Abstract
Theories in personality neuroscience must aim to be consistent with several levels of explanation. If we view personality traits as constructs located only at the psychological level, we must still make their explanations compatible with observations and theories at lower levels, particularly with what we know at the neural level. If we view personality traits as constructs located only at the neural level, we will still need to predict their emergent effects at the psychological level. Personality theory at present treats traits as psychological-level constructs, with even the recent neurally oriented Cybernetic Big Five Theory specified in terms of a “conceptual nervous system” and not requiring complete or immediate translation into neural mechanisms. Here, we argue for the existence of phylogenetically old, neural-level traits that are substantially conserved across many vertebrate species. We first ask what known mechanisms control trait-like properties of neural systems: Focusing on hormones, the GABAA receptor, and amine neurotransmitter systems. We derive from what we know about these sources of neuronal modulation some metatheoretical principles to guide the future development of those aspects of personality theory, starting with neural-level trait constructs and drawing implications for higher-level trait psychology observations. Current descriptive approaches such as the Big Five are an essential precursor to personality neuroscience, but may not map one-to-one to the mechanisms and constructs of a neuroscience-based approach to traits.
Key words: personality, traits, evolution, hormones, monoamines, Anxiety/fear, Psychopathology (general), Hormones, Personality, Systematic review
1. Introduction
1.1. Our goal
Most researchers would agree that personality refers to stable patterns of Affect, Behavior, Cognition, and Desire (ABCD; Revelle, 2007), and that the goal of personality theory is to provide an explanation for these regularities in behavior and experience. The neuroscientist too would accept that long-term patterns of ABCD are the explicanda. However, importantly, when we recognize a repeating pattern of ABCD, or invoke a construct that reliably predicts new sets of repeating patterns, the neuroscientist would expect this to be the result of some set of stable neural causes. This raises a question: Is personality neuroscience just the addition of neuroscience techniques to current personality research? Or, can neuroscience offer new, fundamental, insights as to the nature of at least some personality constructs?
Theories in personality neuroscience must aim to be consistent with multiple levels of explanation. Theoretical constructs located at the psychological level must still be reductively compatible with data and theory at lower levels such as the gene or neuron. Those located at the neural level can only provide useful explanations when we have translated our predictions up to the psychological level. Given both neural and psychological alternatives, it is reasonable to ask: At which level should we locate our primary explanatory constructs? This paper suggests that for some personality constructs at least, the answer is the neural level; 1 and we then look at the implications of this answer for the types of explanation that personality neuroscientists might want to use. The purpose of this review is not to provide a summary of associations between personality traits and neural-level data (for such a summary, we recommend consulting Allen & DeYoung, 2017; Kennis, Rademaker, & Geuze, 2013). Rather, we aim to develop and explicate metatheoretical principles that we believe might helpfully guide theory and research in personality neuroscience.
1.2. Levels of explanation
We can rephrase all scientific explanations at progressively lower levels of analysis until we reach The Standard Model of physics. In genetics, particularly with point mutations, it is all very well to look at whether peas are wrinkled/round or have purple/white flowers and note the pattern of transfer of these superficial characteristics between generations, but it is more useful to go down to the AGCT level of base pairs to provide explanations. However, higher-level disciplines exist because the lower-level analysis is not only often excessively complicated but also often uninformative. Although higher levels ought to be consistent with lower ones, quantum mechanics will not help us understand genetic problems and even biochemistry may not be particularly useful. Likewise, ecology will often not need gene-level detail for its explanations.
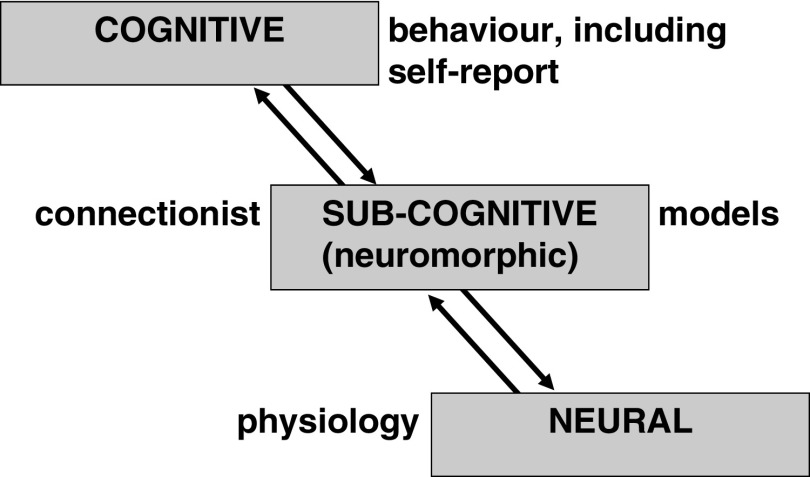
We can see all of psychology as using at least three levels of explanation (Fajkowska, 2018; Polc, 1995; Smolensky, 1988)—all capable in principle of explaining our observed ABCD patterns. As shown in Figure 1, the top level (which is both descriptive and explanatory) is cognitive—here the theoretical constructs will be psychological. The bottom level (which in psychology will be primarily explanatory) is neural, and the theoretical constructs will be physiological (including biochemistry, pharmacology, and particularly systems anatomy). As argued in detail by Smolensky (1988), to link the top and bottom level we also generally need an intermediate level of explanation: The sub-cognitive. Here the theoretical constructs will often be connectionist and embedded in computational models. Critically, the relevant intermediate-level models will involve neuromorphic computing (see, e.g., Economist: Science and Technology, 2013). They will be inspired by, intended to analyze, or intended to mimic, the brain but in a simplified summary form with its own symbology.
Figure 1.
Levels of explanation in psychology as proposed by Smolensky (1988) and their usual associated forms of direct observation (see text). Note that a cognitive construct will genuinely explain behavior and will not be a simple restatement of an observed regularity in observed behavior.
We will not consider other levels of explanation, above and below these three, in detail. The neural level of explanation can itself be reduced to lower levels such as biochemistry and genetics; but, as we will argue below, these (like quantum mechanics) are not useful in explaining personality, as such. The precise surface expression of personality will also depend on an individual’s culture. All the different levels of both biological and social/environmental pathways will contribute to what we observe superficially and describe as personality (Zuckerman, 2005, figure 7-1, p. 246), but we suggest that some kinds of traits may be said to exist at the neural level. These will often also be traits that can be identified across species (Weiss, 2018).
1.3. Conceptualizing traits
What exactly is a personality trait? In the next section, we consider some of the definitions of personality and traits offered by psychologists (see also a recent special issue on the trait concept, Fajkowska & Kreitler, 2018). Largely, these equate the term trait with a pattern of behavior and experience reported verbally by an individual or observer. However, the description of a pattern as, for example, “high trait fearfulness,” can group quite disparate behaviors together within a trait that can also be viewed as an explanatory construct: Used to predict, understand, and model a variety of entities (Ozer & Benet-Martínez, 2006). Importantly, such constructs are amenable to comparative analysis and evolutionary insights into their explanations (Weiss, 2018). In what follows, we will use the term personality trait in this broad predictive and potentially explanatory way. Unless the context explicitly implies a physical trait, wherever we use the word trait by itself we will be referring to a personality trait.
Even when used as fully explanatory constructs, we can see some traits as constructs operating at the cognitive level. However, the neuroscientist is trained to always ask if a higher-level psychological construct can be reduced to a lower-level neural one without loss of explanatory power. The neuroscientist would expect an explanatory trait construct to be the neural-level cause of an observed pattern of behavior and experience. “Something like this sense of ‘trait’ appears to be what lay people often mean when they refer to a trait in conversation; they are attempting to identify the cause of someone’s behaviour” (DeYoung, 2015, p. 37). Importantly such neural-level traits are likely to be similarly distributed in all human populations; to control behavior via phylogenetically old modulatory systems; and to have homologues in nonhuman species (Weiss, 2018). The question then is whether we can identify such explanatory trait constructs at the neural level. Or, must all traits be emergent properties: Dependent on neural interactions but only coherent as unitary explanations at the sub-cognitive level or higher (see also Fajkowska, 2018).
Of course, we must always start with a high level of description. As with an illusion, or color perception, the neuroscientist cannot study a trait if they do not first start with some form of superficial description of the relevant patterns framed by some higher-order taxonomy. We need coherent sets of observations (data structures) before we can try to explain them; individually, data are incoherent. Starting with the megadata of the brain would just lead to confusion—and certainly not give you a good reason to focus your efforts on understanding stable individual differences. So, it is tempting to frame explanations in terms of psychological (e.g., cognitive) trait constructs and use biological tools only to analyze neural correlates.
However, a neuroscientist would expect at least some longer-term, culture-general, individual differences in behavior or cognition or neural firing patterns to result from biological control mechanisms adapted to particular functional situations (see also Matthews, 2018). This expectation is different from the neuroscientist’s view of memory or behavioral plasticity. General mechanisms of neural plasticity require biological explanation (pure association = long-term potentiation; associative reinforcement = dopamine; etc.); but the fact that a particular person speaks English rather than German (or speaks both) requires an appeal to their history rather than their brain. In contrast, an evolved, conserved trait may be identifiable with a particular evolved neural system, with both constrained by a particular adaptive requirement.
If this is true, then personality neuroscience should explain some traits in terms of biological constructs. For these traits, lower-level network properties will transform recurring types of environmental situation to give rise to some degree of ABCD regularity. We will refer to these as neural-level traits, identifying their level of explanation, and distinguish them from cognitive-level traits; with cognitive-, sub-cognitive-, and neural-level personality traits being conflated within the simple term “traits.” In other words, we distinguish between several trait-like mechanisms, which are located at multiple levels of explanation, and which give rise to personality traits as described within taxonomic models such as the “Big Five.” In all these cases we would accept the idea that “trait denotes the underlying, recurrent mechanisms that pattern its structure and account for the stability/variability of individual characteristics” (Fajkowska, 2018, p. 36).
Below we consider what such bottom-up explanations could look like, in principle; what this implies for (complementary) top-down descriptions; and how we could go about assessing the systems concerned. A key conclusion is that such fundamental neural processing regularities can give rise to observed regularities in ABCD that we can dignify with the name of traits. These neural-level causes may produce ABCD variations that have statistical properties that do not map directly to the regularities described within existing taxonomies of traits. The same could be true of sub-cognitive-level traits. The identification of traits at multiple levels of analysis is not a typical approach in current personality research. So, let us explain.
1.4. The psychologist’s view of personality
Let us first consider some definitions of personality, and statements of the goals of personality research, to provide a context. These take us, immediately, beyond any simple pattern of ABCD:
Personality is an abstraction used to explain consistency and coherency in an individual’s pattern of affects, cognitions, desires and behaviors. What one feels, thinks, wants and does changes from moment to moment and from situation to situation but shows a patterning across situations and over time that may be used to recognize, describe and even to understand a person. The task of the personality researcher is to identify the consistencies and differences within and between individuals (what one feels, thinks, wants and does) and eventually to try to explain them in terms of a set of testable hypotheses (why one feels, thinks, wants and does) Revelle (2007, p. 37, our emphasis).
Critically, while the general form of the pattern is common to many people (allowing us to discern it and use it usefully in social interactions), its specific settings vary between individuals of a species. We would not see patterns that vary only between species as reflecting personality. Particularly in humans,
personality is conceived as (a) an individual’s unique variation on the general evolutionary design for human nature, expressed as a developing pattern of (b) dispositional traits, (c) characteristic adaptations, and (d) self-defining life narratives, complexly and differentially situated (e) in culture and social context. … The new trait psychology heralded by the Big Five is arguably the most recognizable contribution personality psychology has to offer today to the discipline of psychology as a whole and to the behavioural and social sciences. But personality psychology should be offering more. Despite its recent revival, personality psychology still falls somewhat short because it continues to retreat from its unique historical mission. That mission is to provide an integrative framework for understanding the whole person … species-typical characteristics of human nature (how the individual person is like all other persons), individual differences in common characteristics (how the individual person is like some other persons), and the unique patterning of the individual life (how the individual person is like no other person) McAdams and Pals (2006, p. 204).
Working within the descriptive paradigm provided by the Big Five (John, Naumann, & Soto, 2008) personality scientists are now moving to satisfy McAdams and Pals’ request for an integrative framework. For example, Cybernetic Big Five Theory (DeYoung, 2015), 2 “attempts to provide a comprehensive, synthetic, and mechanistic explanatory model [via] the study of goal-directed, adaptive systems” and taking the view that “personality traits are probabilistic descriptions of relatively stable patterns of emotion, motivation, cognition, and behavior, in response to classes of stimuli that have been present in human cultures over evolutionary time” (DeYoung, 2015, p. 35, our emphasis).
Even Cybernetic Big Five Theory “does not depend on complete or immediate translation into biological mechanisms for its utility” (DeYoung, 2015, p. 33), despite its focus on evolved adaptive systems. However, it recognizes that “a complete mechanistic theory of personality should encompass the biological basis of the mechanisms responsible for personality… [and] is designed to be fully compatible with the current state of personality neuroscience” (DeYoung, 2015, p. 33). Critically, it also recognizes that we should not expect to find exactly five constructs at lower levels of analysis simply because a five-trait system appears to be psychometrically optimal. This is not simply because it views personality traits are hierarchically structured, with broader “domains” subsuming narrower “aspects” and even narrower “facets” (DeYoung, 2015; Figure 1). Rather, it is reasonable to assume a complex causal mapping, such that multiple causal mechanisms may contribute either independently or in interaction to any given trait.
1.5. The neuroscientist’s view of personality
In practice, personality science has had to start with descriptions of ABCD at the psychological level of analysis. So, personality neuroscience could now be just a matter of determining neuroscientific correlates of agreed descriptions of personality or explicating the neural details from which ABCD regularities emerge. That is, having worked out what personality is and how it can be organized using a system such as the Big Five, we can develop biological explanations of at least some components of our model. If you see personality as just regularities in ABCD then all you need to do is take the relevant type of regularity and ask what, if any, new information can be provided by neuroscience (Allen & DeYoung, 2017; Smillie, 2008)—including “psychophysiology, psychopharmacology, neurology of the brain, and genetics” (Zuckerman, 2005, p. xi), all treated as equally informative.
Of course, the neuroscientist expects biology to inform usefully any mechanistic personality theory. Biological understanding can strongly affect our understanding of mechanisms within high-level theoretical explanations in all areas of psychology. For example, our current understanding of long-term potentiation and activity-dependent facilitation at the synaptic level make it more attractive to include both pure association and reinforcement as learning mechanisms in our theories of cognitive and behavioral plasticity—and, indeed, to further separate reinforcement into two distinct types, stimulus-based and response-based (McNaughton & Corr, 2009, pp. 717–719). This demonstration of distinct learning processes at the neural level is useful as it feeds up to the connectionist at the sub-cognitive level and to the learning theorist at the cognitive level (Figure 1). This usefulness does not make neuroscience necessary for learning theoretic explanations of behavior; but we argue here that, for some personality traits, neuroscience may well be necessary for their correct identification.
In practice, the core business of personality neuroscience is more restricted than McAdams and Pals’ mission, “understanding the whole person” (2006, p. 204). An individual’s personality reflects as they say, “an individual’s unique variation on the general evolutionary design for human nature.” Unique variation includes “how the individual person is like no other person.” With unique differences, it is impossible to frame useful general rules that explain their specific form. In such case, the neuroscience of learning can help us understand in general that learning can produce detailed, synapse-based, individual-specific changes; but this does not help us at all with understanding what this specific individual has learned that makes them unique—for that we need to know their unique history. However, some individuals are like no other person only quantitatively. Here, the neuroscientist would focus on their unique value of a source of variation that McAdams and Pals’ say is “how the individual person is like some other persons” (2006, p. 204). That is the individual person may occupy a particular unique (“unlike”) position but this is in a space defined by a shared source of differences between persons, which we describe in terms of personality traits. However, this shared trait variation in evolutionary design has to be understood in the context of both “species-typical characteristics” and species general ones—both how and why we differ from Bonobos and how and why we share certain aspects of the neural systems contributing to all types of traits with many multicellular species (Krebs, Stephens, & Sutherland, 1983). Neuroscientists (used to comparing across species) would also focus on the biological pathway to traits, seeing them as stable transformations of stimulus input by the individual. They would view the social pathway (Zuckerman, 2005, figure 7-1, p. 246) as a matter of stable culture, and context-dependent input to the individual, not amenable to a primarily neuroscience explanation.
Whether defined at the psychological level or the neuronal level, traits are regularities in state phenomena that ultimately depend on the brain (a key tributary of nature and nurture). The long-term surface-level trait regularities studied by personality scientists must depend on some kind of consistency in the functioning—or at least the outputs—of the neural systems that mediate genetic, epigenetic, and long-term environmental influences on behavior. Even in inbred laboratory mice, genetic and epigenetic variation can contribute to substantial individual differences in correlated suites of behavior (Lathe, 2004). However, neural-level traits are a consistent type of transformation of inputs by neural circuits. For example, an increase in fear can produce either an increase or decrease in risk assessment behavior depending on the level of threat. Under these circumstances, one might see the neural regularities controlling fearfulness as primary—their parametric value consistently distinguishes each individual from another—while the behavior itself is secondary and dependent on circumstances. This is not to trade psychology for neuroscience; as we will see, a purely bottom-up approach is no more viable than a purely top-down one. Both need to proceed in tandem, and this requires us to appreciate the nature of the lower level when discussing the higher level.
If personality theorists are to take biology seriously, they must ask “Why would personality exist?” That is, why would the brain generate identifiable, long-term, individual differences in ABCD? This “why” has two components: The first component is the classic evolutionary question as to what function (in a historical sense) does an adaptation fulfill. For purists reading this, we should note that we are not asking here, “What is its prospective teleology?” (as in “The eye was designed for seeing”). Instead, our “why” asks “What is its retrospective teleonomy,” that is the appearance of design that results from progressive adaptations across phylogenetic history (Pittendrigh, 1958). That said, it can often be convenient to ask design-like questions of the various productions of evolution, viewing it as The Blind Watchmaker (Dawkins, 1986). The second component is the question of how, given a particular functional requirement, evolution has generated a system capable of fulfilling that requirement. A particular series of adaptive mutations must have occurred. As we will see, this does not require that the brain systems that deliver a single apparent superficial functional entity should themselves be unitary. Personality here emerges from the joint requirements of chance and necessity (Monod, 1972) imposed on all products of evolution (Nettle, 2006; Penke, Denissen, & Miller, 2007).
The goal of the neuroscientist, then, is to identify the biological processes that determine the position of an individual within the population trait value space (including values that may reflect psychopathology). This paper explores the kind of features these biological processes might have and the implications of this lower-level analysis for our view of the higher-level regularities that emerge.
2. A metatheoretical analysis
In this section, we examine aspects of biology that seem likely to be important for the types of explanations a neuroscientist would use for traits. Our goal is not a theory of personality but rather to construct a picture of the kind of elements that such a theory would comprise (i.e., a metatheory, cf. Fajkowska, 2018). This should both highlight the specific kinds of biological processes that personality theorists might want to focus on and emphasize the unexpected ways that such processes may deliver the superficial ABCD patterns.
2.1. Evolution, function, adaptations, and genetics
We have already seen that personality theorists have conceived of personality as “an individual’s unique variation on the general evolutionary design for human nature” (McAdams & Pals, 2006, p. 204, our emphasis) with traits embedded in “goal-directed, adaptive systems … that have been present in human cultures over evolutionary time” (DeYoung, 2015, p. 35, our emphasis). Implicit in this phrasing is the idea that traits arise in evolved systems and reflect key adjustments of functional adaptation. Traits such as intelligence or extraversion show concordances of 40%–70% in monozygotic twins that are at least 30% lower in dizygotic twins (Bouchard & McGue, 2003)—suggesting both a strong genetic/epigenetic control delivering increased concordance and a substantial contribution from postnatal environmental influences delivering decreased concordance. This raises two questions. First, what kind of genetic control can we expect to operate on traits? Second, what kind of traits can we expect to arise from natural selection? While we will conclude that genes are at a level of explanation below that at which we would want to locate traits, our consideration of them will lead to our first metatheoretical conclusions about trait explanations.
The most important feature of individual trait variations in evolved features of human nature is that they are variations. That is, a particular population of a particular species shows a broad distribution of relatively stable individual values for each trait. This has an important consequence for a higher-level explanation based on the evidence for genetic control of traits: Traits must reflect settings of control systems where both high and low values present fitness costs. For example, high intelligence depends on a large brain that, in humans, is costly in terms of high energy requirements (Navarrete, van Schaik, & Isler, 2011) and risk of birth complications resulting from increased brain size. Otherwise, the population would rapidly evolve to an extreme with little individual trait variation (cf., Johnson, 2010). That is we expect traits to result from “balancing selection (where selection itself maintains genetic variation)” (Penke, Denissen, & Miller, 2007, p. 554). The nature of trait value variation is also important. There are point mutations that can deliver (like color in sweet peas) trait extremes, as in the attention-deficit-hyperactivity-disorder-like profile of those with phenylketonuria (Stevenson & McNaughton, 2013); but traits that show more graded heritable effects across a population (and show gradual shifts in the population mean in response to selection) must be polygenic (e.g., Holmes et al., 2012). This makes any single gene a poor explanation for a trait, even before we factor in the influence of the environment (Chabris, Lee, Cesarini, Benjamin, & Laibson, 2015). So, while genetics undoubtedly contributes causally to personality (Zuckerman, 2005), genes are not the explanatory level at which we would want to identify trait constructs.
The next important feature of individual trait variations in evolved features of human nature is their evolution. Adaptive selection operates on a series of phenotypes, each of which transmits genes to the next generation. These genes must control traits (and so affect behavior) via a neural function. However, genes have no way of directly changing patterns of behavior, which is also subject to epigenetic, fetal developmental, and later environmental influences. All of these distal causes can only have long-term individual-specific effects on behavior via the nervous system. This gives us positive reasons for preferring neural to genetic explanation of traits.
This decision to focus on neural explanation does not imply that we should ignore the implications of evolution and genetics. There are two useful questions to ask, here. First, what will evolution select for and how can this affect the neural level? Second, what type of mechanism is “The Blind Watchmaker” (Dawkins, 1986) likely to produce to control traits and at what level do we then want place the trait construct?
We can view phenotypic selection as operating directly on a specific ABCD pattern that arises in response to particular classes of stimuli or contexts. The resultant response affects the transmission of the genes to the next generation. Where consistent aspects of ABCD reflect the operation of genes, we can see balancing selection as operating on traits (Penke, Denissen, & Miller, 2007) over phylogenetic time. As with you: Low neuroticism may mean the rat dies before reproducing; low extraversion may mean the bird of paradise fails to obtain a high-quality mate with a resultant impact on survival of their offspring; low agreeableness may mean the chimpanzee fails to maintain social harmony in ways detrimental to reproductive fitness. High values of these traits will also be maladaptive (see Nettle, 2006). It follows that selection of genes that control traits must produce the kind of neural changes that reliably produce these general classes of phenotypic change. It is likely that broad patterns of ABCD will involve neural control at the systems level—and there are certainly modulatory hormonal and neural systems of the type required. We will consider the mechanistic implications of this in the next section.
However, phenotypic selection of a trait must operate in the context of an adaptive requirement that is consistent over evolutionary time scales and via incremental changes in fitness. The implications are easiest to see in the context of the emotions. Each emotion can be viewed as a set of reactions that evolved to fulfill some recurring requirement (McNaughton, 1989). If there were not some recurrent functional aspect of, for example, dangerous situations, then there could be no progressive evolution of the associated adaptive phenotype—your individual survival would not have any implication for survival of your offspring. There are multiple requirements in such situations, each contributing to fitness. Fear involves a range of bodily changes, not all of which would contribute to psychological experience (Cannon, 1936, p. xiv):
increased blood sugar as a source of muscular energy …
increased (adrenaline) in the blood as an antidote to the effects of fatigue …
vascular changes … favorable to supreme muscular exertion …
the value of increased red blood corpuscles …
changes in respiratory function …. favorable to great effort …
the utility of rapid coagulation in preventing the loss of blood
Importantly, these changes are all adaptive in the context of a wide variety of different threats. Crucially, and this is why we have emotional reactions at all, they are all costly and therefore maladaptive in the absence of threat. This also provides us with a reason to expect the existence of a trait capturing individual differences in fearfulness. The reactions will be maladaptive if the level of threat experienced by an individual is greater than is justified for them generally given their capacity to deal with threatening situations.
It is also important to ask how we would expect the brain to instantiate personality. There must be substantial state systems (each fulfilling a recognizable function or set of functions) needing a range of sensitivities to their adequate stimuli between dysfunctional extremes. It is from a deep coherence of neural control systems that any regularity in any of ABCD that we can perceive must emerge. However, we must also be prepared for the regularity in ABCD to reflect a functional rule (that has driven the parallel evolution of multiple neural systems) rather than for it to reflect multiple outputs from a single system. For example, it is theoretically optimal to persist in the face of uncertain food delivery (McNamara & Houston, 1980, p. 687) and a very wide range of species do this (Jenkins & Stanley, 1950; Lewis, 1960). However, the “rule of thumb” actually used can be quite different from the optimal rule (Krebs, Stephens, & Sutherland, 1983) and, with persistence in the face of failure, we have evidence for at least three neurally distinct systems, each delivering its own, independent, rule of thumb in a limited set of circumstances (McNaughton, 1989, Chapter 7). As these all deliver the same pattern of observable behavior, it is likely that many superficially unitary traits are underpinned by multiple separate systems. As a result, we should prefer explanatory pluralism in personality theory, and be vigilant for descriptions at the psychological level that may conflate multiple distinct mechanisms at lower levels.
We can get a sense of the way the packages of ABCD that are our traits resulted from natural selection by looking at cases of artificial selection that, at least superficially, affect trait fear. Let us first look at selection for tameness in silver foxes, and then at selection for emotionality in rats. The silver fox experiment was undertaken to test the assumption that:
because behavior is rooted in biology, selecting for tameness and against aggression means selecting for physiological changes in the systems that govern the body’s hormones and neurochemicals. Those changes, in turn, could have had far-reaching effects on the development of the animals themselves, effects that might well explain why different animals would respond in similar ways when subjected to the same kinds of selective pressures. [In practice this] created a population of tame foxes fundamentally different in temperament and behavior from their wild forebears [with] some striking changes in physiology, morphology and behavior, which mirror the changes known in other domestic animals (Trut, 1999, page number not available in electronic source).
These effects appeared to depend, at least in part, on ontogenetic delays with resultant alterations in the rate of development producing decreases in stress hormones, increases in serotonin, and perhaps even retention of floppy ears into adulthood and changes in coat color (Trut, 1999).
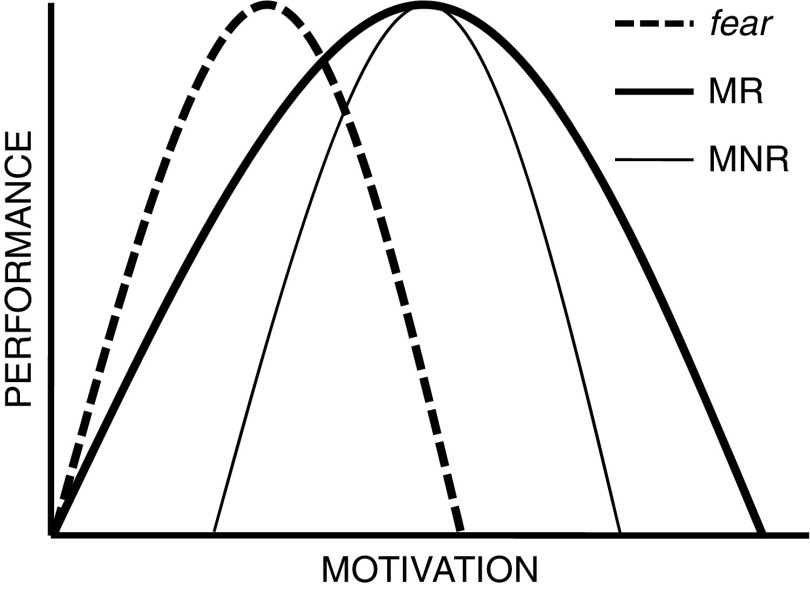
Selection for “emotionality” in rats is particularly interesting. Its key point of difference with the fox experiment is that it involved selection for a single objective measure (number of fecal boli deposited in a single threatening apparatus, the open field), that had face-validity in terms of homology with a human response (Broadhurst, 1960, pp. 36–37). This differential selection separated one originally intermediate rat strain into two (high and low emotional defecation) substrains. Threat-related defecation occurs in a wide variety of species (see McNaughton, 1989, p. 155) including, in humans, 20% of soldiers under bombardment and ~5% of aircrew during combat (Stouffer et al., 1949). The resultant “Maudsley reactive” (MR) rats defecated somewhat more than the parent strain, and the Maudsley nonreactive (MNR) rats did not defecate at all. Importantly, after selection purely for open-field defecation, the strains differed on many other measures (Blizard, 1981; Broadhurst, 1975; Gray, 1987)—but these did not include maze learning, while rats selected for maze learning “showed no difference in the measures of emotionality [from which] it was concluded that emotional reactivity is orthogonal to intelligence in the rat” (Broadhurst, 1960, p. 65). One can ask, here, if emotionality (as selected) reflects fearfulness. That is, if we force rats to learn to swim underwater and subject them to different levels of air deprivation (and so the fear of suffocation) will the two strains differ in a way consistent with MR rats being functionally more air deprived. Broadhurst (1957) obtained results with this procedure that suggest MR emotionality reflects a broader spread of emotional reaction (greater dynamic range) relative to MNR rather than a greater sensitivity to fear (see also Beardslee, Papadakis, Altman, Harrington, & Commissaris, 1989). Figure 2 shows the key aspects of the data diagrammatically. Like the tame foxes, MNR rats show a smaller response to stress than MR (Blizard & Liang, 1979; Buda et al., 1994) but this seems to be linked more to changes in noradrenaline than, as was the case in the foxes, stress hormones or serotonin (Blizard, 1981; Buda et al., 1994).
Figure 2.
Diagrammatic representation of the conclusions to be drawn from Broadhurst (1957). Maudsley reactive (MR) rats (thick line) show a broader spread of performance in relation to motivation than Maudsley nonreactive (MNR) rats (thin line). If MR emotionality were the same as sensitivity to fear they would follow a similar curve to MNR but left shifted (fear). Figure adapted from figure 11.2 in McNaughton (1989).
Both the fox and rat breeding experiments suggest that functional requirements that we can link to concepts such as fearfulness can drive selection. However, the nature of the resultant trait profile will likely depend on genetic constraints related to selectable sources of variation and systemic factors related to available neural targets than can deliver the appropriate variation (teleonomy rather than teleology). With the foxes, Trut (1999) has argued that the phenotype emerging from selection has been constrained by the fact that selecting for polygenes is problematic (likely to produce deleterious changes) and this has biased selection to altering rate of development (and so a range of distinct morphological features simultaneously). With the rats, selected for a single measure in response to a single challenge, the noradrenergic neural system has adapted. At high levels of motivation in the task, which match the levels used for selection, this has resulted in changes of the type we would expect. However, at low levels of motivation in the task, which do not match the conditions of selection, noradrenergic change has resulted in behavioral changes opposite to those we would expect. Taken together these observations lead to our first metaprinciple.
Metaprinciple 1:
The mechanisms of the systems embodying traits have evolved in small steps, with each one constrained by both available mutations and the nature of the pre-existing systems that the mutation is modifying; so a mechanism may be a “Rule of Thumb,” and not directly reflect the superficial features it appears to control or have been selected for.
2.2. Hormonal modulation
Given the evolutionary argument we have made so far for neural-level control of traits, the question arises as to how a trait could be instantiated in the nervous system. If a trait is (as in our earlier quote from Revelle) a consistent pattern of what one feels, thinks, wants, and does, it must reflect common variance in the reactivity of fairly widespread neural systems. An obvious candidate source for such common variance would be modulation by hormones, which after release into the blood stream can target any systems that have acquired the relevant receptor in which common variance will result from their common control by the level of a single hormone.
Evolution of hormonal control over personality traits has two key evolved elements: The system releasing the chemical that controls the long-term sensitivity of the neural systems and the specific set of neural targets that have acquired the appropriate receptors. A particular class of adaptive function will have shaped the evolution of both the chemical system (controlling variation in the sensitivity to inputs of that class) and its current neural targets (that define the set of responses that covary). This will also be true of hormonal controls of states, where the brief release of hormones signals an event requiring a response; for example, adrenaline signals the necessity for urgent action and triggers immediate consequent bodily and mental changes. However, control processes that operate on traits, as opposed to states, would require longer-term, relatively stable, concentrations of the relevant compound changing the sensitivity or capacity of systems for state changes, as with the effects of testosterone on musculature.
Importantly, any chemical control of trait expressions is likely to have some degree of medium-term adjustment—tuning the person to their usual environment. Testosterone “rises, for example, in athletes preparing for a competition and rises even further in the winning athlete, while falling in the losing one. [This rise can effect states such as] confidence and risk taking” even with financial risk taking, where testosterone level is linked, on a daily basis, to an individual’s profit in share-trading (Coates & Herbert, 2008, p. 6167). Given testosterone’s role in development, it is easy to see how it could retain the capacity to modulate a suite of features, controlled by structures already containing testosterone receptors, in adulthood.
Chemical control of traits requires a capacity to adjust the sensitivity of systems to their normal inputs. This is unlike the brief state-level production of specific responses by either neurons or hormones. Testosterone could produce the changes we have already described largely at the morphological level, similar to the dendritic changes observed in response to long-term potentiation (Hosokawa, Rusakov, Bliss, & Fine, 1995) or via related adjustments in neurotransmitter synthesis or breakdown. The same will be true of other hormones. For example, depressive disorder (i.e. extreme trait depressivity) has been characterized as dysregulation of the stress system (Pariante, 2003; Pariante & Miller, 2001). However, there is also evidence for more direct control of the sensitivity of neural function by hormones.
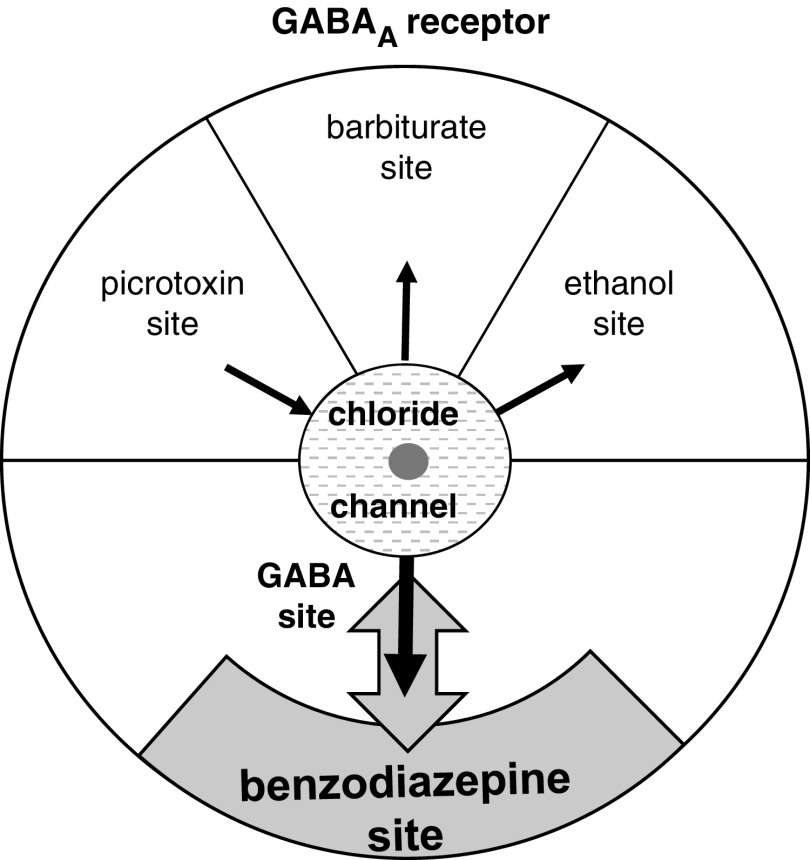
The clearest case of a chemical controlling the sensitivity of a neural system occurs with the GABAA receptor. The GABAA receptor mediates neural inhibition by passing chloride through its channel. This will typically happen when GABA (γ-Aminobutyric acid) binds to the GABA site (Figure 3) pulling the channel open (black arrow) producing changes that operate on a timescale of milliseconds. The channel can also be pulled open (and inhibition immediately produced) at two sites: One that binds barbiturates and one that binds ethanol (producing relaxation and, at high enough doses, death). A site that binds picrotoxin can push it closed (opposite direction black arrow), with loss of inhibition resulting in convulsions. While these direct action sites could directly alter background inhibitory tone (holding it high or low) in the long term they could also, like the GABA site, operate on very short time scales (if they bind compounds coreleased with GABA) or fairly short ones (if they bind hormones that operate in the same way as adrenaline). The GABAA receptor has an additional, unusual, site that is of particular interest in the context of trait control: One that binds benzodiazepines. Unlike the other sites, the benzodiazepine site has no direct effect on the channel. Instead, as shown in Figure 3 it alters the conformation of the GABA site and so changes GABA binding. It thus acts as a kind of amplifier knob for the receptor and, in particular, it can (double-headed gray arrow) increase the effect of GABA (benzodiazepine agonists) or decrease it (benzodiazepine inverse agonists). It is their capacity for long-term fine-tuning of the GABA system that makes benzodiazepines attractive as anxiolytics. The artificial anxiolytic action of exogenous benzodiazepines also demonstrates directly how levels of endogenous ligands could control something akin to “trait anxiety.”
Figure 3.
The GABAA receptor. The neurotransmitter GABA binds to a site that opens the chloride channel to produce inhibition. The picrotoxin, barbiturate, and ethanol sites also directly affect the channel. The benzodiazepine site is different. It only indirectly affects chloride by changing (up or down) the affinity of GABA—acting like an amplifier knob. These sites will have evolved to bind endogenous compounds that modify the effects of GABA; but their naming is pharmaceutical. Thus, the endogenous compounds binding to the benzodiazepine site (Montagna et al., 1995; Skolnick, Marangos, Syapin, Goodwin, & Paul, 1979; Taupin et al., 1994) need not be benzodiazepines. Black arrows indicate the direction of effect on the channel (producing or blocking inhibition). Gray double-headed arrow: Benzodiazepines of different types increase or decrease the effect of GABA when it is released.
We can view the GABAA receptor as a plausible node for medium-to-long-term modulation of trait systems. There may have been selection pressure both for various existing chemicals to acquire sites at which they could modulate the receptor and for various existing neural systems to express the receptor. On both the input and output side, adaptation will have been constrained by the fact that the GABAA receptor mediates the relationship. Let us suppose that a particular chemical signals a particular functional requirement. If a mutation causes a site for that chemical to occur in GABAA receptors in an area that controls the particular requirement and the result is adaptive, then the site will be conserved in future generations. Conversely, only those chemicals that signal the same (or a compatible) requirement will acquire sites on the receptor.
If we can identify the benzodiazepine site, in particular, with control of something akin to trait anxiety, then we can draw some interesting conclusions. Note that this is a hypothetical “if … then …” set of conclusions, not a statement of empirical fact. No one has proved that the site is the basis of a trait; but it clearly exists and has the appropriate properties. We use it here as a model that allows us to arrive at metaprinciples.
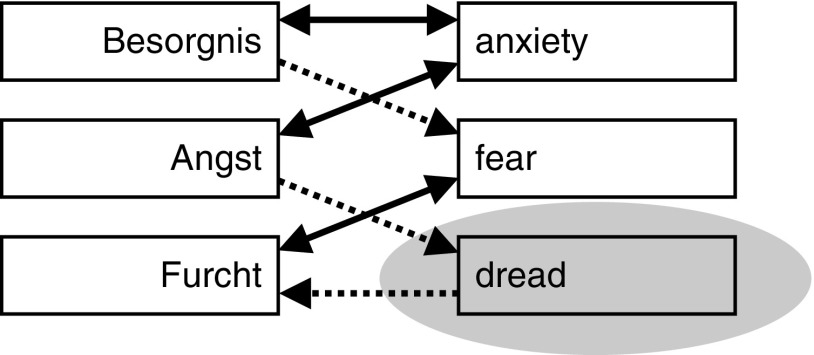
Our first conclusion is in relation to the distinction between anxiety and fear. Many see anxiety and fear as synonyms. As we have discussed elsewhere (McNaughton, 2018), the blurring of their meanings is very obvious when one attempts to translate between two languages (Figure 4). However, pharmacologically there is a clear distinction: Benzodiazepines (and other anxiolytics) affect passive avoidance, risk assessment, stress hormone release, and a range of other reactions that result when threat avoidance conflicts with other goals (which we would link to “anxiety”) but do not affect reactions produced by pure threat (which we would link to “fear”). Thus, rather than viewing fear and anxiety as highly similar terms, we can sharply distinguish them in terms of what has been called defensive withdrawal and defensive approach (in the form of, e.g., risk assessment behavior), respectively. That is, fear is the set of reactions that evolved to allow the individual to get away from danger; anxiety is the set of information-gathering reactions that evolved to allow the individual to face uncertainty/danger and survive (McNaughton & Corr, 2004). Here, biology does not match the lexicon (as exemplified by the links demonstrated in Figure 4). Of course, this does not provide evidence against the fact that people we describe as “anxious” are often the same people we describe as “fearful.” Neither does it make it wrong for these and other lexical descriptors (e.g., “depressivity”) to be organized in terms of a single Big Five construct, that is, neuroticism. However, it does suggest, as we have foreshadowed, that descriptive-level traits will not have one-to-one mappings to neural-level trait parameters. Put differently, the Big Five (and other taxonomies in personality) attempts to specify the major lines of covariation among descriptions of personality traits, but we should not expect them to also represent the organizational structure of the neural systems that may causally influence traits.
Metaprinciple 2:
The systems embodying traits may have evolved in relation to functional (cybernetic in the sense used by DeYoung, 2015 ) constraints not captured by normal language use.
Figure 4.
Translation paths in Collins German dictionary. Note that Angst (anxiety) translates to dread and then dread to Furcht (fear) but there is no translation path in the other direction. Figure and legend from McNaughton (2018).
Our second conclusion is in relation to the evolution of traits. The benzodiazepine receptor appears to have arisen early in vertebrate (but not invertebrate) phylogeny being present in higher vertebrates such as bony fish but not, for example, hagfish (Nielsen, Braestrup, & Squires, 1978) and to have a similar role in behavior control in all vertebrates (Cloninger & Gilligan, 1987, p. 464). The same appears true of stress hormones where studies in zebrafish suggest that human depression, anxiety, and posttraumatic stress disorder appear to involve highly conserved systems (Griffiths et al., 2012). We can expect such conservation with “survival circuits” where lower-level changes can be catastrophic. Of course, higher-level processing, particularly circuits in the cortex that determine what we are anxious or fearful about and precisely how we perceive our anxiety or fear will produce detailed surface differences between species, but these will be superimposed on ancient conserved systems (LeDoux, 2012).
Metaprinciple 3:
Phylogeny will often conserve control processes acting on traits, such that traits reflecting phylogenetically ancient constraints and their basic control mechanism are likely to be similar across vertebrate species.
Corollary:
We can study control processes acting on traits with comparative methods; but detailed surface expression (eliciting stimuli, patterns of ABCD, etc.) may be species-specific.
2.3. Neural modulation
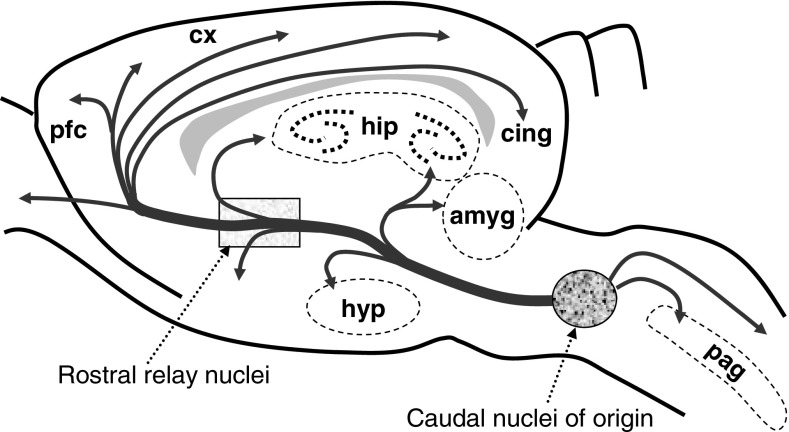
The most obvious modulatory central neural systems involve biogenic amines (e.g., acetylcholine, dopamine, histamine, noradrenaline, and serotonin). The amine systems, to a first approximation, have similar general neuroanatomy (Figure 5). The key point is that very small numbers of caudally placed cells (in phylogenetically ancient nuclei) can effectively spray the relevant neuromodulator over much of the forebrain. Aston-Jones and colleagues have suggested that the central noradrenergic system is, in this respect, an analogue of the peripheral sympathetic nervous system (Aston-Jones, Chiang, & Alexinsky, 1991; Van Bockstaele & Aston-Jones, 1995). This can clearly provide control of the type and timescale required for broad personality traits.
Figure 5.
A schematic overview of the aminergic systems. Nuclei of origin are caudal but may relay rostrally. They have few cells with many collaterals providing diffuse innervation of the forebrain. The dopamine system is most rostral, most differentiated, and least diffuse. The cholinergic system is most caudal, least differentiated, and most diffuse. Abbreviations: amyg=amygdala; cing=cingulate cortex; cx=cortex; hip=hippocampus; hyp=hypothalamus; pag=periaqueductal gray; pfc=prefrontal cortex. From McNaughton (2002).
Let us look at serotonin as an example. Simple manipulation of serotonin by dietary tryptophan depletion in humans has widespread effects compatible with the idea that this depletion has reduced aversive control of learning (Faulkner & Deakin, 2014) particularly in relation to punishment-induced inhibition of responding (Crockett, Clark, & Robbins, 2009). Conversely, we can see the clinical use of specific serotonin re-uptake inhibitors as, albeit slowly, modifying trait depressivity. Similarly, serotonin has been studied in relation to its contribution to a wide range of trait measures, but does not appear to map directly to any one higher-order trait (Carver, Johnson, & Joormann, 2008, p. 924). On one view, however, the fundamental role of serotonin is better understood at a lower level of explanation: It determines the overall level of the nervous system that is, at any point, in control of behavior. On this view, high levels of serotonin bias processing to more recently evolved prefrontal control, which can “override or inhibit lower-level influences on behaviour. A result is that persons with low serotonergic function (and thus diminished executive control) are especially responsive to associative and affective cues of the moment” (Carver, Johnson, & Joormann, 2008, p. 912). It follows that we can view serotonin as a potential source of trait high-level bias that interacts with other neural-level traits, such as reward sensitivity, to generate depressivity. This view suggests that it might be more useful to equate the sensitivity of the serotonin system with a neural-level trait, rather than attempt to explain its contribution to multiple psychological-level traits.
Metaprinciple 4:
The brain contains modulatory systems that produce trait-like control of neural processing; and which we should see as the basis for defining some neural-level traits.
Examples: Tentative mappings on current evidence would include: Serotonin [high-level control], noradrenaline [dynamic range], and dopamine [exploration] (DeYoung, 2013).
Caveats: Changes in available precursors (as in tryptophan depletion) can affect each amine systems as a whole and changes in, for example, monoamine oxidase (MAO) can affect more than one amine system concurrently. However, the serotonin system has two distinct components (arising in the dorsal and median raphe) that innervate somewhat different areas and so reflect safety signaling and behavioral inhibition, respectively (for detailed anatomy, etc., see Gray & McNaughton, 2000, appendix 10); similarly the dopamine system can be divided into distinct value coding and salience coding components that have been linked to extraversion and Openness/Intellect, respectively (DeYoung, 2013). These distinct parts of the main amine systems may generate facets of the higher-order traits, with neither mapping clearly to existing superficial trait and facet measures.
The brain can also directly control hormonal systems and so control the trait-level sensitivity of hormones that then (as discussed in the previous section) determine the nature of trait expression. The clearest example of this is the hippocampus. Despite its apparent role in learning and memory, the hippocampus has an “unusual density and diversity of receptor expression … of more than 60 [hormone-like factors and, e.g.,] the mineralocorticoid receptor is very substantially restricted to, if not almost exclusively located in, the hippocampus” (Lathe, 2001, p. 207). A large body of data suggest that this allows the hippocampus to control body physiology, particularly via negative feedback control of, for example, stress hormones. This form of control is important for the trait psychologist.
Cognitive processes within the brain are known to exert a large degree of control over body physiology. Reflex-like behaviour is common – the presence of a predator elicits the secretion of stress hormones … . Before we dismiss this as [just] a reflex, it must be recognised that the response is to the perception of the predator (Lathe, 2001, p. 219, our emphasis).
Conversely, chronic stress can produce extensive remodeling of the circuitry of the brain, particularly the hippocampus, amygdala, and prefrontal cortex. This remodeling appears to be the basis for the chronic changes we can describe as mood and anxiety disorders (Brown, Rush, & McEwen, 1999; McEwen, Eiland, Hunter, & Miller, 2012), with similar but less extreme changes potentially providing the basis for trait depressivity and trait anxiousness in humans and emotionality in rodents (Costa-Nunes et al., 2014). Stress-related changes in the hippocampus, which controls stress hormones, raises the possibility of a vicious cycle (Sapolsky, 2004, p. 249; but see Yehuda, 2001) that would produce very long-term trait-like changes.
Metaprinciple 5:
The brain can control hormones in a way that we should see as the basis for defining some neural-level traits.
Metaprinciple 6:
Chronic hormonal levels can produce both macroscopic and microscopic changes in the morphology and function of brain systems (as they can bodily systems) allowing expressed traits to involve multiple brain systems.
Corollary to 5 and 6:
Traits may involve reciprocal brain–hormone interactions.
2.4. Trait interactions and nonorthogonality
In this section, we look at the implications for patterns of ABCD (currently identified as traits) of trait constructs that operate at the neural level. In the neural case, a trait’s specific value for any individual will often be the level or sensitivity of a particular biological modulator (hormone or amine system). This stable sensitivity will then deliver particular patterns of ABCD mediated by the neural systems that are controlled by the modulator. We will first consider two sources of superficial nonorthogonality that are inherent in biological systems. Then we will consider some issues that are not specifically biological but where a biological approach may provide clarity.
Our first biological source of nonorthogonality is that neural-level traits with quite independent sensitivities, nonetheless, may interact in their control of behavior. They can meet the criterion of psychometric purity in terms of their biological-level values but generate patterns of behavior that are psychometrically impure. This may potentially help to account for some of the psychometric complexities evident in many personality taxonomies, such as the heterogeneity in the content of Big Five Openness/Intellect (DeYoung, Grazioplene, & Peterson, 2012) and disagreement as to whether aggression/volatility is part of high neuroticism (John, Naumann, & Soto, 2008) or low agreeableness (Ashton, Lee, & de Vries, 2014). It may also account, more generally, for the lack of “simple structure” that characterizes virtually all personality taxonomies.
Neural-level traits potentially interact in various ways to control behavior. Our previous sections treated stress hormones, endogenous benzodiazepine receptor ligands, and serotonin as distinct. It is certainly the case that long-term manipulation of any one of these systems does not produce identical changes to either of the others (e.g., unlike serotonin manipulations, benzodiazepines do not affect depressivity). However, there are situations where all three systems are involved in the control of behavior; and, it turns out, where they interact with each other. Anxiety (but not fear) releases corticosterone/cortisol (CORT) and (as with other stressors) releases serotonin. Activation of serotonin (5HT1A) receptors can contribute to the release of CORT (de Boer, Slangen, & Van der Gugten, 1990; Lorens & Van de Kar, 1987), while activation of benzodiazepine receptors can reduce the release of CORT (de Boer, Slangen, & Van der Gugten, 1990; de Boer, Van der Gugten, & Slangen, 1990). Despite their opposite effects on the release of CORT, both benzodiazepine and 5HT1A receptor ligands can reduce anxiety; while CORT acts, essentially as an anxiolytic antagonist. These effects can combine, in the short term, to control observed anxiety-related behavior (McNaughton, Panickar, & Logan, 1996) and, while demonstrated in rats, are consistent with the clinical dynamics of anxiolytic action in humans, particularly the delayed onset of therapeutic action of 5HT1A receptor agonists. So, while chronic stress can produce specific trait-like changes in the serotonin system (Natarajan, Forrester, Chiaia, & Yamamoto, 2017), the resultant behavioral changes could include indirect changes mediated by the CORT and benzodiazepine systems.
Our second biological source of nonorthogonality is that neural-level traits may not always be completely independent; we may have to treat them as oblique rather than orthogonal factors. This will occur if they share part of their long-term control. (Where two neural/hormonal systems completely share their long-term control, we would view them as controlling different outputs for the same trait.) This conclusion might make us want to revise our views as to the desirability of psychometric purity of trait constructs themselves (independently of the purity of their measurement).
There are a number of possible sources of nonindependence of the traits themselves. We have already emphasized the neuroanatomical similarity of the amine systems. This similarity suggests only that the way they operate is similar. However, the monoamines (dopamine, noradrenaline, and serotonin) have a deeper relationship. The enzyme MAO breaks down all monoamines (hence its name). Genetic variation in MAO, then, would be likely to impact simultaneously on long-term control of dopamine, noradrenaline, and serotonin—and so generate some nonorthogonality in the trait control of those systems. However, this common MAO component is likely to interact with more specific components to determine, for example, specific dopamine turnover (Grigorenko et al., 2010) and so maintain a substantial degree of independence of the three monoamine traits.
Finally, even when independence does not fail for the biological reasons we have already mentioned, we can expect some degree of nonorthogonality both of the trait values and of their expression through morphological features. The important point here is that, within an individual, a biological trait may be a specific independent source of general behavioral control while, across a population (or within an individual’s life span), it may be nonorthogonal to a second biological trait. This will most obviously occur when distinct environmental factors affect two biologically independent traits and those factors are, for some external reason, correlated in the environment (e.g., psychological trauma and famine in a war zone). It will also occur when one trait is a predisposing factor (perhaps interacting with the environment) for changes in some other trait. For example, in Australian male firefighters, neuroticism was a better predictor of “post-traumatic morbidity than the degree of … extreme exposure to a bushfire disaster … or the losses sustained” (McFarlane, 1989, p. 221; see also Ogle, Siegler, Beckham, & Rubin, 2017). Features of interest may also appear jointly controlled when their expression is the result of the combination of two otherwise independent traits. For example, neuroticism (perhaps reflecting low serotonin) is a general predisposing factor (Andrews, Stewart, Morris-Yates, Holt, & Henderson, 1990) for a range of neurotic disorders that we can view as a form of extreme trait change. As we have already noted, neuroticism may interact with environmental stress to generate disorder. However, it may also be interacting with trait anxiety/benzodiazepine-based systems or trait depressivity/stress hormones or trait panic/cholecystokinin-based systems (Bradwejn & Koszycki, 1994; Scherrer et al., 2000; Wang, Valdes, Noyes, Zoega, & Crowe, 1998), with these adjunct systems determining the particular form of neurotic disorder expressed.
Metaprinciple 7:
Neural-level traits with independent values may interact during expression to produce patterns of ABCD which are, from the perspective of existing taxonomic models, factorially complex or “impure.”
Metaprinciple 8:
Biological trait factors may be oblique rather than orthogonal.
Metaprinciple 9:
Independence within the individual of not only biological control but also an expression of personality traits does not guarantee the statistical independence of measurements of expression of those traits across a population.
3. . Conclusions
In the above, we have attempted to suggest what neural-level traits might look like and deduce how they would be expressed in terms of ABCDs. We have not argued that any specific hormonal or neural entity is a personality trait. The important point is that we already know a lot about trait-like control at the biological level. We have therefore been able to say, “This system behaves in this way, so a neural-level trait construct could do this too, in principle,” and deduce metatheoretical principles that we think could refine current, and guide future, theory. However, drawing metatheoretical conclusions based on neuroscience does not mean our approach is completely theoretically neutral at the ABCD level. We obviously incline to a theory where there is a range of distinct traits (e.g., dopaminergic, noradrenergic, serotonergic, and many more) that, at the ABCD level, may be factorially oblique.
We hope it is also obvious that we agree that:
the relationship between personality psychology and neuroscience should be viewed as a two-way street. Personality psychology can help to guide neuroscience hypotheses and to organize and synthesize neuroscience findings. Additionally, however, neuroscience data may influence personality psychologists in their development of trait models … . Using neuroscience methods to study personality has the potential to produce explanatory biological models for trait taxonomies that were at first purely descriptive, and these models may help to realize the goal of a theory of personality as a system of dynamic, interacting elements that generates the ongoing flux of behavior and experience (DeYoung, 2010, p. 1176, our emphasis).
Where we can identify neural-level traits in the way we have suggested, there are implications for current approaches to studying them. We can expect them to show strong homology with other species giving us new powerful tools for the genetic (Broadhurst, 1957), and neural (Gray & McNaughton, 2000) analysis of personality. At the neural level, it has already proved possible to identify key systems in rats, develop human homologs, and so have human biomarkers with which to anchor personality neuroscience (McNaughton, 2018). Importantly, if a neural-level trait comprises some specific long-term modulation of neural processing that we can identify with, for example, serotonin, noradrenaline, or dopamine, and explains the same ABCD regularities as an existing psychological construct, then we may need to revise our interpretations of psychological-level constructs. For example, if “neuroticism” is, at root, the sensitivity of the serotonin system it may be best understood, at the cognitive level, in terms of trait high-level control (Carver, Johnson, & Joormann, 2008, p. 912), rather than in terms of negative emotionality. Similarly, if the noradrenaline system generates something like trait dynamic range or the dopamine system generates trait exploration (DeYoung, 2013) this may lead us to reinterpret the nature of Big Five traits linked with behavioral (extraversion) or cognitive (openness) exploration. More generally, if we start with psychological-level traits as currently defined, we can expect there “to be no one-to-one correspondence or isomorphism between specific traits and specific brain systems” (Matthews, 2018, p. 70).
Conflicts of Interest
All authors have nothing to disclose.
Acknowledgments
We thank the four reviewers of this ms for their detailed suggestions, which greatly improved the original ms.
Inaugural Invited Paper
Footnotes
The arguments presented in this paper have their roots in an “adversarial collaboration” currently in MS form in which we are engaged together with Gerald Matthews and Philip Corr.
We focus here on Cybernetic Big Five Theory for two reasons: First, it is notable in its attempt to synthesize across previous neurally inspired personality theories, explicitly incorporating the more robustly established mechanisms from within those theories, for example, reward-processing circuitry as a mechanism that may partly explain variation in extraversion (Depue & Collins, 1999; Pickering & Gray, 2001; Rammsayer, 1998). Second, most other neurally inspired personality theories posit structures for personality that are not so generally accepted as the Big Five within personality psychology (e.g., Cloninger, Svrakic, & Przybecky, 1993; Davis, Panksepp, & Normansell, 2003; Eysenck, 1967; Panksepp, 1998), and they do not explicitly address the metatheoretical principles that are the subject of this paper.
References
- Allen T. A. DeYoung C. G. (2017). Personality neuroscience and the five factor model In T. A. Widiger (Ed.), Oxford handbook of the five factor model (pp. 319–352). New York: Oxford University Press. [Google Scholar]
- Andrews G., Stewart G., Morris-Yates A., Holt P. Henderson S. (1990). Evidence for a general neurotic syndrome. British Journal of Psychiatry, 157, 6–12. 10.1192/bjp.157.1.6 [DOI] [PubMed] [Google Scholar]
- Ashton M. C., Lee K. de Vries R. E. (2014). The HEXACO honesty-humility, agreeableness, and emotionality factors: A review of research and theory. Personality and Social Psychology Review, 18, 139–152. 10.1177/1088868314523838 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Chiang C. Alexinsky T. (1991). Discharge of noreadrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Progress in Brain Research, 88, 501–520. 10.1016/S0079-6123(08)63830-3 [DOI] [PubMed] [Google Scholar]
- Beardslee S. L., Papadakis E., Altman H. J., Harrington G. M. Commissaris R. L. (1989). Defensive burying behavior in Maudsley reactive (MR/Har) and nonreactive (MNRA/Har) rats. Physiology and Behavior, 45, 449–451. 10.1016/0031-9384(89)90154-6 [DOI] [PubMed] [Google Scholar]
- Blizard D. A. (1981). The Maudsley reactive and nonreactive strains: A North American perspective. Behavior Genetics, 11, 469–489. 10.1007/BF01070004 [DOI] [PubMed] [Google Scholar]
- Blizard D. A. Liang B. (1979). Plasma catecholamines under basal and stressful conditions in rat strains selectively bred for differences in response to stress In E. Usdin, I. J. Kopin, & J. Bachas (Eds.), Catecholamines: Basic and clinical frontiers (pp. 1795–1797). New York: Pergamon. [Google Scholar]
- Bouchard T. J. J. McGue M. (2003). Genetic and environmental influences on human psychological differences. Developmental Neurobiology, 54, 4–45. 10.1002/neu.10160 [DOI] [PubMed] [Google Scholar]
- Bradwejn J. Koszycki D. (1994). The cholecystokinin hypothesis of anxiety and panic disorder. Annals of the New York Academy of Sciences, 713, 273–282. 10.1111/j.1749-6632.1994.tb44075.x [DOI] [PubMed] [Google Scholar]
- Broadhurst P. L. (1957). Emotionality and the Yerkes-Dodson law. Journal of Experimental Psychology, 54, 345–351. 10.1037/h0049114 [DOI] [PubMed] [Google Scholar]
- Broadhurst P. L. (1960). Applications of biometrical genetics to the inheritance of behaviour In H. J. Eysenck (Ed.), Experiments in personality, Vol 1 Psychogenetics and psychopharmacology (pp. 1–102). London: Routledge Kegan Paul. [Google Scholar]
- Broadhurst P. L. (1975). The Maudsley reactive and nonreactive strains of rats: A survey. Behavior Genetics, 5, 299–319. 10.1007/BF01073201 [DOI] [PubMed] [Google Scholar]
- Brown E. S., Rush A. J. McEwen B. S. (1999). Hippocampal remodeling and damage by corticosteroids: Implications for mood disorders. Neuropsychopharmacology, 21, 474–484. 10.1016/S0893-133X(99)00054-8 [DOI] [PubMed] [Google Scholar]
- Buda M., Lachuer J., Devauges V., Barbagli B., Blizard D. Sara S. J. (1994). Central noradrenergic reactivity to stress in Maudsley rat strains. Neuroscience Letters, 167, 33–36. 10.1016/0304-3940(94)91021-9 [DOI] [PubMed] [Google Scholar]
- Cannon W. B. (1936). Bodily changes in pain, hunger, fear and rage. New York: Appleton-Century. [Google Scholar]
- Carver C. S., Johnson S. L. Joormann J. (2008). Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: What depression has in common with impulsive aggression. Psychological Bulletin, 134, 912–943. 10.1037/a0013740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabris C. F., Lee J. J., Cesarini D., Benjamin D. J. Laibson D. I. (2015). The fourth law of behavior genetics. Current Directions in Psychological Science, 24, 304–312. 10.1177/0963721415580430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger C. R. Gilligan S. B. (1987). Neurogenetic mechanisms of learning: A phylogenetic perspective. Journal of Psychiatric Research, 21, 457–472. 10.1016/0022-3956(87)90094-X [DOI] [PubMed] [Google Scholar]
- Cloninger C. R., Svrakic D. M. Przybecky T. R. (1993). A psychobiological model of temperament and character. Archives of General Psychiatry, 50, 975–990. 10.1001/archpsyc.1993.01820240059008 [DOI] [PubMed] [Google Scholar]
- Coates J. M. Herbert J. (2008). Endogenous steroids and financial risk taking on a London trading floor. Proceedings of the National Academy of Sciences, 105, 6167–6172. 10.1073/pnas.0704025105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Nunes J., Zubareva O., Araujo-Correia M., Valenca A., Schroeter C. A., Pawluski J. L., … Strekalova T. (2014). Altered emotionality, hippocampus-dependent performance and expression of NMDA receptor subunit mRNAs in chronically stressed mice. Stress, 17, 108–116. 10.3109/10253890.2013.872619 [DOI] [PubMed] [Google Scholar]
- Crockett M. J., Clark L. Robbins T. W. (2009). Reconciling the role of serotonin in behavioral inhibition and aversion: Acute tryptophan depletion abolishes punishment-induced inhibition in humans. The Journal of Neuroscience, 29, 11993–11999. 10.1523/JNEUROSCI.2513-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K. L., Panksepp J. Normansell L. (2003). The Affective Neuroscience Personality Scales: Normative data and implications. Neuropsychoanalysis, 5, 57–69. 10.1080/15294145.2003.10773410 [DOI] [Google Scholar]
- Dawkins R. (1986). The blind watchmaker. Harlow, England: Longman Scientific & Technical. [Google Scholar]
- de Boer S. F., Slangen J. L. Van der Gugten J. (1990). Effects of chlordiazepoxide and buspirone on plasma catecholamine and corticosterone levels in rats under basal and stress conditions. Endocrinologia Experimentalis, 24, 229–239. [PubMed] [Google Scholar]
- de Boer S. F., Van der Gugten J. Slangen J. L. (1990). Brain benzodiazepine receptor-mediated effects on plasma catecholamine and corticosterone concentrations in rats. Brain Research Bulletin, 24, 843–847. 10.1016/0361-9230(90)90149-T [DOI] [PubMed] [Google Scholar]
- Depue R. A. Collins P. F. (1999). Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation and extraversion. Behavioral and Brain Sciences, 22, 491–569. https://www.cambridge.org/core/journals/behavioral-and-brain-sciences/article/neurobiology-of-the-structure-of-personality-dopamine-facilitation-of-incentive-motivation-and-extraversion/E8AC4097F3DBB5EE4A7EFE822482D8E4 [DOI] [PubMed] [Google Scholar]
- DeYoung C. G. (2010). Personality neuroscience and the biology of traits. Social and Personality Psychology Compass, 4, 1165–1180. 10.1111/j.1751-9004.2010.00327.x [DOI] [Google Scholar]
- DeYoung C. G. (2013). The neuromodulator of exploration: A unifying theory of the role of dopamine in personality. Frontiers in Human Neuroscience, 7, 762 10.3389/fnhum.2013.00762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung C. G. (2015). Cybernetic Big Five theory. Journal of Research in Personality, 56, 33–58. 10.1016/j.jrp.2014.07.004 [DOI] [Google Scholar]
- DeYoung C. G., Grazioplene R. G. Peterson J. B. (2012). From madness to genius: The Openness/Intellect trait domain as a paradoxical simplex. Journal of Research in Personality, 46, 63–78. 10.1016/j.jrp.2011.12.003 [DOI] [Google Scholar]
- Economist: Science and Technology (2013). Neuromorphic computing: The machine of a new soul. The Economist, 407, 67–69. [Google Scholar]
- Eysenck H. J. (1967). The biological basis of personality. Springfield, IL: Charles C. Thomas. [Google Scholar]
- Fajkowska M. (2018). Personality traits: Hierarchically organized systems. Journal of Personality, 86, 36–54. 10.1111/jopy.12314 [DOI] [PubMed] [Google Scholar]
- Fajkowska M. Kreitler S. (2018). Status of the trait concept in contemporary personality psychology: Are the old questions still the burning questions? Journal of Personality, 86, 5–11. 10.1111/jopy.12335 [DOI] [PubMed] [Google Scholar]
- Faulkner P. Deakin J. F. W. (2014). The role of serotonin in reward, punishment and behavioural inhibition in humans: Insights from studies with acute tryptophan depletion. Neuroscience and Biobehavioral Reviews, 46(Pt. 3), 365–378. 10.1016/j.neubiorev.2014.07.024 [DOI] [PubMed] [Google Scholar]
- Gray J. A. (1987). The psychology of fear and stress. London: Cambridge University Press. [Google Scholar]
- Gray J. A. McNaughton N. (2000). The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system (2nd ed.). Oxford: Oxford University Press. [Google Scholar]
- Griffiths B., Schoonheim P. J., Ziv L., Voelker L., Baier H. Gahtan E. (2012). A zebrafish model of glucocorticoid resistance shows serotonergic modulation of the stress response. Frontiers in Behavioral Neuroscience, 6, A68 10.3389/fnbeh.2012.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorenko E. L., De Young C. G., Eastman M., Getchell M., Haeffel G. J., Klinteberg B., … Yrigollen C. M. (2010). Aggressive behavior, related conduct problems, and variation in genes affecting dopamine turnover. Aggressive Behavior, 36, 158–176. 10.1002/ab.20339 [DOI] [PubMed] [Google Scholar]
- Holmes A. J., Lee P. H., Hollinshead M. O., Bakst L., Roffman J. L., Smoller J. W. Buckner R. L. (2012). Individual differences in amygdala-medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. The Journal of Neuroscience, 32, 18087–18100. 10.1523/JNEUROSCI.2531-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa T., Rusakov D. A., Bliss T. V. P. Fine A. (1995). Repeated confocal imaging of individual dendritic spines in the living hippocampal slice: Evidence for changes in length and orientation associated with chemically induced LTP. The Journal of Neuroscience, 15, 5560–5573. http://www.jneurosci.org/content/15/8/5560/tab-article-info [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins W. O. Stanley J. C. (1950). Partial reinforcement. A review and critique. Psychological Bulletin, 47, 193–234. 10.1037/h0060772 [DOI] [PubMed] [Google Scholar]
- John O. P., Naumann L. P. Soto C. J. (2008). Paradigm shift to the integrative Big Five trait taxonomy: History: measurement, and conceptual issues In O. P. John, R. W. Robins, & L. A. Pervin (Eds.), Handbook of personality: Theory and research (pp. 114–158). New York: Guilford Press. [Google Scholar]
- Johnson W. (2010). Understanding the genetics of intelligence: Can height help? Can corn oil? Current Directions in Psychological Science, 19, 177–182. 10.1177/0963721410370136 [DOI] [Google Scholar]
- Kennis M., Rademaker A. R. Geuze E. (2013). Neural correlates of personality: An integrative review. Neuroscience and Biobehavioral Reviews, 37, 73–95. 10.1016/j.neubiorev.2012.10.012 [DOI] [PubMed] [Google Scholar]
- Krebs J. R., Stephens D. W. Sutherland W. J. (1983). Perspectives in optimal foraging In G. A. Clark, & A. H. Brush (Eds.), Perspectives in ornithology (pp. 165–221). Cambridge: Cambridge University Press. [Google Scholar]
- Lathe R. (2001). Hormones and the hippocampus. Journal of Endocrinology, 169, 205–231. 10.1677/joe.0.1690205 [DOI] [PubMed] [Google Scholar]
- Lathe R. (2004). The individuality of mice. Genes, Brain and Behavior, 3, 317–327. 10.1111/j.1601-183X.2004.00083.x [DOI] [PubMed] [Google Scholar]
- LeDoux J. (2012). Rethinking the emotional brain. Neuron, 73, 653–676. 10.1016/j.neuron.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. J. (1960). Partial reinforcement: A selective review of the literature since 1950. Psychological Bulletin, 57, 1–28. [DOI] [PubMed] [Google Scholar]
- Lorens S. A. Van de Kar L. D. (1987). Differential effects of serotonin (5-HT1A and 5-HT2) agonists and antagonists on renin and corticosterone secretion. Neuroendocrinology, 45, 305–310. 10.1159/000124754 [DOI] [PubMed] [Google Scholar]
- Matthews G. (2018). Cognitive-adaptive trait theory: A shift in perspective on personality. Journal of Personality, 86, 69–82. 10.1111/jopy.12319 [DOI] [PubMed] [Google Scholar]
- McAdams D. P. Pals J. L. (2006). A new Big Five: Fundamental principles for an integrative science of personality. American Psychologist, 61, 204–217. 10.1037/0003-066X.61.3.204 [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Eiland L., Hunter R. G. Miller M. M. (2012). Stress and anxiety: Structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology, 62, 3–12. 10.1016/j.neuropharm.2011.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane A. C. (1989). The aetiology of post-traumatic morbidity: Predisposing, precipitating and perpetuating factors. British Journal of Psychiatry, 154, 221–228. [DOI] [PubMed] [Google Scholar]
- McNamara J. Houston A. (1980). The application of statistical decision theory to animal behaviour. Journal of Theoretical Biology, 85, 673–690. 10.1016/0022-5193(80)90265-9 [DOI] [PubMed] [Google Scholar]
- McNaughton N. (1989). Biology and emotion. Cambridge: Cambridge University Press. [Google Scholar]
- McNaughton N. (2002). Aminergic transmitter systems In H. D’Haenen, J. A. Den Boer, H. Westenberg, & P. Willner (Eds.), Textbook of biological psychiatry (pp. 895–914). Chichester: John Wiley & Sons. [Google Scholar]
- McNaughton N. (2018). What do you mean “anxiety”? Developing the first anxiety syndrome biomarker. Journal of the Royal Society of New Zealand, 48, 177–190. 10.1080/03036758.2017.1358184. [DOI]
- McNaughton N. Corr P. J. (2004). A two-dimensional neuropsychology of defense: Fear/anxiety and defensive distance. Neuroscience and Biobehavioral Reviews, 28, 285–305. 10.1016/j.neubiorev.2004.03.005 [DOI] [PubMed] [Google Scholar]
- McNaughton N. Corr P. J. (2009). Central theories of motivation and emotion In G. G. Berntson, & J. T. Cacioppo (Eds.), Handbook of neuroscience for the behavioural sciences (Vol. 2, pp. 710–730). Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- McNaughton N., Panickar K. S. Logan B. (1996). The pituitary-adrenal axis and the different behavioral effects of buspirone and chlordiazepoxide. Pharmacology, Biochemistry and Behavior, 54, 51–56. 10.1016/0091-3057(95)02129-9 [DOI] [PubMed] [Google Scholar]
- Monod J. (1972). Chance and necessity: An essay on the natural philosophy of modern biology (A. Wainhouse, Trans.) London: Collins. [Google Scholar]
- Montagna P., Sforza E., Tinuper P., Provini F., Plazzi G., Cortelli P., … Lugaresi E. (1995). Plasma endogenous benzodiazepine-like activity in sleep disorders with excessive daytime sleepiness. Neurology, 45, 1783–1783. 10.1212/WNL.45.9.1783 [DOI] [PubMed] [Google Scholar]
- Natarajan R., Forrester L., Chiaia N. L. Yamamoto B. K. (2017). Chronic-stress-induced behavioral changes associated with subregion-selective serotonin cell death in the dorsal raphe. The Journal of Neuroscience, 37, 6214–6223. 10.1523/jneurosci.3781-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete A., van Schaik C. P. Isler K. (2011). Energetics and the evolution of human brain size. Nature, 480, 91–93. 10.1038/nature10629 [DOI] [PubMed] [Google Scholar]
- Nettle D. (2006). The evolution of personality variation in humans and other animals. American Psychologist, 61, 622–631. 10.1037/0003-066X.61.6.622 [DOI] [PubMed] [Google Scholar]
- Nielsen M., Braestrup C. Squires R. F. (1978). Evidence for a late evolutionary appearance of brain-specific benzodiazepine receptors: An investigation of 18 vertebrate and 5 invertebrate species. Brain Research, 141, 342–346. 10.1016/0006-8993(78)90203-2 [DOI] [PubMed] [Google Scholar]
- Ogle C. M., Siegler I. C., Beckham J. C. Rubin D. C. (2017). Neuroticism increases PTSD symptom severity by amplifying the emotionality, rehearsal, and centrality of trauma memories. Journal of Personality, 85, 702–715. 10.1111/jopy.12278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer D. J. Benet-Martínez V. (2006). Personality and the prediction of consequential outcomes. Annual Review of Psychology, 57, 401–421. 10.1146/annurev.psych.57.102904.190127 [DOI] [PubMed] [Google Scholar]
- Panksepp J. (1998). Affective neuroscience: The foundations of human and animal emotions. New York: Oxford University Press. [Google Scholar]
- Pariante C. M. (2003). Depression, stress and the adrenal axis. Neuro-Endocrinology Briefings Retrieved from http://www.neuroendo.org.uk/Portals/0/Briefings/briefing_19.pdf [DOI] [PubMed]
- Pariante C. M. Miller A. H. (2001). Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biological Psychiatry, 49(5), 391–404. 10.1016/S0006-3223(00)01088-X [DOI] [PubMed] [Google Scholar]
- Penke L., Denissen J. J. A. Miller G. F. (2007). The evolutionary genetics of personality. European Journal of Personality, 21, 549–587. 10.1002/per.629 [DOI] [Google Scholar]
- Pickering A. D. Gray J. A. (2001). Dopamine, appetitive motivation, and the neuropsychology of human learning: An individual differences approach In A. Eliasz & A. Angleitner (Eds.), Advances in individual differences research (pp. 113–149). Lengerich, Germany: PABST Science. [Google Scholar]
- Pittendrigh C. S. (1958). Adaptation, natural selection and behaviour In A. Roes & G. G. Simpson (Eds.), Behaviour and evolution (Vol. 1, pp. 390–416). New Haven: Yale University Press. [Google Scholar]
- Polc P. (1995). Involvement of endogenous benzodiazepine receptor ligands in brain disorders: Therapeutic potential for benzodiazepine antagonists. Medical Hypotheses, 44, 439–446. 10.1016/0306-9877(95)90504-9 [DOI] [PubMed] [Google Scholar]
- Rammsayer T. H. (1998). Extraversion and dopamine: Individual differences in response to changes in dopaminergic activity as a possible biological basis of extraversion. European Psychologist, 3, 37–50. 10.1027/1016-9040.3.1.37 [DOI] [Google Scholar]
- Revelle W. (2007). Experimental approaches to the study of personality In B. Robins, R. C. Fraley & R. F. Krueger (Eds.), Handbook of research methods in personality psychology (pp. 37–61). New York: Guilford Press. [Google Scholar]
- Sapolsky R. M. (2004). Why zebras don’t get ulcers (3rd ed.). New York: Henry Holt and Company. [Google Scholar]
- Scherrer J. F., True W. R., Xian H., Lyons M. J., Eisen S. A., Goldberg J., … Tsuang M. T. (2000). Evidence for genetic influences common and specific to symptoms of generalized anxiety and panic. Journal of Affective Disorders, 57, 25–35. [DOI] [PubMed] [Google Scholar]
- Skolnick P., Marangos P. J., Syapin P., Goodwin F. K. Paul S. M. (1979). CNS benzodiazepine receptors: Physiological studies and putative endogenous ligands. Pharmacology, Biochemistry and Behavior, 10, 815–823. https://www.sciencedirect.com/journal/pharmacology-biochemistry-and-behavior/vol/10/issue/5 [DOI] [PubMed] [Google Scholar]
- Smillie L. D. (2008). What is reinforcement sensitivity? Neuroscience paradigms for approach-avoidance process theories of personality. European Journal of Personality, 22, 359–384. 10.1002/per.674 [DOI] [Google Scholar]
- Smolensky P. (1988). On the proper treatment of connectionism. Behavioral and Brain Sciences, 11, 1–41. [Google Scholar]
- Stevenson M. McNaughton N. (2013). A comparison of phenylketonuria with attention deficit hyperactivity disorder: Do markedly different aetiologies deliver common phenotypes? Brain Research Bulletin, 99, 63–83. 10.1016/j.brainresbull.2013.10.003 [DOI] [PubMed] [Google Scholar]
- Stouffer S. A., Lumsdaine A. A., Lumsdaine M. H., Williams R. M., Smith M. B., Janis I. L., … Cottrell L. S. (1949). Studies in social psychology in World War II: Vol 2. The American soldier: Combat and its aftermath. Princeton, NJ: Princeton University Press, cited by Broadhurst, P. L. (1960) page 37.
- Taupin V., Toulmond S., Benavides J., Gogusev J., Descamps-Latscha B. Zavala F. (1994). Regulation of neural and peripheral cytokine production by benzodiazepines and endogenous anxiogenic peptides. Advances in Experimental Medicine and Biology, 355, 101–106. 10.1007/978-1-4615-2492-2_17 [DOI] [PubMed] [Google Scholar]
- Trut L. (1999). Early canid domestication: The farm-fox experiment. American Scientist, 87. Retrieved from https://web.archive.org/web/20110623153014/http://www.americanscientist.org:20110623153080/issues/id.20110623153813,y.20110623151999,no.20110623153012,content.true,page.20110623153012,css.print/issue.aspx
- Van Bockstaele E. J. Aston-Jones G. (1995). Integration in the ventral medulla and coordination of sympathetic, pain and arousal functions. Clinical and Experimental Hypertension, 17, 153–165. 10.3109/10641969509087062 [DOI] [PubMed] [Google Scholar]
- Wang Z. W., Valdes J., Noyes R., Zoega T. Crowe R. R. (1998). Possible association of a cholecystokinin promotor polymorphism (CCK-36CT) with panic disorder. American Journal of Medical Genetics, 81, 228–234. [DOI] [PubMed] [Google Scholar]
- Weiss A. (2018). Personality traits: A view from the animal kingdom. Journal of Personality, 86, 12–22. 10.1111/jopy.12310 [DOI] [PubMed] [Google Scholar]
- Yehuda R. (2001). Are glucocortoids responsible for putative hippocampal damage in PTSD? How and when to decide. Hippocampus, 11, 85–89. 10.1002/hipo.1025 [DOI] [PubMed] [Google Scholar]
- Zuckerman M. (2005). Psychobiology of personality. Cambridge: Cambridge University Press. [Google Scholar]