Early oxygen supplementation, conservative fluid management, and prompt identification for the need of more intense respiratory support are crucial in the management of pregnant patients with coronavirus disease 2019 (COVID-19).
Abstract
The present coronavirus disease 2019 (COVID-19) pandemic is affecting pregnant patients worldwide. Although it appears that the severity of disease is reduced in pregnant patients, some are likely to develop severe disease. Our objective is to summarize the basic initial respiratory support interventions recommended for pregnant patients with infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Despite the fact that most pregnant patients with coronavirus disease 2019 (COVID-19) infection are likely to have only mild disease, some may require some immediate form of respiratory support.1
OXYGEN THERAPY
The initial intervention for hypoxemic pregnant patients with COVID-19 infection is administration of oxygen therapy. This may be accomplished by the use of a conventional nasal cannula or a facemask. Commonly used oxygen-delivery devices are described in Table 1. Current guidelines in nonpregnant individuals suggest starting oxygen therapy with peripheral SpO2 levels below 92% and recommend its use when levels are below 90%.2 Based on physiologic changes in pregnancy (eg, increased oxygen demand and a physiologic increase in partial pressure of oxygen), we suggest starting oxygen therapy in pregnant patients when SpO2 values fall below 94%. Therapy should be titrated to avoid SpO2 levels above 96%.2 Once oxygen supplementation is initiated, obstetricians should involve personnel expert in airway management (eg, an anesthesiologist) in case endotracheal intubation is required later. Together with oxygen therapy, asking the patient to lay down in bed prone (awake self-prone position) appears to improve oxygenation (likely by anterior displacement of the mediastinum and improved posterior lung recruitment).3 The latter may be considered in pregnant patients at less than 20 weeks of gestation.
Table 1.
Conventional Methods of Oxygen Delivery
FLUID THERAPY
It is common practice to limit fluids in patients with respiratory failure. In patients with acute hypoxemic respiratory failure, a conservative fluid strategy, as opposed to liberal fluid administration, has been shown to reduce the number of days on mechanical ventilation and the number of days in the intensive care unit.4 Current guidelines suggest a conservative fluid strategy in patients with COVID-19 infection, aiming for a daily negative balance of 0.5–1 L.2 During pregnancy, the risk of pulmonary edema in the setting of lung inflammation may be increased secondary to increased blood volume and lower oncotic pressure. We recommend that maintenance fluids be avoided in pregnant patients with acute COVID-19 infection and oxygen desaturation (SpO2 less than 94%). If daily positive fluid balances are present combined with worsening respiratory status, the use of furosemide (10–20 mg intravenously every 12 hours) may be indicated.
HIGH-FLOW NASAL CANNULA
High-flow nasal cannula has emerged as an attractive alternative to treat patients with acute hypoxemic respiratory failure whose respiratory status is not improving despite conventional oxygen therapy and who do not have an immediate indication for endotracheal intubation. Patients should be hemodynamically stable and able to protect the airway (normal mentation, good cough reflex with adequate clearance of secretions).
High-flow nasal cannula is similar to a conventional nasal cannula; however, oxygen flows as high as 60 L/min may be provided (air is heated and humidified). FiO2 may also be titrated more accurately than with a conventional nasal cannula. Commonly used initial parameters are a flow of 50–60 L/min with an FiO2 of 1.0 (100% oxygen). Once improvement is noted, the FiO2 should be weaned before the flow is decreased, because the flow provides alveolar recruitment (high flow of air results in 3–5 cmH2O of positive pressure ventilation, keeping more alveoli open). We recommend that, once the FiO2 is 0.4–0.5, the flow may be weaned gradually by decreases of 5–10 L/min every 4–6 hours as tolerated to maintain the SpO2 level above 94%.
The efficacy of high-flow nasal cannula compared with conventional oxygen therapy and noninvasive positive pressure ventilation in the form of continuous positive airway pressure or bilevel positive airway pressure has been addressed previously.5,6 One study compared the three modalities in patients with acute hypoxemic respiratory failure. Although there was no difference in intubation rates, mortality was lower in the high-flow nasal cannula group.5 A recent systematic review and meta-analysis concluded that high-flow nasal cannula resulted in lower rates of intubation compared with conventional oxygen therapy and no difference when compared with noninvasive positive pressure ventilation.6 In summary, current evidence suggests that high-flow nasal cannula is superior to conventional oxygen therapy and comparable with noninvasive positive pressure ventilation in patients with hypoxemic respiratory failure who do not respond to initial oxygen supplementation.
Unlike noninvasive positive pressure ventilation, high-flow nasal cannula does not appear to increase the risk of respiratory virus transmission (including coronavirus) compared with conventional oxygen therapy.2 This has made high-flow nasal cannula a primary therapeutic option for patients with COVID-19 infection.7 Once high-flow nasal cannula is initiated, it is paramount to closely observe such patients for further deterioration; failure to improve respiratory status after 30–60 minutes of high-flow nasal cannula (or noninvasive positive pressure ventilation) treatment should warrant immediate reevaluation and consideration of endotracheal intubation and invasive mechanical ventilation. Delays in recognizing early failure of high-flow nasal cannula or noninvasive positive pressure ventilation may result in life-threatening hypoxemia at the time of induction and intubation (especially in pregnant patients with difficult airway anatomy).
An adequate response usually involves an improvement in dyspnea, decreased tachypnea, and improvement in oxygen saturation. Figure 1 depicts a high-flow nasal cannula device.
Fig. 1. High-flow nasal cannula.

Pacheco. Respiratory Support for Pregnant Patients With COVID-19. Obstet Gynecol 2020.
NONINVASIVE POSITIVE PRESSURE VENTILATION
Noninvasive positive pressure ventilation is another modality commonly used in patients for whom conventional oxygen therapy fails but who are not “sick enough” to require tracheal intubation and invasive mechanical ventilation. Noninvasive positive pressure ventilation is ideal for patients with cardiogenic pulmonary edema or chronic obstructive pulmonary disease exacerbations with respiratory acidosis. Unlike high-flow nasal cannula, the use of noninvasive positive pressure ventilation may be associated with an increased risk of disease transmission to health care professionals owing to its aerosol-generating properties.8 This makes high-flow nasal cannula the first-line option for patients not responding to conventional oxygen therapy but who are not yet candidates for endotracheal intubation. In resource-limited areas where high-flow nasal cannula is not available, the use of noninvasive positive pressure ventilation may be considered.2
NEBULIZED TREATMENTS
The use of unnecessary nebulized treatments and sputum-inducing agents should be minimized in patients with COVID-19 infection, because they increase the risk of transmission to health care professionals.2 Needless to say, if such treatment is required, ideally it should be performed in an airborne-infection isolation room, and all individuals in the room should be wearing full personal protective equipment.
FETAL MONITORING AND DELIVERY CONSIDERATIONS
It is commonly accepted, based on extremely limited data, that delivery does not improve the respiratory status of pregnant patients with acute respiratory failure.9 Before 23–24 weeks of gestation, we do not recommend electronic fetal monitoring for pregnant patients with COVID-19–related respiratory failure given that the risks of an emergent cesarean delivery outweigh fetal benefit. Even after this gestational age, the decision to monitor the fetus needs to be individualized, because emergent cesarean delivery under general anesthesia carries a significant risk to the mother as well as to the health care professionals.
For pregnant patients who are stable on either conventional oxygen therapy or high-flow nasal cannula, we suggest a daily nonstress test as opposed to continuous monitoring in an attempt to limit repetitive exposure of nursing personnel (eg, needing to adjust monitors after displacement with maternal movements).
In pregnant patients who require endotracheal intubation and mechanical ventilation, we suggest continuous monitoring after 28 weeks of gestation. From 24 to 28 weeks of gestation, the decision to monitor may be based on estimated fetal weight, neonatology capability, maternal body habitus, and availability of personal protective equipment, among other factors. If the pregnant patient's respiratory status deteriorates, requiring maximal ventilatory settings, especially after 28 weeks of gestation, we recommend proceeding with a controlled delivery (likely cesarean) instead of awaiting fetal distress from refractory hypoxemia and needing an emergent delivery in the intensive care unit.
Despite having no data on the use of steroids for fetal lung maturity in the setting of COVID-19 infection, current guidelines suggest the use of steroids in patients with COVID-19 infection and acute respiratory distress syndrome. Until more data are available, we believe that the use of a single course of steroids to induce fetal lung maturity may be reasonable when indicated. Figure 2 summarizes our recommendations.
Fig. 2. Initial respiratory management of a pregnant patient with coronavirus disease 2019 (COVID-19) infection (personal protective equipment must be used for any interaction with the patient).
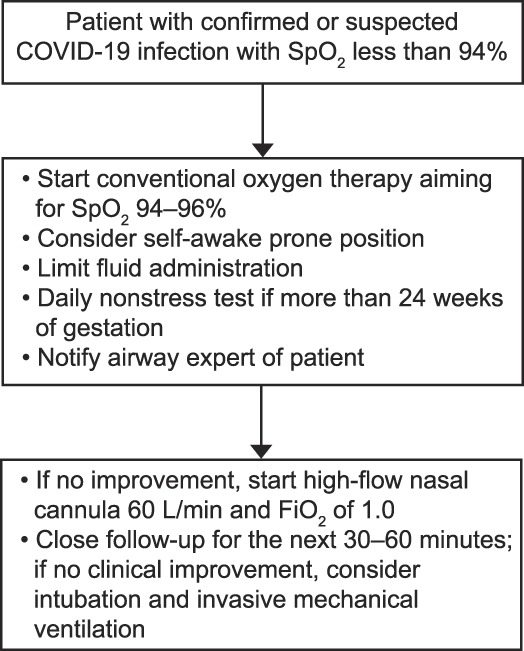
Pacheco. Respiratory Support for Pregnant Patients With COVID-19. Obstet Gynecol 2020.
Footnotes
Financial Disclosure The authors did not report any potential conflicts of interest.
Editing services were provided by LeAnne Garcia in the Publication, Grant, and Media Support Office of the Department of Obstetrics & Gynecology, the University of Texas Medical Branch at Galveston. These services are provided to the authors at no cost as part of their affiliation with the department.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/B886.
REFERENCES
- 1.Breslin N, Baptiste C, Gyamfi-Bannerman C, Miller R, Martinez R, Bernstein K, et al. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis Campaign: guideline on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care 2020;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354:2564–75. [DOI] [PubMed] [Google Scholar]
- 5.Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015;372:2185–96. [DOI] [PubMed] [Google Scholar]
- 6.Ni YN, Luo J, Yu H, Liu D, Ni Z, Cheng J, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: a systematic review and meta-analysis. Chest 2017;151:764–75. [DOI] [PubMed] [Google Scholar]
- 7.Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One 2012;7:e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapinsky SE. Management of acute respiratory failure in pregnancy. Semin Respir Crit Care Med 2017;38:201–7. [DOI] [PubMed] [Google Scholar]



