Abstract
Background
To examine changes in hair cortisol concentrations (HCC) in children with chronic physical illness and identify patterns of association between HCC and mental comorbidity.
Methods
A sample of 50 children aged 6 to 16 years were recruited within six months of being diagnosed with a chronic physical illness. Data were collected via hair samples, structured interviews, and behavioral checklists.
Results
There was no change in HCC over six months. Baseline HCC was associated with internalizing—odds ratio (OR) = 1.29 (90% confidence interval (CI): 1.01–1.66)—and externalizing disorders—OR = 1.32 (90% CI: 1.07–1.64). Externalizing disorder at six months was associated with elevated baseline—OR = 1.25 (90% CI: 1.02–1.53)—and six-month HCC—OR = 1.25 (90% CI: 1.02–1.54). Associations between HCC and mental disorder weakened over time, and for internalizing disorders, changed direction (i.e., inverse association), albeit not significantly.
Conclusion
Results provide preliminary evidence that physiological stress, measured using HCC, may be implicated in the relationship between physical and mental illness, and these associations align with attenuated stress responses over time.
Keywords: adolescent, chronic disease, externalizing, internalizing, psychiatric disorder, stress
Introduction
Mental disorder is common among children with a chronic physical illness (CPI), with reports suggesting 35% to 60% of children affected.1,2 The occurrence of mental disorder in children with CPI is concerning, as this form of multimorbidity has been shown to negatively impact child mortality/morbidity and psychosocial and health management outcomes.3–5 As a result, research aimed at understanding mechanisms of physical-mental comorbidity and identifying at-risk children is critical to ensure optimal outcomes for children.6 Identifying these vulnerable children requires exploration of the link between physical and mental illness, including psychosocial and biological outcomes.7 Research surrounding the human stress response, particularly as it relates to chronic levels of cortisol, is an emerging area of investigation in this population of children.
Cortisol, a human glucocorticoid hormone, is released by the hypothalamic–pituitary–adrenal (HPA) axis in response to stressors.8 Traditionally, cortisol levels measured in blood, saliva, and urine have been used to study the stress responses.9 However, cortisol extracted from these biological specimens provide transient measures of physiological stress, limiting their utility in understanding chronic physiological stress in children with long-term physical and mental illnesses.9 Hair cortisol concentration (HCC) has recently been identified as a potentially valuable biomarker for chronic physiological stress, averaging daily fluctuations in circulating cortisol levels.10–13 Hair sampling represents a noninvasive method to measure chronic physiological stress and reduces the impact of individual and contextual differences associated with circulating cortisol.14,15
Current research using HCC in children has explored stress resulting from maltreatment,9,16 childcare arrangements,17 posttraumatic stress disorder,18 and externalizing disorders.19 Similar to findings from previous research using salivary cortisol,20 lower HCC was found to be associated with symptom severity in preschool boys with attention-deficit hyperactivity disorder (ADHD).21 Associations were more pronounced in children experiencing family adversity, and it was suggested that lower HCC may reflect HPA dysregulation due to compounding adversities in boys.21 Having a CPI in childhood is considered a type of childhood adversity7 that can negatively impact children and families.22–27 It is therefore not uncommon for children diagnosed with CPIs to experience higher rates of mental disorder.28,29 However, little is known about the underlying physiological links between physical and mental illness. It is plausible that HPA axis dysregulation may play a role in the pathogenesis of mental disorder in children with CPI and thus necessitating research to explore these specific relationships.30
Although research using hair cortisol is relatively new, studies using salivary cortisol have suggested that the physiological patterns of HPA axis dysregulation underlying internalizing and externalizing mental disorders may differ.31 Lower basal levels of salivary cortisol have been found to correlate with externalizing disorders,32 while higher basal levels with internalizing disorders.31
Children with CPI are at increased risk of both internalizing and externalizing disorders.33 Examining patterns of HCC in this population may provide valuable insight into the differential effects of HPA axis dysregulation on their pathogenesis. It has been suggested that HCC could provide a useful biomarker to identify individuals who are at risk for adverse psychiatric outcomes following exposure to stressors.34 Emerging research shows the utility of basal HCC in predicting stress-related disorder symptomology, but results are mixed—some finding positive associations,35 whereas others finding inverse associations.18,36 The current study aims to expand the knowledge base by exploring whether HCC following the diagnosis of a CPI is associated with current and future mental disorder in children. Although stress and inflammatory markers are naturally elevated in children with CPI, patterns of HCC in this group of vulnerable children remain largely unknown, and to the best of our knowledge, no prior studies have examined the extent to which HCC may be associated with mental disorder in this population. Findings may provide evidence for the etiological processes surrounding cooccurring CPI and mental disorder as well as determine if HCC may serve as a predictor of mental disorder in these children.
The objectives of this study were to examine changes in HCC over a six-month follow-up in children newly diagnosed with a CPI and to quantify the extent to which HCC was associated with mental disorder at diagnosis (baseline) and six months later. Given that HCC is already elevated in children with CPI, we do not expect associations typically observed between HCC and mental disorder in children without CPI.
Methods
Sample
Details of this study have been described previously.1 Briefly, data come from a prospective pilot study assessing the mental health of children aged 6 to 16 years newly diagnosed with one of five CPIs (asthma, diabetes, epilepsy, food allergy, or juvenile arthritis) recruited from two pediatric hospitals. Children were ineligible if their illness duration was >6 months, they had a degenerative neurological disorder, or their parents could not read English. Parents completed telephone interviews and mailed surveys at baseline and six months. Health professionals identified 62 eligible children, and 50 (83%) participated in the study. Six (12%) were lost to attrition. Parents and children provided informed consent/assent. Ethical approval was granted from the Hamilton Integrated Research Ethics Board and Western Research Ethics Board.
Data Collection and Hair Processing
Parents were provided with detailed written instructions for collecting hair samples. Approximately 50 to 60 strands of hair were cut as close to the scalp as possible from the posterior vertex of the head, an area shown to have high cortisol concentration and low interindividual variability.13 Parents used a paper clip to secure the hair sample to a piece of folded cardstock and indicated the end closest to the scalp. The sample was then placed in a sealed plastic bag and returned to investigators via mail with the study survey. Along with hair samples, parents reported on factors hypothesized to affect HCC: current medications,37 household smoke exposure,38 chemical treatments,12 and other relevant information. Hair samples were obtained at the baseline and six-month assessments. Hair typically grows at a rate of approximately 1 cm per month39; therefore, the 3 cm samples collected reflect the three months prior to the date of data collection. Previous research has found no significant differences in the measurement of HCC between samples collected by participants at home compared to samples collected in clinic.40
Hair samples were processed using a standardized protocol for hair washes, steroid extraction, and cortisol assays. The first 3 cm of each hair sample (proximal to the scalp) was cut with scissors and placed into a Falcon 50 mL Conical Centrifuge Tube. Hair samples were washed twice with 12 mL of isopropanol. Samples were shaken for 2 min by hand, and the isopropanol was discarded. Tubes were left open to air dry over 48 h to ensure complete evaporation of the isopropanol. Dried samples were placed in a grinding jar with four stainless steel ball bearings and pulverized using the Retsch CryoMill at 25 Hz for 3 min. Next, 30 to 35 mg of ground hair powder was measured and transferred to a 2 mL Eppendorf tube, where 1 mL of 100% ethanol was added before it was shaken by hand and rotated at 45 r/min on the RPI Mix-All Laboratory Tube Mixer for 24 to 72 h at room temperature. Samples were then vortexed for 5 s and centrifuged at 2800 r/min for 15 min after which 0.8 mL of supernatant was aliquoted into a new 2 mL Eppendorf tube (supernatant tube). The supernatant was left to air dry for 48 h to ensure complete evaporation of the ethanol. Another 1 mL of 100% ethanol was added to the sample tube, and it was rotated at 45 r/min on the RPI Mix-All Laboratory Tube Mixer for 24 to 72 h at room temperature. Samples were vortexed for 5 s and centrifuged at 2800 r/min for 15 min. During this final extraction stage, 1 mL of the supernatant was aliquoted into the supernatant tube, and it was left to air dry for 48 h. The supernatant was reconstituted with 150 µL of Salimetrics Salivary Cortisol Assay Diluent, vortexed for 5 s, and centrifuged for 10 min. Samples were then assayed in duplicate by ELISA using the High Sensitivity Salivary Cortisol Immunoassay Kit (Cat# 1-3002, Salimetrics, USA), as per manufacturer instructions. Cortisol levels are expressed as pg/mg hair. Intra- and interassay coefficients of variance were below 10% in this study.
Study Measures
Mental Disorder
The Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID), a structured diagnostic interview was used to assess current mental disorder according to the DSM-IV in children aged 6 to 17 years.41 The most common mental disorders affecting children were measured42,43: major depressive disorder, separation anxiety disorder, social or specific phobia, generalized anxiety disorder (internalizing), ADHD, oppositional defiant disorder, and conduct disorder (externalizing). The MINI-KID has a high degree of sensitivity (0.61–1.00) and specificity (0.73–1.00) for measuring mental disorder in children.44,45
Psychiatric Symptoms
The Ontario Child Health Study Emotional Behavioural Scales (OCHS-EBS) is a self-administered checklist used to assess symptoms of mental disorder over the past six months in children aged 4 to 17 years.46,47 Items are scored 0, 1, or 2, indicating responses of “never or not true,” “sometimes or somewhat true,” and “often or very true,” respectively. The raw scores are summed to calculate a scale score for each disorder as well as total severity score and scores for internalizing and externalizing disorders. The OCHS-EBS demonstrates robust psychometric properties46,47 and internal consistencies ranged from α = 0.79 to 0.91 in this study.
Sociodemographic Characteristics
Parents provided information regarding age and sex (parent and child), relationship status, educational attainment, and household income.
Analysis
McNemar and paired t-tests compared changes in the proportion of mental disorder and mean psychiatric symptom scores over time, respectively. The Wilcoxon test compared HCC from baseline to six months and correlation between the assessments using Spearman rank correlation. The Kruskal–Wallis test examined differences in HCC across the different CPI. Logistic regression calculated odds ratios (ORs) and associated confidence intervals for the association between HCC and mental disorder as measured using the MINI-KID. Separate models for the presence of any DSM-IV mental disorder, internalizing disorder, and externalizing disorder were computed. Due to low cell counts, logistic models were not computed for individual mental disorders; instead, linear regression was used to examine associations between HCC and psychiatric symptoms measured using the OCHS-EBS. Models quantified the following associations: baseline HCC and mental disorder; baseline HCC and six-month mental disorder; and six-month HCC and mental disorder. All models adjusted for child age, sex, type of CPI, and use of corticosteroids. Although our data showed no difference in HCC between children who used corticosteroids, analyses were also conducted with these children excluded (n = 14). Inferences remained consistent and thus these children were retained in the analyses presented. Given the pilot nature of the data and relatively small sample size, type I error was α = 0.10 a priori.1 Data were analyzed using SAS 9.4.
Results
Hair samples were provided by 42 (84%) children at baseline and 41 (82%) at six months. Although the reasons for not providing a hair sample were unknown, there were no significant sociodemographic or health-related differences between children who did and did not provide a sample. The mean age of children was 11.6 (3.0) years and approximately half (47%) were male. Asthma was the most common CPI (28%), followed by juvenile arthritis (21%), diabetes (19%), epilepsy (14%), and food allergy (14%). Medication use was reported by 93% of the sample at baseline and 83% at follow-up. Specifically, corticosteroids were used by 19% and 9% of the sample at baseline and follow-up. Sixty percent of the sample screened positive for any mental disorder, this number declined to 44% at six months (p = 0.092). A similar decline was seen for internalizing (55% vs. 30%; p = 0.096), but not externalizing disorders (20% vs. 16%; p = 0.625).
The mean age of parents was 44.1 (5.8) years and most were female (91%). Eighty percent had completed postsecondary education, 79% had a partner (married or common-law), and 68% had annual household incomes ≥$75,000 (median household income from 2016 Canadian Census). Nearly one-quarter (23%) of parents were immigrants.
HCC and CPI
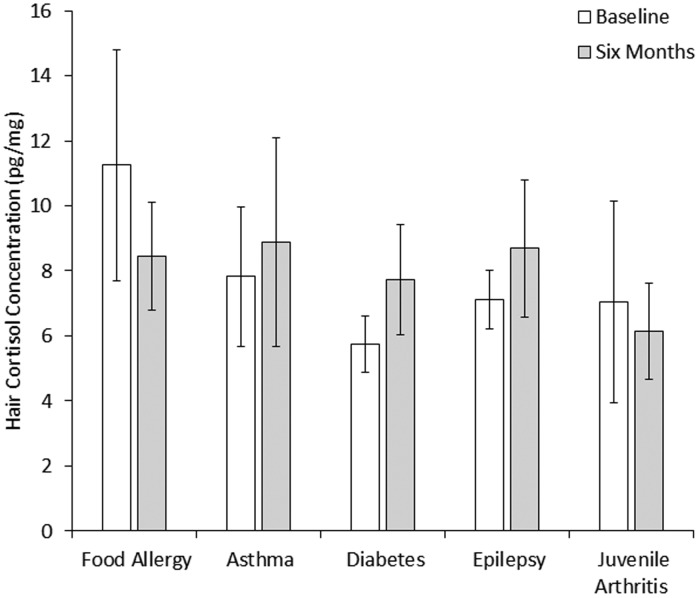
HCC was relatively consistent from baseline to six months (7.63 (6.48) vs. 7.79 (6.96); Z = −0.23, p = 0.820) and the correlation between assessments was moderate in magnitude (ρ = 0.47; p = 0.003). As shown in Figure 1, there was no evidence to suggest that HCC differed across CPIs at baseline (χ2 = 5.00; p = 0.287) or six months (χ2 = 2.60; p = 0.628). On average, children washed their hair 3.8 (1.9) times per week, 26% had a hair treatment (e.g., coloring), and 26% lived with a smoker. None of these factors, nor age or sex, correlated with HCC.
Figure 1.
Mean hair cortisol concentration by type of chronic physical illness. Error bars represent the standard error.
HCC and Mental Disorder
HCC was not significantly associated with the presence of any mental disorder at baseline or six months (OR ranging from 1.21 to 1.27) in the adjusted analyses (Table 1). Baseline HCC was associated with having an internalizing disorder at baseline—OR = 1.29 (90% confidence interval (CI): 1.01–1.66). No associations were found between baseline and six-month HCC with six-month internalizing disorders. Significant associations were found for externalizing disorders at baseline—OR = 1.32 (90% CI: 1.07–1.64)—and six months—OR = 1.25 (90% CI: 1.02–1.54).
Table 1.
Associations between HCC and mental disorder assessed using the MINI-KID.
| Baseline mental disorder | Six-month mental disorder | |
|---|---|---|
| Any mental disorder | ||
| Baseline HCC | 1.27 (0.99–1.62) | 1.21 (0.96–1.52) |
| Six-month HCC | – | 1.22 (0.96–1.55) |
| Internalizing disorder | ||
| Baseline HCC | 1.29 (1.01–1.66) | 0.86 (0.72–1.03) |
| Six-month HCC | – | 0.98 (0.89–1.09) |
| Externalizing disorder | ||
| Baseline HCC | 1.32 (1.07–1.64) | 1.25 (1.02–1.53) |
| Six-month HCC | – | 1.25 (1.02–1.54) |
Note: Results are reported as OR (90% CI) for the association between HCC (pg/mg) and mental disorder. Adjusted models controlled for age, sex, type of chronic physical illness, and use of corticosteroids. HCC: hair cortisol concentration. Statistically significant associations are shown in bold.
HCC and Psychiatric Symptoms
As shown in Table 2, models of HCC and psychiatric symptoms as measured using the OCHS-EBS were aligned with those that examined mental disorder. No associations were found for HCC and total symptoms. HCC and internalizing symptoms were significantly associated at baseline (β = 0.32, p = 0.027), but not at six months (β = −0.10, p = 0.758). Consistent associations were found for baseline HCC and symptoms, baseline HCC and six-month symptoms, and six-month HCC and symptoms (β range: 0.24–0.58, p < 0.10 for all). Condition-specific associations were found for symptoms related to ADHD (β = 0.25, p = 0.046), oppositional defiant disorder (β = 0.18, p = 0.044), and conduct disorder (β = 0.14, p = 0.066). At six months, associations between HCC and symptoms of ADHD and oppositional defiant disorder remained significant (β = 0.16, p = 0.024 and β = 0.12, p = 0.094, respectively). Notably, for all models tested, estimates of association between HCC and psychiatric outcomes decreased from baseline to six months.
Table 2.
Associations between HCC and symptoms of mental disorder assessed using the OCHS-EBS.
| Baseline HCC and symptoms | Baseline HCC and six-month symptoms | Six-month HCC and symptoms | |
|---|---|---|---|
| Total symptoms | 0.89 (0.59) | 0.03 (0.55) | 0.22 (0.42) |
| Internalizing | 0.32 (0.18) | −0.21 (0.40) | −0.10 (0.32) |
| Major depression | 0.18 (0.13) | 0.04 (0.12) | −0.03 (0.10) |
| Separation anxiety | −0.15 (0.10) | −0.15 (0.09) | −0.05 (0.08) |
| Phobia | 0.09 (0.09) | −0.05 (0.09) | −0.05 (0.07) |
| Generalized anxiety | 0.19 (0.15) | −0.05 (0.15) | 0.02 (0.12) |
| Externalizing | 0.58 (0.26) | 0.24 (0.14) | 0.32 (0.15) |
| Attention-deficit hyperactivity | 0.25 (0.12) | 0.09 (0.10) | 0.16 (0.07) |
| Oppositional defiant | 0.18 (0.09) | 0.09 (0.09) | 0.12 (0.07) |
| Conduct | 0.14 (0.07) | 0.05 (0.06) | 0.04 (0.05) |
Note: Results are reported as beta coefficients (standard error) for the association between HCC (pg/mg) and symptoms of mental disorder. Adjusted models controlled for age, sex, type of chronic physical illness, and use of corticosteroids. Statistically significant coefficients (p < 0.10) are shown in bold. HCC: hair cortisol concentration.
Discussion
In this pilot study examining mental disorder in children newly diagnosed with a CPI, findings showed that baseline and six-month HCC were moderately correlated, and there were no differences in HCC across different CPIs. Findings also showed that HCC was positively associated with reports of internalizing and externalizing disorders at baseline, but only with externalizing disorders at six months.
In contrast to the findings from this study, meta-analyses have found an inverse association between cortisol levels and externalizing disorder in children,31,32 including ADHD.20,21 This discrepancy may be attributable to the timing of the effects under study. The experience of high levels of stress over long periods of time (i.e., allostatic load) may lead to a down regulation of the HPA axis and lower cortisol levels—hypoactivity48— which is positively associated with time since the onset of the stressor.8,34 The diagnosis of a CPI in childhood is often considered a traumatic event/stressor7; however, given that this was an incident study of newly diagnosed CPI with a relatively short follow-up, it is possible that the children in this sample were still in a state of cortisol hyperactivity and the hypoactive response had not yet initiated.8 As a result, reported symptoms of externalizing disorder were higher in children with elevated HCC.
There was no evidence from our study that CPI affects the association between HCC and mental disorder in children—findings are consistent with a previous study of preschoolers which showed that increased salivary cortisol reactivity correlated with increased HCC and externalizing disorders.19 This suggests that HCC captures patterns of HPA axis dysregulation resulting from increased cortisol reactivity and that this dysregulation contributes to the etiology of externalizing disorders. Dysregulation may also mediate the relationship between CPI and the development of mental disorder. Long-term studies are needed to determine if this declining trend is consistent with an attenuated response.
It is unlikely that the source of cortisol (saliva, urine, blood, hair) underlie differences in the nature of the associations between cortisol level and externalizing disorders found in this study relative to previous reports. Past studies linking HCC and externalizing disorders found lower HCC to be associated with externalizing disorders16,21,49; however, the cross-sectional nature of previous research is unable to examine how cortisol levels change in response to a stressor.
Our study was not designed to comprehensively examine whether HPA axis dysregulation manifests differently for internalizing versus externalizing disorders. However, our findings suggested some consistency in the association between HCC and internalizing and externalizing disorders in children with CPI. Specifically, we found positive associations between HCC and mental disorder at baseline, the magnitude of which decreased over time. Although it is unclear whether children with CPI demonstrate an attenuated response (transitioning from a state of hyper- to hypoactivity), findings from the current study provide preliminary evidence to this effect. Associations between HCC and all psychiatric symptoms and disorders declined from baseline to six months. Specifically, estimates of association changed from positive to negative at six months for internalizing disorders, suggesting lower HCC were associated with elevated symptoms of depression, separation anxiety, and phobia, albeit, not significantly at six months. Our finding of increased odds of internalizing disorders among children with elevated HCC was consistent with a previous study showing that children with separation anxiety have increased cortisol secretion compared to healthy controls.50 In addition, evidence suggests that children with anxiety disorders often experience exacerbated stress, worrying, and tension, which can lead to a dysregulated HPA axis over time as posited by an attenuated response.51 The prospective design of the current study and our finding that the association between HCC and mental disorder/symptoms declined over time is an incremental step in providing evidence for the tenability of the attenuated hypothesis in children with CPI. Divergent directions and magnitudes of association between HCC and internalizing and externalizing disorders suggest the need to examine these outcomes independently.
Our findings must be considered in the context of the following limitations. First, the small sample size prevented examination of condition-specific associations between HCC and mental disorder. This challenge was mitigated by examining psychiatric symptoms on a continuous scale using the OCHS-EBS. Likewise, associations within each CPI could not be examined. Instead, the validated noncategorical approach was adopted.52 This was appropriate given that HCC was not significantly different across the types of CPI and that physical illness was adjusted for in our analytic models. In a related vein, information on the manifestation of mental disorder prior to the diagnosis of the CPI was not collected. Second, the relatively short follow-up period and lack of a control group in this pilot study prevented the investigation of long-term effects and whether the nature of these associations remained consistent over time. Likewise, investigation of the potential for HCC to mediate the effect of CPI on mental disorder was not possible. Although evidence in child populations is lacking, CPI is associated with HPA axis dysregulation.53–56 Evidence also suggests that there are both biological57,58 and psychosocial mechanisms26,59 that link CPI and mental disorder. The extent to which HCC mediates the association between CPI and mental disorder is unknown. Third, the composition of our sample likely overestimated two-parent, high-socioeconomic status families, and losses to follow-up may have introduced attrition bias potentially limiting the generalizability of the findings. Fourth, body mass index was not assessed and could not be included in statistical models. Although past research in adult populations has found positive associations between HCC and body mass index,60 no such findings have been reported in child populations.21 Fifth, the use of pharmacological or psychological treatments for the mental health of children were not measured and the extent to which such treatment may have influenced the decline in children screening positive for mental disorder over time could not be ascertained.
To our knowledge, this study was the first to explore patterns of HCC in children with CPI and investigate whether HCC is associated with psychiatric outcomes. Elevated HCC levels at diagnosis were associated with symptoms of externalizing disorders at diagnosis and six months and with internalizing disorders at diagnosis only. Our findings provide preliminary evidence in support of an attenuated response and some insight into HPA axis dysregulation that may contribute to the development of mental disorder in children with CPI. Further research with larger samples and longer follow-ups are needed to advance this research agenda.
Acknowledgments
The authors thank the children, parents, health professionals, and clinical staff for their participation in this study. The authors also especially thank Jessica Zelman for coordinating the study and Jane Terhaerdt for assisting with ethical approval.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study received funding from the Canadian Institute of Health Research (MOP-133645). Dr. Ferro holds the Canada Research Chair in Youth Mental Health and Early Researcher Award from the Ministry of Research, Innovation and Science. The funders had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or, in the decision to submit the article for publication.
References
- 1.Butler A, Van Lieshout RJ, Lipman EL, et al. Mental disorder in children with physical conditions: a pilot study. BMJ Open 2018; 8: e019011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tegethoff M, Belardi A, Stalujanis E, et al. Association between mental disorders and physical diseases in adolescents from a nationally representative cohort. Psychosom Med 2015; 77: 319–332. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein CM, Stockwell MS, Gallagher MP, et al. Mental health issues in adolescents and young adults with type 1 diabetes: prevalence and impact on glycemic control. Clin Pediatr (Phila) 2013; 52: 10–15. [DOI] [PubMed] [Google Scholar]
- 4.Kwon HJ, Kim YL, Lee SM. Relation between functional ability and health-related quality of life of children with juvenile rheumatoid arthritis. J Phys Ther Sci 2015; 27: 837–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Wang Y, Wang LB, et al. A comparison of quality of life in adolescents with epilepsy or asthma using the Short-Form Health Survey (SF-36). Epilepsy Res 2012; 101: 157–165. [DOI] [PubMed] [Google Scholar]
- 6.Calam R, Gregg L, Goodman R. Psychological adjustment and asthma in children and adolescents: the UK nationwide mental health survey. Psychosom Med 2005; 67: 105–110. [DOI] [PubMed] [Google Scholar]
- 7.Pao M, Bosk A. Anxiety in medically ill children/adolescents. Depression Anxiety 2011; 28: 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull 2007; 133: 25–45. [DOI] [PubMed] [Google Scholar]
- 9.Short SJ, Stalder T, Marceau K, et al. Correspondence between hair cortisol concentrations and 30-day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology 2016; 71: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bates R, Salsberry P, Ford J. Measuring stress in young children using hair cortisol: the state of the science. Biol Res Nurs 2017; 19: 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer JS, Novak MA. Minireview: hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology 2012; 153: 4120–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staufenbiel SM, Penninx BWJH, de Rijke YB, et al. Determinants of hair cortisol and hair cortisone concentrations in adults. Psychoneuroendocrinology 2015; 60: 182–194. [DOI] [PubMed] [Google Scholar]
- 13.Wosu AC, Valdimarsdottir U, Shields AE, et al. Correlates of cortisol in human hair: implications for epidemiologic studies on health effects of chronic stress. Ann Epidemiol 2013; 23: 797–811.e792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson EL, Checkley S, Papadopoulos A, et al. Increased salivary cortisol reliably induced by a protein-rich midday meal. Psychosom Med 1999; 61: 214–224. [DOI] [PubMed] [Google Scholar]
- 15.Adam EK, Hawkley LC, Kudielka BM, et al. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci U S A 2006; 103: 17058–17063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White LO, Ising M, von Klitzing K, et al. Reduced hair cortisol after maltreatment mediates externalizing symptoms in middle childhood and adolescence. J Child Psychol Psychiatry 2017; 58: 998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vermeer HJ, Groeneveld MG. Children’s physiological responses to childcare. Curr Opin Psychol 2017; 15: 201–206. [DOI] [PubMed] [Google Scholar]
- 18.Straub J, Klaubert LM, Schmiedgen S, et al. Hair cortisol in relation to acute and post-traumatic stress symptoms in children and adolescents. Anxiety Stress Coping 2017; 30: 661–670. [DOI] [PubMed] [Google Scholar]
- 19.Kao K, Doan SN, St John AM, et al. Salivary cortisol reactivity in preschoolers is associated with hair cortisol and behavioral problems. Stress 2018; 21: 28–35. [DOI] [PubMed] [Google Scholar]
- 20.Scassellati C, Bonvicini C, Faraone SV, et al. Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses. J Am Acad Child Psychiatry 2012; 51: 1003–1019. [DOI] [PubMed] [Google Scholar]
- 21.Pauli-Pott U, Schloss S, Ruhl I, et al. Hair cortisol concentration in preschoolers with attention-deficit/hyperactivity symptoms-roles of gender and family adversity. Psychoneuroendocrinology 2017; 86: 25–33. [DOI] [PubMed] [Google Scholar]
- 22.Qadeer RA, Shanahan L, Ferro MA. Chronic disruptive pain in emerging adults with and without chronic health conditions and the moderating role of psychiatric disorders: evidence from a population-based cross-sectional survey in Canada. Scand J Pain 2017; 17: 30–36. [DOI] [PubMed] [Google Scholar]
- 23.Ferro MA, Van Lieshout RJ, Ohayon J, et al. Emotional and behavioral problems in adolescents and young adults with food allergy. Allergy 2016; 71: 532–540. [DOI] [PubMed] [Google Scholar]
- 24.Ferro MA. Major depressive disorder, suicidal behaviour, bipolar disorder, and generalised anxiety disorder among emerging adults with and without chronic health conditions. Epidemiol Psychiatr Sci 2016; 25: 462–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferro MA, Gorter JW, Boyle MH. Trajectories of depressive symptoms during the transition to young adulthood: the role of chronic illness. J Affect Disord 2015; 174: 594–601. [DOI] [PubMed] [Google Scholar]
- 26.Ferro MA, Boyle MH. The impact of chronic physical illness, maternal depressive symptoms, family functioning, and self-esteem on symptoms of anxiety and depression in children. J Abnorm Child Psychol 2015; 43: 177–187. [DOI] [PubMed] [Google Scholar]
- 27.Ferro MA, Speechley KN. Examining clinically relevant levels of depressive symptoms in mothers following a diagnosis of epilepsy in their children: a prospective analysis. Soc Psychiatry Psychiatr Epidemiol 2012; 47: 1419–1428. [DOI] [PubMed] [Google Scholar]
- 28.Pinquart M. Psychological health of children with chronic physical illness and their parents—results from meta-analyses. Prax Kinderpsychol Kinderpsychiatr 2017; 66: 656–671. [DOI] [PubMed] [Google Scholar]
- 29.Ferro MA, Boyle MH. Self-concept among children and adolescents with a chronic illness: a meta-analytic review. Health Psychol 2013; 32: 839–848. [DOI] [PubMed] [Google Scholar]
- 30.Goodwin RD, Bandiera FC, Steinberg D, et al. Asthma and mental health among youth: etiology, current knowledge and future directions. Expert Rev Respir Med 2012; 6: 397–406. [DOI] [PubMed] [Google Scholar]
- 31.Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones Behav 2006; 50: 632–639. [DOI] [PubMed] [Google Scholar]
- 32.Alink LR, van Ijzendoorn MH, Bakermans-Kranenburg MJ, et al. Cortisol and externalizing behavior in children and adolescents: mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Dev Psychobiol 2008; 50: 427–450. [DOI] [PubMed] [Google Scholar]
- 33.Pinquart M, Shen YH. Behavior problems in children and adolescents with chronic physical illness: a meta-analysis. J Pediatr Psychol 2011; 36: 1003–1016. [DOI] [PubMed] [Google Scholar]
- 34.Staufenbiel SM, Penninx BWJH, Spijker AT, et al. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology 2013; 38: 1220–1235. [DOI] [PubMed] [Google Scholar]
- 35.Pacella ML, Hruska B, Steudte-Schmiedgen S, et al. The utility of hair cortisol concentrations in the prediction of PTSD symptoms following traumatic physical injury. Soc Sci Med 2017; 175: 228–234. [DOI] [PubMed] [Google Scholar]
- 36.Schalinski I, Elbert T, Steudte-Schmiedgen S, et al. The cortisol paradox of trauma-related disorders: lower phasic responses but higher tonic levels of cortisol are associated with sexual abuse in childhood. PLoS One 2015; 10: e0136921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smy L, Shaw K, Smith A, et al. Hair cortisol as a novel biomarker of HPA suppression by inhaled corticosteroids in children. Pediatr Res 2015; 78: 44–47. [DOI] [PubMed] [Google Scholar]
- 38.Feller S, Vigl M, Bergmann MM, et al. Predictors of hair cortisol concentrations in older adults. Psychoneuroendocrinology 2014; 39: 132–140. [DOI] [PubMed] [Google Scholar]
- 39.LeBeau MA, Montgomery MA, Brewer JD. The role of variations in growth rate and sample collection on interpreting results of segmental analyses of hair. Forensic Sci Int 2011; 210: 110–116. [DOI] [PubMed] [Google Scholar]
- 40.Ouellet-Morin I, Laurin M, Robitaille MP, et al. Validation of an adapted procedure to collect hair for cortisol determination in adolescents. Psychoneuroendocrinology 2016; 70: 58–62. [DOI] [PubMed] [Google Scholar]
- 41.Sheehan DV, Sheehan KH, Shytle RD, et al. Reliability and validity of the mini international neuropsychiatric interview for children and adolescents (MINI-KID). J Clin Psychiatry 2010; 71: 313–326. [DOI] [PubMed] [Google Scholar]
- 42.Comeau J, Georgiades K, Duncan L, et al. Changes in the prevalence of child and youth mental disorders and perceived need for professional help between 1983 and 2014: evidence from the Ontario Child Health Study. Can J Psychiatry 2019; 64: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Georgiades K, Duncan L, Wang L, et al. Six-month prevalence of mental disorders and service contacts among children and youth in Ontario: evidence from the 2014 Ontario Child Health Study. Can J Psychiatry 2019; 64: 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyle MH, Duncan L, Georgiades K, et al. Classifying child and adolescent psychiatric disorder by problem checklists and standardized interviews. Int J Methods Psychiatr Res 2017; 26: e1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duncan L, Georgiades K, Wang L, et al. Psychometric evaluation of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). Psychol Assess 2018; 30: 916–928. [DOI] [PubMed] [Google Scholar]
- 46.Boyle MH, Duncan L, Georgiades K, et al. The 2014 Ontario Child Health Study Emotional Behavioural Scales (OCHS-EBS) Part II: psychometric adequacy for categorical measurement of selected DSM-5 disorders. Can J Psychiatry 2019; 64: 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duncan L, Georgiades K, Wang L, et al. The 2014 Ontario Child Health Study Emotional Behavioural Scales (OCHS-EBS) Part I: a checklist for dimensional measurement of selected DSM-5 disorders. Can J Psychiatry 2019; 64: 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fries E, Hesse J, Hellhammer J, et al. A new view on hypocortisolism. Psychoneuroendocrinology 2005; 30: 1010–1016. [DOI] [PubMed] [Google Scholar]
- 49.Koss KJ, Mliner SB, Donzella B, et al. Early adversity, hypocortisolism, and behavior problems at school entry: a study of internationally adopted children. Psychoneuroendocrinology 2016; 66: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brand S, Wilhelm FH, Kossowsky J, et al. Children suffering from separation anxiety disorder (SAD) show increased HPA axis activity compared to healthy controls. J Psychiatr Res 2011; 45: 452–459. [DOI] [PubMed] [Google Scholar]
- 51.Steudte S, Stalder T, Dettenborn L, et al. Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry Res 2011; 186: 310–314. [DOI] [PubMed] [Google Scholar]
- 52.Stein RE, Silver EJ. Operationalizing a conceptually based noncategorical definition: a first look at US children with chronic conditions. Arch Pediatr Adolesc Med 1999; 153: 68–74. [DOI] [PubMed] [Google Scholar]
- 53.Montero-Lopez E, Santos-Ruiz A, Gonzalez R, et al. Analyses of hair and salivary cortisol for evaluating hypothalamic-pituitary-adrenal axis activation in patients with autoimmune disease. Stress 2017; 20: 541–548. [DOI] [PubMed] [Google Scholar]
- 54.Wester VL, van Rossum EF. Clinical applications of cortisol measurements in hair. Eur J Endocrinol 2015; 173: M1–M10. [DOI] [PubMed] [Google Scholar]
- 55.van Aken M, Oosterman J, van Rijn T, et al. Hair cortisol and the relationship with chronic pain and quality of life in endometriosis patients. Psychoneuroendocrinology 2018; 89: 216–222. [DOI] [PubMed] [Google Scholar]
- 56.Savas M, Wester VL, Dykgraaf RHM, et al. Long-term cortisol exposure and associations with height and comorbidities in Turner syndrome. J Clin Endocrinol Metab 2019; 104: 3859–3867. [DOI] [PubMed] [Google Scholar]
- 57.Besser MJ, Ganor Y, Levite M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNF alpha or both. J Neuroimmunol 2005; 169: 161–171. [DOI] [PubMed] [Google Scholar]
- 58.Buske-Kirschbaunn A, Schmitt J, Plessow F, et al. Psychoendocrine and psychoneuroimmunological mechanisms in the comorbidity of atopic eczema and attention deficit/hyperactivity disorder. Psychoneuroendocrinology 2013; 38: 12–23. [DOI] [PubMed] [Google Scholar]
- 59.Ferro MA. Mediated moderation of the relation between maternal and adolescent depressive symptoms: role of adolescent physical health. Soc Psychiatry Psychiatr Epidemiol 2015; 50: 1743–1751. [DOI] [PubMed] [Google Scholar]
- 60.Stalder T, Steudte-Schmiedgen S, Alexander N, et al. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology 2017; 77: 261–274. [DOI] [PubMed] [Google Scholar]