to possibly be driven by particular outlier cases. For example, one participant was a statistical outlier on multiple questionnaire measures, including Depression, which might indicate that this person did not complete the measures accurately, or is potentially outside the spectrum of “typical” healthy individual differences. With this in mind, we have chosen to be on the conservative side and focus on the results without these cases. Nevertheless, as noted above, future research further targeting possible dissociations between anxiety and depression is needed.
4. Conclusion
In summary, the current study showed that greater latent PFC volume in healthy participants was associated with greater latent trait Resilience, which in turn was associated with lower Anxiety. In addition, latent trait Resilience mediated the indirect relation between the latent PFC volume and Anxiety. These results build upon and advance previous findings regarding the roles of the PFC and trait Resilience in the integration and control of emotion to protect against affective challenges. The present findings have valuable implications for the development of future tools targeting the reduction of anxiety, as well as the promotion of enhanced emotional well-being, in both healthy and clinical populations.
Conflicts of Interest:
The authors have nothing to disclose.
Acknowledgments:
This work was conducted in part at the Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign (UIUC-BI). The authors wish to thank members of the Dolcos Lab for assisting with data collection. To access analysis scripts, please visit https://github.com/mmoore16/paper-persneuro
Authors’ Contributions: S.D. and F.D. conceived the study; S.D. contributed to data collection; M.M., S.C., S.D., and F.D. contributed to the analytical approach, with feedback from K.L.P. and T.J.S.; M.M. performed the analyses; M.M., S.D., and F.D. wrote the manuscript. All authors provided feedback to, and approved the content of the manuscript.
Financial Support: This research was partially supported by the National Alliance for Research on Schizophrenia and Depression (currently, the Brain & Behavior Research Foundation), the Canadian Psychiatric Research Foundation (currently, Healthy Minds Canada), the Canadian Institutes of Health Research, the University of Alberta Hospital Foundation, and the University of Illinois. During the preparation of this manuscript, F.D. was supported by a Helen Corley Petit Scholarship in Liberal Arts and Sciences and an Emanuel Donchin Professorial Scholarship in Psychology from the University of Illinois. M.M. was supported by a Beckman Institute Pre-Doctoral Fellowship.
Cite this article: Moore M, Culpepper S, Phan KL, Strauman TJ, Dolcos F, Dolcos S. (2018) Neurobehavioral Mechanisms of Resilience Against Emotional Distress: An Integrative Brain-Personality-Symptom Approach Using Structural Equation Modeling. Personality Neuroscience. Vol 1: e8, 1–10. doi: 10.1017/pen.2018.11
Inaugural Invited Paper
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/pen.2018.11.
click here to view supplementary material
References
- Alexander-Bloch A., Giedd J. N. Bullmore E. T. (2013). Imaging structural co-variance between human brain regions. Nature Reviews Neuroscience, 14, 322–336. 10.1038/nrn3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anxiety and Depression Association of America. (2016). Facts & statistics Retrieved from https://adaa.org/about-adaa/press-room/facts-statistics.
- Arbuckle J. (2016). 24.0 User’s guide. Chicago, IL: SPSS. [Google Scholar]
- Ashby F. G., Isen A. M. Turken A. U. (1999). A neuropsychological theory of positive affect and its influence on cognition. Psychological Review, 106, 529–550. 10.1037/0033-295X.106.3.529 [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers A. R., Neumann C. S., Cope L. M. Kiehl K. A. (2016). Latent-variable modeling of brain gray-matter volume and psychopathy in incarcerated offenders. Journal of Abnormal Psychology, 125, 811–817. 10.1037/abn0000175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. T., Ward C. H., Mendelson M., Mock J. Erbaugh J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–571. 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- Bentler P. M. (1990). Comparative fit indexes in structural models. Psychological Bulletin, 107, 238–246. 10.1037/0033-2909.107.2.238 [DOI] [PubMed] [Google Scholar]
- Bishop S. Forster S. (2013). Trait anxiety, neuroticism and the brain basis of vulnerability to affective disorder. In J. Armony & P. Vuilleumier (Eds.), The Cambridge handbook of human affective neuroscience (pp. 553–574). New York, NY: Cambridge University Press.
- Bora E., Fornito A., Pantelis C. Yucel M. (2012). Gray matter abnormalities in major depressive disorder: A meta-analysis of voxel based morphometry studies. Journal of Affective Disorders, 138, 9–18. 10.1016/j.jad.2011.03.049 [DOI] [PubMed] [Google Scholar]
- Bremner J. D., Vythilingam M., Vermetten E., Nazeer A., Adil J., Khan S., … Charney D. S. (2002). Reduced volume of orbitofrontal cortex in major depression. Biological Psychiatry, 51, 273–279. 10.1016/S0006-3223(01)01336-1 [DOI] [PubMed] [Google Scholar]
- Buhle J. T., Silvers J. A., Wager T. D., Lopez R., Onyemekwu C., Kober H., … Ochsner K. N. (2013). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex, 24, 2981–2990. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E. T. Sporns O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience, 10, 186–198. 10.1038/nrn2575 [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P. Posner M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4, 215–222. 10.1016/S1364-6613(00)01483-2 [DOI] [PubMed] [Google Scholar]
- Bütefisch C. M., Davis B. C., Wise S. P., Sawaki L., Kopylev L., Classen J., & Cohen L. G. (2000). Mechanisms of use-dependent plasticity in the human motor cortex. Proceedings of the National Academy of Sciences of the United States of America, 97, 3661–3665. 10.1073/pnas.050350297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T., Desmond J. E., Zhao Z., Glover G. Gabrieli J. D. E. (1998). Hemispheric asymmetry for emotional stimuli detected with fMRI. NeuroReport, 9, 3233–3239. 10.1097/00001756-199810050-00019 [DOI] [PubMed] [Google Scholar]
- Carver C. S., Scheier M. F. Segerstrom S. C. (2010). Optimism. Clinical Psychology Review, 30, 879–889. 10.1016/j.cpr.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Yu S. C., McQuoid D. R., Messer D. F., Taylor W. D., Singh K., … Payne M. E. (2011). Reduction of dorsolateral prefrontal cortex gray matter in late-life depression. Psychiatry Research, 193, 1–6. 10.1016/j.pscychresns.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. C., Maydeu-Olivares A. D’Zurilla T. J. (1997). Optimism and pessimism as partially independent constructs: Relationship to positive and negative affectivity and psychological well-being. Personality and Individual Differences, 23, 433–440. 10.1016/S0191-8869(97)80009-8 [DOI] [Google Scholar]
- Colibazzi T., Zhu H. T., Bansal R., Schultz R. T., Wang Z. S. Peterson B. S. (2008). Latent volumetric structure of the human brain: Exploratory factor analysis and structural equation modeling of gray matter volumes in healthy children and adults. Human Brain Mapping, 29, 1302–1312. 10.1002/hbm.20466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette F., Van der Linden M., Laureys S., Delfiore G., Degueldre C., Luxen A., & Salmon E. (2005). Exploring the unity and diversity of the neural substrates of executive functioning. Human Brain Mapping, 25, 409–423. 10.1002/hbm.20118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P. T. McCrae R. R. (1992). Revised NEO Personality Inventory and NEO Five Factor Inventory: Professional manual. Odessa, FL: Psychological Assessment. [Google Scholar]
- Curtis C. E. D’Esposito M. (2003). Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences, 7, 415–423. 10.1016/S1364-6613(03)00197-9 [DOI] [PubMed] [Google Scholar]
- Cuthbert B. N. Insel T. R. (2013). Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Medicine, 11, 126. 10.1186/1741-7015-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. J. Irwin W. (1999). The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences, 3, 11–21. https://doi.org/S1364-6613(98)01265-0 [DOI] [PubMed] [Google Scholar]
- Davidson R. J. McEwen B. S. (2012). Social influences on neuroplasticity: Stress and interventions to promote well-being. Nature Neuroscience, 15, 689–695. 10.1038/nn.3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkova E., Dolcos S. Dolcos F. (2015). Neural correlates of “distracting” from emotion during autobiographical recollection. Social Cognitive and Affective Neuroscience, 10, 219–230. 10.1093/scan/nsu039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R. S., Segonne F., Fischl B., Quinn B. T., Dickerson B. C., Blacker D., … Killiany R. J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31, 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Destrieux C., Fischl B., Dale A. Halgren E. (2010). Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage, 53, 1–15. 10.1016/j.neuroimage.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung C. G., Hirsh J. B., Shane M. S., Papademetris X., Rajeevan N. Gray J. R. (2010). Testing predictions from personality neuroscience: Brain structure and the Big Five. Psychological Science, 21, 820–828. 10.1177/0956797610370159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F., Kragel P., Wang L. McCarthy G. (2006). Role of the inferior frontal cortex in coping with distracting emotions. NeuroReport, 17, 1591–1594. 10.1097/01.wnr.0000236860.24081.be [DOI] [PubMed] [Google Scholar]
- Dolcos F., LaBar K. S. Cabeza R. (2004). Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: An event-related fMRI study. NeuroImage, 23, 64–74. 10.1016/j.neuroimage.2004.05.015 [DOI] [PubMed] [Google Scholar]
- Dolcos S., Hu Y., Iordan A. D., Moore M. Dolcos F. (2016). Optimism and the brain: Trait optimism mediates the protective role of the orbitofrontal cortex gray matter volume against anxiety. Social Cognitive and Affective Neuroscience, 11, 263–271. 10.1093/scan/nsv106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N. U., Fair D. A., Miezin F. M., Cohen A. L., Wenger K. K., Dosenbach R. A., … Petersen S. E. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104, 11073–11078. 10.1073/pnas.0704320104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N. U., Visscher K. M., Palmer E. D., Miezin F. M., Wenger K. K., Kang H. C., … Petersen S. E. (2006). A core system for the implementation of task sets. Neuron, 50, 799–812. 10.1016/j.neuron.2006.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant E. M., McRae K., Manuck S. B., Hariri A. R. Gross J. J. (2009). Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biological Psychiatry, 65, 367–373. 10.1016/j.biopsych.2008.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B., Gaser C., Kempermann G., Kuhn H. G., Winkler J., Buchel C., & May A. (2006). Temporal and spatial dynamics of brain structure changes during extensive learning. The Journal of Neuroscience, 26, 6314–6317. 10.1523/JNEUROSCI.4628-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B. May A. (2008). Training-induced structural changes in the adult human brain. Behavioural Brain Research, 192, 137–142. 10.1016/j.bbr.2008.02.015 [DOI] [PubMed] [Google Scholar]
- Dreisbach G. Goschke T. (2004). How positive affect modulates cognitive control: Reduced perseveration at the cost of increased distractibility. Journal of Experimental Psychology: Learning Memory and Cognition, 30, 343–353. 10.1037/0278-7393.30.2.343 [DOI] [PubMed] [Google Scholar]
- Duncan J., Johnson R., Swales M. Freer C. (1997). Frontal lobe deficits after head injury: Unity and diversity of function. Cognitive Neuropsychology, 14, 713–741. 10.1080/026432997381420 [DOI] [Google Scholar]
- Eddington K. M., Dolcos F., Cabeza R., Krishnan K. R. Strauman T. J. (2007). Neural correlates of promotion and prevention goal activation: An fMRI study using an idiographic approach. Journal of Cognitive Neuroscience, 19, 1152–1162. 10.1162/jocn.2007.19.7.1152 [DOI] [PubMed] [Google Scholar]
- Fava M., Rankin M. A., Wright E. C., Alpert J. E., Nierenberg A. A., Pava J., & Rosenbaum J. F. (2000). Anxiety disorders in major depression. Comprehensive Psychiatry, 41, 97–102. 10.1016/S0010-440x(00)90140-8 [DOI] [PubMed] [Google Scholar]
- Fischl B. (2012). FreeSurfer. NeuroImage, 62, 774–781. 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman N. P. Miyake A. (2017). Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex, 86, 186–204. 10.1016/j.cortex.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S. J. Burgess P. W. (2008). Executive function. Current Biology, 18, R110–R114. 10.1016/j.cub.2007.12.014 [DOI] [PubMed] [Google Scholar]
- Giuliani N. R., Drabant E. M., Bhatnagar R. Gross J. J. (2011). Emotion regulation and brain plasticity: Expressive suppression use predicts anterior insula volume. NeuroImage, 58, 10–15. 10.1016/j.neuroimage.2011.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani N. R., Drabant E. M. Gross J. J. (2011). Anterior cingulate cortex volume and emotion regulation: Is bigger better? Biological Psychology, 86, 379–382. 10.1016/j.biopsycho.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P. R., McRae K., Ramel W. Gross J. J. (2008). The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry, 63, 577–586. 10.1016/j.biopsych.2007.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. R., Braver T. S. Raichle M. E. (2002). Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 99, 4115–4120. 10.1073/pnas.062381899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. J. John O. P. (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85, 348–362. 10.1037/0022-3514.85.2.348 [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. (2003). Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology, 40, 838–848. 10.1111/1469-8986.00121 [DOI] [PubMed] [Google Scholar]
- Hebb D. O. (1949). The organization of behavior: A neuropsychological theory. New York, NY: Wiley. [Google Scholar]
- Hu Y. Dolcos S. (2017). Trait anxiety mediates the link between inferior frontal cortex volume and negative affective bias in healthy adults. Social Cognitive and Affective Neuroscience, 12, 775–782. 10.1093/scan/nsx008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Moore M., Bertels Z., Phan K. L., Dolcos F. Dolcos S. (2018). Smaller amygdala volume and increased neuroticism predict anxiety symptoms in healthy subjects: A volumetric approach using manual tracing. Neuropsychologia 10.1016/j.neuropsychologia.2017.11.008. [DOI] [PubMed]
- Kalisch R. (2009). The functional neuroanatomy of reappraisal: Time matters. Neuroscience and Biobehavioral Reviews, 33, 1215–1226. 10.1016/j.neubiorev.2009.06.003 [DOI] [PubMed] [Google Scholar]
- Kim J., Zhu W., Chang L., Bentler P. M. Ernst T. (2007). Unified structural equation modeling approach for the analysis of multisubject, multivariate functional MRI data. Human Brain Mapping, 28, 85–93. 10.1002/hbm.20259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline R. B. (1998). Principles and practice of structural equation modeling. New York, NY: Guilford Press. [Google Scholar]
- Kringelbach M. L. (2005). The human orbitofrontal cortex: Linking reward to hedonic experience. Nature Reviews Neuroscience, 6, 691–702. 10.1038/nrn1747 [DOI] [PubMed] [Google Scholar]
- Kruger G. H. J. (2011). Executive functioning and positive psychological characteristics: A replication and extension. Psychological Reports, 108, 477–486. 10.2466/04.09.21.PR0.108.2.477-486 [DOI] [PubMed] [Google Scholar]
- Lai T. J., Payne M. E., Byrum C. E., Steffens D. C. Krishnan K. R. R. (2000). Reduction of orbital frontal cortex volume in geriatric depression. Biological Psychiatry, 48, 971–975. 10.1016/S0006-3223(00)01042-8 [DOI] [PubMed] [Google Scholar]
- Lazarus R. S. Alfert E. (1964). Short-circuiting of threat by experimentally altering cognitive appraisal. Journal of Abnormal and Social Psychology, 69, 195–205. 10.1037/H0044635 [DOI] [PubMed] [Google Scholar]
- Limbrick-Oldfield E. H., van Holst R. J. Clark L. (2013). Fronto-striatal dysregulation in drug addiction and pathological gambling: Consistent inconsistencies? NeuroImage: Clinical, 2, 385–393. 10.1016/j.nicl.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn N., Dolcos S., Iordan A. D., Rudolph K. D. Dolcos F. (2013). Reappraisal and suppression mediate the contribution of regulatory focus to anxiety in healthy adults. Emotion, 13, 610–615. 10.1037/a0032568 [DOI] [PubMed] [Google Scholar]
- Marsh H. W., Ludtke O., Muthen B., Asparouhov T., Morin A. J. S., Trautwein U., & Nagengast B. (2010). A new look at the Big Five factor structure through exploratory structural equation modeling. Psychological Assessment, 22, 471–491. 10.1037/a0019227 [DOI] [PubMed] [Google Scholar]
- Martin R. C. Dahlen E. R. (2005). Cognitive emotion regulation in the prediction of depression, anxiety, stress, and anger. Personality and Individual Differences, 39, 1249–1260. 10.1016/j.paid.2005.06.004 [DOI] [Google Scholar]
- May A. (2011). Experience-dependent structural plasticity in the adult human brain. Trends in Cognitive Sciences, 15, 475–482. 10.1016/j.tics.2011.08.002 [DOI] [PubMed] [Google Scholar]
- Mayberg H. S. (1997). Limbic-cortical dysregulation: A proposed model of depression. The Journal of Neuropsychiatry & Clinical Neurosciences, 9, 471–481. 10.1176/jnp.9.3.471 [DOI] [PubMed] [Google Scholar]
- Mayberg H. S. (2006). Defining neurocircuits in depression. Psychiatric Annals, 36, 259–268. 10.1016/j.biopsych.2007.01.013 [DOI] [Google Scholar]
- McRae K., Jacobs S. E., Ray R. D., John O. P. Gross J. J. (2012). Individual differences in reappraisal ability: Links to reappraisal frequency, well-being, and cognitive control. Journal of Research in Personality, 46, 2–7. 10.1016/j.jrp.2011.10.003 [DOI] [Google Scholar]
- Mechelli A., Friston K. J., Frackowiak R. S. Price C. J. (2005). Structural covariance in the human cortex. The Journal of Neuroscience, 25, 8303–8310. 10.1523/Jneurosci.0357-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. K. (2000). The prefontral cortex and cognitive control. Nature Reviews Neuroscience, 1, 59–65. 10.1038/35036228 [DOI] [PubMed] [Google Scholar]
- Miyake A., Friedman N. P., Emerson M. J., Witzki A. H., Howerter A. Wager T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- Moore M., Hu Y., Woo S., O’Hearn D., Iordan A. D., Dolcos S. Dolcos F. (2014). A comprehensive protocol for manual segmentation of the medial temporal lobe structures. Journal of Visualized Experiments, 89, e50991 10.3791/50991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M., Iordan A. D., Hu Y., Kragel J. E., Dolcos S. Dolcos F. (2016). Localized or diffuse: The link between prefrontal cortex volume and cognitive reappraisal. Social Cognitive and Affective Neuroscience, 11, 1317–1325. 10.1093/scan/nsw043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Mental Health. (2016). Major depression among adults. Retrieved from https://www.nimh.nih.gov/health/statistics/prevalence/major-depression-among-adults.shtml
- Nes L. S. Segerstrom S. C. (2006). Dispositional optimism and coping: A meta-analytic review. Personality and Social Psychology Review, 10, 235–251. 10.1207/s15327957pspr1003_3 [DOI] [PubMed] [Google Scholar]
- Nudo R. J., Milliken G. W., Jenkins W. M. Merzenich M. M. (1996). Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. The Journal of Neuroscience, 16, 785–807. 10.1523/JNEUROSCI.16-02-00785.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K. N. Gross J. J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9, 242–249. 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Ochsner K. N., Silvers J. A. Buhle J. T. (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–E24. 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J., Bastiaansen A., Riese H., Bos E. H., Servaas M., Ellenbogen M., … Aleman A. (2013). The biological and psychological basis of neuroticism: Current status and future directions. Neuroscience and Biobehavioral Reviews, 37, 59–72. 10.1016/j.neubiorev.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Osborne J. W. Overbay A. (2004). The power of outliers (and why researchers should always check for them). Practical Assessment, Research & Evaluation, 9, 1–12. [Google Scholar]
- Pollack M. H. (2005). Comorbid anxiety and depression. Journal of Clinical Psychiatry, 66, 22–29. [PubMed] [Google Scholar]
- Power J. D., Cohen A. L., Nelson S. M., Wig G. S., Barnes K. A., Church J. A., … Petersen S. E. (2011). Functional network organization of the human brain. Neuron, 72, 665–678. 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J. D. Petersen S. E. (2013). Control-related systems in the human brain. Current Opinion in Neurobiology, 23, 223–228. 10.1016/j.conb.2012.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y. (2012). lavaan: An R package for structural equation modeling. Journal of Statistical Software, 48, 1–36. [Google Scholar]
- Sanz J. Garcia-Vera M. (2007). A psychometric analysis of the short forms of the 1978 version of the Beck Depression Inventory (BDI-IA). Psicologia Conductual, 15, 191–214. [Google Scholar]
- Scheier M. F., Carver C. S. Bridges M. W. (1994). Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology, 67, 1063–1078. 10.1037/0022-3514.67.6.1063 [DOI] [PubMed] [Google Scholar]
- Seeley W. W., Menon V., Schatzberg A. F., Keller J., Glover G. H., Kenna H., … Greicius M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience, 27, 2349–2356. 10.1523/jneurosci.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P. J., Ebmeier K. P., Glabus M. F. Goodwin G. M. (1998). Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression – Controlled magnetic resonance imaging study. British Journal of Psychiatry, 172, 527–532. 10.1192/bjp.172.6.527 [DOI] [PubMed] [Google Scholar]
- Shang J., Fu Y. C., Ren Z. J., Zhang T., Du M. Y., Gong Q. Y., … Zhang W. (2014). The common traits of the ACC and PFC in anxiety disorders in the DSM-5: Meta-analysis of voxel-based morphometry studies. PLoS ONE, 9, e93432 10.1371/journal.pone.0093432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K. E., Sauder C., Beauchaine T. P. Gatzke-Kopp L. M. (2009). Disrupted effective connectivity between the medial frontal cortex and the caudate in adolescent boys with externalizing behavior disorders. Criminal Justice and Behavior, 36, 1141–1157. 10.1177/0093854809342856 [DOI] [Google Scholar]
- Sharot T., Kanai R., Marston D., Korn C. W., Rees G. Dolan R. J. (2012). Selectively altering belief formation in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 109, 17058–17062. 10.1073/pnas.1205828109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T., Riccardi A. M., Raio C. M. Phelps E. A. (2007). Neural mechanisms mediating optimism bias. Nature, 450, 102–105. 10.1038/nature06280 [DOI] [PubMed] [Google Scholar]
- Siegle G. J., Ghinassi F. Thase M. E. (2007). Neurobehavioral therapies in the 21st century: Summary of an emerging field and an extended example of cognitive control training for depression. Cognitive Therapy and Research, 31, 235–262. 10.1007/s10608-006-9118-6 [DOI] [Google Scholar]
- Spielberg J. M., Stewart J. L., Levin R. L., Miller G. A. Heller W. (2008). Prefrontal cortex, emotion, and approach/withdrawal motivation. Social and Personality Psychology Compass, 2, 135–153. 10.1111/j.1751-9004.2007.00064.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C. D., Gorsuch R. L. Lushene R. E. . (1970). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Talati A., Pantazatos S. P., Schneier F. R., Weissman M. M. Hirsch J. (2013). Gray matter abnormalities in social anxiety disorder: Primary, replication, and specificity studies. Biological Psychiatry, 73, 75–84. 10.1016/j.biopsych.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuber H. L. (1972). Unity and diversity of frontal lobe functions. Acta Neurobiologiae Experimentalis, 32, 615–656. [PubMed] [Google Scholar]
- van Tol M. J., van der Wee N. J. A., van den Heuvel O. A., Nielen M. M. A., Demenescu L. R., Aleman A., … Veltman D. J. (2010). Regional brain volume in depression and anxiety disorders. Archives of General Psychiatry, 67, 1002–1011. 10.1001/archgenpsychiatry.2010.121 [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L. A. Carey G. (1988). Positive and negative affectivity and their relation to anxiety and depressive-disorders. Journal of Abnormal Psychology, 97, 346–353. 10.1037/0021-843x.97.3.346 [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L. A. Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2017). Depression and other common mental disorders: Global health estimates. Geneva: World Health Organization. [Google Scholar]
- Wu G., Feder A., Cohen H., Kim J. J., Calderon S., Charney D. S., & Mathe A. A. (2013). Understanding resilience. Frontiers in Behavioral Neuroscience, 7, 10 10.3389/fnbeh.2013.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh P. H., Zhu H. T., Nicoletti M. A., Hatch J. P., Brambilla P. Soares J. C. (2010). Structural equation modeling and principal component analysis of gray matter volumes in major depressive and bipolar disorders: Differences in latent volumetric structure. Psychiatry Research: Neuroimaging, 184, 177–185. 10.1016/j.pscychresns.2010.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B. T., Krienen F., Chee M. Buckner R. (2014). Estimates of segregation and overlap of functional connectivity networks in the human cerebral cortex. NeuroImage, 88, 212–227. 10.1016/j.neuroimage.2013.10.046 [DOI] [PMC free article] [PubMed]
- Yeo B. T., Krienen F., Sepulcre J., Sabuncu M., Lashkari D., Hollinshead M., ... Buckner R. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/pen.2018.11.
click here to view supplementary material