Abstract
Background
Somatostatin Analogues (SSAs) are used to treat Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) and acromegaly. Side effects of SAAs usually include biliary disorders, gastrointestinal disorders, injection-site pain and hyperglycemia. Exocrine Pancreatic Insufficiency (EPI) is often misdiagnosed as disease progression or failure to SAAs or diagnosed after a delay in patients receiving SAAs. We present our experience with EPI developing in patients following use of SAAs.
Methods
We reviewed chart and pharmacy records of 110 GEP-NETs patients who received SSAs. Data was collected including demographics, pathology, stage, dose/duration of long and short-acting SA, use of antidiarrheal, pancreatic enzyme replacement (PER), proton pump inhibitors (PPI) or H2 blockers). Laboratory data include chromogranin-A (CgA), urine 5-HIAA and quantitative fecal fat test (QFFT) or fecal elastase test (FE). EPI was defined by a FE below normal level OR by a reduction of ≥ 21.2% or steatorrhea on QFFT. Patients who were identified to develop EPI were treated with pancreatic exocrine replacement therapy (PERT).
Results
Among, 110 GEP-NETs patients, 104 received LA Octreotide and 6 Somatuline Depot Injection. Of these, 23 received short-acting SSA for worsening diarrhea, 96 had intensification of antidiarrheal and 1 got telotristat ethyl. QFFT confirmed EPI in 19, 11 based on clinical symptoms, and 16 had sample error or refusal to collect specimen. CTCAE 4.0 grades of EPI were: grade 2(69%), grade 3(22%) and grade 4(9%). Median time to development of EPI was 12 months (95%CI 3 – 23). Except 1, all patients received PERT either with concomitant PPI (13) or later if no improvement with PERT (6) and 2 on H2 blockers. 37% of the patients had improvement in EPI within 4–8 weeks. Deficiency of vitamins and trace elements was found in 11 of 19 patients, who received supplementation.
Conclusions
Our experience constitutes the first and the largest study addressing EPI as a rare but serious complication of chronic use of SAAs. Although SAAs are used to treat diarrhea, paradoxically they can worsen diarrhea secondary to EPI. Early recognition and diagnosis of this under-diagnosed and under-reported side effect of SAAs, such as EPI, can improve not only diarrhea and weight loss in these patients but also can reduce cost of using short-acting SAAs and antidiarrheal.
Keywords: Exocrine pancreatic insufficiency, Chronic pancreatitis, Gastric surgery, Pancreatic surgery, Pancreatic neoplasms, Risk factors, Clinical studies, Lanreotide, Gastroenteropancreatic neuroendocrine tumors (GEP-NET), Somatostatin
Introduction
Somatostatin Analogues (SSAs) (OCT: octreotide; LAN: lanreotide) are the most commonly used agents in the treatment of Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) for both symptom control and to improve disease free survival (DFS) [1,2]. Due to the long course of disease and the urgency presented by the symptoms secondary to the hormonal secretion, management of the syndrome often becomes the primary treatment focus. Furthermore, other treatment options for GEP-NETs, such as chemotherapy and targeted agents may not necessarily translate into improvements in syndrome control [3,4]. There are many over-the-counter medications available to individuals experiencing chronic diarrhea. However, the mechanism by which these agents act does not address the mechanisms underlying the diarrhea induced in carcinoid syndrome. Though SSAs are effective in reducing the frequency of bowel movements and episodes of flushing, but we’re often forced to increase SSA dose and frequency well above label or use supplemental short-acting doses to help maintain control. Further, many patients develop tachyphylaxis. Despite all these efforts, many patients do not experience improved syndrome control.
The most commonly reported adverse events include injection-site discomfort and erythema, gastrointestinal (GI) disturbances such as diarrhea, abdominal pain, nausea and vomiting, biliary sludge or gallstones, and abnormal glucose metabolism [1,2]. As these agents mimic Somatostatin, they can inhibit pancreatic hormones, leading to Exocrine Pancreatic Insufficiency (EPI) [5–7]. The diagnosis of EPI may be under-estimated due to difficulties in distinguishing carcinoid syndrome-related diarrhea. However, this under-published condition is easy to diagnose and treat [8].
Review of literature clearly indicated that the prevalence of EPI is as high as 65% fat malabsorption and 50% protein malabsorption in pancreatic exocrine cancer patients [9]. The causes of the EPI are obviously secondary to the obstruction of and/or the loss of the pancreatic parenchyma. On the other hand, SSAs are used in the treatment of GEP-NETs and are investigated as treatment options in many diseases. These agents mimic somatostatin effect which is inhibitory to pancreatic hormones. The most common side effects are biliary dysfunction and gastroenterological disorders. EPI is a common adverse effect of the medication which is explained by the direct inhibition of pancreatic hormones responsible to stimulate the production and excretion of pancreatic enzymes [8,10]. Lack of recognition or delay in diagnosis of this side effect often leads to increasing use of short-acting agents, escalation of the dose of long-acting octreotide, use of higher doses or addition of other anti-diarrheal agents or performing more testing or imaging to assess for disease progression [8].
We performed a retrospective chart review to identify incidence of EPI in patients receiving SSAs for well-differentiated neuroendocrine tumors GEP-NETs, diagnosis and management in these population. Moreover, we also assessed the clinical and laboratory manifestations of EPI in these patients receiving SSAs for GEP-NETs.
Patients & Methods
Retrospective chart and pharmacy review of patients with histologically confirmed advanced well-differentiated GEP-NETs (6/2009 – 6/2017) was performed. Major eligibility criteria included histological diagnosis of well-differentiated-NET (any site), WHO grade 1 or 2, who received SSA with palliative intent (any line of treatment) and with available follow-up and radiological response data and tumor markers. Patients with poorly differentiated grade 3 neuroendocrine carcinoma (NEC) were excluded.
Data collected included demographics, symptoms including diarrhea, weight loss, flatulence, etc., dose/duration of long and short-acting SSAs, use of antidiarrheal medications including over the counter (OTC), use of pancreatic enzyme replacement (PER), use of proton pump inhibitors (PPI) or H-2 blockers, and laboratory data, particularly tumor related markers chromogranin-A (CgA), urine 5-HIAA, along with radiological imaging (CT scan, MRI, Octreotide scan).
Diarrhea was defined as >2 stools/day in addition to chronic baseline diarrhea while on SAs and weight loss >10% of baseline despite adequate caloric intake. Steatorrhea was suspected when the patient has large, “greasy”, and foul-smelling stools. Currently the gold standard test to diagnose steatorrhea remains the 72-hours fat balance method (normal output is <7 gram of fat per 24-hours period).
Regarding EPI, records were searched for any consults made with the nutritionist or a gastroenterologist or both, and laboratory results if available on quantitative fecal fat, blood levels of fat-soluble vitamins, such as Vitamins A, D, E, K and elements including Magnesium, Potassium, Zinc, Chromium, Selenium, Iron, and Iodine {e.g. Vitamin D (serum 25-hydroxyvitamin D [(25(OH)D]) <20 ng/mL}.
EPI was graded according to the Pancreas exocrine enzyme deficiency according to CTCAE) Version 4.0 (Table 1) [11].
Table 1:
Grades of pancreas exocrine enzyme deficiency per (CTCAE) 4.0.
| Grade | 1 | 2 | 3 | 4 | 5 |
| - | Increase in stool frequency, bulk or odor, steatorrhea | Sequelae of absorption deficiency (e.g. weight loss) | Life-threatening consequences | Death |
Results
Demographics
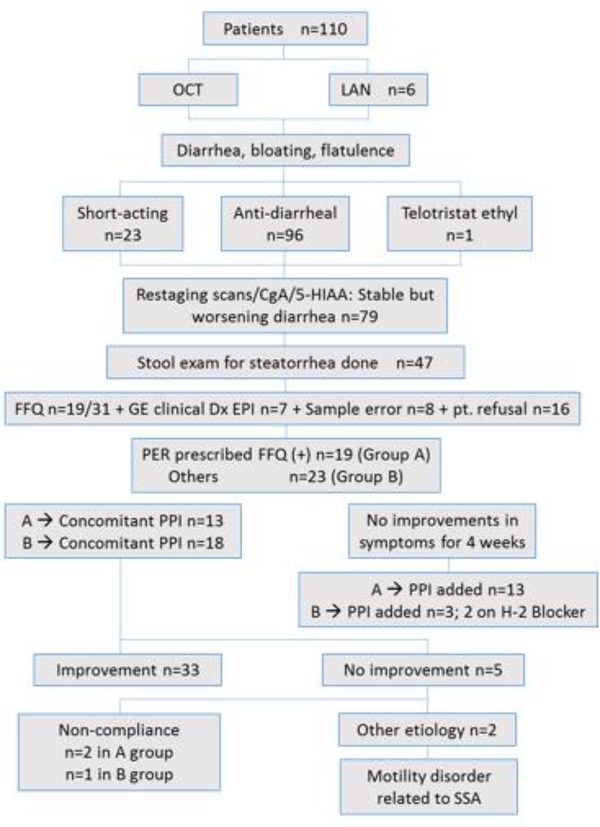
The study schema and demographic features are shown listed in figure 1 and table 2. Based on the previous data including perfusion studies, PER was dosed to administer 25,000 USP to 50,000 USP units of lipase per meal to achieve normal fat digestion and absorption [12]. Total 110 patients with histologically confirmed GEP-NETs with a median age of 56 years were identified. Out of 110, 104 patients received LA Octreotide acetate and 6 Somatuline Depot Injection. Of these, 23 received SA octreotide for worsening diarrhea, 96 had intensification of antidiarrheal and 1 got telotristat ethyl. Seventy-nine patients were formally evaluated by nutritionist and/or gastroenterologist. QFFT was performed in 47 patients with worsening diarrhea despite stable or improved CgA/urine 5-HIAA. Diagnosis of EPI was based on QFFT in 19 patients, 11 of clinical suspicion, and 16 had sample error or refusal to collect stool.
Figure 1:
Allocation of patients.
Table 2:
Patient demographics.
| Total No. of Patients | 110 |
| Median Age (Range) | 61 (29 – 85) |
| Male:Female | 41:69 |
| Ethnicity | W: 75, AA: 7, HS. 11, AS. 5, UK: 12 |
| Site of Primary Tumor | Small Bowel: 68, Unknown: 16, Pancreas: 11, Gastric: 7, Rectal: 5, Colon: 3 |
| Stage at diagnosis | III (17)/ IV (59)/ UK (34) |
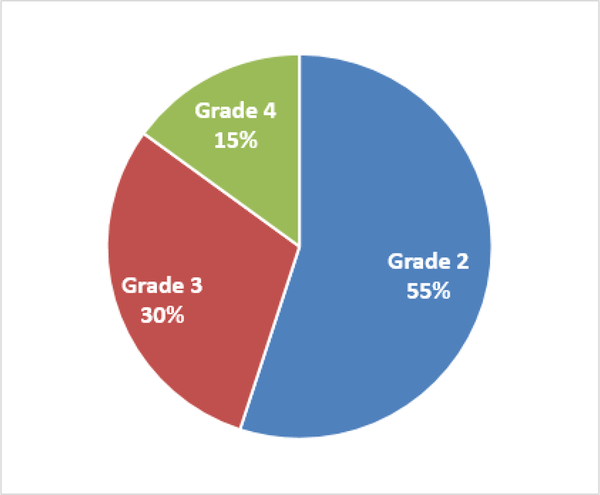
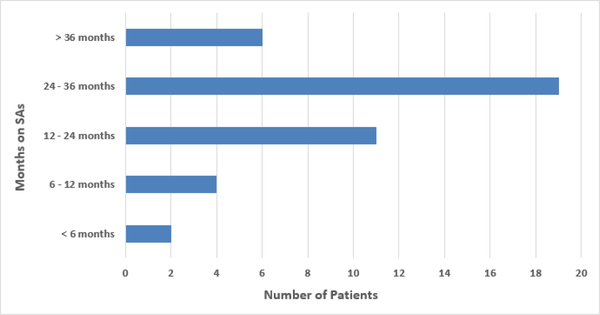
Forty two patients (38%) developed SSA-related EPI. The grades of EPI at diagnoses were G2(55%), G3(30%), and G4(15%) (Figure 2). Median time to development of EPI was 6.5 months (95%CI 2.0 - CI 19) (Figure 3). Diagnosis of EPI was based on QFFT in 19 patients, 11 on clinical suspicion and 16 had sample error or refusal to collect stool.
Figure 2:
Grades of EPI.
Figure 3:
Duration of SAAs till EPI.
Efficacy
Nineteen patients had evidence of steatorrhea and received PER at a dose of 72,000 lipase units per meal. Thirteen patients received PPI concomitantly while 6 started when symptoms did not improve with PER. In addition, a low-fat diet was recommended. Fourteen of nineteen had improvement in diarrhea within 4 weeks - 8 weeks. Two patients were and three were found to have motility disorders. Deficiency of vitamins and trace elements was found in 11 patients of 19 patients, who received supplementation as summarized in Table 3.
Table 3:
Vitamin and mineral deficiency.
| Vitamin/Mineral Deficiency * | N 11/42 (26%) |
| A | 2 (18%) |
| D | 7 (64%) |
| E | 2 (18%) |
| K | 1 (9%) |
| B12 | 3 (27%) |
| Mg | 3 (27%) |
| Se | 4 (36%) |
| K | 1 (9%) |
| Zn | 2 (18%) |
| Cr | 4 (36%) |
| Fe | 3 (27%) |
| I | 0 |
Note:
Based on either laboratory data or clinical features.
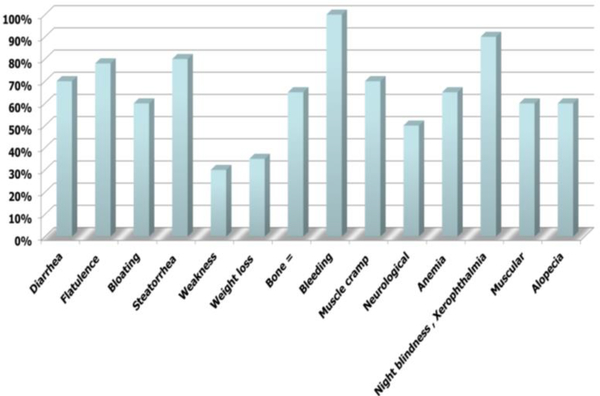
Overall response to PER was 78% including diarrhea in 65%, flatulence in 78%, bloating in 55%, and steatorrhea in 75% (Figure 4).
Figure 4:
Improvement in EPI manifestations.
Toxicity
SSA-related toxicities were similar to our previous experience and mainly grade 1 and grade 2. Grade 3 and grade 4 toxicities were uncommon, and included grade 3 hyperglycemia in 1 patient and grade 2 nausea in 1 patient. No grade 4 toxicities were identified.
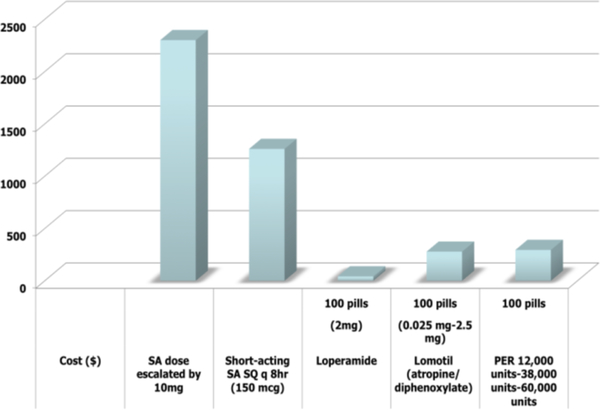
Financial comparison
We also performed a rudimentary financial comparison to other agents used to treat diarrhea in these patients as shown in Figure, which further supported the need to diagnosis and treat EPI (Figure 5).
Figure 5:
Cost analysis.
Discussion
Our study showed that SSA-induced EPI occurs in 4 out of 10 patients treated with SSAs (38%). EPI is likely to be misdiagnosed as carcinoid-syndrome related diarrhea in patients with well-differentiated NETs. Clinicians should actively identify and treat this adverse-event.
EPI is a rare but serious complication of octreotide analogs. Our study series constitutes the largest study addressing the fact that SSAs can induce EPI. Although SSAs are used to treat diarrhea in an unlabeled manner, paradoxically, they cause another type of diarrhea secondary to EPI. This may lead to escalating the dose of the SSA thinking that the primary disease (neuroendocrine tumor) is not controlled while, on the contrary, causing pancreatic dysfunction by more inhibition to the pancreatic hormone secretion, mainly secretin and cholecystokinin (CCK). This results in more diarrhea related to fat malabsorption. Our experience alerts doctors treating neuroendocrine tumors to be aware of this common and forgotten side effect of the octreotide analogs, resulting in better patient management and cost reduction.
Later, another retrospective study was presented at ESMO in 2017 by our colleagues in the UK and their data further confirmed the results of our study [12].
Previously, many animal and human volunteer studies have investigated this association. In a dog’s study, following the administration of SSA 201–995, an analogue of somatostatin, pancreatic exocrine secretion was investigated. SMS 201–995 inhibited significantly the pancreatic secretion and release of hormones, including secretin, CCK, pancreatic polypeptide (PP), and motilin in a dose-dependent manner [13].
The effect of the long-acting SSA (SMS 201–995) on intestinal absorption and propagation (mouth-to-caecum transit time; MCTT), pancreatic secretion and gall bladder contraction after direct (secretin-pancreozymin test) and indirect stimulation (Lundh meal), and meal-induced responses of gastrointestinal regulatory peptides was investigated in a double-blind cross-over study by Creutzfeldt W et al. [14]. Nine healthy volunteers completed two 7-day periods with subcutaneous injections of either placebo or 25 micrograms SMS 201–995 twice daily. Mean fecal fat excretion was increased to 19.2 g/day and MCTT was three times longer during the SMS period. After duodenal infusion of a mixture containing D-galactose, D-xylose and triglycerides, SMS 201–995 significantly reduced the serum concentrations of D-galactose but increased serum levels of D-xylose. After 6 days of pretreatment, SMS 201–995 completely suppressed duodenal trypsin, lipase and bilirubin increases in response to endogenous stimulation by a Lundh meal. Concomitantly, CCK release and gall bladder contraction were almost abolished. Compared with placebo, SMS 201–995 significantly diminished pancreatic amylase, trypsin and lipase output after stimulation with CCK, while the secretion of fluid and bicarbonate in response to secretin was unchanged. This inhibition of enzyme response was significantly more marked after a single injection of the analogue than after pretreatment for 7 days and did not reach the level of exocrine pancreatic insufficiency. CCK-induced gall bladder contraction was significantly inhibited by a single dose of 25 micrograms SMS 201–995 but not after 7 days of pretreatment with the somatostatin analogue.
In another double-blind, placebo-controlled study in healthy subjects by Misumi A et al. [15]. SMS 201–995 was administered subcutaneously at a dose of 25 micrograms twice daily, and all tests were performed 30 minutes after the morning injection. Pancreatic enzyme secretion, gall bladder contraction, and CCK response after a Lundh meal were completely inhibited by SMS, while pancreatic enzyme secretion elicited by intravenous injection of secretin and pancreozymin was suppressed by 80%. The inhibitory effect of SMS on endogenous CCK release was fully operative on the sixth day of injection treatment, whereas the inhibitory effect on exogenous cholecystokinin injection significantly decreased after SMS administration for seven days, indicating desensitization of the end organ by somatostatin. Witt K et al. investigated the effect of SMS 201–995 on secretagogue-stimulated human exocrine pancreatic secretion in 18 healthy volunteers [16]. During secretin stimulation, amylase was inhibited between 41% and 59%, trypsin between 28% and 72%, chymotrypsin between 55% and 70%, and bicarbonate between 0% and 31% with 5, 20 and 80 micrograms/h octreotide. During secretin and ceruletide stimulation, amylase was significantly inhibited by 84%, 78%, 81%, trypsin by 76%, 55%, 52%, chymotrypsin by 77%, 55%, 60%, and bicarbonate by 25%, 11%, 19% with 5, 20, and 80 micrograms/h octreotide, respectively. They found that the maximal inhibitory potency of octreotide was achieved at a dose of only 5 micrograms/hr.
Nakamura T et al. studied the effect of SMS 201–995 on fat assimilation in 6 healthy subjects in the double-blinded, randomized, crossover study, using 14C-triolein and 3H-oleic acid as tracers of dietary triglycerides and free fatty acids, respectively, and 51CrCl3 as non-absorbable marker [17]. Following SMS 201–995 versus placebo, fecal 14C-triolein excretion increased to 75% versus 1%, 3H-oleic acid excretion increased to 82% versus 5%, fecal fat excretion increased to 22 g/day versus 4 g/day. The fecal 14C-triolein/3H-oleic acid test showed triglycerides and free fatty acids to be equally malassimilated following SMS, consistent with malabsorption. In another study conducted in 5 healthy volunteers showed significant reduced secretion of amylase following the initial dose that remain persistent even after 7 days injections [18]. Trypsin and chymotrypsin were decreased and bile acid concentration, bicarbonate and lipase excretions in the octreotide group decreased to 1/3 – 1/4 that of controls. Fecal fat excretion increased; obvious steatorrhea occurred in 2 cases. SMS 201–995 completely abolished release of PPP. Interestingly, ultrasound-measured pancreatic size was not decreased.
Clinical manifestations of EPI may comprise of increase in daily bowel movements, fatty, bulky stools which are difficult to flush away (mainly after high fat containing meals), steatorrhea occurs after meals, it typically happens 2 to 3 times a day in individuals with a normal lipid-content diet, weight loss, and anorexia [19]. Physical Examination may reveal temporal scalloping, interosseous wasting, and lack of subcutaneous fat (weight loss). Nail leukonychia due to hypoalbuminemia may be present in the late stages of chronic malabsorption. Signs of liposoluble vitamin lack may appear, such as ecchymoses due to clotting abnormalities in the case of vitamin K deficiency, ataxia and peripheral neuropathy resembling Friedreich ataxia due to vitamin E deficiency, abnormalities of night blindness and xerophthalmia (dry corneas) due to vitamin A deficiency and contraction or muscle spasms, osteomalacia and osteoporosis due to hypocalcemia.
Many tests are present to aid the diagnosis of pancreatic insufficiency [12,19]. Some of the tests are hormone (secretin, CCK) stimulants, other are serum measurements of enzymes. The cheapest and easiest test to confirm the diagnosis is fecal testing. Steatorrhea from pancreatic maldigestion implies a loss of greater than 90 percent of normal enzyme secretory output. Qualitative measurement (Sudan stain) of a spot stool specimen is easier to perform but less reliable than a quantitative 72-hours collection. These tests are easy to perform and require clinical suspicion of the drug side effects. Nutritional status, including Mg, Albumin, pre-albumin, retinol binding protein, ferritin, and hemoglobin is complementary to the diagnosis of EPI. Early referral to a gastroenterologist might help aid and lead with the diagnosis and treatment. This underdiagnosed and under-published side effect of octreotide analogs is easy to treat when thought about with PER.
As mentioned above that malabsorption of lipids can result in several micronutrient deficiencies, especially fat-soluble vitamins as also observed in our study. It is postulated that unabsorbed fats trap fat-soluble vitamins, such as A, D, E, and K and may also prevent mineral absorption. Absorption of cobalamin (vitamin B12) requires pepsin, which is released through stimulation of gastrin, and thus prolonged malabsorption can result in cobalamin deficiency, especially in individuals with other risk factors, such as those who have a history of antacid use. This could lead to development of anemia, however, iron and RBC folate should be monitored in addition to cobalamin in these patients.
Similarly, trace element deficiencies have been documented in patients with chronic pancreatitis and could occur in this population as seen in our study [20].
In summary, our experience constitutes first study addressing EPI as a rare but serious complication of chronic use of SAAs. Although SAAs are used to treat diarrhea, paradoxically they can worsen diarrhea secondary to EPI. Early recognition and diagnosis of this under-diagnosed and under-reported side effect of SAAs can improve not only diarrhea and weight in these points. But PER can also reduce the cost of using short-acting SA and antidiarrheal. We believe this is an extremely topic of interest to internists, gastroenterologists, oncologists and surgeons, who need to be fully aware of this under-diagnosed toxicity associated with chronic use of SSAs. Future studies to study EPI associated with SSA is warranted [21].
Conclusion
Our data showed that SSA-induced EPI occurs in 4/10 of points treated (38%) and constitutes the first and the largest study addressing this under-diagnosed but serious complication of chronic use of SA which is likely to be misdiagnosed as carcinoid-syndrome related diarrhea. This toxicity is expected as SA may inhibit secretion and release of amylase, trypsin, lipase, secretin, CCK, motilin, bile acid. Clinicians should actively identify and treat this adverse-event to improve not only diarrhea and weight in these points but also use of PERT can reduce cost of using short-acting SA and antidiarrheal, leading to increasing SSAs dosage, use of short acting or additional medications that not only burden the quality of life of these patients or cause additional adverse events but also add to cost of treatment.
References
- 1.Rinke A, Muller HH, Schade-Brittinger C, et al. (2009) Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. Journal of Clinical Oncology 27(28): 4656–4663. [DOI] [PubMed] [Google Scholar]
- 2.Caplin ME, Pavel M, Ćwikła JB, et al. (2014) Lanreotide in metastatic enteropancreatic neuroendocrine tumors. New England Journal of Medicine 371(3): 224–233. [DOI] [PubMed] [Google Scholar]
- 3.Gao L, Natov NS, Daly KP, et al. (2018) An update on the management of pancreatic neuroendocrine tumors. Anti-Cancer Drugs 29(7): 597–612. [DOI] [PubMed] [Google Scholar]
- 4.Papaxoinis G, Syrigos K, Saif MW (2016) Novel therapeutic approaches and mechanisms in neuroendocrine tumors: The role of targeted agents. Discovery Medicine 21(117): 391–402. [PubMed] [Google Scholar]
- 5.Kemmer TP, Malfertheiner P, Büchler M, et al. (1992) Inhibition of human exocrine pancreatic secretion by the long-acting somatostatin analogue octreotide (SMS 201–995). Alimentary Pharmacology & Therapeutics 6(1): 41–50. [DOI] [PubMed] [Google Scholar]
- 6.Liessum PV, Hopman WPM, Pieters GFFM, et al. (1990) Postprandial exocrine pancreatic function during long-term treatment with the somatostatin analogue SMS 201–995 in acromegalic patients. European Journal of Clinical Investigation 20(4): 348–353. [DOI] [PubMed] [Google Scholar]
- 7.Friess H, Bordihn K, Ebert M, et al. (1994) Inhibition of pancreatic secretion under long-term octreotide treatment in humans. Digestion 55(Suppl. 1): 10–15. [DOI] [PubMed] [Google Scholar]
- 8.Saif MW (2010) Chronic octreotide therapy induce PI: A common but under-recognized adverse effect. Expert Opinion on Drug Safety 9(6): 867–873. [DOI] [PubMed] [Google Scholar]
- 9.Bruno MJ, Haverkort EB, Tytgat GN, et al. (1995) Maldigestion associated with exocrine pancreatic insufficiency: Implications of gastrointestinal physiology and properties of enzyme preparations for a cause-related and patient-tailored treatment. American Journal of Gastroenterology 90(9): 1383–13930. [PubMed] [Google Scholar]
- 10.Bank S (1986) Chronic pancreatitis: Clinical features and medical management. The American Journal of Gastroenterology 81(3): 153–167. [PubMed] [Google Scholar]
- 11. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5×11.pdf .
- 12.Lamarca A, McCallum L, Nuttall C, et al. (2018) Somatostatin analogue-induced pancreatic exocrine insufficiency in patients with neuroendocrine tumors: Results of a prospective observational study. Expert Review of Gastroenterology & Hepatology 12(7): 723–731. [DOI] [PubMed] [Google Scholar]
- 13.Lamarca A, McCallum L, Nuttall C, et al. (2017) 450PPancreatic exocrine insufficiency (PEI) in patients (pts) with well-differentiated neuroendocrine tumours (wd-NETs) treated with somatostatin analogues (SSAs): Incidence and impact on quality of life. Annals of Oncology 28(suppl_5). [Google Scholar]
- 14.Misumi A, Shiratori K, Lee KY, et al. (1988) Effects of SMS 201–995, a somatostatin analogue, on the exocrine pancreatic secretion and gut hormone release in dogs. Surgery 103(4): 450–455. [PubMed] [Google Scholar]
- 15.Creutzfeldt W, Lembcke B, Folsch UR, et al. (1987) Effect of somatostatin analogue (SMS 201–995, Sandostatin) on pancreatic secretion in humans. The American Journal of Medicine 82(5): 49–54. [DOI] [PubMed] [Google Scholar]
- 16.Köhler E, Beglinger C, Dettwiler S, et al. (1986) Effect of a new somatostatin analogue on pancreatic function in healthy volunteers. Pancreas 1(2): 154–159. [DOI] [PubMed] [Google Scholar]
- 17.Witt K, Pedersen NT (1989) The long-acting somatostatin analogue SMS 201–995 causes malabsorption. Scandinavian Journal of Gastroenterology 24(10): 1248–1252. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T, Kudoh K, Takebe K, et al. (1994) Octreotide decreases biliary and pancreatic exocrine function, and induces steatorrhea in healthy subjects. Internal Medicine 33(10): 593–596. [DOI] [PubMed] [Google Scholar]
- 19.Lembcke B, Creutzfeldt W, Schleser S, et al. (1987) Effect of the somatostatin analogue sandostatin (SMS 201–995) on gastrointestinal, pancreatic and biliary function and hormone release in normal men. Digestion 36(2): 108–124. [DOI] [PubMed] [Google Scholar]
- 20.Pezzilli R, Andriulli A, Bassi C, et al. (2013) Exocrine pancreatic insufficiency in adults: A shared position statement of the Italian Association for the Study of the Pancreas. World Journal of Gastroenterology: WJG 19(44): 7930–7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bank S (1986) Chronic pancreatitis: Clinical features and medical management. The American Journal of Gastroenterology 81(3): 153–167. [PubMed] [Google Scholar]