Abstract
Depression is a heterogeneous disease with many different subtypes. Patients with the anxious depression—a common subtype of major depression—are at an increased risk for treatment-resistance to standard antidepressants, with resultant increases in morbidity. However, the underlying pathophysiology of anxious depression remains unknown. Without such knowledge, the development of targeted treatments towards this specific depression subtype will likely remain elusive. One method by which research into the neurobiology of anxious depression may prove fruitful is with the research domain criteria (RDoC). RDoC provides a framework for investigation into the underlying pathophysiology of mental illness. By studying disorders in terms of RDoC constructs—such as the sustained threat construct of the negative valence system—new insights may be gained into neurobiological mechanisms of disease. These mechanisms may be useful for the development of novel antidepressants that are based on specific brain targets. Specifically, we review the impact that sustained threat—or chronic stress—has on the eventual development of depression (especially anxious depression) through pathological changes to molecules, cells, neurocircuitry, physiology, and behavior.
Keywords: chronic stress, anxious depression, research domain criteria, hypothalamic–pituitary–adrenal axis, anxiety, depression, negative valence
Introduction
Psychiatric providers recognize anxious depression as a subtype of major depressive disorder. Yet, the scientific study of anxious depression is complex—in part, due to difficulty with its definition. Though the definition of anxious depression varies throughout the literature, it is often characterized as either (a) the combination of major depressive disorder plus an anxiety disorder (as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM)),1,2 or (b) major depressive disorder plus symptoms of anxiety, as measured by symptom rating scales.3 These definitions certainly provide a common language for clinicians to use for the care of patients; for example, a patient with major depressive disorder and anxiety symptoms will be less likely to respond to currently approved antidepressant medications compared to depressed patients without anxiety.4 However, the purely descriptive definitions tend to “box” patients into categories that can be misleading.
Alternatively, the Hamilton Depression Rating Scale Anxiety Somatization Factor Score is useful for dividing patients into anxious and nonanxious depression, in that those with a score of 7 or greater meet criteria for “anxious depression.” But what does this mean for patients with a score of 6? Clearly, they endorse anxiety symptoms on this scale, but do not officially meet criteria for “anxious depression.” Furthermore, our current definitions do not take into account the neurobiological signatures that help characterize patients with both anxiety and depression. For these reasons, it is time to rethink our definitions of anxious depression.
One potential solution is to consider a paradigm shift for the study of mental illness. The research domain criteria (RDoC) set forth by the National Institute of Mental Health5 may provide such an avenue. Anxious depression, in particular, may stand to benefit from utilization of the proposed RDoC, as they provide a novel way of characterizing psychiatric disorders for research purposes. Instead of defining mental illness by lists of subjective symptoms, RDoC aims to incorporate pathophysiological mechanisms to explain mental illness across a wide range of traditional DSM disorders. For example, instead of defining anxious depression symptomatically as depressed mood with symptoms of anxiety (e.g., worry, somatic symptoms, and psychomotor agitation), RDoC requires defining anxious depression based on aberrant neural circuitry, physiologic biomarkers, and genetic signatures, in combination with patient reports and behaviors. Through this, RDoC may offer a more precise way of studying anxious depression, by describing illness based on neurobiological mechanisms rather than purely clinical characterizations. This would enable the elucidation of the pathophysiology of anxious depression, which could offer improved diagnostics and prognostics for our patients, as well as open a world of discovery towards novel, targeted therapeutics. Given that currently approved medications are not effectively targeting patients with anxious depression to the same extent as those with nonanxious depression,4 it is clear that a change in our research methods is necessary.
An RDoC-based hypothesis would consider that the development of anxious depression may arise, at least in part, from changes in the body that occur during chronic stress. Chronic stress is represented in the RDoC matrix as the “sustained threat” construct, within the negative valence domain. As defined by RDoC, chronic stress is an “aversive emotional state caused by prolonged (i.e., weeks to months) exposure to internal and/or external condition(s), state(s), or stimuli that are adaptive to escape or avoid. The exposure may be actual or anticipated; the changes in affect, cognition, physiology, and behavior caused by sustained threat persist in the absence of the threat, and can be differentiated from those changes evoked by acute threat.”6 By definition, stress is a disruption in equilibrium.
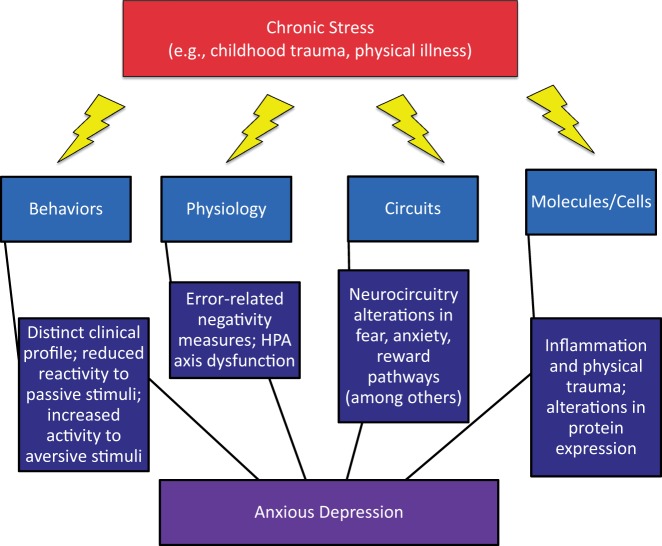
Though this disturbance in equilibrium may be acutely important for survival—for example, the “fight or flight” response to danger—chronic stress may contribute to psychopathology. The effects of the molecular mechanisms involved in the human response to stress can impact human behavior and biology in a number of contexts—from molecular mechanisms to neurocircuitry—with resultant depression, anxiety, and anxious depression, in some people. Within this context, we will provide an overview on chronic stress as it applies to anxious depression. We start with behavior—the most clinical of all RDoC units of analyses. Then, we review physiology, circuits, molecules, and cells, with application of their strengths in characterizing the role of chronic stress in anxious depression. Finally, we will end with a discussion of limitations, future directions, and implications for studying the role of chronic stress in anxious depression (Figure 1).
Figure 1.
Chronic stress and anxious depression. Chronic stress may lead to anxious depression through a number of mechanisms. Here, we present levels from the Research Domain Criteria (RDoC) framework as they may relate to the development of anxious depression.
Behavior
Studying the behaviors of patients with anxious depression (especially compared to those with nonanxious depression and healthy volunteers) through RDoC modules can provide key insights into the pathophysiology of disease, thereby directing human-inspired translational and basic research investigations. Patients with anxious depression typically have higher measures on depression rating scales for symptoms of anxiety; for example, on the Hamilton Depression Rating Scale Anxiety Somatization Factor Score, patients with anxious depression have higher scores on items for psychic and somatic anxiety, somatic and gastrointestinal symptoms, hypochondriasis, and insight.7 Clinical studies suggest that, compared to depressed patients without anxiety, patients with anxious depression have significantly longer depressive episodes, earlier age of depression onset, more medical comorbidities, are more likely to endorse and attempt suicide, and are less likely to respond and remit to currently approved antidepressant treatments (as previously3,4 reviewed). Interestingly, anhedonia appears to play more of a role in depression than anxiety,8 implying that reward reinforcements are less relevant in the anxious component of anxious depression.
Behavioral study from a psychophysiological standpoint may also be important for understanding underlying neurobiological processes. For example, in order to describe the reduced reactivity observed in patients with depression, Rottenberg et al.9 proposed the emotion context insensitivity hypothesis. In this theory, patients with depression (regardless of anxiety status) reported reduced reactivity to passive tasks, such as viewing pictures with positive and negative emotional stimuli. In contrast to the emotion context insensitivity hypothesis, another study demonstrated that when exposed to a more aversive stimulus—i.e., threat of shock—patients with depression had elevated anxious anticipation and startle responses.10 These data suggest that when an aversive stimulus is used (as opposed to passive tasks like picture viewing), depression is associated with enhanced defensive reactivity—not emotional blunting—during harm anticipation. Furthermore, depression and anxiety may be mutually reinforcing, as depressed mood can enhance anxiety responses during threat of shock paradigms.10 Indeed, aversive stimuli (compared to passive tasks) may be more representative of the types of “real world” stress that patients are exposed to—thus implicating a common mechanism for both depression and anxiety in susceptible individuals.
Physiology
Error-related Negativity
Everyone makes mistakes; for this reason, detection of and response to errors is essential for the successful navigation of the world. The magnitude of the neural networks that responds to errors—known as error-related negativity (ERN)—can be measured and are generated by the anterior cingulate cortex (ACC).11 Patients with certain disorders, such obsessive-compulsive disorder—a disease characterized, in part, by the excessive and sustained threat of making errors—consistently demonstrate enhanced ERN magnitudes (as recently reviewed12). Furthermore, ERN measurements may differ in patients with anxiety alone, depression alone, and anxious depression—representing a potentially useful physiologic biomarker to distinguish diseases. For example, Weinberg et al.13 demonstrated differences in ERN magnitudes between diagnostic groups. They measured ERN in medication-free female patients with generalized anxiety disorder (GAD; n = 26), anxious depression (n = 23), and healthy volunteers (n = 36). Compared to healthy volunteers, enhanced ERN magnitudes were found in the GAD group, but not in the anxious depression group. Similarly, a more recent, larger study14 measured ERNs in several groups of patients, including those with GAD (n = 57), depression (n = 62), and healthy volunteers (n = 56); a subset of patients had anxious depression (n = 27). Only patients with GAD were characterized by higher ERN measurements compared to healthy controls. Because there were no significant differences between patients with depression (regardless of anxiety status) and healthy volunteers, the authors concluded that the addition of depression onto anxiety might eliminate the higher ERN observed in patients with anxiety only. Though more research is needed to parse through the diagnostic heterogeneity of mood and anxiety disorders, data from the ERN literature underscores the importance of a transdiagnostic phenotype approach to illness that is not captured when categorical criteria are used.
Dysregulated Hypothalamic–Pituitary–Adrenal Axis
The hypothalamic–pituitary–adrenal (HPA) axis is central in mediating the human stress response. At times of stress, corticotrophin-releasing hormone (CRH) is secreted by the hypothalamus and acts on the anterior pituitary gland. In turn, this stimulates the release of adrenocorticotropic hormone (ACTH), which increases circulating cortisol from the adrenals. In response to acute stress, increased cortisol is critical for the mobilization of energy resources, such as increased gluconeogenesis. During high-stress situations, these resources may be important for immediate escape and/or problem solving—skills critical to survival.
Clinically, chronic stress is measured by sustained exposure to life events. Most notably, early life stressors may alter future responses to stress that are mediated through the HPA axis. For example, women with trauma early in life (a risk factor for the development of depression) exhibit the greatest abnormalities in ongoing HPA-mediated stress response.15 During chronic stress, adaptation normally occurs to the triggered cortisol release.16 Elegant negative feedback mechanisms are in place to regulate HPA axis activity through cortisol binding to central mineralocorticoid receptors (MRs; primarily localized to the limbic system/hippocampus) with high affinity and to widely distributed glucocorticoid receptor (GRs) with lower affinity.17 After the acute stressor, MRs are activated at the onset of the stressor and are involved in appraisal. GRs, on the other hand, terminate the acute stress response and aid in recovery, facilitating the return to homeostatic balance until called upon for the next stressor.16
In certain genetically predisposed individuals, there is evidence to support that exposure to chronic stress leads to HPA axis dysfunction with resultant depression.18 Indeed, studies of patients with depression reveal hyperactivity of the HPA axis.19 Specifically, hypercortisolemia is observed in upwards of 55% of patients with major depression.20 Furthermore, data from a small study (n = 12)21 suggest that patients with major depression exhibit an inappropriately increased functional activity of the MR system due to an imbalance in the MR/GR ratio that favors MR activation; this, in turn, results in a pathological activation of the stress response. The authors go onto suggest that this imbalance may indirectly have a negative effect on central serotonin levels, thereby leading to depressed mood.21 It is important to underscore that these data are from a small sample and are in need of replication. However, the negative impact of stress on serotonin may be a clue to why medications that modulate central serotonin signaling—such as selective serotonin reuptake inhibitors (SSRIs)—are effective antidepressant treatments for many patients.
Such “stress-induced depression” may result in anxious depression, due to failed homeostatic regulation of the HPA axis secondary to chronic stressors. Indeed, patients with anxious depression demonstrate abnormal HPA axis functioning, even compared to those with depression without significant anxiety. For example, one small study showed that, compared to patients with nonanxious depression (n = 11) and healthy volunteers (n = 27), patients with anxious depression (n = 14) had significantly attenuated ACTH and cortisol responses following exogenous CRH challenge.22 Furthermore, evidence from a dexamethasone challenge—which works similarly to cortisol through negative feedback to suppress the release of ACTH—found that 50% of women with anxious depression (n = 17) exhibited impaired cortisol suppression compared to 37% with anxiety only (n = 9) and 18% with major depression only (n = 12).23 After being challenged with the notoriously stressful Trier Social Stress Test (which is known to activate the HPA axis), patients with anxious depression (n = 18) had significantly elevated levels of ACTH and cortisol compared to those with an anxiety disorder only (n = 15), major depression only (n = 15), and health volunteers (n = 48).24 Taken together, these data suggest that dysregulation of the HPA axis—in part, due to chronic stress—may be an important part of the pathogenesis of anxious depression. However, we must emphasize that these data are from small samples and only represent a minor portion of the literature; for more information, there are several review articles written on the HPA axis and its relationship to depressive disorders.25–27
Circuits
Perhaps one of the most important discoveries in psychiatric research is the use of neuroimaging to further understand brain structure and function. Structural, functional, and diffusion tensor magnetic resonance imaging (MRI), positron emission tomography, and magnetoencephalography techniques, among others, have allowed researchers to objectively identify areas of the brain that are involved in normal and pathological states—including depression and anxiety. Assuming that structure affects function, neuroscientists can begin to map both the normal circuitry of the human brain, as well as the changes that occur in psychiatric illness, in order to pinpoint aberrant circuits that may be critical to the propagation of disease. Ultimately, these discoveries may improve the diagnosis and prognosis of mental illness, as well as help to guide providers in choosing individualized treatments for their patients based on objective findings.
Activation of certain areas of the brain is necessary for a normal response to fear (i.e., amygdala),28 anxiety (i.e., bed nucleus of the stria terminalis; BNST; “extended amygdala”),29 and reward/motivation (i.e., nucleus accumbens).30 However, chronic stress likely plays a role in the formation of pathologic neurocircuitry, especially during times of brain development, such as early childhood. Critically, resultant changes in neuronal networks from sustained threats—perhaps through enhancement of connections involved in the production of depression/anxiety and reduction of circuitry associated with reward and pleasure—may lead to the experience of depression, anxiety, or both, as previously reviewed.8,31,32
Insights into the neurocircuitry of anxious depression (as it related to stress) can be studied though the examination of the extent to which the brain changes during sustained threats in healthy volunteers. Herrmann et al.33 used fMRI to study healthy volunteers (n = 38) presented with different visual cues to indicate an unpredictable aversive (i.e., human screams) or neutral (i.e., neutral sounds) sound event. Activations were observed in the amygdala, ACC, and ventrolateral prefrontal cortex (PFC) during the onset of the aversive stimuli. However, during the sustained response to the threatening stimuli, activations were observed in the BNST, insula, dorsolateral PFC, ACC, cuneus, posterior cingulate cortex, and periaqueductal grey area. These results support the theory that the amygdala is primarily involved in the acute response to threats, whereas the BNST (among other areas) is primarily responsible for response to sustained threats. Indeed, another study of healthy volunteer responses to acute and sustained threats found that activations in the brain evolve from the amygdala to the BNST in acute versus sustained threats, respectively.34 Because the BNST provides input to the HPA axis through direct projections to the hypothalamus,35 underscoring the connection between systems responsible for stress and anxiety.
Through the examination of neuroimaging studies of depressed patients, regardless of exposure to sustained threat, several patterns have emerged (as recently reviewed36). Specifically, disruptions of the connections between the medial PFC (mPFC) and orbital PFC (oPFC)—as well as other related circuits, including connections to limbic structures and regions involved in reward (e.g., the ventral tegmental area)—have been implicated in the resultant emotional dysregulation and anhedonia observed in depression.36,37 Furthermore, activity in the default mode network (networks in the brain that are active at “rest” but relatively inactive during active task performance) appears to be increased in patients with depression, possibly representative of the increased introspection observed in depression. Instead of shifting attention towards rewards and pleasurable events, the depressed brain may stay focused on internal negative experiences. A study in depressed elderly patients with high anxiety (age ≥ 65) demonstrated a dissociative pattern in the default mode network (increased connectivity in the posterior regions (occipital and parietal association areas) and decreased connectivity in the anterior regions (rostral ACC, medial prefrontal, and orbitofrontal cortex)) compared to depressed elderly with low anxiety.38 Indeed, the decreased ability to control default mode activity in depression may hinder attention shifts from introspective to external tasks, thereby resulting in the impairments observed in depression.36,37 In other words, patients with anxious depression may exhibit hypervigilant brain networks, even at rest, compared to those with low anxiety.
How does sustained threat, in particular, change neuronal connections in a way that may result in anxious depression? Neurocircuitry changes in vulnerable individuals may help to identify those at risk for the development of depression and anxiety after exposure to sustained threats. Towards this end, one experiment prospectively studied healthy volunteers (n = 24) before and after active military deployment, in order to assess the effects of chronic war stress towards the development of anxiety.39 Soldiers completed an interactive game that consisted of risky and rewarding behaviors pre- and post-deployment. Data from fMRI indicated that in those with increased symptoms of post-traumatic stress disorder after deployment, greater response to risk was observed in the amygdala pre- and post-deployment; decreased response to reward (as measured by activation of the nucleus accumbens) was observed post-deployment. This study provides evidence that stress vulnerability may be marked by an imbalance in neural response to risk and reward after an exposure to sustained threats.39
A commonality that may help to explain the neurocircuitry changes observed in both anxiety and depression states may be linked through the effects that chronic stress has on the structural integrity of neurons. Indeed, structural changes within the neurons themselves also occur during times of sustained threats. As reviewed by Duman et al.,36 reduced volumes in the PFC and hippocampus are consistently linked to depression in humans; rodents exposed to chronic stress exhibit similar pathologies. It is possible that remodeling of the circuitry through stress-induced neuronal and synaptic changes results in dysfunctional circuitry that manifests as psychiatric disease.
Molecules/Cells
Neuronal communication is affected by interactions within the cell (e.g., protein expression), as well as outside of the cell (e.g., the hormonal milieu and synaptic plasticity changes). In the setting of disease, communication between neurons (and glia34) is altered in some fundamental way, speeding up, slowing down, or altogether stopping the flow of information carried by electrical and chemical activity; this results in behavioral abnormalities. Physical trauma can cause this through mechanically disrupting the information flow, as in traumatic brain injury, where axons are physically distorted. Similarly, emotional trauma can alter the functionality of neurons by changing the signaling milieu, forcing neurons to adapt in kind. Depending on baseline protein expression, which directly affects neuronal function, this change can physically alter the neuronal circuitry and activity, and thereby change brain function and behavior. It is telling that many of the gene and molecular candidates of interest in the propagation of mental illness—including depression and anxiety—are important in cell architecture and remodeling, such as GSK3 and brain-derived neurotrophic factor40–43 or in cell capacity to make changes in protein expression, such as FKBP5 and DICER1.44–46
These synaptic architecture and protein expression changes underlie the flexibility of the brain’s response to stress. As discussed above, normal physiologic and psychogenic stressors lead to acute activation of the autonomic nervous system and the HPA axis. The sympathetic nervous system very quickly (on the order of seconds) leads to release of epinephrine and norepinephrine from the adrenal medulla. The HPA axis is a slower (on the order of minutes) route that requires expression of hypothalamic releasing hormones, such as CRH, to promote emission of pituitary ACTH to the periphery, which acts on the adrenal cortex to secrete glucocorticoids (corticosterone in rodents and cortisol in humans) into circulation. The monoamines of sympathetic activation stimulate the HPA axis, as does serotonin, when released from a particular subpopulation of brainstem neurons.47 This facilitates and maintains the physiologic and behavioral response to stress, but can complicate that response when stress is a regular, continuous, or unpredictable occurrence. Though it is difficult to correlate the changes in circulating cortisol with clinical findings,20 in rodent models of chronic stress, there is a decrease in synaptic connections between the mPFC and hippocampus with the paraventricular nucleus of the hypothalamus, as well as a reduction in GRs,48,49 in response to prolonged exposure to corticosterone.50 This leads to reactive changes in the excitability of the HPA axis and these upstream regulatory regions (mPFC, hippocampus, and extended amygdala). In contrast, chronic stress also leads to an increase in synaptic density originating within the amygdala,51 promoting further excitability of the HPA axis. These changes may be due to epigenetic modifications or transcriptional alterations that predispose an affected person to greater reactivity of the HPA axis and could relate to the anxious or hyperstartle phenotype of anxious depression.52–54
There is increasing evidence that the changes in neuronal circuitry seen in chronic stress, depression, and anxiety involve maladaptive communication between neurons and the immune system.55 In healthy tissues, resident macrophages monitor homeostasis and support tissue function.56 Microglia—the brain’s macrophages—are particularly suited to support neurons and play an active role in synaptic plasticity, pruning synapses during development, and secreting neurotrophic factors.57 Perceived environmental or internal stress leads to changes in tissue function and energy metabolism that are detected by macrophages and microglia both in the periphery and the brain; these immune cells then initiate an inflammatory response, including production of cytokines such as IL-1b, TNFa, and IL-6, recruitment of additional monocytes from the circulation, and concurrent induction of negative regulators including glucocorticoids that help return the system to baseline.55 In parallel to the activation of the HPA discussed above, limited, acute stress leads to an acute inflammatory response: cytokines mobilize tissue resources to fight potential infection, repair tissues, regulate energy metabolism, and induce sickness behavior (social isolation, increased sleep, and decreased mobility).58 However, chronic stress precipitates chronic inflammation—leading to a “primed” state, where further inflammation is easily triggered and normal tissue function is compromised. In the brain, for example, microglia switch from supplying neurotrophic factors to proinflammatory cytokines, and become more phagocytic, inducing neuronal atrophy, remodeling, and synaptic plasticity in vulnerable areas such as the hippocampus.55 Thus, chronic inflammation and chronic stress form a vicious cycle, in which stress leads to inflammation, which leads to more tissue stress and further inflammation—ultimately leading to structural damage that may result in depression.
Through chronic stress and inflammation, the way in which neurochemicals—including monoamines—are altered in depression may have impacts on the treatment of depressed patients. Though patients with anxious depression do not respond as well to treatment with traditional monoaminergic antidepressants compared to patients with nonanxious depression, they remain first line treatment. Monoamines can both facilitate and mitigate the HPA axis signal, and for serotonin, this is likely through different anatomic populations and receptor systems.47,59 SSRIs and serotonin/norepinephrine reuptake inhibitors do not have a direct effect on synaptic remodeling in animal studies, but do reverse synaptic architecture changes in the setting of stress induction models of depression like behavior.60–62 They also induce neurogenesis, though it remains unclear how critical this is for positive behavior outcomes.63 Given their utility, clinicians have recently turned to non-traditional psychoactive medications, such as the glutamatergic N-methyl-D-aspartate-receptor antagonist ketamine. Unlike the response to SSRIs, patients with anxious depression respond better to ketamine compared to those with nonanxious depression.64 N-methyl-D-aspartate receptors play a significant role in synaptic strength and plasticity, and ketamine has been shown in rodent models to increase the density of synaptic dendritic spines.65 Intriguingly, it is this activity that is likely responsible for the antidepressant quality; administration of ketamine in a chronic unpredictable stress model in rats blocks both the synaptic and behavior changes seen in the depression-like model state.66 This holds promise for the translation of these data into clinical correlates towards the treatment of patients with anxious depression.
Putting It All Together: A Case for RDoC Towards the Treatment of Anxious Depression
As discussed, anxious depression is more resistant to current antidepressant treatments compared to nonanxious depression.4 Even when patients with anxious depression do respond to antidepressants, they experience more side effects and relapse sooner, highlighting the heterogeneity of depressive illness. Furthermore, all approved medications for depression likely exert their primary mechanism through monoaminergic modulation. Though these mechanisms may be useful for treating certain types of depression, this highlights the need for antidepressants with novel mechanisms of action to help those patients resistant to the current standards—such as those with anxious depression. Perhaps one way towards these discoveries is through RDoC-inspired investigations.
Part of the problem is that antidepressant discovery is largely the result of serendipity. Thus far, no antidepressants were created based on targets towards underlying neurobiological changes that occur in depression. Furthermore, all currently approved antidepressant medications are thought to enact their mechanism of action through monoaminergic (e.g., serotonin, norepinephrine, and dopamine) modulation. This may be difficult, given the current diagnostic criteria for depression (as outlined by subjective symptoms in the current standard psychiatric diagnostic tool, the DSM-51). In this convention, two patients can meet criteria for depression with two completely different sets of symptoms. Therefore, it is problematic to consider that both patients would respond well to the same treatments. Here is how RDoC may help: through the utilization of neurobiological findings to cut across diagnostic heterogeneity, we will base psychiatric illness classification on dimensions—ranging from behaviors to genes—rather than via a subjective grouping of symptoms. This classification system has the potential to uncover neurobiological differences among patients with depression. For example, a recent report found that childhood abuse (occurring at ≤7 years old) predicted poorer outcomes after 8 weeks of antidepressants in patients with heterogeneously diagnosed depression.67 By understanding the underlying pathophysiology of sustained threats (such as childhood trauma) that leads to anxious depression with resultant treatment-resistance, future drug research can be aimed towards treatment of these most vulnerable groups (through targeting specific aberrant pathologies—whether it be neurocircuitry, dysregulated physiology, genetics, etc.). Ultimately, doctors may someday be able to choose one treatment over another based on a patient’s RDoC “signature” (instead of the current standard, in which treatments are chosen largely based on tolerability and safety rather than efficacy).
Towards this end, several RDoC-inspired projects on the neurobiology of anxious depression using psychophysiological and neuroimaging experiments are underway (ClinicalTrials.gov unique IDs: NCT02669043; NCT02544607). Ketamine (a glutamatergic modulator that has rapid (within hours) and robust (across many symptoms) antidepressant effects) can be utilized to specifically probe differences in the brains of depressed patients with and without anxiety pre- and post-ketamine treatment, because patients with anxious depression respond better to ketamine than depressed patients without an anxiety component.64 Through the use of ketamine as a probe, neurobiological differences that distinguish anxious and non-anxious groups pre- and post-ketamine may be useful for disentangling the heterogeneity of depression. Therefore, we propose ketamine as a tool to study brain differences in patients with and without anxious depression by examining changes pre- and post-treatment. New research tools—such as RDoC—may help to cut through barriers to advancements in treatment that would otherwise not be possible with the standard classification systems.
Discussion
Though we know anxious depression to be a clinically important phenomenon, the consensus on a common definition (by current classification standards) remains elusive.3 This inconsistency in defining anxious depression is due, in part, to a lack of objective data. The result is a wide heterogeneity of studies from which conclusions can be difficult to draw. In contrast, the RDoC organization provides a new theory to guide the study of complex mental illness; instead of relying solely on subjective symptoms, researchers can use a framework to cut through the heterogeneity observed in subjective diagnoses, thereby uncovering common neurobiological underpinnings of disease. In this paper, we reviewed the effects of the sustained threat construct on the pathophysiology of anxious depression—from behaviors to molecules. Through the utilization of this framework, the elucidation of the neurobiology of anxious depression is beginning.
What is anxious depression in terms of the sustained threat construct of RDoC? Perhaps we should consider anxious depression as a disease of enhanced defensive reactivity, based on behavioral findings. Furthermore, dysregulation in HPA responses are also important factors to the development of anxiety and depression, and may be useful to distinguish anxious depression from other depression subtypes. Literature on changes in neurocircuitry, cells, and molecules that are the result of chronic stress also provide insights into why anxious depression occurs. For example, dysregulation in the reward circuitry related to reward and anhedonia (that is the result of the effects of chronic stress on neural structures) may result in depression. Eventually, patients may be able to have a neurobiological profile of their disease based on individual results from RDoC constructs; this, in turn, may help with the personalization of the diagnosis, prognosis, and treatment of mental illness.
Though RDoC provides a novel framework for shaping the definition of anxious depression, there are a few limitations. For example, RDoC does not explicitly take into consideration feeding behaviors associated with depression. Depressed patients can experience either hypo or hyperphagia in relation to their other symptoms. However, this problem can be circumvented by taking into consideration the role of feeding behaviors in other domains of RDoC, namely, reward pathways. Even though RDoC overlooks certain clinical observations associated with disease, the pathology can still be dissected through use of the constructs.
As psychiatric neuroscience advances from the subjective to the objective, it is unlikely that one pathway (e.g., neurocircuitry changes, alternations in genetic expressions, variations in brain-derived neurotrophic factor expression, etc.) will fully explain the pathophysiological processes underlying depression. However, through the consideration of all aspects of the RDoC framework, we may begin to parse through the heterogeneity of depression towards a richer understanding of the processes that underlie psychiatric disease. Our field is already starting to cut through diagnoses in order to better understand pathology. For example, Smoller et al.68 discovered common genetic variations at the same four chromosomal sites in autism, attention deficit hyperactivity disorder, bipolar disorder, major depression, and schizophrenia—psychiatric disorders that are traditionally thought to be distinct from one another. More recently, clinical, neuroimaging, and blood data from the recently completed Establishing Moderators and Biosignatures of Antidepressant Response in Clinical Care trial are expected to provide moderators and mediators for antidepressant responses in patients with depression, regardless of subtype.69 These investigations exemplify what is necessary to parse through the current heterogeneity of mental illness, towards a more complete understanding of both normal and abnormal neurobiological processes.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Ross is funded by an appointed KL2 award from Harvard Catalyst, NIH award KL2 TR001100, and a BIDMC Department of Psychiatry Junior Faculty fund. Dr. Foster is funded by a K12 award from the NICHD (2K12HD051959-11) and a postdoctoral enrichment award from the Burroughs Wellcome Fund. Dr. Ionescu is funded by a K23 award from the NIMH (K23-MH107776), a NARSAD Young Investigator Award through the Brain and Behavior Research Foundation, and through the Massachusetts General Hospital Executive Committee on Research (ECOR).
References
- 1.American Psychiatric Association APADSMTF. Diagnostic and statistical manual of mental disorders: DSM-5. 2013.
- 2.American Psychiatric Association and American Psychiatric Association. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV, 4th ed Washington, DC: American Psychiatric Association, 1994. p.xxv, p.886. [Google Scholar]
- 3.Ionescu DF, Niciu MJ, Henter ID, et al. Defining anxious depression: a review of the literature. CNS Spectr 2013; 18: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ionescu DF, Niciu MJ, Richards EM, et al. Pharmacologic treatment of dimensional anxious depression: a review. Prim Care Companion CNS Disord 2014; 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry 2014; 13: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Construct: Sustained Threat. RDoC Classification. NIMH2016.
- 7.Cleary P, Guy W. Factor analysis of Hamilton depression scale. Drugs Exp Clin Res 1977; 1: 115–120. [Google Scholar]
- 8.Dillon DG, Rosso IM, Pechtel P, et al. Peril and pleasure: an rdoc-inspired examination of threat responses and reward processing in anxiety and depression. Depress Anxiety 2014; 31: 233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. J Abnorm Psychol 2005; 114: 627–639. [DOI] [PubMed] [Google Scholar]
- 10.Robinson OJ, Overstreet C, Letkiewicz A, et al. Depressed mood enhances anxiety to unpredictable threat. Psychol Med 2012; 42: 1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter CS, Braver TS, Barch DM, et al. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 1998; 280: 747–749. [DOI] [PubMed] [Google Scholar]
- 12.Weinberg A, Dieterich R, Riesel A. Error-related brain activity in the age of RDoC: a review of the literature. Int J Psychophysiol 2015; 98: 276–299. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg A, Klein DN, Hajcak G. Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. J Abnorm Psychol 2012; 121: 885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinberg A, Kotov R, Proudfit GH. Neural indicators of error processing in generalized anxiety disorder, obsessive-compulsive disorder, and major depressive disorder. J Abnorm Psychol 2015; 124: 172–185. [DOI] [PubMed] [Google Scholar]
- 15.Nemeroff CC. Early-Life Adversity, CRF Dysregulation, and Vulnerability to Mood and Anxiety Disorders. Psychopharmacol Bull 2004; 38: 14–20. [PubMed] [Google Scholar]
- 16.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci 2005; 6: 463–475. [DOI] [PubMed] [Google Scholar]
- 17.Patel PD, Lopez JF, Lyons DM, et al. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J Psychiatr Res 2000; 34: 383–392. [DOI] [PubMed] [Google Scholar]
- 18.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 2000; 23: 477–501. [DOI] [PubMed] [Google Scholar]
- 19.Owens MJ, Nemeroff CB. The role of corticotropin-releasing factor in the pathophysiology of affective and anxiety disorders: laboratory and clinical studies. Ciba Found Symp 1993; 172: 296–308. discussion-16. [DOI] [PubMed] [Google Scholar]
- 20.Murphy BE. Steroids and depression. J Steroid Biochem Mol Biol 1991; 38: 537–559. [DOI] [PubMed] [Google Scholar]
- 21.Young EA, Lopez JF, Murphy-Weinberg V, et al. Mineralocorticoid receptor function in major depression. Archives of General Psychiatry 2003; 60: 24–28. [DOI] [PubMed] [Google Scholar]
- 22.Meller WH, Kathol RG, Samuelson SD, et al. CRH challenge test in anxious depression. Biol Psychiatry 1995; 37: 376–382. [DOI] [PubMed] [Google Scholar]
- 23.Rao ML, Vartzopoulos D, Fels K. Thyroid function in anxious and depressed patients. Pharmacopsychiatry 1989; 22: 66–70. [DOI] [PubMed] [Google Scholar]
- 24.Cameron OG. Anxious-depressive comorbidity: effects on HPA axis and CNS noradrenergic functions. Essent Psychopharmacol 2006; 7: 24–34. [PubMed] [Google Scholar]
- 25.Jacobson L. Hypothalamic-pituitary-adrenocortical axis: neuropsychiatric aspects. Compr Physiol 2014; 4: 715–738. [DOI] [PubMed] [Google Scholar]
- 26.Juruena MF, Werne Baes CV, Menezes IC, et al. Early life stress in depressive patients: role of glucocorticoid and mineralocorticoid receptors and of hypothalamic-pituitary-adrenal axis activity. Curr Pharm Des 2015; 21: 1369–1378. [DOI] [PubMed] [Google Scholar]
- 27.Gold PW. The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry 2015; 20: 32–47. [DOI] [PubMed] [Google Scholar]
- 28.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci 2000; 23: 155–184. [DOI] [PubMed] [Google Scholar]
- 29.Avery SN, Clauss JA, Blackford JU. The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology 2016; 41: 126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knutson B, Gibbs SE. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl) 2007; 191: 813–822. [DOI] [PubMed] [Google Scholar]
- 31.Lener MS, Iosifescu DV. In pursuit of neuroimaging biomarkers to guide treatment selection in major depressive disorder: a review of the literature. Ann N Y Acad Sci 2015; 1344: 50–65. [DOI] [PubMed] [Google Scholar]
- 32.Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry 2016; 3: 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herrmann MJ, Boehme S, Becker MP, et al. Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Hum Brain Mapp 2016; 37: 1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMenamin BW, Langeslag SJ, Sirbu M, et al. Network organization unfolds over time during periods of anxious anticipation. J Neurosci 2014; 34: 11261–11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SY, Adhikari A, Lee SY, et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 2013; 496: 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science 2012; 338: 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips ML, Chase HW, Sheline YI, et al. Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: neuroimaging approaches. Am J Psychiatry 2015; 172: 124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andreescu C, Wu M, Butters MA, et al. The default mode network in late-life anxious depression. Am J Geriatr Psychiatry 2011; 19: 980–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Admon R, Lubin G, Rosenblatt JD, et al. Imbalanced neural responsivity to risk and reward indicates stress vulnerability in humans. Cereb Cortex 2013; 23: 28–35. [DOI] [PubMed] [Google Scholar]
- 40.Cymerman IA, Gozdz A, Urbanska M, et al. Structural plasticity of dendritic spines requires GSK3alpha and GSK3beta. PloS One 2015; 10: e0134018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S, Sun N, Xu Y, et al. Possible association of the GSK3beta gene with the anxiety symptoms of major depressive disorder and P300 waveform. Genet Test Mol Biomarkers 2012; 16: 1382–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry 2006; 59: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 43.Magarinos AM, Li CJ, Gal Toth J, et al. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus 2011; 21: 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holz NE, Buchmann AF, Boecker R, et al. Role of FKBP5 in emotion processing: results on amygdala activity, connectivity and volume. Brain Struct Funct 2015; 220: 1355–1368. [DOI] [PubMed] [Google Scholar]
- 45.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 2009; 34(Suppl 1): S186–S195. [DOI] [PubMed] [Google Scholar]
- 46.Wingo AP, Almli LM, Stevens JS, et al. DICER1 and microRNA regulation in post-traumatic stress disorder with comorbid depression. Nat Commun 2015; 6: 10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol 2002; 14: 911–923. [DOI] [PubMed] [Google Scholar]
- 48.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 1995; 69: 89–98. [DOI] [PubMed] [Google Scholar]
- 49.Radley JJ, Rocher AB, Rodriguez A, et al. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol 2008; 507: 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson RM, Glanz RM, Johnson SB, et al. Prolonged corticosterone exposure induces dendritic spine remodeling and attrition in the rat medial prefrontal cortex. J Comp Neurol 2016; 524: 3729–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vyas A, Mitra R, Shankaranarayana Rao BS, et al. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 2002; 22: 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Francis D, Diorio J, Liu D, et al. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 1999; 286: 1155–1158. [DOI] [PubMed] [Google Scholar]
- 53.Murgatroyd C, Patchev AV, Wu Y, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci 2009; 12: 1559–1566. [DOI] [PubMed] [Google Scholar]
- 54.Yehuda R, Flory JD, Bierer LM, et al. Lower methylation of glucocorticoid receptor gene promoter 1F in peripheral blood of veterans with posttraumatic stress disorder. Biol Psychiatry 2015; 77: 356–364. [DOI] [PubMed] [Google Scholar]
- 55.Wohleb ES, Franklin T, Iwata M, et al. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci 2016; 17: 497–511. [DOI] [PubMed] [Google Scholar]
- 56.Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol 2016; 17: 9–17. [DOI] [PubMed] [Google Scholar]
- 57.Hong S, Stevens B. Microglia: phagocytosing to clear, sculpt, and eliminate. Dev Cell 2016; 38: 126–128. [DOI] [PubMed] [Google Scholar]
- 58.Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, Damjanoska KJ, Carrasco GA, et al. Evidence that 5-HT2A receptors in the hypothalamic paraventricular nucleus mediate neuroendocrine responses to (-)DOI. J Neurosci 2002; 22: 9635–9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ampuero E, Rubio FJ, Falcon R, et al. Chronic fluoxetine treatment induces structural plasticity and selective changes in glutamate receptor subunits in the rat cerebral cortex. Neuroscience 2010; 169: 98–108. [DOI] [PubMed] [Google Scholar]
- 61.Bessa JM, Ferreira D, Melo I, et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 2009; 14: 764–773, 739. [DOI] [PubMed] [Google Scholar]
- 62.Hajszan T, Dow A, Warner-Schmidt JL, et al. Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol Psychiatry 2009; 65: 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.David DJ, Samuels BA, Rainer Q, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 2009; 62: 479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ionescu DF, Luckenbaugh DA, Niciu MJ, et al. Effect of baseline anxious depression on initial and sustained antidepressant response to ketamine. J Clin Psychiatry 2014; 75: e932–e938. [DOI] [PubMed] [Google Scholar]
- 65.Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li N, Liu RJ, Dwyer JM, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 2011; 69: 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams LM, Debattista C, Duchemin AM, et al. Childhood trauma predicts antidepressant response in adults with major depression: data from the randomized international study to predict optimized treatment for depression. Transl Psychiatry 2016; 6: e799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cross-Disorder Group of the Psychiatric Genomics C. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 2013; 381: 1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trivedi MH, McGrath PJ, Fava M, et al. Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): rationale and design. J Psychiatr Res 2016; 78: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]