COVID-19 is now established in all but a handful of small countries. Each city and each country are experiencing their own outbreak based on their preparedness, capacities, resources, and interventions put in place. Estimates are that communities and health-care settings will need to adapt for some years. Countries in lockdown will likely have less trauma, but accidents and injuries will still occur. It is inevitable that we orthopaedic surgeons will need to operate on patients with suspected or confirmed infections. Although the literature is replete with information guiding our internist colleagues in the optimal medical management of patients with COVID-19, guidelines on the appropriate surgical management of patients with COVID-19 and protection of the surgical team are few and far between. What are the surgical considerations and protocols when operating on such high-risk patients? How do we best protect ourselves and our orthopaedic teams in the operating room (OR), including our anesthesiology colleagues, while providing the most effective surgical care? In our previous article, we briefly discussed key surgical considerations in the preoperative, intraoperative, and postoperative management of general orthopaedic patients during this COVID-19 pandemic1. Preoperative patients are thoroughly screened and undergo a surgical procedure only when strictly necessary. Intraoperatively, surgical teams are minimized, as is the duration of the operation. Postoperatively, patients are discharged at the earliest possible setting (preferably within the same day) to minimize the risk of nosocomial infections. In this article, we share important surgical considerations and protocols when operating on orthopaedic patients who have suspected or confirmed COVID-19 infections. These guidelines have become more pertinent and useful as we battle a resurgence of COVID-19 infections in Singapore, imported largely from local residents returning to our shores from COVID-19 hotspots2. We believe that these guidelines should be an integral part of every orthopaedic surgeon’s armamentarium as we brace ourselves for an unrelenting battle with COVID-19.
Previously1, we described 3 main overarching principles for any operation during this pandemic, namely (1) clinical urgency, (2) patient and health-care worker protection, and (3) conservation of health-care resources. These principles remain unchanged when managing high-risk patients with COVID-19 or those with suspected COVID-19. Adherence to strict guidelines in the perioperative period is required to mitigate against inadvertent occupational exposure to COVID-19. Effective surgical management of patients with COVID-19 mandates a collaborative effort across services and disciplines from porter and security staff to our nursing and anesthesiology colleagues. Precautions that are taken before and after anesthetic induction are crucial in the prevention of COVID-19 transmission to the surgical team. Any lapse potentially can result in the entire surgical team being compromised, with profound repercussions.
Preoperative Considerations
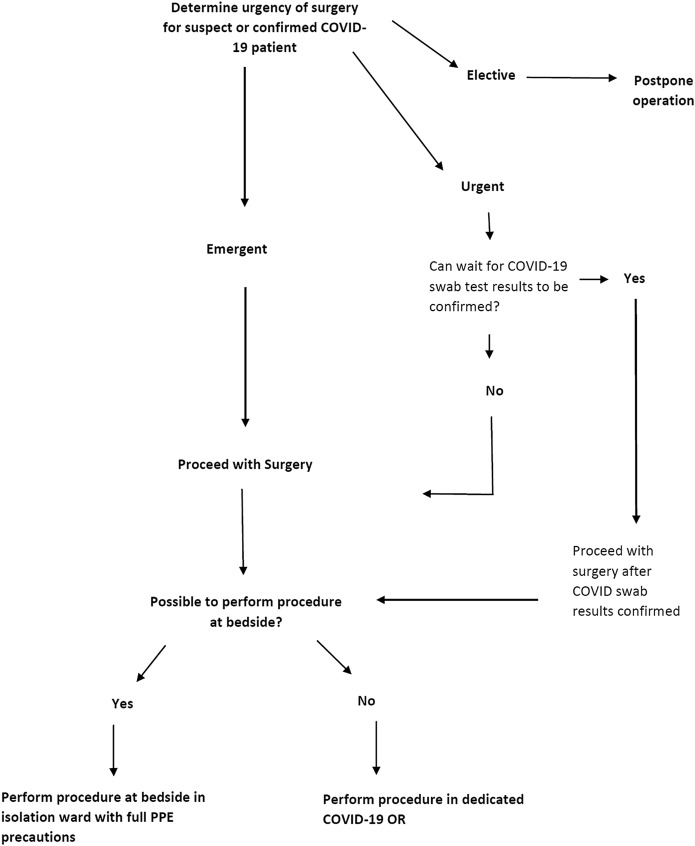
In the preoperative setting, the rationalizing of the indications, timing, and location in which a surgical procedure is to be performed is of primary importance (Fig. 1). Where possible, patients should be managed conservatively without unduly compromising clinical care3. Most upper-limb fractures, including clavicle, humeral, and wrist fractures, have high rates of union and can be managed conservatively4-7, although some patients may eventually require late reconstruction8. Even in the lower extremity, the nonoperative management of tibial fractures can be considered9,10. Ligamentous injuries of the knee can also be managed with bracing in preference to early ligament reconstruction11,12.
Fig. 1.
Decisional flowchart rationalizing the indication, timing, and location of orthopaedic surgical procedures performed in patients with COVID-19.
When a surgical procedure is indicated, deciding on the optimal timing of a surgical procedure is crucial. This is where our first principle of clinical urgency applies. In our institution, COVID-19 tests are processed by the laboratory 4 to 5 times a day, and it takes, on average, 4 hours before results are known. In extremely urgent cases in which a 4-hour wait for COVID-19 results may not be tenable, surgical procedures proceed with the surgical team donned in full protective gear. This consists of the N95 mask, goggles, caps, shoe covers, gowns, and gloves. Powered air-purifying respirators (PAPRs) are worn if involvement in aerosol-generating procedures is anticipated. Emergency cases include patients with life and limb-threatening injuries (e.g., high-grade open fractures with gross contamination, fractures with vascular compromise or compartment syndrome, cauda equina, or infections such as necrotizing fasciitis). In less urgent cases, patients are conscientiously evaluated to rule out the presence of concomitant COVID-19 infection. When suspicion arises, we should have a low threshold for performing COVID-19 swab tests. In our institution, guidance on the decision to perform COVID-19 swab tests may also be sought from our dedicated COVID-19 infectious diseases team, 24 hours a day, 7 days a week. This is in line with our second principle: patient and health-care worker protection. Within a permitted surgical time frame, we should strive to ensure that COVID-19 swab results are known before proceeding with a surgical procedure.
As health-care resources are being stretched, it is imperative that orthopaedic surgeons, in each institution, come to a consensus with regard to the urgency of orthopaedic cases. What defines essential and nonessential orthopaedic surgery13? Examples of essential urgent cases include epidural abscesses, spinal trauma with neurological deficits, and grossly contaminated open fractures. Nonessential surgical procedures would include benign bone tumors (e.g., osteochondroma) and chronic degenerative joint disease. There is no cookie-cutter, 1-size-fits-all approach, and these definitions need to be reviewed regularly, with adjustments tailored to each hospital’s manpower and COVID-19 situation.
In patients with COVID-19, the decision to operate should also be based on the patient’s clinical status, in particular his or her respiratory function. A fine balance needs to be struck between mitigating potential fracture-related complications (e.g., fat embolism from long bone fractures14) with worsening of respiratory function in patients who are COVID-19-positive from the added stresses of anesthesia and an expedient surgical procedure. In some instances, patients with fractures that would usually require surgical fixation may need to be treated conservatively until they are fit enough for a surgical procedure at a later date.
Surgical procedures that can reasonably be performed by the bedside may be carried out in the isolation ward in full personal protective equipment (PPE). Examples include the bedside debridement and irrigation of grade-I open fractures in pediatric patients. This has been demonstrated to be effective in reducing infection rates15. In managing patients preferentially by the bedside instead of the OR, we minimize inadvertent exposure of staff to COVID-19.
Anesthetic and Operative Considerations
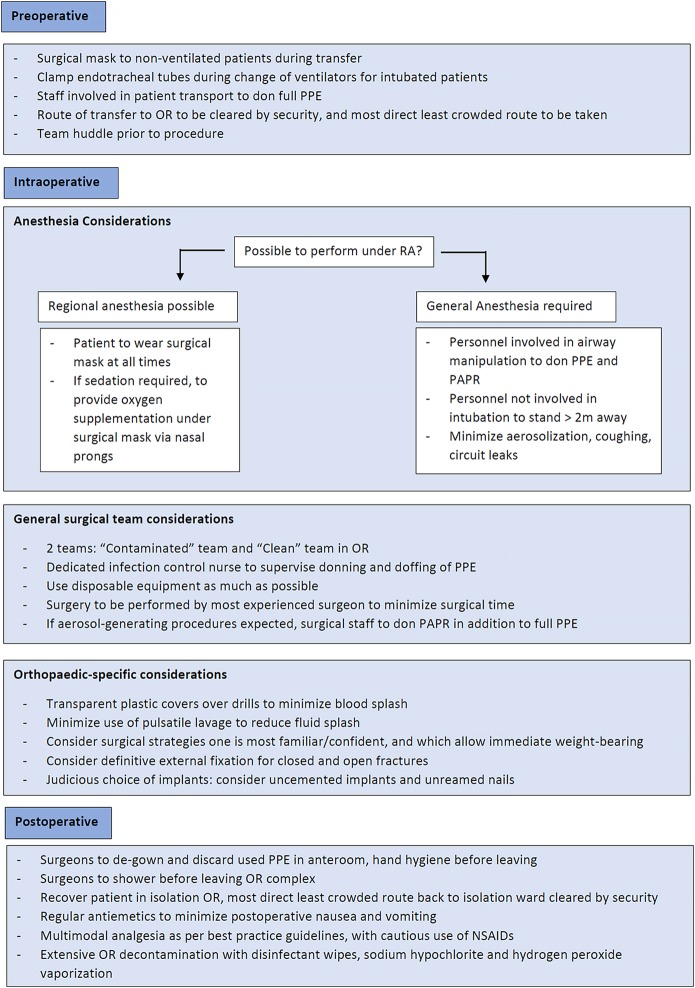
Once the decision, timing, and location of the surgical procedure have been decided, surgical planning starts right from when the patient is transferred from the isolation ward to the OR. Each step needs to be planned and performed methodically in the preoperative, intraoperative, and postoperative settings to avoid inadvertent COVID-19 exposure (Fig. 2). Surgical masks need to be worn for all nonintubated patients during the transfer process, and it is mandatory for all staff accompanying patients to be wearing full PPE including N95 masks, visors, or goggles. For intubated patients coming from the intensive care unit, a dedicated transport ventilator must be used. Staff must be mindful to clamp endotracheal tubes when changing ventilators during the transfer process to avoid aerosolization. A specific route that is the most direct and least crowded must be taken from the isolation ward to the OR. This route, including elevators, must be clearly sign-posted and cleared by security staff prior to transfer.
Fig. 2.
Perioperative surgical considerations in the management of patients with COVID-19. RA = regional anesthesia.
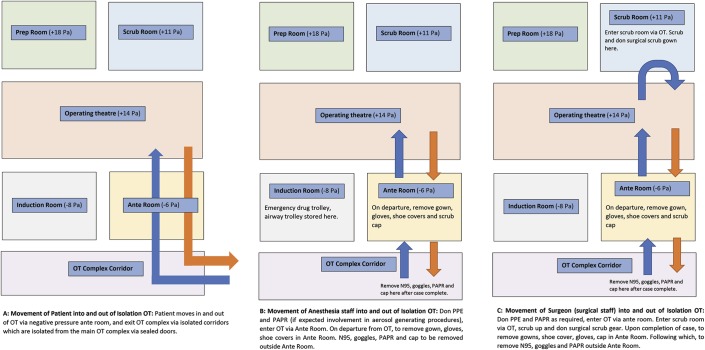
It is preferable for dedicated isolation ORs with separate access to be used for patients with COVID-19. These ORs should each have a separate air-conditioning and humidification unit with individual atmospheric air inlet and exhaust systems. In our institution, this takes the form of 5 interconnecting rooms of which the OR itself and the preparation and scrub rooms are positively pressurized and allow for 20 air changes per hour. This is aimed at reducing surgical site infections16. The anterooms and induction rooms are negatively pressurized; the anteroom is used for the donning and removal of PPE including PAPRs. Because of the availability of a negative-pressure anteroom, we have elected to keep the OR itself positively pressurized. When a negative-pressure anteroom is not available, it may be advisable to operate in neutral or negative pressure, as recommended by some jurisdictions. Improvisations can even be made to modify existing ORs, as was done locally in Tan Tock Seng Hospital in Singapore during our severe acute respiratory syndrome (SARS) crisis in 200317. All doors that led into the OR were locked and were sealed with tape. Existing pressure-relief valves opening from the OR into the corridors and adjacent rooms were sealed. In this way, the induction room was converted to effectively function as an anteroom and air lock. It is extremely important that orthopaedic surgeons have a thorough knowledge of the airflows within their specific ORs to minimize the risk of infection to both themselves and their surgical teams.
Similar to SARS, COVID-19 is predominantly spread through respiratory droplets18. Aerosol transmission can also occur from exposure to elevated aerosol concentrations in enclosed spaces19 (e.g., during intubation). Staff who performed aerosol-generating procedures such as endotracheal intubations were 6.6 times more likely to be infected compared with staff who did not20. In light of this, if there are no contraindications, we would advocate for the use of regional anesthesia techniques. Central axial or peripheral nerve blockades are effective for the majority of orthopaedic procedures and can potentially reduce aerosolization and transmission of COVID-19 droplets, can avoid worsening existing COVID-19-related respiratory compromise due to general anesthesia, and can prevent postoperative nausea and vomiting21-23. After a regional blockade, surgical masks must be placed on patients at all times. Careful attention must also be given to placing nasal prongs under the patients’ surgical masks if sedation is concurrently administered to minimize aerosolization. This further mitigates the risk of surgical teams being exposed to COVID-19 droplets during the surgical procedure itself.
In the event that airway manipulation is deemed necessary (e.g., from surgical necessity or failure of regional blockade), all personnel involved in intubation must don full PPE including PAPRs24,25. Induction and reversal should only take place within the main OR, where COVID-19-dedicated anesthesia machines are located. All potentially required drug and airway equipment is taken from the main drug trolley and is placed in the OR in dedicated trays. A separate, fully stocked drug and airway trolley is also available in the induction room. If additional drugs or equipment are required urgently, the anesthetic nurse may change gloves and gown and perform hand hygiene before entering the induction room and retrieving the items.
Staff not involved in intubation (including the orthopaedic surgical team) should stay at least 2 m away, preferably outside the OR. Aerosol generation must be minimized with specific anesthetic interventions (e.g., rapid sequence induction, avoidance of high-flow nasal cannulas and bag valve mask ventilation, proper securing of endotracheal tubes to avoid air leaks, and minimizing patient coughing on emergence)26,27. Laryngeal mask airways are avoided for these patients, given their high propensity for leaking and absence of a closed circuit28,29. These recommendations have been adopted against the backdrop of evidence supporting dispersion of droplets from exhaled air up to 30 cm away with the use of bag valve mask ventilators and up to 1 m away with coughing30. All anesthetic interventions should be completed before the surgical team enters the OR for patient positioning and the subsequent surgical procedure.
Along with the routine surgical timeout, it is extremely important that a team huddle takes place prior to a surgical procedure to familiarize the surgical team with the anesthetic and surgical plans. This ensures that all necessary drugs and equipment are prepared and minimizes unnecessary movement into and out of the OR to bring in additional drugs or implants. This is even more pertinent given potential difficulties with communication intraoperatively after the donning of PPE and PAPR.
Intraoperatively, the surgical team should accordingly be divided into 2 main teams: (1) a “contaminated team” with direct patient contact (donning, at the minimum, N95 masks with full PPE including at least goggles and with PAPRs preferable when performing aerosol-generating procedures), and (2) a “clean team” providing ancillary support to deliver equipment and instruments to and from the contaminated team should the need arise. Equipment will be left in the anteroom for the contaminated team to retrieve. The same process in reverse will be used when sending out specimens. This will be particularly pertinent in musculoskeletal tumor surgical procedures in which frozen sections are commonly sent intraoperatively for histopathological review.
A dedicated infection control nurse with the chief responsibility of ensuring that all infection control measures within the OR are strictly adhered to should be deployed. This nurse is also responsible for supervising the appropriate donning and doffing of PPE and the decontamination and sterilization of used instruments and equipment. This is particularly pertinent given the risk of self-contamination during the doffing process31,32. All contact episodes between staff and patients are conscientiously recorded, so that contact tracing and isolation efforts can be facilitated expediently if required. The movement of patients and the anesthetic and surgical staff into and out of the OR complex needs to be tightly regulated to ensure health-care worker and patient protection and to minimize nosocomial COVID-19 spread (Fig. 3).
Fig. 3.
Suggested flowchart of patient, anesthetic, and surgical team movement within the operating theatre (OT) complex. Blue arrows depict ingress, and orange arrows depict egress.
As far as possible, disposable instruments, equipment, and drapes should be used. Intraoperatively, surgical times should be kept as short as possible and teams should be kept as small as possible. Resident training is secondary to the imperative that surgical procedures are performed as expediently as possible by the most experienced surgeon to shorten surgical times33. As orthopaedic surgeons, we can undertake specific orthopaedic interventions to mitigate our intraoperative risk of inadvertent COVID-19 exposure. General PPE and PAPR guidelines must be strictly adhered to. The donning of space suits with open fan systems is not recommended because of the potential risk of drawing contaminated submicron particles into the suit system34.
Equipment and Implant Considerations
Sporadic reports have emerged raising concerns of the potential airborne transmission of COVID-1919. COVID-19 aerosols have been reported to be able to linger in the air for up to 3 hours19. However, conflicting articles have also demonstrated the absence of COVID-19 RNA in air samples35,36. Although the World Health Organization (WHO) still recommends precautions against droplets and contact, the U.S. Centers for Disease Control and Prevention (CDC) has recommended additional precautions against airborne transmission for any situation involving the care of patients with COVID-1937. The nature of our job entails the frequent handling of power tools such as high-speed drills and hammers and sharp equipment such as reamers and Kirschner wires38,39. Direct contact with infectious secretions, even blood, can potentially predispose one to COVID-19 infection30. High-speed drilling and blood spatters can potentially exacerbate the aerosolization of COVID-19. Although this theory still remains unproven, we would do well to err on the side of caution. Practical pointers to reduce blood and fluid splatter include the placement of transparent plastic covers over wounds when drilling and minimizing the use of pulsatile lavage40. When possible, to minimize surgical times, we should consider surgical approaches and strategies in which we are most confident and with which we are familiar. For some surgeons, this may involve opting for replacement over fixation options, particularly when operating on complex fractures in elderly patients41,42. Replacement allows for immediate weight-bearing postoperatively. This facilitates rehabilitation and minimizes further complications (e.g., urinary tract infections) from prolonged bed rest in patients with COVID-1943,44. When managing fractures (both closed and open), definitive external fixation should be considered given their minimal invasiveness and the relative ease in performing them45-47. The judicious choice of implants is of utmost importance as well. Uncemented implants (e.g., in bipolar hemiarthroplasties) and unreamed nails should be considered given their shorter surgical timings and potential advantage in reducing respiratory complications in patients who are infected with COVID-19 and have respiratory compromise. In doing so, we can potentially avoid inducing further cardiorespiratory insult from bone cement implantation syndrome48,49 and fat embolism during reaming50,51. By taking these precautions, we protect both ourselves and our patients during surgical procedures.
Postoperative Considerations
Postoperatively, surgeons should remove and discard their used gowns, shoe covers, and gloves in the negative-pressure anteroom and should perform hand hygiene before leaving the anteroom. PAPRs (if used), N95 masks, and goggles are removed outside the anteroom on departure from the OR. PAPRs are placed in a dedicated area for disinfection. All staff should also shower prior to leaving the OR complex16. Patients should recover within the OR itself and should be brought back to the isolation ward directly from the OR by the same dedicated porter team. This is different from the routine practice in which patients are first recovered in the post-anesthesia care unit (PACU) and are brought back to their wards by a common pool of porter staff17. Again, the route back to the isolation ward should be cleared by the hospital security team, and a face mask should always be placed on the patient prior to transfer. Patients should be given regular antiemetics (e.g., ondansetron to reduce postoperative nausea and vomiting), hence minimizing potential aerosolization. Fang et al. reported in The Lancet Respiratory Medicine that the consumption of nonsteroidal anti-inflammatory drugs (NSAIDs) (e.g., ibuprofen) can potentially worsen symptoms in patients with COVID-1952. This is attributed to their increased expression of angiotensin-converting enzyme 2 (ACE-2), which enables SARS-CoV-2 (the coronavirus responsible for COVID-19) to bind to its target cells in the lungs53. This theory is still highly controversial and is as yet unproven. However, it might be prudent to opt for alternative classes of analgesia when managing patients with COVID-19.
A minimum of 1 hour should be planned between cases to allow for OR staff to properly decontaminate the OR and all equipment. Studies have shown that human coronaviruses (e.g., SARS-CoV-2) can persist on inanimate surfaces but can be effectively inactivated by surface disinfection procedures, such as 70% ethanol or 0.5% hydrogen peroxide54-56. In our institution, we routinely disinfect all medical devices, surfaces, and OR equipment with quaternary ammonium chloride disinfectant wipes. The OR is cleaned with sodium hypochlorite; this is followed by hydrogen peroxide vaporization as an added precaution16,57.
Conclusions
In the surgical and perioperative management of patients with COVID-19, the general principles of clinical urgency, patient and health-care worker protection, and conservation of health-care resources need to be similar applied in the preoperative, intraoperative, and postoperative settings to minimize inadvertent COVID-19 occupational exposure. We need to be cognizant of specific nuances with regard to orthopaedics when surgically managing patients with COVID-19. Among these include the consideration of uncemented and unreamed implants to avoid respiratory compromise, and the employment of surgical strategies with which one is most familiar and in which one is most confident to shorten operative times. In addition to surgical precautions, abiding by strict peri-anesthetic precautions is equally, if not more, important. As orthopaedic surgeons, we are leaders of the surgical team. It is imperative that we familiarize ourselves with the key considerations as discussed, to ensure the safety of ourselves, our surgical team members, and our patients as we battle this COVID-19 pandemic while delivering the most effective care for our patients.
Footnotes
Investigation performed at the Department of Orthopaedic Surgery, National University of Singapore, National University Health System, Singapore
Disclosure: The authors indicated that no external funding was received for any aspect of this work. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJS/F869).
References
- 1.Liang ZC, Wang W, Murphy D, Po Hui JH. Novel coronavirus and orthopaedic surgery: early experiences from Singapore. J Bone Joint Surg Am. 2020. May 6;102(9). [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goh T. 70 new coronavirus patients in Singapore, of which 41 are imported cases. 2020. Mar 28, https://www.straitstimes.com/singapore/health/70-new-coronavirus-patients-in-singapore-of-which-41-are-imported-cases Accessed 2020 Apr 6. [Google Scholar]
- 3.National Health Service (NHS). Clinical guide for the management of trauma and orthopaedic patients during the coronavirus pandemic. 2020. March 16 Accessed 2020 Apr 6 https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/specialty-guide-orthopaedic-trauma-and-coronavirus-v1-16-march-2020.pdf [Google Scholar]
- 4.Sarmiento A, Zagorski JB, Zych GA, Latta LL, Capps CA. Functional bracing for the treatment of fractures of the humeral diaphysis. J Bone Joint Surg Am. 2000. April;82(4):478-1-8. [DOI] [PubMed] [Google Scholar]
- 5.Koch PP, Gross DF, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg. 2002. Mar-Apr;11(2):143-1-8. [DOI] [PubMed] [Google Scholar]
- 6.Waldmann S, Benninger E, Meier C. Nonoperative treatment of midshaft clavicle fractures in adults. Open Orthop J. 2018. January 17;12:1-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JK, Park I, Baek E, Han SH. Clinical outcomes of conservative treatment for distal radius fractures with or without ulnar styloid fractures. Arch Hand Microsurg. 2019;24(1):32-1-8 Epub 2019 Mar 1. [Google Scholar]
- 8.Mathews AL, Chung KC. Management of complications of distal radius fractures. Hand Clin. 2015. May;31(2):205-1-8 Epub 2015 Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey BK, Manandhar RR, Sharma S, Pradhan RL, Lakhey S, Rijal KP. Conservative treatment of nonarticular fractures of distal third tibia. JNMA J Nepal Med Assoc. 2009. Oct-Dec;48(176):292-1-8. [PubMed] [Google Scholar]
- 10.Ghosh S, Adak S, Chaudhuri A, Datta S, Roy D, Chaudhuri S. Management of closed isolated tibial shaft fracture: a dilemma in a rural set up of a developing country. Medical J DY Patil Univ. 2014;7(6):738-1-8. [Google Scholar]
- 11.Paterno MV. Non-operative care of the patient with an ACL-deficient knee. Curr Rev Musculoskelet Med. 2017. September;10(3):322-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Graziano J, Williams RJ, 3rd, Jones KJ. Nonoperative treatment of PCL injuries: goals of rehabilitation and the natural history of conservative care. Curr Rev Musculoskelet Med. 2018. June;11(2):290-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American College of Surgeons. COVID-19: elective case triage guidelines for surgical care. 2020. March 24 Accessed 2020 Apr 6 https://www.facs.org/covid-19/clinical-guidance/elective-case [Google Scholar]
- 14.Hughes R. Fat embolism syndrome in long bone fractures. JBJS J Orthop Physician Assist. 2016;4(2):5-1-8. [Google Scholar]
- 15.Godfrey J, Choi PD, Shabtai L, Nossov SB, Williams A, Lindberg AW, Silva S, Caird MS, Schur MD, Arkader A. Management of pediatric type I open fractures in the emergency department or operating room: a multicenter perspective. J Pediatr Orthop. 2017. March 10 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Ti LK, Ang LS, Foong TW, Ng BSW. What we do when a COVID-19 patient needs an operation: operating room preparation and guidance. Can J Anaesth. 2020. March 6 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chee VW, Khoo ML, Lee SF, Lai YC, Chin NM. Infection control measures for operative procedures in severe acute respiratory syndrome-related patients. Anesthesiology. 2004. June;100(6):1394-1-8. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Centers for Disease Control and Prevention (CDC). How COVID-19 spreads. Accessed 2020 Apr 6 https://www.cdc.gov/coronavirus/2019-ncov/prepare/transmission.html [Google Scholar]
- 19.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020. March 17 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(4):e35797 Epub 2012 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu SE, Irwin MG. Regional anaesthesia for orthopaedic procedures. Anaesth Intensive Care Med. 2018. April;19(4):164-1-8. [Google Scholar]
- 22.Singelyn FJ, Capdevila X. Regional anaesthesia for orthopaedic surgery. Curr Opin Anaesthesiol. 2001. December;14(6):733-1-8. [DOI] [PubMed] [Google Scholar]
- 23.Grauman S, Boethius J, Johansson J. Regional anaesthesia is associated with shorter postanaesthetic care and less pain than general anaesthesia after upper extremity surgery. Anesthesiol Res Pract. 2016;2016:6308371 Epub 2016 Nov 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020. February 12 Epub 2020 Feb 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng PWH, Ho PL, Hota SS. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth. 2020. February 27 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong J, Goh QY, Tan Z, Lie SA, Tay YC, Ng SY, Soh CR. Preparing for a COVID-19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Can J Anaesth. 2020. March 11 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tompkins BM, Kerchberger JP. Special article: personal protective equipment for care of pandemic influenza patients: a training workshop for the powered air purifying respirator. Anesth Analg. 2010. October;111(4):933-1-8 Epub 2010 Sep 1. [DOI] [PubMed] [Google Scholar]
- 28.Weiler N, Latorre F, Eberle B, Goedecke R, Heinrichs W. Respiratory mechanics, gastric insufflation pressure, and air leakage of the laryngeal mask airway. Anesth Analg. 1997. May;84(5):1025-1-8. [DOI] [PubMed] [Google Scholar]
- 29.Hönemann CW, Hahnenkamp K, Möllhoff T, Baum JA. Minimal-flow anaesthesia with controlled ventilation: comparison between laryngeal mask airway and endotracheal tube. Eur J Anaesthesiol. 2001. July;18(7):458-1-8. [DOI] [PubMed] [Google Scholar]
- 30.Chan MTV, Chow BK, Lo T, Ko FW, Ng SS, Gin T, Hui DS. Exhaled air dispersion during bag-mask ventilation and sputum suctioning - implications for infection control. Sci Rep. 2018. January 9;8(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chughtai AA, Chen X, Macintyre CR. Risk of self-contamination during doffing of personal protective equipment. Am J Infect Control. 2018. December;46(12):1329-1-8 Epub 2018 Jul 17. [DOI] [PubMed] [Google Scholar]
- 32.Suen LKP, Guo YP, Tong DWK, Leung PHM, Lung D, Ng MSP, Lai TKH, Lo KYK, Au-Yeung CH, Yu W. Self-contamination during doffing of personal protective equipment by healthcare workers to prevent Ebola transmission. Antimicrob Resist Infect Control. 2018. December 22;7(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brindle M, Gawande A. Managing COVID-19 in surgical systems. Ann Surg. 2020. March 23 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derrick JL, Gomersall CD. Surgical helmets and SARS infection. Emerg Infect Dis. 2004. February;10(2):277-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng VCC, Wong SC, Chen JHK, Yip CCY, Chuang VWM, Tsang OTY, Sridhar S, Chan JFW, Ho PL, Yuen KY. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect Control Hosp Epidemiol. 2020. March 5:1-1-8 Epub 2020 Mar 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020. March 4 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. 2020. March 29 Accessed 2020 Apr 6 https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations
- 38.Tokars JI, Chamberland ME, Schable CA, Culver DH, Jones M, McKibben PS, Bell DM; The American Academy of Orthopaedic Surgeons Serosurvey Study Committee. A survey of occupational blood contact and HIV infection among orthopedic surgeons. JAMA. 1992. July 22-29;268(4):489-1-8. [PubMed] [Google Scholar]
- 39.Quebbeman EJ, Telford GL, Hubbard S, Wadsworth K, Hardman B, Goodman H, Gottlieb MS. Risk of blood contamination and injury to operating room personnel. Ann Surg. 1991. November;214(5):614-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tellier R, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis. 2019. January 31;19(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosen AL, Strauss E. Primary total knee arthroplasty for complex distal femur fractures in elderly patients. Clin Orthop Relat Res. 2004. August;425:101-1-8. [DOI] [PubMed] [Google Scholar]
- 42.Wolfensperger F, Grüninger P, Dietrich M, Völlink M, Benninger E, Schläppi M, Meier C. Reverse shoulder arthroplasty for complex fractures of the proximal humerus in elderly patients: impact on the level of independency, early function, and pain medication. J Shoulder Elbow Surg. 2017. August;26(8):1462-1-8 Epub 2017 Mar 31. [DOI] [PubMed] [Google Scholar]
- 43.Bettin CC, Weinlein JC, Toy PC, Heck RK. Distal femoral replacement for acute distal femoral fractures in elderly patients. J Orthop Trauma. 2016. September;30(9):503-1-8. [DOI] [PubMed] [Google Scholar]
- 44.Chen F, Li R, Lall A, Schwechter EM. Primary total knee arthroplasty for distal femur fractures: a systematic review of indications, implants, techniques, and results. Am J Orthop (Belle Mead NJ). 2017. May-Jun;46(3):E163-1-8. [PubMed] [Google Scholar]
- 45.Beltsios M, Savvidou O, Kovanis J, Alexandropoulos P, Papagelopoulos P. External fixation as a primary and definitive treatment for tibial diaphyseal fractures. Strategies Trauma Limb Reconstr. 2009. October;4(2):81-1-8 Epub 2009 Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alhammoud A, Maaz B, Alhaneedi GA, Alnouri M. External fixation for primary and definitive management of open long bone fractures: the Syrian war experience. Int Orthop. 2019. December;43(12):2661-1-8 Epub 2019 Mar 23. [DOI] [PubMed] [Google Scholar]
- 47.Scaglione M, Fabbri L, Dell’ Omo D, Goffi A, Guido G. The role of external fixation in the treatment of humeral shaft fractures: a retrospective case study review on 85 humeral fractures. Injury. 2015. February;46(2):265-1-8 Epub 2014 Sep 16. [DOI] [PubMed] [Google Scholar]
- 48.Olsen F, Kotyra M, Houltz E, Ricksten SE. Bone cement implantation syndrome in cemented hemiarthroplasty for femoral neck fracture: incidence, risk factors, and effect on outcome. Br J Anaesth. 2014. November;113(5):800-1-8 Epub 2014 Jul 16. [DOI] [PubMed] [Google Scholar]
- 49.Donaldson AJ, Thomson HE, Harper NJ, Kenny NW. Bone cement implantation syndrome. Br J Anaesth. 2009. January;102(1):12-1-8. [DOI] [PubMed] [Google Scholar]
- 50.Giannoudis PV, Tzioupis C, Pape HC. Fat embolism: the reaming controversy. Injury. 2006. October;37(Suppl 4):S50-1-8. [DOI] [PubMed] [Google Scholar]
- 51.Högel F, Gerlach UV, Südkamp NP, Müller CA. Pulmonary fat embolism after reamed and unreamed nailing of femoral fractures. Injury. 2010. December;41(12):1317-1-8 Epub 2010 Sep 17. [DOI] [PubMed] [Google Scholar]
- 52.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020. April;8(4):e21 Epub 2020 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020. March 17;94(7):e00127-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020. March;104(3):246-1-8 Epub 2020 Feb 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hulkower RL, Casanova LM, Rutala WA, Weber DJ, Sobsey MD. Inactivation of surrogate coronaviruses on hard surfaces by health care germicides. Am J Infect Control. 2011. June;39(5):401-1-8 Epub 2011 Jan 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kariwa H, Fujii N, Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology. 2006;212(Suppl 1):119-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pottage T, Richardson C, Parks S, Walker JT, Bennett AM. Evaluation of hydrogen peroxide gaseous disinfection systems to decontaminate viruses. J Hosp Infect. 2010. January;74(1):55-1-8 Epub 2009 Nov 20. [DOI] [PubMed] [Google Scholar]