Abstract
Recently emerging evidence indicates accelerated age-related changes in the structure and function of the brain in schizophrenia, raising a question about its potential consequences on cognitive function. Using a large sample of schizophrenia patients and controls and a battery of tasks across multiple cognitive domains, we examined whether patients show accelerated age-related decline in cognition and whether an age-related effect differ between females and males. We utilized data of 1,415 schizophrenia patients and 1,062 healthy community collected by the second phase of the Consortium on the Genetics of Schizophrenia (COGS-2). A battery of cognitive tasks included the Letter-Number Span Task, two forms of the Continuous Performance Test, the California Verbal Learning Test, Second Edition, the Penn Emotion Identification Test and the Penn Facial Memory Test. The effect of age and gender on cognitive performance was examined with a general linear model. We observed age-related changes on most cognitive measures, which was similar between males and females. Compared to controls, patients showed greater deterioration in performance on attention/vigilance and greater slowness of processing social information with increasing age. However, controls showed greater age-related changes in working memory and verbal memory compared to patients. Age-related changes (η2p of 0.001 to .008) were much smaller than between-group differences (η2p of 0.005 to .037). This study found that patients showed continued decline of cognition on some domains but stable impairment or even less decline on other domains with increasing age. These findings indicate that age-related changes in cognition in schizophrenia are subtle and not uniform across multiple cognitive domains.
Introduction
While schizophrenia is considered a neurodevelopmental disorder with fixed early deficits, emerging evidence also supports the idea that schizophrenia patients may show both an early brain dysfunction along with progressive neural and/or behavioral deterioration over the course of the illness. For example, schizophrenia patients showed greater age-related reduction in the brain gray matter volumes than controls after early adulthood [1, 2]. This pattern was disproportionately greater in the clinically important frontal and temporal regions [2]. Schizophrenia patients also showed faster age-related deterioration in white matter integrity, emerging after age 35, and this deterioration was focused in the anterior corpus callosum and the temporal aspects of the superior longitudinal fasciculus, also areas implicated in schizophrenia [2–4]. Interestingly, age-related deterioration in both gray and white matter appears to be faster in males than females [2], consistent with the idea that schizophrenia pathology may show sexual dimorphism with males showing greater impairment across multiple neurobiological and clinical domains [5, 6]. Patients also showed greater age-related decline in functional connectivity in several neural networks including the frontal-parietal network and cingulo-opercular network compared with healthy control subjects (HCS) [7]. Thus, existing evidence suggests that schizophrenia patients show faster age-related decline in the structure and function of the brain compared to healthy controls.
The evidence for accelerated aging in the structure and function of the brain in schizophrenia raises questions about its potential consequences for cognitive function. Longitudinal studies with recent-onset psychotic patients shows that impaired cognitive performance was largely stable up to 20 years past onset [8–11]. However, because most studies did not assess healthy controls at follow-up, the stable pattern of cognitive performance in patients does not necessarily rule out that possibility that patients show greater age-related deterioration. Further, most studies on cognitive changes over the course of illness did not assess multiple cognitive domains nor did they examine whether female and male patients show different patterns of performance over time. In addition, most studies have examined only non-social cognition. A recent finding of stable social cognitive performance over 5 years in recent-onset schizophrenia [12] suggests, but did not demonstrate, that age-related changes in cognition in schizophrenia may be similar across several cognitive domains.
To examine these questions regarding stability versus progression of cognitive deficits with age in schizophrenia patients versus healthy controls, this study utilized a large between-subjects data set from a large case-control study of endophenotypes collected by the second phase of the Consortium on the Genetics of Schizophrenia (COGS-2) [13]. Specifically, this study included a large battery of cognitive tasks tapping into multiple cognitive domains to examine 1) whether schizophrenia patients show age-related changes in cognition that differ from those in healthy controls, and 2) if age-related effects differ between males and females.
Materials and methods
Participants included 1,415 patients diagnosed with schizophrenia or schizoaffective disorder, depressed type, via a Structured Clinical Interview for DSM-IV (SCID) and 1,062 healthy community controls from the COGS-2 study. The NIH-funded COGS-2 included data collection from five sites: University of California at Los Angeles (UCLA), University of California at San Diego (UCSD), Mount Sinai School of Medicine (MSSM), University of Pennsylvania (PENN), and University of Washington (UW). The COGS-2 study also included Stanford University as a non-data-collecting site. Details of the recruitment, the selection criteria for participants and clinical assessments are available in the Supplement and elsewhere [13]. We decided to recruit patients with schizophrenia or schizoaffective disorder, depressed type, for the following reasons. First, findings from genetic studies suggest that schizoaffective disorder, depressed type, is more genetically similar to schizophrenia compared to schizoaffective disorder, bipolar type [14, 15]. Second, schizoaffective disorder, depressed type, showed illness course similar to that of schizophrenia, compared to schizoaffective disorder, bipolar type [16, 17]. The local Institutional Review Boards of each site approved the study, and all participants provided informed consent and were compensated for their participation. For both patients and controls, the Global Assessment of Function Scale (GAF) [18] was administered to characterize samples. Additional clinical assessments for patients included a modified version of the Scale for the Assessment of Negative Symptoms (SANS) [19] and the Scale for the Assessment of Positive Symptoms (SAPS) [20].
Assessments on cognition
The following cognitive tasks from the COGS-2 study were included in this analysis: the Letter-Number Span Task (LNS) [21, 22] for working memory; two forms of the Continuous Performance Test (CPT) [23–25] for attention/vigilance; the California Verbal Learning Test, Second Edition (CVLT-II) [26, 27] for verbal memory; and the Penn Emotion Identification Test (PEIT) and PENN Facial Memory Test (PEMT) of the Penn Computerized Neurocognitive Battery (CNB) [28, 29] for facial affect recognition and facial memory, respectively. As details of each paradigm are described in the references cited, brief descriptions are provided below.
The LNS [30] employed a set of intermixed letters and digits that experimenters verbally presented at a rate of one per second and consisted of two conditions, the “Forward” and “Reorder” conditions. In the Forward condition, participants were asked to repeat the letters and numbers in the same order as they were presented. In the Reorder condition, participants were asked to repeat the digits first in ascending order and then letters in alphabetical order. The primary measure for each condition was the total number of correctly recalled sequences (i.e., maximum score for each condition = 21).
The two forms of CPT involved computerized versions of the Degraded Stimulus CPT (DS-CPT) and the CPT-Identical Pairs (CPT-IP) [23]. The DS-CPT employed a quasi-random series of blurred single digits that were presented for 29-ms each at a rate of one digit per second. Participants were asked to detect each target “0”. The CPT-IP employed a series of digits presented for 50 ms in a quasi-random sequence at a rate of one digit per second and had two conditions: a 3-digit condition and a 4-digit condition. Participants were asked to respond whenever the same stimulus occurred twice in a row. For both the DS-CPT and CPT-IP, the primary measure was d prime (d’), a signal/noise discrimination index reflecting attention and vigilance from signal detection theory [31, 32].
The CVLT-II employed a 16-item word list that was presented verbally over five learning trials, followed by a single presentation of a second list of 16-item words [26]. Participants were asked to recall as many words as possible from the first list immediately after each list presentation, after the second list was presented, and then after a 20-minute delay. The primary measure was Trials 1–5 Free Recall Correct, Short-Delay Free Recall, and Long-Delay Free Recall.
The PENN Emotion Recognition Test (PEIT) of the Penn computerized neurocognitive battery (CNB) [28, 29] was employed as a measure of emotion identification. The PEIT employed 40 facial stimuli expressing one of 4 emotions (happy, sad, anger, fear) or neutral facial expressions that were presented one at a time. Participants were asked to identify the emotion displayed. The primary measures were accuracy and reaction time. The PENN Face Memory Test (PEMT) [28, 29] was employed to assess episodic memory for faces. In the first part of the task, the participants were shown a series of 20 faces one at a time and asked to memorize them. During the recognition part, the participants were shown 40 faces, 20 of which were the faces they were shown, and the other 20 were distractors. For each face stimulus, participants were asked to indicate whether they have seen the face before. The primary measures were accuracy and reaction time.
Statistical analysis
For demographic and clinical characteristics, differences by diagnostic group and gender were examined with ANOVA for continuous variables and chi-square tests for categorical variables. The effect of age and gender on cognitive performance in schizophrenia was examined with a general linear model. Specifically, for each dependent measure of the cognitive tasks, a general linear model was conducted, with age, gender and group as fixed factors. Age was included as a continuous variable and gender and group were included as categorical variables. In addition to the linear effect of age, we also examined whether age has a non-linear effect on cognition by adding the quadratic effect of age in the model. The addition of the non-linear effect of age did not improve the model fit, and we report findings from a general linear model with the linear effect of age below. Effect sizes are provided for any significant effects (i.e., partial eta square [η2p]s: η2p of 0.01 represents a small; η2p of 0.06 represents a medium; and η2p of 0.14 represents a large effect size [33].
Results
Demographic and clinical characteristics of the participants
Table 1 presents demographic and clinical characteristics of the participants. For age, we observed a significant effect of group (F(1,2473) = 252.88, p < .001, η2p = .093) and a significant group by sex interaction (F(1,2473) = 14.52, p < .001, η2p = .006). Overall, controls were younger than patients (p < .001). Females were younger than males in the control group (p < .001), but not in the patient group (p = .055). For personal education, we observed a significant effect of group (F(1,2472) = 745.44, p < .001, η2p = .232), a significant effect of sex (F(1,2472) = 13.35, p < .001, η2p = .005) and a significant group by sex interaction (F(1,2472) = 7.79, p < .01, η2p = .003). Females had higher personal education levels than males in the control group (p < .001), but not in the patient group (p = .52). For parental education, we found a significant effect of group (F(1,2269) = 146.62, p < .001, η2p = .061) and a significant group by sex interaction (F(1,2269) = 6.32, p < .05, η2p = .003). Males had higher levels of parental education than females in the patient group (p < .05), but not in the control group (p = .14). For GAF in the past month, we observed a significant effect of group (F(1,2454) = 15438.32, p < .001, η2p = .863) and a significant effect of sex (F(1,2454) = 33.95, p < .001, η2p = .014). Patients had lower GAF than controls (p < .001), and across both groups, females had higher GAF than males (p < .001). Within the patient group, males reported younger onset of psychotic symptoms than females (F(1,1399) = 12.79, p < .001, η2p = .009). Male and female patients did not differ on positive or negative symptoms (p = .15 and p = .46, respectively).
Table 1. Demographic and clinical characteristics of participants.
| Patients | Controls | |||
|---|---|---|---|---|
| Female (n = 436) | Male (n = 979) | Female (n = 540) | Male (n = 522) | |
| Age | 47.2 (10.5) | 45.9 (11.2) | 37.3 (13.4) | 39.8 (12.7) |
| Personal Edu. | 12.5 (2.2) | 12.5 (2.0) | 15.2 (2.1) | 14.7 (2.2) |
| Parental Edu. | 12.1 (3.1) | 12.5 (2.9) | 14.1 (2.9) | 13.8 (3.1) |
| % Past Sub Dis | ||||
| GAF | 44.7 (8.7) | 43.1 (7.8) | 87.5 (7.6) | 85.2 (8.5) |
| Age of Onset | 23.2 (3.1) | 21.8 (6.2) | ||
| Global_SANS | 10.8 (6.0) | 11.1 (5.5) | ||
| Global_SAPS | 6.7 (4.1) | 7.0 (3.9( | ||
** GAF: GAF of the last month
The effect of age and sex on cognitive performance
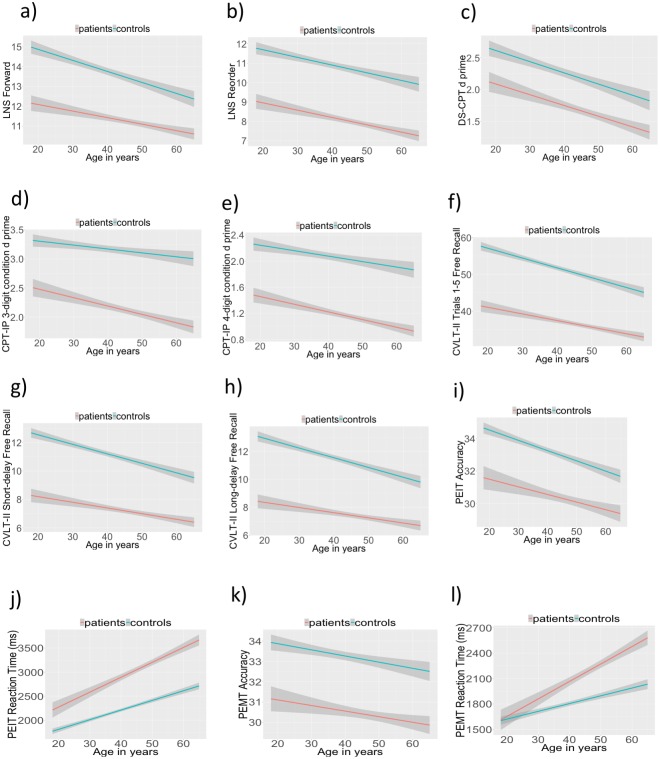
For LNS Forward (Fig 1a), we observed a significant effect of age (F(1,2415) = 74.33, p < .001, η2p = .019), a significant effect of group (F(1,2415) = 55.07, p < .001, η2p = .022), and a significant group by age interaction (F(1,2415) = 5.81, p < .05, η2p = .002). No other effect was significant. Patients showed poorer performance than controls. While both groups showed the decline in performance with increasing age, this decline was steeper in the control group. For LNS Reorder (Fig 1b), we observed a significant effect of age (F(1,2410) = 55.89, p < .001, η2p = .022) and a significant effect of group (F(1,2410) = 46.82, p < .001, η2p = .019), but no other effect was significant. Patients showed poorer performance than controls and both groups showed the decline in performance with increasing age.
Fig 1. Cognitive performance in patients (red line) and controls (blue line).
Lines show fitted value of cognitive performance with increasing age, and shaded areas correspond to 95% confidence interval.
For DS-CPT (Fig 1c), we found a significant effect of age (F(1,2184) = 70.73, p < .001, η2p = .031) and a significant effect of group (F(1,2187) = 12.46, p < .001, η2p = .005). No other effect was significant. In both groups, performance declined with increasing age. Patients performed poorer than controls. For the CPT-IP 3-digit condition (Fig 1d), we found a significant effect of age (F(1,2241) = 32.12, p < .001, η2p = .014), a significant effect of group (F(1,2241) = 18.88, p < .001, η2p = .008) and a significant group by age effect (F(1,2241) = 4.46, p < .05, η2p = .001). Patients showed poorer performance than controls. While both groups showed the decline in performance with increasing age, this effect was greater in the patient group than the control group. For CPT-IP with a 4 digit condition (Fig 1e), we found a significant effect of age (F(1,2193) = 38.57, p < .001, η2p = .017) and a significant effect of group (F(1,2193) = 34.77, p < .001, η2p = .015). Both groups showed the decline in performance with increasing age and patients showed poorer performance than controls.
For CVLT-II Trials 1–5 Free Recall Correct (Fig 1f), an effect of age and an effect of group were significant (F(1,2382) = 129.10, p < .001, η2p = .051; and F(1,2382) = 93.62, p < .001, η2p = .037, respectively). In both groups, performance declined with increasing age. Patients showed poorer performance. For CVLT-II short-delay free recall (Fig 1g), we found a significant effect of age (F(1,2382) = 86.43, p < .001, η2p = .035), a significant effect of group (F(1,2382) = 82.64, p < .001, η2p = .033), and a significant group by age interaction (F(1,2382) = 3.97, p < .05, η2p = .001). Patients showed poorer performance than controls. Both groups showed the decline in performance with increasing age, and this aging effect was larger in the control group. Similarly, for CVLT-II long-delay free recall (Fig 1h), we found a significant effect of age (F(1,2382) = 76.75, p < .001, η2p = .031), a significant effect of group (F(1,2382) = 85.44, p < .001, η2p = .034), and a significant group by age interaction (F(1,2382) = 5.34, p < .05, η2p = .002). Patients again showed poorer performance than controls. Both groups showed the decline in performance with increasing age, with the steeper decline in the control group.
For PEIT accuracy (Fig 1i), we found a significant effect of age (F(1,2121) = 53.69, p < .001, η2p = .024) and a significant effect of group (F(1,2121) = 22.67, p < .001, η2p = .010). Both groups showed poorer performance with increasing age, and patients showed poorer performance than controls. For PEIT reaction time (Fig 1j), we found a significant effect of age (F(1,2121) = 262.49, p < .001, η2p = .110) and a significant group by age interaction (F(1,2121) = 12.71, p < .001, η2p = .005). Both groups showed slower reaction time with increasing age, and this effect was larger in the patient group. For accuracy of the PEMT (Fig 1k), we observed a significant effect of age (F(1,2157) = 16.44, p < .001, η2p = .007) and a significant effect of group (F(1,2157) = 17.78, p < .001, η2p = .008). Patients showed poorer performance than controls. In both groups performance declined with increasing age. For reaction time in the PEMT task (Fig 1l), we found a significant effect of age (F(1,2157) = 144.02, p < .001, η2p = .062) and a significant effect age by group interaction (F(1,2157) = 18.91, p < .001, η2p = .008). Both groups showed slower reaction time with increasing age and this effect was greater in the patient group.
Discussion
In this study, using a large sample of chronic, medicated outpatients and controls of COGS2 study, we examined whether schizophrenia patients and healthy controls show differential age-related differences in cognition and whether age-related changes differ between male and female participants. For most cognitive measures, we observed selective age-related differences between controls and patients that did not differ between male and female participants. Specifically, compared to controls, schizophrenia patients showed greater deteriorated performance with increasing age on attention/vigilance and greater slowness for processing social information. Compared to patients, controls showed greater aging effect on working memory and verbal memory. Further, age-related changes in these cognitive measures were subtle as evidenced by small effect sizes (η2p of 0.001 to .008), and much smaller than the prominent between-group differences (i.e., patients showing poorer performance than controls; η2p of 0.005 to .037). This rather subtle effect of accelerated aging on cognition could be detected because of the large sample size.
Previous longitudinal studies with recent-onset psychotic patients showed stable cognitive performance after the onset of illness [8–11]. However, because most studies had small samples and did not assess healthy controls at follow-up, it remained unclear whether schizophrenia patients showed age-related decline in cognition over time and, if so, whether this decline differs from that of controls. We observed greater age-related cognitive deterioration of patients on some cognitive domains, suggesting a subtle progressive degenerative process. However, on other cognitive domains, we observed smaller age-related effects in schizophrenia compared to controls, but there was still substantial impairment in schizophrenia patients across different ages. This pattern suggests relatively stable levels of cognitive impairment after the onset of illness. Given the critical role of cognitive deficits for functional outcome in schizophrenia [34], our finding of continued decline of cognition along with substantial impairment during adulthood suggests that pharmacological and psychosocial interventions to improve cognitive deficits could benefit schizophrenia patients in all phases of illness.
These findings raise the question of which factors contribute to accelerated cognitive aging in schizophrenia when it is seen. One possibility is that the cognitive decline could be due to accelerated brain aging of schizophrenia patients [1, 2, 7, 35]. Faster brain aging based on neuroimaging in schizophrenia was observed across multiple brain regions, which is consistent with the observation from the current study that patients showed accelerated cognitive aging on attention/vigilance and information processing speed. However, accelerated cognitive aging of patients seen in this study was smaller than one might expect based on the effect sizes of the age-related changes at the neural level. We also did not find greater age-related changes on working memory and verbal memory, even though other studies have found that age-related reduction in the brain gray matter volume in schizophrenia was greater in the frontal and temporal regions [2]. It remains to be determined whether other personal factors (e.g., educational level, cognitive reserve) may play a role of protecting performance from age-related changes in schizophrenia.
The changes for social cognition with age in schizophrenia were observed only in reaction time, not accuracy. In studies with healthy adults, declines with aging appear first in processing speed-based measures, followed by accuracy-based measures [36, 37]. Given that age-related decline was observed with accuracy measures on non-social cognitive tasks in this study, one may wonder whether non-social cognition is more vulnerable to age-related changes than social cognition in schizophrenia. However, we did not measure reaction time for non-social cognitive measures, so we cannot directly test this possibility.
This study had several limitations. It employed a cross-sectional design, and it was not possible to determine whether the same pattern of findings would be seen with a longitudinal study. This study included performance-based cognitive measures, and it remains to be determined whether a similar pattern of accelerated aging would be seen in neural measures of cognition. While this study included individuals over a wide range of ages, we did not include individuals older than 60. Thus, we do not know if a similar level of age-related cognitive decline would be present among patients older than 60 years old.
In summary, with a large sample of schizophrenia patients and controls, this study examined the stability versus progression of cognitive deficits in schizophrenia. Schizophrenia patients showed small, but significantly greater, age-related cognitive decline on attention/vigilance and social cognition (i.e., reaction time) but less age-related changes on working memory and verbal memory, compared to controls. Further, this age-related change in cognitive performance in schizophrenia was much smaller than the substantial between-group difference and was similar across both male and female patients. The current findings indicate that age-related changes in cognition in schizophrenia are subtle and are not uniform across multiple cognitive domains.
Supporting information
(CSV)
(DOCX)
Acknowledgments
Dr. Larry J. Seidman passed away before the submission of the final version of this manuscript. Dr. Junghee Lee accepts responsibility for the integrity and validity of the data collected and analyzed.
Data Availability
Data are contained within Supporting Information File.
Funding Statement
This study was supported by grants R01-MH065571, R01-MH065588, R01-MH065562, R01-MH065707, R01-MH065554, R01-MH065578, R01-MH065558, R01-MH86135, and K01-MH087889 from the National Institute of Mental Health. Other than providing support, the National Institute of Mental Health does not have any further role in this manuscript.
References
- 1.Koutsouleris N, Davatzikos C, Borgwardt S, Gaser C, Bottlender R, Frodl T, et al. Accelerated brain aging in schizophrenia and beyond: a neuroanatomical marker of psychiatric disorders. Schizophr Bull. 2014;40(5):1140–53. 10.1093/schbul/sbt142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cropley VL, Klauser P, Lenroot RK, Bruggemann J, Sundram S, Bousman C, et al. Accelerated Gray and White Matter Deterioration With Age in Schizophrenia. Am J Psychiatry. 2017;174(3):286–95. 10.1176/appi.ajp.2016.16050610 [DOI] [PubMed] [Google Scholar]
- 3.Hawco C, Voineskos AN, Radhu N, Rotenberg D, Ameis S, Backhouse FA, et al. Age and gender interactions in white matter of schizophrenia and obsessive compulsive disorder compared to non-psychiatric controls: commonalities across disorders. Brain Imaging Behav. 2017;11(6):1836–48. 10.1007/s11682-016-9657-8 [DOI] [PubMed] [Google Scholar]
- 4.Kochunov P, Ganjgahi H, Winkler A, Kelly S, Shukla DK, Du X, et al. Heterochronicity of white matter development and aging explains regional patient control differences in schizophrenia. Hum Brain Mapp. 2016;37(12):4673–88. 10.1002/hbm.23336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergh S, Hjorthoj C, Sorensen HJ, Fagerlund B, Austin S, Secher RG, et al. Predictors and longitudinal course of cognitive functioning in schizophrenia spectrum disorders, 10years after baseline: The OPUS study. Schizophr Res. 2016;175(1–3):57–63. 10.1016/j.schres.2016.03.025 [DOI] [PubMed] [Google Scholar]
- 6.Allen DN, Strauss GP, Barchard KA, Vertinski M, Carpenter WT, Buchanan RW. Differences in developmental changes in academic and social premorbid adjustment between males and females with schizophrenia. Schizophr Res. 2013;146(1–3):132–7. 10.1016/j.schres.2013.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheffield JM, Repovs G, Harms MP, Carter CS, Gold JM, MacDonald AW 3rd, et al. Evidence for Accelerated Decline of Functional Brain Network Efficiency in Schizophrenia. Schizophr Bull. 2016;42(3):753–61. 10.1093/schbul/sbv148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rund BR, Barder HE, Evensen J, Haahr U, ten Velden Hegelstad W, Joa I, et al. Neurocognition and Duration of Psychosis: A 10-year Follow-up of First-Episode Patients. Schizophr Bull. 2016;42(1):87–95. 10.1093/schbul/sbv083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonner-Jackson A, Grossman LS, Harrow M, Rosen C. Neurocognition in schizophrenia: a 20-year multi-follow-up of the course of processing speed and stored knowledge. Compr Psychiatry. 2010;51(5):471–9. 10.1016/j.comppsych.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoff AL, Sakuma M, Wieneke M, Horon R, Kushner M, DeLisi LE. Longitudinal neuropsychological follow-up study of patients with first-episode schizophrenia. Am J Psychiatry. 1999;156(9):1336–41. [DOI] [PubMed] [Google Scholar]
- 11.Gold S, Arndt S, Nopoulos P, O'Leary DS, Andreasen NC. Longitudinal study of cognitive function in first-episode and recent-onset schizophrenia. American Journal of Psychiatry. 1999;156(9):1342–48. [DOI] [PubMed] [Google Scholar]
- 12.McCleery A, Lee J, Fiske AP, Ghermezi L, Hayata JN, Hellemann GS, et al. Longitudinal stability of social cognition in schizophrenia: A 5-year follow-up of social perception and emotion processing. Schizophr Res. 2016;176(2–3):467–72. 10.1016/j.schres.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swerdlow NR, Gur RE, Braff DL. Consortium on the Genetics of Schizophrenia (COGS) assessment of endophenotypes for schizophrenia: an introduction to this Special Issue of Schizophrenia Research. Schizophr Res. 2015;163(1–3):9–16. 10.1016/j.schres.2014.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maj M, Starace F, Pirozzi R. A family study of DSM-III-R schizoaffective disorder, depressive type, compared with schizophrenia and psychotic and nonpsychotic major depression. Am J Psychiatry. 1991;148(5):612–6. 10.1176/ajp.148.5.612 [DOI] [PubMed] [Google Scholar]
- 15.Cardno AG, Rijsdijk FV, West RM, Gottesman II, Craddock N, Murray RM, et al. A twin study of schizoaffective-mania, schizoaffective-depression, and other psychotic syndromes. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(2):172–82. 10.1002/ajmg.b.32011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreasen NC, Rice J, Endicott J, Coryell W, Grove WM, Reich T. Familial rates of affective disorder. A report from the National Institute of Mental Health Collaborative Study. Arch Gen Psychiatry. 1987;44(5):461–9. 10.1001/archpsyc.1987.01800170083011 [DOI] [PubMed] [Google Scholar]
- 17.Keshavan MS, Morris DW, Sweeney JA, Pearlson G, Thaker G, Seidman LJ, et al. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr Res. 2011;133(1–3):250–4. 10.1016/j.schres.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36(3):267–75. 10.1016/S0033-3182(95)71666-8 [DOI] [PubMed] [Google Scholar]
- 19.Andreasen NC. The scale for the assessment of negative symptoms (SANS). Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- 20.Andreasen NC. The scale for the assessment of positive symptoms (SAPS). Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- 21.Wechsler D. Wechsler Memory Scale. 3rd ed San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 22.Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54(2):159–65. 10.1001/archpsyc.1997.01830140071013 [DOI] [PubMed] [Google Scholar]
- 23.Nuechterlein KH, Green MF, Calkins ME, Greenwood TA, Gur RE, Gur RC, et al. Attention/vigilance in schizophrenia: performance results from a large multi-site study of the Consortium on the Genetics of Schizophrenia (COGS). Schizophr Res. 2015;163(1–3):38–46. 10.1016/j.schres.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Res. 1988;26(2):223–38. 10.1016/0165-1781(88)90076-5 [DOI] [PubMed] [Google Scholar]
- 25.Nuechterlein KH, Parasuraman R, Jiang Q. Visual sustained attention: Image degradation produces rapid sensitivity decrement over time. Science. 1983;220:327–29. 10.1126/science.6836276 [DOI] [PubMed] [Google Scholar]
- 26.Stone WS, Mesholam-Gately RI, Braff DL, Calkins ME, Freedman R, Green MF, et al. California Verbal Learning Test-II performance in schizophrenia as a function of ascertainment strategy: comparing the first and second phases of the Consortium on the Genetics of Schizophrenia (COGS). Schizophr Res. 2015;163(1–3):32–7. 10.1016/j.schres.2014.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delis DC, Kramer J, Kaplan E, Ober BA. California Verbal Learning Test, 2nd Edition San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 28.Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, et al. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: Standardization and initial construct validation. J Neurosci Methods. 2010;187(2):254–62. 10.1016/j.jneumeth.2009.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gur RC, Braff DL, Calkins ME, Dobie DJ, Freedman R, Green MF, et al. Neurocognitive performance in family-based and case-control studies of schizophrenia. Schizophr Res. 2015;163(1–3):17–23. 10.1016/j.schres.2014.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J, Green MF, Calkins ME, Greenwood TA, Gur RE, Gur RC, et al. Verbal working memory in schizophrenia from the Consortium on the Genetics of Schizophrenia (COGS) study: the moderating role of smoking status and antipsychotic medications. Schizophr Res. 2015;163(1–3):24–31. 10.1016/j.schres.2014.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nuechterlein KH. Vigilance in schizophrenia and related disorders In: Steinhauer SR, Gruzelier JH, Zubin J, editors. Handbook of schizophrenia. Neuropsychology, Psychophysiology and Information Processing. 5 Amsterdam: Elsevier; 1991. p. 397–433. [Google Scholar]
- 32.Green DM, Swets JA. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- 33.Cohen J, Milles J, Shevlin M. Applying regression and correlations: A guide for students and researchers. London: Sage; 2001. [Google Scholar]
- 34.Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67 Suppl 9:3–8; discussion 36–42. [PubMed] [Google Scholar]
- 35.Shahab S, Mulsant BH, Levesque ML, Calarco N, Nazeri A, Wheeler AL, et al. Brain structure, cognition, and brain age in schizophrenia, bipolar disorder, and healthy controls. Neuropsychopharmacology. 2019;44(5):898–906. 10.1038/s41386-018-0298-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starns JJ, Ratcliff R. The effects of aging on the speed-accuracy compromise: Boundary optimality in the diffusion model. Psychol Aging. 2010;25(2):377–90. 10.1037/a0018022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaportzis E, Georgiou-Karistianis N, Stout JC. Dual task performance in normal aging: a comparison of choice reaction time tasks. PLoS One. 2013;8(3):e60265 10.1371/journal.pone.0060265 [DOI] [PMC free article] [PubMed] [Google Scholar]