Short abstract
Background
Many research papers claim that patients with specific psychiatric disorders (major depressive disorder, posttraumatic stress disorder, borderline personality disorder, alcohol use disorder, and others) have smaller hippocampi, but most of those reports compared patients to healthy controls. We hypothesized that if psychiatrically matched controls (psychiatric control, matched for demographics and psychiatric comorbidities) were used, much of the biomarker literature in psychiatric research would not replicate. We used hippocampus and amygdala volume only as examples, as these are very commonly replicated results in psychiatry biomarker research. We propose that psychiatry biomarker research could benefit from using psychiatric controls, as the use of healthy controls results in data that are not disorder-specific.
Method
Hippocampus/amygdala volumes were compared between major depressive disorder, sex-/age-/race-matched healthy control, and psychiatric control (N = 126/group). Similar comparisons were performed for posttraumatic stress disorder (N = 67), borderline personality disorder (N = 111), and alcohol use disorder (N = 136).
Results
Major depressive disorder patients had smaller left (p = 8.79 × 10−3) and right (p = 3.13 × 10−3) hippocampal volumes than healthy control. Posttraumatic stress disorder had smaller left (p = 0.018) and right (p = 8.64 × 10−4) hippocampi than healthy control. Borderline personality disorder had smaller right hippocampus (p = 7.90 × 10−3) and amygdala (p = 1.49 × 10−3) than healthy control. Alcohol use disorder had smaller right hippocampus (p = 0.034) and amygdala (p = .024) than healthy control. No differences were found between any of the four diagnostic groups and psychiatric control.
Conclusion
When psychiatric controls were used, there was no difference in hippocampal or amygdalar volume between any of the diagnoses studied and controls. This strategy (keeping all possible relevant variables matched between experimental groups) has been used to advance science for hundreds of years, and we propose should also be used in biomarker psychiatry research.
Keywords: brain morphometry, hippocampus volume, psychiatry research, major depressive disorder, alcohol use disorder, borderline personality disorder, posttraumatic stress disorder, amygdala volume, comorbidity
Introduction
Magnetic resonance imaging (MRI) brain volumetry may be an important approach to discovering biomarkers of mental disorders. Structural alterations in subcortical regions have been demonstrated in many disorders, including major depressive disorder (MDD), posttraumatic stress disorder (PTSD), borderline personality disorder (BPD), and alcohol use disorder (AUD). In MDD, PTSD, BPD, and AUD, the hippocampus and amygdala are commonly studied due to their central role in memory and emotional regulation.1–4 Identifying the disease-specific structural changes that accompany common psychiatric conditions provides useful insight for understanding pathology and developing targeted therapies.
Reduction of hippocampal volume in MDD is a widely replicated finding5 and recently, an Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) consortium study confirmed this on a very large sample.6 They evaluated volumetric subcortical data from 20 study sites, comparing 1902 MDD adult patients to 7658 healthy controls (HCs). Interestingly, this study could not replicate previous reports of amygdalar volume reductions when comparing MDD to HC. In fact, the hippocampus was the only subcortical structure they found to be significantly reduced in their MDD sample. Reduced hippocampal volume is also the most commonly reported volumetric finding in PTSD patients compared to HC7–19 including in another massive ENIGMA consortium effort.20
The hippocampus and other limbic regions are popularly studied in conjunction with several other psychiatric disorders as well. However, this literature has been somewhat inconsistent. For example in BPD, several studies found significant bilateral volume reductions in the hippocampus compared to HC21–28 as well as significant bilateral volume reductions in the amygdala.22,24–27 However, there have also been several dissenting studies: three found no significant volume reductions in the hippocampus29–31 and six found no significant reduction in amygdala volumes29,30,32–35 in BPD versus HC.
Additionally, several studies have shown subcortical volumetric decreases in AUD. The hippocampus is particularly susceptible to atrophy in both adolescents36 and adults.36,37 Similar trends emerge for amygdala gray matter volume.4,37–39 While the relationship between structural changes and AUD is well-documented, studies are often confounded by psychiatric comorbidity. Several psychiatric illnesses may act synergistically with AUD to exacerbate reduction in cortical and subcortical brain volumes.40 For example, AUD patients with comorbid anxiety have smaller subcortical volumes than those without.41 This phenomenon is corroborated by a meta-analysis that found less gray matter loss in cases of AUD without comorbidity.42 These findings, as well as the reports of hippocampal reductions across psychiatric disorders, suggest these reductions may not be diagnosis-specific. It is possible that volumetric reductions may be the result of some traits many of these patients have in common or that the etiologies are different, but the end result (e.g., reduced hippocampal size) are the same.
Psychiatry research has been severely criticized as the slowest advancing field in medical research. The research domain criteria (RDoC43) has been established to help with this problem by changing psychiatry research from categorical to dimensional and from diagnoses-based to symptom-based. We propose that using controls matched for all possible known or suspected variables that can affect the measure of interest is also important. Thus, we propose it would be advantageous to use psychiatrically matched controls when studying psychiatric disorders. In clinical investigations, control populations minimize potential confounding effects on a measured outcome. Usually, affected patients are carefully matched to HC across many dimensions (age, sex, etc.) to isolate the effects of a single variable. However, in psychiatric research, patients and controls are generally not matched for comorbid psychiatric illnesses, or more commonly comorbidities are excluded for within the study. Consequently, many studies are not studying ecologically valid samples, as most patients with a psychiatric illness are likely to have another. Also, when comparing to HC, one cannot account for the many heterogeneous features a person with mental illness often has such as specific symptoms, childhood trauma, eating problems, sleep problems, and so on. This problem of using HC is present in the recent ENIGMA study of MDD.6 While its large sample size lends validity to the finding of decreased hippocampal volume in MDD, controls were not matched for psychiatric comorbidity. Therefore, volume reduction of the hippocampus may be generalizable across many diagnoses and not correlate with MDD specifically. We propose that this is a common methodological error that should be avoided in psychiatry research, as it may produce misleading results that perpetuate an incomplete understanding of mental illness.
In this study, we investigated volumetric differences previously described in MDD, BPD, PTSD, and AUD. We studied populations of ecologically valid MDD (n = 126), BPD (n = 111), PTSD (n = 67), and AUD (n = 136) patients. To account for the high prevalence of comorbidities, we utilized psychiatric control (PC) groups that were matched for age, gender, and race as well as all comorbid psychiatric disorders. For comparison to previous studies, we also included HC groups matched for age, sex, and race. We compared the average hippocampal and amygdalar volumes of these groups using a standardized imaging analysis method (Freesurfer 6.0, http://surfer.nmr.mgh.harvard.edu) with the hypothesis: Hippocampal and amygdalar volume reductions may be observed between MDD, BPD, PTSD, and AUD patients and HC, but this difference will not exist when patients with each diagnosis are compared to controls matched for psychiatric comorbidities.
Methods
Healthy Controls
HC were recruited from the community (N = 141). They had no history or current diagnosis of mental illness as confirmed by the MINI International Neuropsychiatric Interview and had no contraindications for MRI.44 The average age of the HC was 33.0 ± 12.0 years with 57.4% being males (81/141) and 80.9% being Caucasian (114/141).
Psychiatric Patients and Clinical Measures
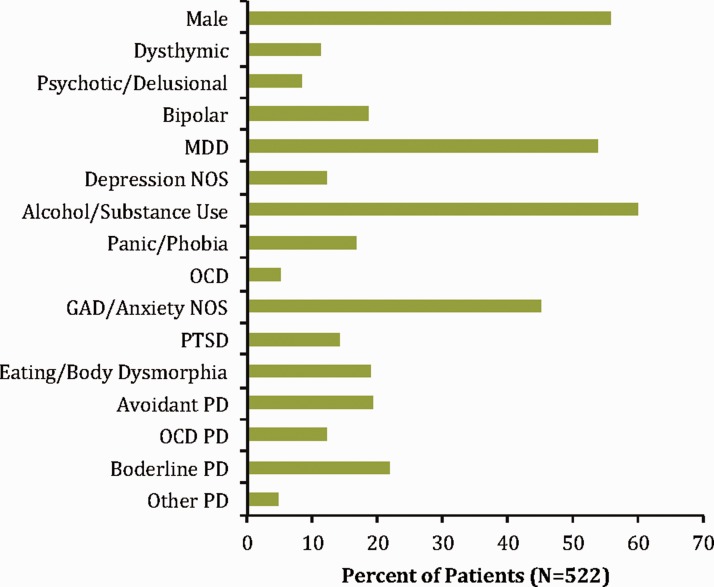
Psychiatric patients (PPs; N = 518) were recruited from the Menninger Clinic in Houston, TX, as a part of the McNair Initiative for Neuroscience Discovery—Menninger/Baylor (MIND-MB) research study.45–48 All PPs were eligible if they were mentally stable enough to participate, with no contraindications for MRI. All participants gave signed, informed consent (procedures were approved by the institutional review board). PP had a variety of psychiatric conditions including mood, anxiety, personality, and substance use disorders, with over 80% diagnosed with comorbid psychiatric disorders. They remained at the clinic for several weeks while receiving treatment (medication, psycho-educational groups, 24-h nursing care, individual and group psychotherapy, addictions management, and structured interpersonal and recreational activities). Note that treatment was not relevant to this study. The MIND-MB study collected demographic, clinical, and neuroimaging data. Demographic data relevant to our study (age, gender, and race) and psychiatric diagnoses from the Structured Clinical Interview for DSM-IV disorders axis I49 and II50 were collected. We also collected assessments on several psychiatric characteristics including (1) depression (Patient Health Questionnaire module for depression51 (PHQ-9)), (2) anxiety (generalized anxiety disorder scale52 (GAD-7)), (3) emotion regulation (difficulties in emotion regulation scale53), (4) history of trauma (Stressful Life Events Screening Questionnaire54), and (5) alcohol/substance use (World Health Organization Alcohol, Substance, and Smoking Involvement Test55 (ASSIST)). The PHQ-9 and GAD-7 specifically assess symptoms over the past two weeks and ASSIST assesses use over the past three months. All of these measures represent total scores (higher scores indicate a more severe rating) from respective scales and were obtained near the time of scanning. The average age of PP was 31.0 ± 12.3 years with 56.0% being males (290/518), and 87.3% being Caucasian (452/522; Figure 1).
Figure 1.
Characteristics of psychiatric patients. Broad categories of psychiatric disorders from the Structured Clinical Interview for the DSM-IV are shown to demonstrate the general prevalence of disorders within or psychiatric sample (note this is just for visualization as groups were matched on all possible psychiatric disorders, not just those shown). GAD: generalized anxiety disorder; MDD: major depressive disorder; NOS: not otherwise specified; OCD: obsessive compulsive disorder; PD: personality disorder; PTSD: posttraumatic stress disorders.
Matching Control Groups to Patient Groups of Interest
Four patient groups of interest were formed based only on the diagnosis of a specific disorder of interest: MDD (single or recurrent; N = 126), BPD (N = 111), AUD (alcohol abuse or dependence disorder; N = 136), and PTSD (N = 67). On average, patients in each of these groups had two other psychiatric disorders in addition to the diagnosis of interest. Note that some patients in these groups could, therefore, be in more than one group. For each patient group of interest, a HC group of the same size was matched based on demographic characteristics (age, sex, and race). A group of PCs of the same size was also matched for demographic characteristics and all other past and current diagnoses of any psychiatric disorder. Group matching for both HC and PC was performed using a Euclidean distance-matching algorithm. This algorithm matched patients to controls (HC and PC separately) in a one-to-one manner, calculating the Euclidean distance between each pair (in multidimensional space) such that the sum of all paired distances was minimized. The number of dimensions in the algorithm was equal to the number of features desired to match for. Features used in distance matching were demographic characteristics when matching to HC and demographic characteristics plus psychiatric diagnoses (past and current) when matching to PC. Prior to placing the features in the algorithm, they were normalized using z-scores (there was no missing data). All matching was coded in Python (version 3); code is available upon request to authors. Following matching, we analyzed between group differences on all features using t tests for age and chi-squared tests for all other variables to ensure the groups were not significantly different for any feature (p > 0.05, no multiple comparisons corrections). Any feature that was still significantly different between groups was controlled for during statistical analysis. The end result was 12 total groups (with some participants in more than one group) because each of 4 groups of interest had a matching HC group and a matching PC group. For each group of interest, HC, and PC, the Euclidean distance-matching algorithm produces samples that do not significantly differ in any feature (each feature being either a demographic characteristic or a diagnosis). There was one exception to this (depression no otherwise specified (Depress)) which was dealt with by using a covariate.
Neuroimaging Acquisition and Analysis
Participants were scanned in a 3T Siemens Trio MR scanner in the Center for Advanced Magnetic Resonance Imaging at the Baylor College of Medicine in Houston, TX, as close to admission to the clinic as possible. A ∼4.5-min structural MPRAGE sequence (echo time = 2.66 ms, repetition time = 1200 ms, flip angle = 12°, 256 × 256 matrix, 160 one mm axial slices at 1 × 1 × 1 mm voxels) was collected. FreeSurfer version 6.0 (http://surfer.nmr.mgh.harvard.edu) was used to perform all preprocessing and automated volumetric segmentation using the T1-weighted structural images. FreeSurfer segments regions of interest (ROIs) with probabilistic brain mapping based on the Aseg atlas.56 The Aseg atlas was used to obtain bilateral amygdalar and hippocampal ROIs as well as total intracranial volume (ICV). We controlled for ICV by dividing each patient’s individual ROI volume (mm3) by his or her total ICV.
Statistics
Student’s t tests were used to compare groups of interest to HC and to PC on volumetric measures of the right and left hippocampus and amygdala. Volumetric measures were also compared in the same fashion for HC and PC. The exception to this was when comparing MDD to PC because they were statistically different for a diagnosis of depression not otherwise specified (Depress), so an analysis of covariance (ANCOVA) was used with Depress as the covariate. Since we had a strong a priori hypothesis of lower hippocampal and amygdalar volumes between groups of interest and HC, no multiple comparisons were made to account for comparisons across these four ROIs.
Additional correlations (Pearson’s) were explored between hippocampal and amygdalar volumes in PPs (groups of interest and each PC group) for the other five clinical measures listed above (depression, anxiety, emotion regulation, history of trauma, and alcohol/substance use) to investigate the effects of these common psychiatric characteristics. Partial correlations were performed to control for age and sex. Volumes were divided by ICV prior to statistical analysis. We also investigated these correlations across our entire PP population. All statistical analyses were performed in SPSS (SPSS, Inc., Chicago, IL). We consider these correlations an exploratory analysis, as no multiple comparison corrections were performed.
Results
Group Matching
Each group of interest was matched to HC such that they were not significantly different for any demographic characteristic. The group of interest was matched to PCs such that they were not significantly different for any demographic characteristic or psychiatric diagnosis (past or current). The only group that had any characteristic that was significantly different between matched groups was MDD versus their matched PC group. These groups were significantly different for a diagnosis of depression not otherwise specified (Depress: χ2 = 65.31; p = 6.41 × 10−16; PC: 56/126; MDD: 2/126).
They were also significantly different for current mood alcohol disorder (χ2 = 4.07; p = 0.044; PC: 4/126; MDD: 0/126).
Group Comparisons for Hippocampal and Amygdalar Volumes
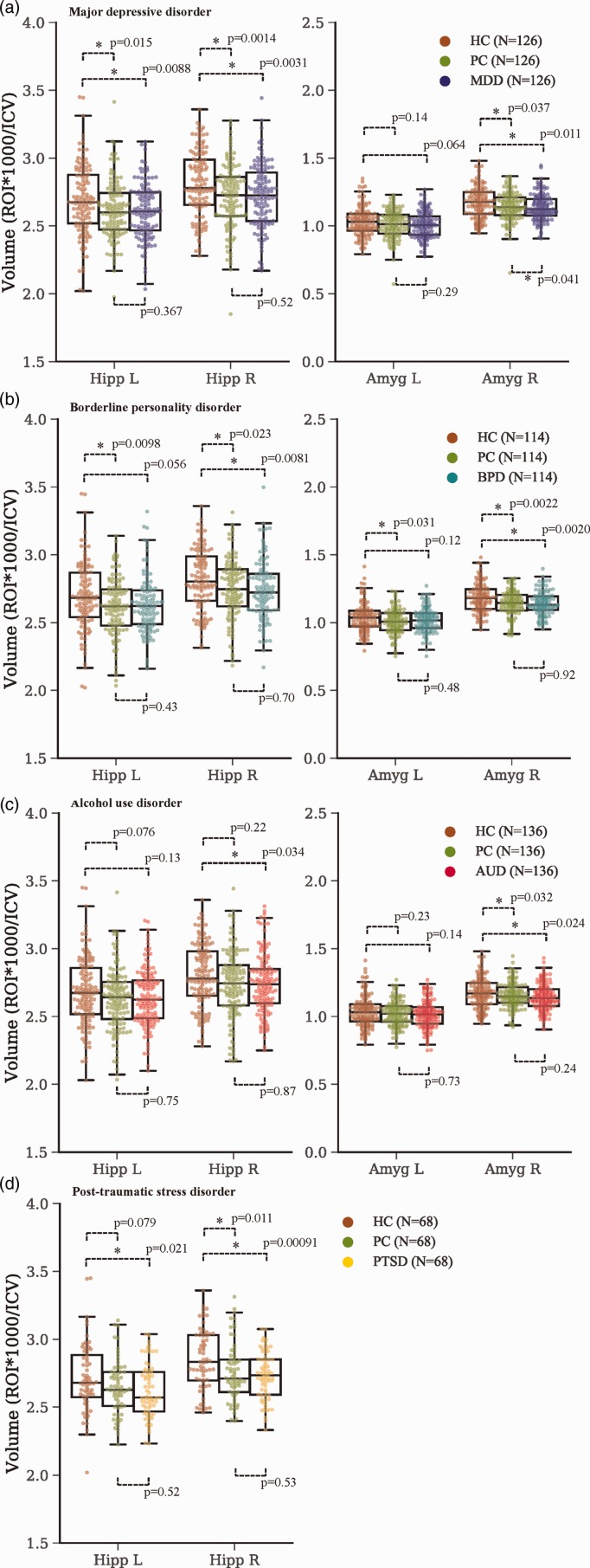
Figure 2 shows results from t tests between groups. eTable 1 gives the full statistical results in more detail. Note that MDD versus PC used Depress as a covariate in an ANCOVA. Current mood alcohol disorder was not included as a covariate because there were only 4 of 126 individuals with the diagnosis, and we did not believe there was enough power to affect the result. The effect of Depress is shown in eFigure 1. There was a significant effect of Depress for the left hippocampus. However, the individuals with Depress had higher volume averages for the left hippocampus, indicating that the lower volume found in MDD and PC groups was not due to the diagnosis of Depress. Note the higher volumes in Depress patients were not due to age. Importantly, no significant differences were found between PC and group of interest.
Figure 2.
Hippocampal and amygdalar volumes for all groups. (a) Major depressive disorder; (b) Borderline personality disorder; (c) Alcohol use disorder and (d) Post-traumatic stress disorder. Boxplots show median value as horizontal line within boxes, the top line of the boxes is the upper quartile (75% of the data lies below this line), the bottom line of the boxes is the lower quartile (25% of the data lies below this line), whiskers represent the rest of the range of the data that is outside the interquartile. Points that are outside the whiskers are considered outliers. All data points are shown jittered within the boxplots. *p < 0.05. Amyg: amygdala; AUD; alcohol use disorder; BPD: borderline personality disorder; HC: healthy control; Hipp: hippocampus; ICV: total intracranial volume; L: left; MDD: major depressive disorder; PC: psychiatric control; PTSD: posttraumatic stress disorder; R: right; ROI: region of interest.
Correlations Between Psychiatric Traits and Volumes
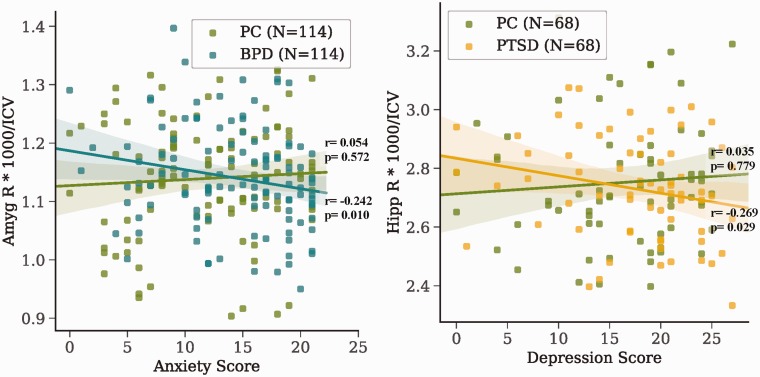
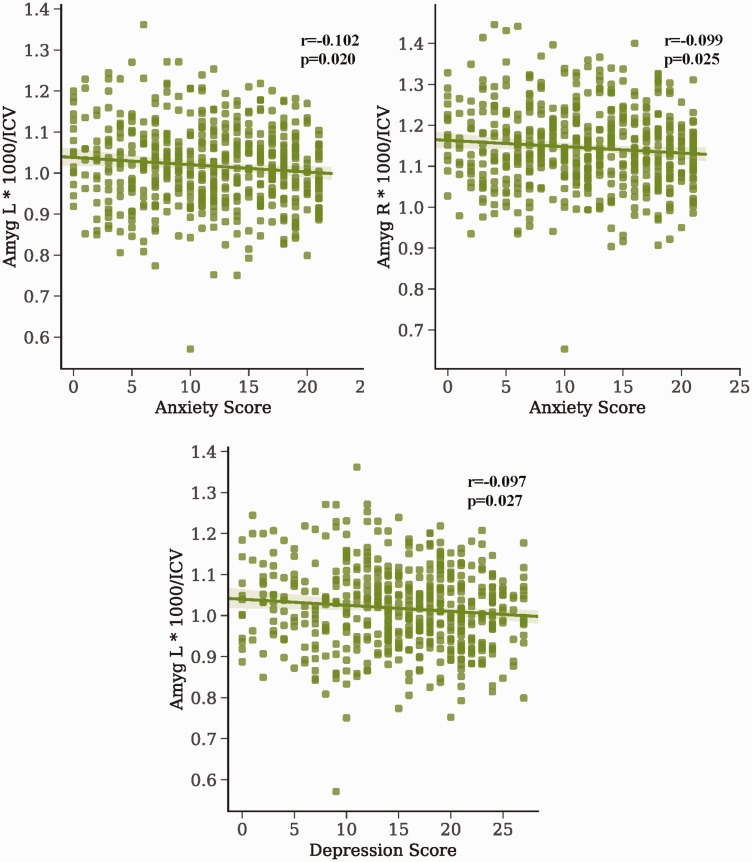
Because there were no meaningful differences in volume between PC and groups of interest and both groups seemed to be generally lower than HC, we investigated whether these differences were due to some common psychiatric traits we had measures for (full results shown in eTables 2 to 5). Exploratory correlations showed only two nominally significant correlations between groups of interest and volumes: right amygdala/anxiety in BPD and right hippocampus/depression in PTSD (Figure 3). One correlation was also shown for PC group (controlling for BPD) between the right hippocampus and depression (r = 0.192, p = 0.042). To investigate correlations between PC, we performed the same correlations but for all PPs (full results shown in eTable 6). Significant negative correlations were found for the amygdala and depression/anxiety scores (Figure 4). Note these findings are preliminary, as they would not survive multiple comparisons corrections.
Figure 3.
Correlations between psychiatric traits and volumes within each patient group. Pearson correlation r and p values are shown for each line of best fit (p < 0.05, no multiple corrections). Volumes were divided by ICV and scaled by 1000. Amyg: amygdala; BPD: borderline personality disorder; Hipp: hippocampus; ICV: total intracranial volume; PC: psychiatric control; PTSD: posttraumatic stress disorder; R: right.
Figure 4.
Correlations between psychiatric traits and volumes across psychiatric patients. Correlations between psychiatric traits and volumes were performed for all psychiatric patients (N = 518). Pearson correlation r and p values are shown for each line of best fit (p < 0.05, no multiple corrections). Volumes were divided by ICV and scaled by 1000. Amyg: amygdala; ICV: total intracranial volume; L: left; R: right.
Discussion
One reason we studied hippocampal size is that the available data are extremely robust, and it is not our intention to try to “refute” previous findings. However, we showed that when compared to PC matched for not only sex, age, and race but also psychiatric comorbidities, there is no difference in hippocampal volume between groups of interest (MDD, PTSD, BPD, and AUD) and controls. This should not be too surprising since the PC control groups are expected to have several patients with psychiatric diagnoses known to have smaller hippocampi than HC. The take-home message from our work is that we cannot say, for example, “MDD patients have smaller hippocampi than controls” without possible incurring in a flaw: Such statement is only true if the controls are not matched for psychiatric comorbidities.
Psychiatry has arguably been the slowest growing branch of medicine for a long time. For example, while most causes of death tend to decrease over time, death by suicide rates have stayed steady or even increased.57 There are many reasons why this may be the case, and the National Institute of Mental Health has established the RDoC43 as a general strategy to try to help solve these problems. The RDoC approach is based on symptoms instead of diagnoses and on dimensional instead of categorical approaches. We propose an additional approach that we believe has the potential to help accelerate psychiatry research using controls matched not only for demographics but for comorbidities (or alternatively, psychiatric symptoms).
Studies in psychiatry research use several approaches for comparing populations. One is to carefully recruit patients suffering from a specific illness (we can use MDD as an example), but that are not comorbid with any other psychiatric illness (e.g., recruiting MDD patients with no anxiety, personality, or substance use disorders). One problem arising from such approach is that the studied samples are unlikely to be ecologically valid, and psychiatrists are unlikely to see such “clean” patients in the clinic. A second problem from that approach is that perhaps there are features shared among different psychiatric diagnoses (or symptoms) such that MDD patients may have, for example, smaller hippocampi, but also do other PPs that are not depressed. Another approach is to compare patients with MDD to HC. In this case, the MDD sample may be representative, but any conclusions may be the result of variables other than MDD. This approach is still widely used and in our view causes enormous confusion in the field. A better approach that is also used is to compare patients with one condition (such as MDD) to patients with another condition (such as bipolar disorder), or to compare, for example, suicidal patients to nonsuicidal patients that are equally depressed. However, these can still be confounded by comorbidities.
There is a widely accepted scientific tenet that states: When comparing two groups for a variable of interest, keep all other possible relevant variables matched between the two groups. Psychiatry research has not commonly followed such a tenet, resulting in confusing conclusions that, we believe, are one of the many reasons why psychiatry research advances slower than other medical fields. Thus, we strive to compare groups in which one variable of interest is different between both groups while keeping all other possibly relevant variables not significantly different between groups (or at least statistically accounted for).
We have shown that when all psychiatric comorbidities are accounted for, neither MDD nor BPD, PTSD, or AUD show decreased hippocampal size. However, when the psychiatric illness groups were compared to HC, we did find smaller hippocampal volume as expected from the vast literature available. In our view, given our results, it is perhaps incorrect to state that MDD is associated with decreased hippocampal volume: No one in other scientific fields would accept such a conclusion if the observed difference disappears when relevant variables are matched between groups. No one would say “MDD patients are shorter than controls”: We know males are in average taller than females and significantly less likely to be MDD patients, so the statement may somehow be true but probably meaningless. We think the statement “MDD patients have smaller hippocampi than controls” is similarly flawed even if data are clearly very strong, as we have shown that to find that result one must use a control group not matched for variables known (e.g., PTSD) or hypothesized (e.g., being a PP) to be important.
Two limitations must be noted. First, we used hippocampal size in MDD (one of the most replicated results in psychiatry biomarker research) just as an obvious example of what we believe is a widespread problem in psychiatry biomarker research. However, the generalizability of our results is unknown, and this will need additional studies. In that sense, we propose that it would be safer to match groups as we did, but it is possible that other biomarkers are less sensitive to choice of controls. Second, although we believe that matching using diagnoses is far superior to comparison with HC, we believe that it would likely be even better to match groups using dimensional symptoms instead of diagnoses.
Thus, we postulate that future studies could use psychiatrically matched controls (for diagnoses as we did in this report, or probably better, for dimensional symptoms) to be more relevant.
Supplemental Material
Supplemental material, CCS906799 Supplementary Data for Hippocampal Volume in Psychiatric Diagnoses: Should Psychiatry Biomarker Research Account for Comorbidities? by Savannah N. Gosnell, Matthew J. Meyer, Cassandra Jennings, Danna Ramirez, Jake Schmidt, John Oldham and Ramiro Salas in Chronic Stress
Acknowledgments
The authors thank the Core for Advanced MRI at the Baylor College of Medicine, Dr Charles Neblett, and research participants.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the McNair Medical Institute, American Foundation for Suicide Prevention (SRG-2-125-14), and the Veteran Health Administration (VHA5I01CX000994 and VHA1I21RX002588). This research was partially supported by the Menninger Clinic Foundation, The Brown Foundation, Inc. of Houston, Texas, the George and Mary Josephine Hamman Foundation, and the Toomim Family Fund. This material is partly the result of work supported with resources and the use of facilities at the Michael E. DeBakey VA Medical Center, Houston, TX.
ORCID iD
Ramiro Salas https://orcid.org/0000-0002-1105-566X
Supplemental Material
Supplemental material for this article is available online.
References
- 1.De Bellis MD, Clark DB, Beers SR, et al. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000; 157(5): 737–744. [DOI] [PubMed] [Google Scholar]
- 2.Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter F, Schocke M, Marksteiner J. A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007; 78(6): 610–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segobin SH, Chételat G, Le Berre AP, et al. Relationship between brain volumetric changes and interim drinking at six months in alcohol-dependent patients. Alcohol Clin Exp Res. 2014; 38(3): 739–748. [DOI] [PubMed] [Google Scholar]
- 4.Wrase J, Makris N, Braus DF, et al. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008; 165(9): 1179–1184. [DOI] [PubMed] [Google Scholar]
- 5.Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT. Corpus callosum damage in heavy marijuana use: preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage. 2008; 41(3): 1067–1074. [DOI] [PubMed] [Google Scholar]
- 6.Schmaal L, Veltman DJ, van Erp TG, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 2016; 21(6): 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apfel BA, Ross J, Hlavin J, et al. Hippocampal volume differences in Gulf War veterans with current versus lifetime posttraumatic stress disorder symptoms. Biol Psychiatry. 2011; 69(6): 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonne O, Vythilingam M, Inagaki M, et al. Reduced posterior hippocampal volume in posttraumatic stress disorder. J Clin Psychiatry. 2008; 69(7): 1087–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bossini L, Tavanti M, Calossi S, et al. Magnetic resonance imaging volumes of the hippocampus in drug-naive patients with post-traumatic stress disorder without comorbidity conditions. J Psychiatr Res. 2008; 42(9): 752–762. [DOI] [PubMed] [Google Scholar]
- 10.Bremner JD, Randall P, Vermetten E, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biol Psychiatry. 1997; 41(1): 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emdad R, Bonekamp D, Sondergaard HP, et al. Morphometric and psychometric comparisons between non-substance-abusing patients with posttraumatic stress disorder and normal controls. Psychother Psychosom. 2006; 75(2): 122–132. [DOI] [PubMed] [Google Scholar]
- 12.Felmingham K, Williams LM, Whitford TJ, et al. Duration of posttraumatic stress disorder predicts hippocampal grey matter loss. Neuroreport. 2009; 20(16): 1402–1406. [DOI] [PubMed] [Google Scholar]
- 13.Gurvits TV, Shenton ME, Hokama H, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996; 40(11): 1091–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedges DW, Allen S, Tate DF, et al. Reduced hippocampal volume in alcohol and substance naive Vietnam combat veterans with posttraumatic stress disorder. Cogn Behav Neurol. 2003; 16(4): 219–224. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Chen S, Liu J, Zhang J, He Z, Lin X. Magnetic resonance imaging and magnetic resonance spectroscopy study of deficits in hippocampal structure in fire victims with recent-onset posttraumatic stress disorder. Can J Psychiatry. 2006; 51(7): 431–437. [DOI] [PubMed] [Google Scholar]
- 16.Lindauer RJ, Vlieger EJ, Jalink M, et al. Smaller hippocampal volume in Dutch police officers with posttraumatic stress disorder. Biol Psychiatry. 2004; 56(5): 356–363. [DOI] [PubMed] [Google Scholar]
- 17.Villarreal G, Hamilton DA, Petropoulos H, et al. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry. 2002; 52(2): 119–125. [DOI] [PubMed] [Google Scholar]
- 18.Vythilingam M, Luckenbaugh DA, Lam T, et al. Smaller head of the hippocampus in Gulf War-related posttraumatic stress disorder. Psychiatry Res. 2005; 139(2): 89–99. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Tan Q, Yin H, et al. Decreased gray matter volume in the left hippocampus and bilateral calcarine cortex in coal mine flood disaster survivors with recent onset PTSD. Psychiatry Res. 2011; 192(2): 84–90. [DOI] [PubMed] [Google Scholar]
- 20.Logue MW, van Rooij SJH, Dennis EL, et al. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry. 2018; 83(3): 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brambilla P, Soloff PH, Sala M, Nicoletti MA, Keshavan MS, Soares JC. Anatomical MRI study of borderline personality disorder patients. Psychiatry Res. 2004; 131(2): 125–133. [DOI] [PubMed] [Google Scholar]
- 22.Driessen M, Herrmann J, Stahl K, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry. 2000; 57(12): 1115–1122. [DOI] [PubMed] [Google Scholar]
- 23.Irle E, Lange C, Sachsse U. Reduced size and abnormal asymmetry of parietal cortex in women with borderline personality disorder. Biol Psychiatry. 2005; 57(2): 173–182. [DOI] [PubMed] [Google Scholar]
- 24.Schmahl CG, Vermetten E, Elzinga BM, Douglas Bremner J. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. 2003; 122(3): 193–198. [DOI] [PubMed] [Google Scholar]
- 25.Soloff PH, Pruitt P, Sharma M, Radwan J, White R, Diwadkar VA. Structural brain abnormalities and suicidal behavior in borderline personality disorder. J Psychiatr Res. 2012; 46(4): 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tebartz van Elst L, Hesslinger B, Thiel T, et al. Frontolimbic brain abnormalities in patients with borderline personality disorder: a volumetric magnetic resonance imaging study. Biol Psychiatry. 2003; 54(2): 163–171. [DOI] [PubMed] [Google Scholar]
- 27.Weniger G, Lange C, Sachsse U, Irle E. Reduced amygdala and hippocampus size in trauma-exposed women with borderline personality disorder and without posttraumatic stress disorder. J Psychiatry Neurosci. 2009; 34(5): 383–388. [PMC free article] [PubMed] [Google Scholar]
- 28.Zetzsche T, Preuss UW, Frodl T, et al. Hippocampal volume reduction and history of aggressive behaviour in patients with borderline personality disorder. Psychiatry Res. 2007; 154(2): 157–170. [DOI] [PubMed] [Google Scholar]
- 29.Aguilar-Ortiz S, Salgado-Pineda P, Marco-Pallarés J, et al. Abnormalities in gray matter volume in patients with borderline personality disorder and their relation to lifetime depression: a VBM study. PLoS One. 2018; 13(2): e0191946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chanen AM, Velakoulis D, Carison K, et al. Orbitofrontal, amygdala and hippocampal volumes in teenagers with first-presentation borderline personality disorder. Psychiatry Res. 2008; 163(2): 116–125. [DOI] [PubMed] [Google Scholar]
- 31.Kreisel SH, Labudda K, Kurlandchikov O, et al. Volume of hippocampal substructures in borderline personality disorder. Psychiatry Res. 2015; 231(3): 218–226. [DOI] [PubMed] [Google Scholar]
- 32.Denny BT, Fan J, Liu X, et al. Brain structural anomalies in borderline and avoidant personality disorder patients and their associations with disorder-specific symptoms. J Affect Disord. 2016; 200: 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhlmann A, Bertsch K, Schmidinger I, Thomann PA, Herpertz SC. Morphometric differences in central stress-regulating structures between women with and without borderline personality disorder. J Psychiatry Neurosci. 2013; 38(2): 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmahl C, Berne K, Krause, et al. Hippocampus and amygdala volumes in patients with borderline personality disorder with or without posttraumatic stress disorder. J Psychiatry Neurosci. 2009; 34(4): 289–295. [PMC free article] [PubMed] [Google Scholar]
- 35.Zetzsche T, Frodl T, Preuss UW, et al. Amygdala volume and depressive symptoms in patients with borderline personality disorder. Biol Psychiatry. 2006; 60(3): 302–310. [DOI] [PubMed] [Google Scholar]
- 36.Brooks SJ, Dalvie S, Cuzen NL, Cardenas V, Fein G, Stein DJ. Childhood adversity is linked to differential brain volumes in adolescents with alcohol use disorder: a voxel-based morphometry study. Metab Brain Dis. 2014; 29(2): 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Berre AP, Rauchs G, La Joie R, et al. Impaired decision-making and brain shrinkage in alcoholism. Eur Psychiatry. 2014; 29(3): 125–133. [DOI] [PubMed] [Google Scholar]
- 38.Makris N, Oscar-Berman M, Jaffin SK, et al. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008; 64(3): 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zois E, Kiefer F, Lemenager T, Vollstädt-Klein S, Mann K, Fauth-Bühler M. Frontal cortex gray matter volume alterations in pathological gambling occur independently from substance use disorder. Addict Biol. 2017; 22(3): 864–872. [DOI] [PubMed] [Google Scholar]
- 40.Mon A, Durazzo TC, Abe C, et al. Structural brain differences in alcohol-dependent individuals with and without comorbid substance dependence. Drug Alcohol Depend. 2014; 144: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fein G, Fein D. Subcortical volumes are reduced in short-term and long-term abstinent alcoholics but not those with a comorbid stimulant disorder. Neuroimage Clin. 2013; 3: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, Tian F, Zhang H, et al. Cortical and subcortical gray matter shrinkage in alcohol-use disorders: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2016; 66: 92–103. [DOI] [PubMed] [Google Scholar]
- 43.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010; 167(7): 748–751. [DOI] [PubMed] [Google Scholar]
- 44.Sheehan D, Janavs J, Baker R, Harnett-Sheehan K, Knapp E, Sheehan M. M.I.N.I.: MINI INTERNATIONAL NEUROPSYCHIATRIC INTERVIEW: English Version 5.0.0 DSM-IV. Tampa, FL: University of South Florida; 2006. [Google Scholar]
- 45.Ambrosi E, Arciniegas DB, Madan A, et al. Insula and amygdala resting-state functional connectivity differentiate bipolar from unipolar depression. Acta Psychiatr Scand. 2017; 136: 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curtis K, Viswanath H, Velasquez KM, et al. Increased habenular connectivity in opioid users is associated with an alpha5 subunit nicotinic receptor genetic variant. Am J Addict. 2017; 26(7): 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridgewell C, Bray A, Curtis K, et al. Enhanced olfactory cortex connectivity in a patient with PTSD with olfactory hallucinations. J Neuropsychiatry Clin Neurosci. 2015; 27(1-2): e170–e171. [DOI] [PubMed] [Google Scholar]
- 48.Viswanath H, Velasquez KM, Savjani R, et al. Interhemispheric insular and inferior frontal connectivity are associated with substance abuse in a psychiatric population. Neuropharmacology. 2015; 92: 63–68. [DOI] [PubMed] [Google Scholar]
- 49.First MB, Spitzer RL, Gibbon Met al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP). New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 50.First MB.Gibbon MB, Spitzer RL, et al. Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II). Washington, DC: American Psychiatric Press, Inc; 1997. [Google Scholar]
- 51.Spitzer RL. Patient Health Questionnaire: PHQ. New York: New York State Psychiatric Institute; 1999. [Google Scholar]
- 52.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006; 166(10): 1092–1097. [DOI] [PubMed] [Google Scholar]
- 53.Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: development, factor structure, and initial validation of the difficulties in emotion regulation scale. J Psychopathol Behav Assess. 2004; 26(1): 41–54. [Google Scholar]
- 54.Goodman L, Corcoran C, Turner K, Yuan N, Green BL. Assessing traumatic event exposure: general issues and preliminary findings for the Stressful Life Events Screening Questionnaire. J Trauma Stress. 1998; 11(3): 521–542. [DOI] [PubMed] [Google Scholar]
- 55.Henry-Edwards S., Humeniuk R, Ali R, Poznyak V, Monteiro M. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): Guidelines for Use in Primary Care (Draft Version 1.1 for Field Testing). Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 56.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002; 33(3): 341–355. [DOI] [PubMed] [Google Scholar]
- 57.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013; 382(9904): 1575–1586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, CCS906799 Supplementary Data for Hippocampal Volume in Psychiatric Diagnoses: Should Psychiatry Biomarker Research Account for Comorbidities? by Savannah N. Gosnell, Matthew J. Meyer, Cassandra Jennings, Danna Ramirez, Jake Schmidt, John Oldham and Ramiro Salas in Chronic Stress