Abstract
Allopregnanolone and pregnanolone—neurosteroids synthesized from progesterone in the brain, adrenal gland, ovary and testis—have been implicated in a range of neuropsychiatric conditions including seizure disorders, post-traumatic stress disorder, major depression, post-partum depression, pre-menstrual dysphoric disorder, chronic pain, Parkinson’s disease, Alzheimer’s disease, neurotrauma, and stroke. Allopregnanolone and pregnanolone equipotently facilitate the effects of gamma-amino-butyric acid (GABA) at GABAA receptors, and when sulfated, antagonize N-methyl-D-aspartate receptors. They play myriad roles in neurophysiological homeostasis and adaptation to stress while exerting anxiolytic, antidepressant, anti-nociceptive, anticonvulsant, anti-inflammatory, sleep promoting, memory stabilizing, neuroprotective, pro-myelinating, and neurogenic effects. Given that these neurosteroids are synthesized de novo on demand, this review details the molecular steps involved in the biochemical conversion of cholesterol to allopregnanolone and pregnanolone within steroidogenic cells. Although much is known about the early steps in neurosteroidogenesis, less is known about transcriptional, translational, and post-translational processes in allopregnanolone- and pregnanolone-specific synthesis. Further research to elucidate these mechanisms as well as to optimize the timing and dose of interventions aimed at altering the synthesis or levels of these neurosteroids is much needed. This should include the development of novel therapeutics for the many neuropsychiatric conditions to which dysregulation of these neurosteroids contributes.
Keywords: allopregnanolone, steroidogenesis, biosynthesis, transcriptional regulation, post-traumatic stress disorder
Introduction
Allopregnanolone (3α-hydroxy-5α-pregnan-20-one) and pregnanolone (3α-hydroxy-5β-pregnan-20-one) are neurosteroid metabolites of progesterone that equipotently and positively modulate the action of gamma-amino-butyric acid (GABA) at GABAA synaptic and extrasynaptic receptors. At nanomolar doses, these stereoisomers increase the effects of GABA on GABAA receptor-mediated chloride ion influx ∼7 to 10 times, thus markedly enhancing the inhibitory impact of GABA on neuronal firing.1–6 In contrast, when sulfated, allopregnanolone and pregnanolone potently antagonize N-methyl-D-aspartate (NMDA) receptors. In addition, allopregnanolone and pregnanolone play a role in regulating GABAA receptor number and subunit composition, further shaping the dynamic balance between inhibitory and excitatory neuronal signaling.1
The effects of allopregnanolone and pregnanolone on GABA neurotransmission are thought to be responsible for their potent antidepressant, anxiolytic, anti-conflict, anticonvulsant, anti-nociceptive, anesthetic, sleep-promoting, and memory modulating effects. In addition to these pharmacological effects, these neurosteroids can suppress inflammation, reduce apoptosis, and promote neurogenesis and myelination.7–11 At a higher translational level, such neuroprotective effects manifest as reduced infarct volume and cerebral edema after cerebral ischemia, delays in neuronal death after traumatic brain injury or in the context of Niemann-Pick type C disease,2,12 and lastly as interruption and reversal of neurodegenerative processes associated with Parkinson’s and Alzheimer’s disease.13,14
However, preclinical and clinical research suggests that high as well as low levels of these neurosteroids may be associated with phenotypically similar neuropsychiatric disorders.15–17 Even apparently normal fluctuations in these neurosteroids that induce changes in GABAA receptor subunit composition can be associated with dysphoria and depressive symptoms during the post-partum period or luteal phase of the menstrual cycle,18–21 while subnormal increases of their synthesis in response to stress occur across the menstrual cycle in women with post-traumatic stress disorder (PTSD),15 which shares many clinical symptoms with premenstrual dysphoric disorder (PMDD). PTSD is also associated with substantially increased risk for PMDD.9
Given the manifold neurophysiological roles of allopregnanolone and pregnanolone, as well as their dysregulation in a range of disorders for which treatments warrant improvement, a review of the complex molecular pathways by which these GABAergic neurosteroids are synthesized and released may highlight opportunities for the development of novel precision medicine-based interventions for these disorders. This review and series of graphics thus were developed from the extant literature characterizing molecular regulatory factors and key enzymes that interact in steroidogenic cells from a variety of tissues, including the adrenal gland, brain, ovary, and testis, to produce allopregnanolone and pregnanolone.
Overview of Steroid Biosynthesis Leading to Allopregnanolone and Pregnanolone
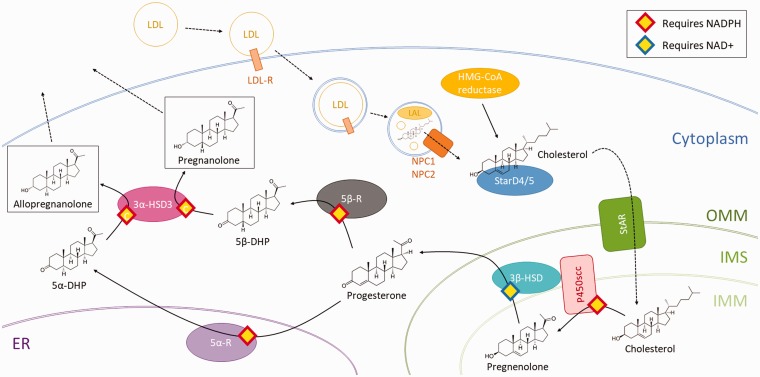
Due to their lipophilic nature, steroids such as allopregnanolone and pregnanolone cannot be stored in steroidogenic cells for later release; thus, nearly all circulating steroid hormones are synthesized de novo and diffuse out of the cell. This accounts for the delayed peaking of blood and brain steroid levels in response to tissue-specific stimulation of neurosteroidogenesis by adrenocorticotropic hormone (ACTH), luteinizing hormone (LH), or NMDA receptor activation22,23—as cells must activate and increase their steroidogenic capacity on demand. Therefore, understanding the mechanisms by which allopregnanolone is synthesized is necessary to understand when and how allopregnanolone and pregnanolone function. The schematic in Figure 1 summarizes the events of allopregnanolone synthesis, which are otherwise described below. Synthesis of pregnanolone follows similar steps with some differences described under the section “5β-reductase.”
Figure 1.
Schematic depicting the steps in the steroidogenesis of allopregnanolone and pregnanolone starting with uptake or synthesis of cholesterol and ending with allopregnanolone and pregnanolone diffusion out of the cell. Dashed lines represent movement, whereas solid lines represent a catalytic reaction. OMM: outer mitochondrial membrane; IMM: inner mitochondrial membrane; IMS: intermembrane space; 5α-DHP: 5α-dihydroprogesterone; 5β-DHP: 5α-dihydroprogesterone.
Cholesterol Synthesis and Import
All steroids are synthesized from a common cholesterol precursor. Human steroidogenic cells source their cholesterol from two processes: uptake of cholesterol from low density lipoproteins (LDL) via receptor-mediated endocytosis or de novo cholesterol synthesis.24
It is thought that the majority of the cholesterol used in steroid biosynthesis originates from LDLs.24–26 Steroidogenic cells activated by trophic hormones upregulate the presence of LDL receptors on their cellular surface in order to endocytose a greater volume of LDLs, which are rich in cholesteryl ester.27,28 These LDLs are broken down in lysosomes to release the cholesteryl esters, which are further processed by lysosomal acid lipase (LAL) into cholesterol for use in steroidogenesis.24,27 Free cholesterol is then transferred from lysosomes via the soluble glycoprotein Niemann Pick type C 2 (NPC2) to the transmembrane protein Niemann Pick type C 1 (NPC1), which inserts the cholesterol into the lysosomal membrane for eventual delivery throughout the cytoplasm.28–30
Alternatively, cells may synthesize cholesterol de novo from three acetyl CoA molecules.31 This reaction, catalyzed by 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase, represents the committed, rate-limiting step for cholesterol biosynthesis; activated steroidogenic cells upregulate this enzyme.24,28 Although LDL endocytosis is conventionally thought to be the main source of cholesterol for steroidogenesis, patients with low LDL levels—such as those with congenital abetalipoproteinemia—have normal basal cortisol production and normal, or mildly impaired cortisol secretion in response to ACTH.24,32 This suggests that de novo cholesterol synthesis may be adequate to provide the precursors for steroidogenesis under normal conditions; it is not clear whether such individuals maintain adequate cortisol production during extreme or chronic stress, conditions under which compensation for impaired steroid production at the single cell level may be compensated by hyperplasia of the steroidogenic tissue.
Intracellular Transport of Cholesterol to Mitochondria
Steroidogenic acute regulatory protein (StAR)-related lipid transfer (START) domain proteins are related to the steroidogenic acute regulatory protein (StAR, described below) and contain a domain of 210 conserved amino acids capable of binding lipids.24,33 Among the 15 proteins in the human START family, StarD4 and StarD5 are cytosolic proteins that bind cholesterol with high specificity and affinity in steroidogenic cells.24,28 StarD4 has been co-localized with Acyl-CoA cholesterol acyltransferase (ACAT), which is responsible for catalyzing the reversible conversion of free cholesterol into cholesterol ester, suggesting a role for these closely related START domain proteins in transporting cholesterol throughout the cytosol, including to the outer mitochondrial membrane (OMM).24,28,34 In addition, activation of steroidogenic cells induces changes in mitochondrial morphology that enable a greater number of close contacts between mitochondria and the endoplasmic reticulum.35–37 The resulting domains of the endoplasmic reticulum, called mitochondria-associated membrane (MAM), are necessary for cholesterol transfer to steroidogenic mitochondria.35,38
Transport of Cholesterol Across the Mitochondrial Membrane
The exact mechanism by which cholesterol is transported from the OMM to the inner mitochondrial membrane (IMM) is not fully understood, but some of the major steps and factors involved have been elucidated.
One of the most important proteins involved is StAR, which performs its cholesterol-transporting role only when anchored to the OMM.35,39 StAR is first inserted into the OMM and then internalized into the mitochondrial matrix where it is degraded by mitochondrial proteases.35,40,41 The half-life of a StAR molecule taking part in the one-way transport from the OMM to the mitochondrial matrix is around 4 to 5 h.35,40 Structural analyses have identified a pocket in StAR capable of binding a single molecule of cholesterol.35,42 However, each molecule of StAR supposedly transports hundreds of molecules of cholesterol, rather than the single cholesterol molecule suggested by its structural stoichiometry, suggesting yet unknown mechanisms by which StAR mediates transport of cholesterol.24,43 StAR has been shown to interact with voltage-dependent anion channels 1 and 2 (VDAC1 and VDAC2) which are thought to regulate the processing of StAR as it passes from the OMM to the mitochondrial matrix.44,45 Another protein in the σ-receptor family named σ-1 is thought to tether StAR and VDAC2 together to allow for this interaction in the MAM of the OMM and is essential to the proper action of StAR.46
Less is known about a controversial protein formerly proposed to play a role in mitochondrial cholesterol transport, the 18 kDa translocator protein (TSPO) originally characterized as the peripheral benzodiazepine receptor (PBR).47 TSPO is anchored to the OMM and is estimated to constitute 5% to 10% of all proteins in the OMM of steroidogenic cells.48–50 TSPO contains a cholesterol recognition/interaction amino acid consensus (CRAC) domain on its C-terminus, which extends out into the cytosol.28,49,51 TSPO is enriched at contact sites between the OMM and IMM.28 Although TSPO is initially inserted into the OMM as a monomer, it is often found in polymeric form, in complex with many other proteins.28,49,52
One hypothesis suggests that the aforementioned proteins involved in cholesterol transport collaborate: StarD4/5 delivers cholesterol to the OMM where StAR is located.53 Subsequently, StAR binds this cholesterol and was thought to transfer it to TSPO.28 As TSPO is localized to OMM-IMM contact sites, TSPO could move cholesterol from the OMM to the IMM, a hypothesis established by studies in vitro as well as a single in vivo embryonic lethal knockout mouse study.28,52,54 However, more recent studies with TSPO knockout mice demonstrated that loss of TSPO did not affect steroidogenesis or any gross aspect of development or behavior in comparison to wild-type mice, suggesting that TSPO does not play an essential role in this process.55–57 However, further research has demonstrated that a reduction in TSPO does not affect gonadal steroidogenesis but significantly impairs adrenal steroidogenesis.58 Given its intracellular localization to the OMM, high expression in steroidogenic tissue, and conflicting results from multiple research groups, further research will need to be done to elucidate the purpose of TSPO.
Nevertheless, once inserted into the IMM by a now uncertain mechanism, the pools of cholesterol mobilized for steroidogenesis remain segregated from that for structural cholesterol28,59,60 and undergo the first committed step for steroidogenesis described below.28,60
P450 Side Chain Cleavage
P450 side chain cleavage (P450scc; also known as CYP11A1), located on the IMM, catalyzes the first committed step of steroidogenesis. P450scc transforms cholesterol into pregnenolone via a series of three distinct chemical reactions eventuating in loss of the hydrophobic six-carbon side chain of cholesterol (hence the name of this enzyme).28,61 P450scc is so crucial in committing cells to steroidogenesis that cells are defined as steroidogenic when they contain this protein.24
A pair of electrons is required for each of the three chemical reactions performed by P450scc.24 P450scc activities thus require two co-factors: ferredoxin reductase, which receives electrons from NADPH (nicotinamide adenine dinucleotide phosphate), and ferredoxin, which shuttles electrons from ferredoxin reductase to P450scc.24,28 The first reaction involves 22-hydroxylation of cholesterol bound to P450scc.24 Due to the time required for the sequence of electron transport events, the binding of cholesterol, and the 22-hydroxylation of cholesterol, this first P450scc reaction constitutes the rate-limiting step in steroidogenesis in which approximately 20 molecules of cholesterol are processed by each P450scc molecule per second.24,61 In the second step, the product of the first reaction undergoes 20-hydroxylation.24 This is followed by the third step during which the C20-22 bond is cleaved to produce isocaproaldehyde and pregnenolone (Figure 1).24
3β Hydroxysteroid Dehydrogenase Type 1 and Type 2
In the context of allopregnanolone and pregnanolone synthesis, 3β hydroxysteroid dehydrogenase (3β-HSD) is responsible for transforming pregnenolone into progesterone.62 3β-HSD exists in two isoforms (3β-HSD1 and 3β-HSD2), which share 93% homology and a similar quaternary structure except for key residues that confer different ligand specificities.63 The 3β-HSD2 isoform is thought to be the most relevant for steroid biosynthesis, as it is expressed in the adrenals, ovaries, and testes—all traditionally steroidogenic tissues.63 However, the 3β-HSD1 isoform is found in non-steroidogenic tissues63 and therefore may play a role in steroidogenesis in the central nervous system (CNS). This is important as 3β-HSD1 has a significantly higher affinity for common substrates, coenzymes, and inhibitors than 3β-HSD264 and thus may have the ability to convert substantially lower concentrations of pregnenolone to progesterone as might be seen in the CNS. Both isoforms likely have a similar mechanism of action given the high similarity in structure; 3β-HSD2 is discussed here, as it is more widely studied and has a well-delineated mechanism of action.
3β-HSD2 is localized to the IMM in close association with P450scc but performs its catalytic function in the intermembrane space because it requires a low pH environment to function.63,65,66 3β-HSD2 first performs a dehydrogenation reaction that converts NAD+ (nicotinamide adenine dinucleotide) to NADH.62 NADH induces a conformational change in 3β-HSD2, thus creating a catalytic site for the isomerization reaction that converts pregnenolone to progesterone.62,67 The catalytic part of the protein can exist in three forms: a highly folded form at physiological pH, a partially folded form (molten globule with a high degree of secondary structure but no defined tertiary structure) at about pH 4.5, or a completely unfolded form at about pH 3.5.63,66 3β-HSD2 is most active when partially unfolded, a state stabilized by chaperone proteins that prevent 3β-HSD2 aggregation (in the case of denaturation) and facilitate recycling of the protein back to the less-active highly folded state during cell quiescence.63,66,68 On both the OMM and IMM, 3β-HSD is tightly associated with P450scc and mitochondrial translocase proteins (Tom22, Tim50, and Tim23) that seem to be necessary for 3β-HSD2 function.63,65 After synthesis, progesterone crosses the OMM into the cytosol via passive diffusion.28
5α-Reductase Type 1 and Type 2 (5α-R2)
5α-Reductase (5α-R) exists in three isoforms.69 Type 1 (SRD5A1 or 5α-R1) is the most abundant isoform in brain, but is also found in peripheral tissues, including the adrenal gland.70,71 In the brain, 5α-R1 has been co-localized with 3α-hydroxysteroid dehydrogenase type 3 (also known as AKR1C2) (3α-HSD3) (see below) in: (a) glutamatergic principal output neurons of the hippocampus, olfactory bulb, amygdala, thalamus and cortex, including pyramidal neurons of the prefrontal cortex (PFC) that project to the amygdala as well as (b) GABAergic principal output neurons such as cerebellar Purkinje cells, striatal medium spiny neurons, and reticular thalamic neurons.72,73 5α-R2 plays a prominent role in the male reproductive system, but also has been identified in brain, including the PFC (specifically pyramidal cells), the pituitary gland (specifically prolactin producing cells), hypothalamus, and hippocampus.74–78 5α-R2 is also expressed at high levels in spinal motor neurons and in the glomerulosa layer of the adrenal gland,79 where it is likely involved in the synthesis of tetrahydrodeoxycorticosterone (Figure 1), another potent GABAergic neuroactive steroid released into the circulation in response to stress.23 5α-R3 (SRD5A3) appears to be primarily responsible for mediating the N-glycosylation of proteins and does not seem to be active in steroidogenesis.69
Differing physiological characteristics of 5α-R1 and 5α-R2 allow these enzymes to play distinct but overlapping roles in steroid regulation. Both are involved in catalyzing the reduction of testosterone into its more potent metabolite 5α-dihydrotestosterone (5α-DHT) and progesterone into 5α-dihydroprogesterone (5α-DHP), the immediate precursor for allopregnanolone.69 5α-R1 has micromolar affinity for its steroid substrates (progesterone > testosterone > androstenedione > glucocorticoids) and is localized to the endoplasmic reticulum.79,80 There it plays an important neuroprotective role by catabolizing the large quantities of steroids produced under conditions of stress81 and certain reproductive phases in females, such as the luteal phase of the menstrual cycle and pregnancy.77 In male rodents, 5α-R1 activity increases markedly as testosterone levels decrease75—as occurs during intense stress in men.82 In female rodents, testosterone has no effect on 5α-R1 expression in the PFC, whereas 5α-DHT markedly increases 5α-R1 levels. In contrast, 5α-R2 has nanomolar affinity for its substrates and thus may be critical in maintaining adequate resting allopregnanolone levels. In male rodents, 5α-R2 levels fall markedly when testosterone levels decrease (as occurs during stress) and normalize when testosterone levels return to normal.75 In contrast, a preliminary unconfirmed report suggests that 5α-R2 expression in hippocampus may increase during acute stress.77 Testosterone and 5α-DHT have lesser effects on 5α-R2 levels in females.76 Of note, potential effects of estradiol on 5α-R levels have not been reported.
The mechanisms by which 5α-R1 and 5α-R2 reduce their steroid substrates have not been well elucidated. The hydrophobic nature of 5α-R1 makes it difficult to solubilize in a form that retains metabolic activity83; thus, crystallography studies have not been possible. 5α-R1 also lacks homology with other NADPH/steroid-binding enzymes.84 Studies have, however, determined that the N-terminal part of this protein binds to steroid substrates, whereas the C-terminal portion containing a glycine-rich region binds NADPH.83,84 5α-R1 works best at pH 5.0 to 8.0 and has a half-life of approximately 20 to 30 h.69 Less is known about the chemical conditions under which 5α-R2 activity is optimized.
5β-Reductase
5β-Reductase (5β-R; also known as AKR1D1) is a monomeric cytoplasmic protein that plays a role in steroid metabolism as well as in cholic acid and chenodeoxycholic bile acid synthesis.85,86 It shares homology with other AKR family members with a similar mechanism of action (e.g., 3α-HSD, described below).85 Northern blot studies on human tissues demonstrated abundant 5β-R in the liver in two mRNA isoforms, smaller amounts in the testes in two isoforms, and trace amounts in all other tissues including the brain in a single isoform.86 This likely reflects a significant role for 5β-R in the inactivation of steroid hormones, given that two-thirds of these hormones, by mass, undergo transformation by 5β-R before excretion.87
Among other reactions, 5β-R catalyzes the reduction of progesterone to 5β-dihydroprogesterone (5β-DHP or 5β-pregnane-3-20-dione), the immediate precursor for pregnanolone and a potent activator of pregnane-X receptor (PXR, discussed later).88 5β-R requires NADPH, which induces a conformational change, and allows for binding of substrate.85 Although the binding and release of NADPH and NADP+ are generally the slowest steps in 5β-R-mediated reactions, 5β-R efficiency also depends on the substrate involved.85 Human 5β-R demonstrates high efficiency for the reduction of progesterone, androstenedione, 17α-hydroxyprogesterone, and testosterone; reduced efficiency for reduction of aldosterone and corticosterone; and poor efficiency for reduction of cortisol.86
3α-Hydroxysteroid Dehydrogenase Type 3
3α-HSD exists in four different isoforms (types 1, 2, 3, and 20a). Type 3 (associated with the AKR1C2 gene) is a cytosolic protein89 found in steroidogenic tissues and the central nervous system.11,73,90,91 Although 3α-HSD3 has been primarily studied with regard to its role in converting 5α-DHT to 5α-androstane-3α,17β-diol (3α-diol), a very weak androgen and moderately potent GABAergic neuroactive steroid, it is also responsible for the conversion of 5α-DHP and 5β-DHP to the potent GABAergic neurosteroid stereoisomers, allopregnanolone and pregnanolone, respectively.91
Among the 3α-HSD family proteins, 3α-HSD3 has the lowest catalytic efficiency, but the highest specificity for reducing 3-ketosteroids.91,92 3α-HSD3 exists as a monomeric α/β barrel with large loops on one side that confer high substrate binding specificity due to their unique orientation and a conserved catalytic tetrad that mediates the reduction reaction.91,92 3α-HSD3 binds NADPH before binding its steroid substrates; it then catalyzes the reduction of the substrate before releasing the steroid product.91 The rate-limiting step in the 3α-HSD3 conversion of substrate into product is the dissociation of NADP+ from the enzyme.91,93
Diffusion and Receptor Engagement of Allopregnanolone and Pregnanolone
Once allopregnanolone and pregnanolone have been synthesized, they can bind to various receptors to mediate their effects. If synthesized in the CNS, they may travel in an unbound state short distances intraneuronally or through the interstitial fluid to act in an autocrine or paracrine manner.73 A vascular transport protein has not been identified for the endocrine action of allopregnanolone, although there are a few likely candidates. Transcortin, also known as corticosteroid-binding globulin, is synthesized by the liver and plays a major role in transporting many adrenal steroid products throughout the body.94,95 Albumin, the major serum protein, also carries a significant proportion of steroid hormones and has been shown to bind progesterone.96 A less likely binding carrier is sex hormone-binding globulin, as it has high binding affinity only for 17β-hydroxysteroid hormones.97
The binding of allopregnanolone to the GABAA receptor hypersensitizes or prolongs the opening time of the chloride ion channel in the presence of GABA.1,2 At a high enough concentration, allopregnanolone can also act as a direct agonist at this receptor.98 Studies have identified allopregnanolone binding sites in the transmembrane domain of the GABAA receptor and suggest that allopregnanolone may access the receptor from an intracellular approach.1,73,98 Allopregnanolone also binds to membrane progesterone receptors—putative G-protein-coupled receptors that activate intracellular second messenger signaling systems—to mediate neuroprotective actions similar to those mediated by progesterone binding.2,99 Finally, the nuclear PXR represents the only non-plasma membrane receptor associated with allopregnanolone function, as allopregnanolone does not bind to the nuclear progesterone receptor.2,100,101 Other potential signaling partners for allopregnanolone have yet to be identified.
Common Pathways for Activating Steroidogenic Enzymes
Steroidogenic Stimulation by Trophic Hormones
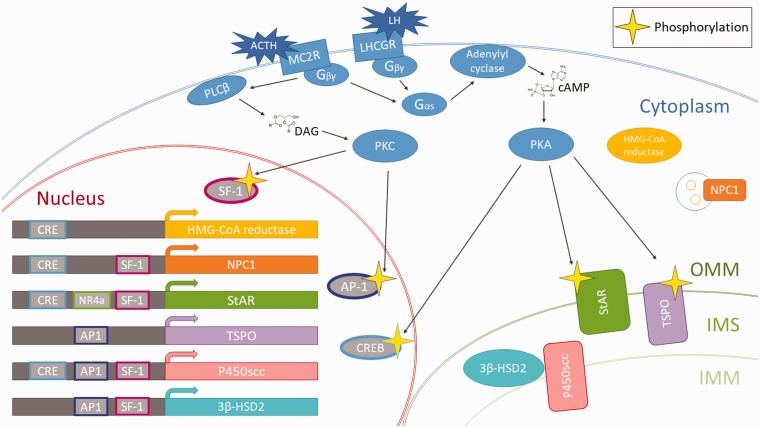
The release of trophic hormones—such as ACTH and LH from the pituitary gland—is the most prominent and studied stimulus for the activation of steroidogenic cells in the periphery. The downstream messaging subsequent to ACTH or LH receptor binding is discussed here as a potential common pathway upon which other activating signals may converge (Figure 2).
Figure 2.
Schematic depicting the activation of a steroidogenic cell with ACTH or LH. In the adrenal gland, ACTH binds to the GPCR melanocortin 2 receptor (MC2R), whereas in the gonads, LH binds to the GCPR LH/choriogonadotropin receptor (LHCGR).103 Upon ligand binding to MC2R or LHCGR, these receptors mediate a signaling cascade through their associated G-protein. The Gαs subunit of the cell membrane-bound G-protein binds guanosine triphosphate (GTP) and dissociates from the G-protein in order to activate adenylyl cyclase which converts ATP to 3′,5′-cyclic AMP (cAMP). The increase in cAMP levels activates protein kinase A (PKA). Meanwhile, the Gβγ subunit of the membrane-bound G-protein activates phospholipase C β (PLCβ), which cleaves diacylglycerols (DAG) from the plasma membrane. The increase in DAG levels activates PKC. Acute changes secondary to this activation include the phosphorylation of StAR and TSPO, which allows for a higher influx of cholesterol across the mitochondrial membranes allowing for increased steroidogenesis. PKA phosphorylates and activates cAMP responsive element binding protein (CREB), which binds to cAMP response elements (CREs) to promote transcription of several downstream genes involved in neurosteroidogenesis.102 CREs have been identified in the promoters of HMG-CoA reductase, NPC1, StAR, and P450scc.49,102,104,105 PKC also has been shown to activate two transcription factors: steroidogenic factor 1 (SF-1) and activating protein 1 (AP-1).102 SF-1 binding domains have been found in the promoters of NPC1, StAR, P450scc, and 3β-HSD2.102,106,107 AP-1 binding domains have been found in the promoters of TSPO, P450scc and 3β-HSD2.49,62,102 The Nuclear Receptor 4A (NR4A) family of transcription are involved in inflammation and have been shown to upregulate StAR and 3β-HSD2.108–111 Long-term changes include activation of transcription factors which upregulate the transcription of genes associated with allopregnanolone synthesis. OMM: outer mitochondrial membrane; IMM: inner mitochondrial membrane; IMS: intermembrane space; HMG-CoA: 3-hydroxy-3-methylglutaryl CoA; NPC1: Niemann Pick type C1.
The steroidogenic effect of ACTH or LH can be parsed into an acute response that occurs within seconds and a long-term response that occurs on the order of minutes to days.102 Acutely, protein kinase A (PKA) phosphorylates StAR at Ser195, which doubles its enzymatic activity in transporting cholesterol from the OMM to the IMM.28,35 TSPO is also a PKA substrate; phosphorylation of TSPO changes its ligand binding affinity and subsequently increases its activity.49,112 Overall, this acute response seems to drive the influx of cholesterol precursor into the mitochondria—whether by the action of StAR or TSPO—to saturate catalytic sites available for the first committed step in steroidogenesis mediated by P450scc. The long-term response to ACTH/LH activation occurs via PKA or protein kinase C (PKC) phosphorylation of several transcription factors that regulate steroidogenic enzymes, thereby increasing the intracellular steroidogenic machinery capacity.
Allopregnanolone and pregnanolone are just two of many end products generated as steroidogenic cells are activated to higher rates of steroid production. Although regulation of P450scc and 3β-HSD2 has been fairly well characterized due to their role in all steroid synthesis pathways, the regulatory mechanisms of 5α-R, 5β-R, and 3α-HSD3 are less well known. Recent research has shown that steroidogenic factor 1 (SF-1) regulates 5α-R and 3α-HSD differently: male murine SF-1 knockout models demonstrate decreased 5α-R mRNA levels and, surprisingly, increased 3α-HSD mRNA levels.113 A few studies have demonstrated that male rat glial cells respond to increased cyclic adenosine monophosphate (cAMP) levels by enhancing de novo increases in 5α-R mRNA levels and activity.114,115Similar experiments in male rodents show that 3α-HSD does not increase in response to increases in cAMP.114 However, these experiments were not conducted in other steroidogenic tissues or in females. Moreover, the activity of these enzymes is determined not only by their abundance in the cell but also by the changing redox state of the cell and the related availability of NADPH, a cofactor required for the reductive activities of these enzymes.83,91 The regulatory mechanisms behind dedicated allopregnanolone production and the means by which production might change in response to stress (or the many means used by individuals to cope with stress) are likely to be highly complex and require further investigation in both sexes. For example, binge alcohol consumption over a month followed by abrupt alcohol withdrawal in male rodents decreased levels of both 5α-R1 and 3α-HSD in brain.116—perhaps by increasing the activity of NADPH oxidase and reducing availability of NADPH.
Role of Allopregnanolone in Psychophysiological and Cellular Stress Responses
Exposing male rodents to intense physical stress, prolonged social isolation, or drugs that reduce the activity of enzymes in the allopregnanolone synthesis pathway reduces serum and brain allopregnanolone levels in association with increases in anxiety and aggression, enhancement of contextual fear conditioning, decreases in the rate of fear extinction, and deficits in extinction retention.117–121 In contrast, compounds that induce neurosteroidogenesis or otherwise increase brain allopregnanolone levels reduce these negative behavioral outcomes.9,122 Effects of stress on allopregnanolone synthesis have been less well studied in female rodents. For example, while isolation stress appeared to reduce brain allopregnanolone levels only in ovariectomized, testosterone-replaced female rodents, potential effects of the diestrus cycle in the normal females were not taken into account, possibly obscuring the effects of isolation. Other stressor types have not been investigated in female rodents. In humans, cross-sectional investigations have demonstrated an inverse relationship between the sum of allopregnanolone and pregnanolone level in cerebrospinal fluid (CSF) and PTSD severity as well as sex differences in the enzyme sites at which allopregnanolone synthesis appears to be blocked in PTSD.15,123–130 It is unclear whether deficits in allopregnanolone synthesis precede trauma exposure or result from trauma exposure or both. Longitudinal studies in humans exposed to severe stressors such as intense military training exercises or deployment would help to answer these important questions.
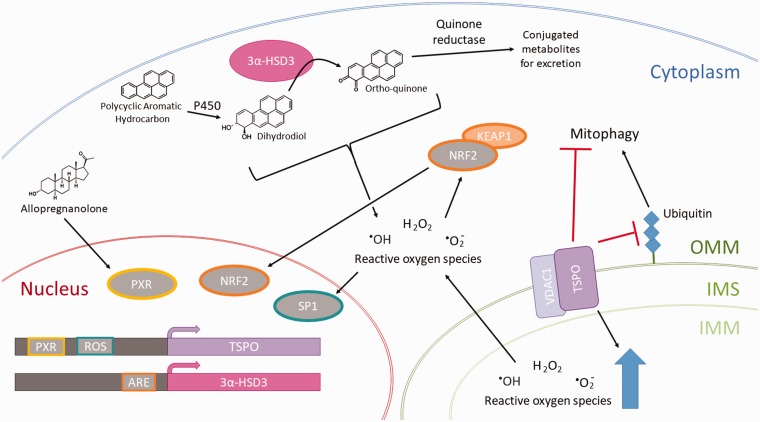
As illustrated in Figure 3, several enzymes involved in allopregnanolone biosynthesis, as well as allopregnanolone itself, also moderate the impact of cellular stressors such as xenobiotics or reactive oxygen species (ROS) requiring clearance. For example, TSPO expression increases in response to pro-pathology stressors and likely protects against apoptosis while preserving cellular mitochondrial metabolic and signaling capabilities in the face of increasing cellular ROS.49 3α-HSD3 plays a role in phase II detoxification of polycyclic aromatic hydrocarbons (PAHs), particulate pollutants released into the air by the burning of fossil fuels.131–133Allopregnanolone is one of the many ligands for PXR, a nuclear receptor that binds promiscuously to both endogenous ligands and xenobiotic ligands of a hydrophobic nature.100,137 When bound to a ligand, PXR binds regulatory elements to the promoter of a variety of genes responsible for the metabolism and clearance of xenobiotics.137 A well-studied and important effect of PXR is on the CYP3A family of enzymes responsible for the biotransformation of more than half of all prescription medications.137 Additionally, PXR drives the expression of phase II detoxification proteins such as glutathione-S-transferase as well as drug transporters such as organic anion-transporting polypeptide 2 (OATP2) and multidrug resistance-associated protein 2 (MRP2), which are responsible for excreting xenobiotic metabolites into the biliary system.137 Changes in PXR activity—such as those associated with elevations in allopregnanolone induced by stress, exercise, or the luteal phase of the menstrual cycle—thus may significantly alter drug metabolism and complicate drug dosing. PXR also upregulates TSPO, potentially creating positive feedback for allopregnanolone synthesis—which may be necessary to preserve adequate allopregnanolone levels in the face of increased PXR-mediated allopregnanolone metabolism.100,138 It is not known whether 3α-HSD3 is a target for PXR-induced upregulation, despite its role as a phase II detoxification protein.
Figure 3.
Schematic depicting the response of steroidogenic cells to stressors such as polycyclic aromatic hydrocarbons and ROS. TSPO responds to stress-related increases in ROS by working with voltage-dependent anion-selective channel protein 1 (VDAC1) to increase the level of ROS in the mitochondria, thereby amplifying ROS signaling in the cell, driving the upregulation of cyclooxygenases, nitric oxide synthases, lipoxygenases, and NAPDH oxidase.49,108,134 It also has been shown to play a role in blocking ubiquitination of the mitochondria by other factors, thereby blocking mitophagy.134 Among the regulatory elements that drive expression of the TSPO gene is an ROS response element in the promoter that binds specificity protein 1 (SP1), which accumulates in the nucleus upon activation by ROS. Additionally, a PXR binding site in the TSPO gene promoter plays a part in the allopregnanolone stress response.100 On the other hand, 3α-HSD3 plays a role in detoxification of polycyclic aromatic hydrocarbons. PAH first undergoes a phase I detoxification reaction by the P450 family enzymes (but not P450scc, which strictly plays a role in steroidogenesis; this family is also known as the CYP enzymes), to form a dihydrodiol.131–133 3α-HSD3 then converts the dihydrodiol into an ortho-quinone, which is further conjugated to glutathione by a quinone reductase, thus producing a nontoxic metabolite for excretion from the body.131–133 Both the dihydrodiol and the ortho-quinone intermediates are capable of producing ROS and are considered genotoxic.131,132 However, the reaction pathway through 3α-HSD3 is the major route for eliminating PAHs, making the synthesis of these intermediates necessary.135 3α-HSD3 is regulated by the nuclear factor E2-related factor 2 (Nrf2), which dissociates from its repressor Kelch-like ECH associated protein 1 (Keap1) upon exposure to increased ROS levels in the cell.132,136 Nrf2 binds to the antioxidant response element (ARE) in the promoter of 3α-HSD3 and upregulates its expression to meet the detoxification demands of the cell. Both TSPO and 3α-HSD3—enzymes responsible for allopregnanolone synthesis—are upregulated in response to these stressors, highlighting a role for allopregnanolone in stress response. OMM: outer mitochondrial membrane; IMM: inner mitochondrial membrane; IMS: intermembrane space; 3α-HSD3: 3α-hydroxysteroid dehydrogenase type 3 (aka AKR1C2); PXR: pregnane X receptor
Clinical Implications
Phenotypes Associated With Defects Along the Allopregnanolone Synthesis Pathway
Various defects can arise in the steroidogenic pathway synthesizing allopregnanolone, many of which are common to steroidogenesis in general. Some defects are related to specific severe medical disorders that may overshadow comorbid psychiatric conditions, while others so far have been linked primarily to psychiatric disorders despite increasing evidence that they contribute to comorbid medical and substance abuse disorders. Thus, further research will be important to characterize the full impact of these defects.
At the start of the steroid synthetic process, cells may have deficiencies that interfere with obtaining, synthesizing, or storing cholesterol precursor. Defects in LAL result in a range of phenotypes.24,26 Wolman disease, one rare genetic disorder affecting LAL function, may lead to multi-organ failure and death in early childhood.24,26 Cholesteryl ester storage disease is a milder, later-onset form of Wolman disease.24,26 Even so, Wolman disease has less effect on steroidogenesis than congenital adrenal hyperplasia (CAH), likely due to the ability of steroidogenic cells to obtain free cholesterol from other sources.24 Niemann-Pick disease type C results from the mutation of NPC1, resulting in an inability to shuttle digested LDL products out of lysosomes.24,27 Lysosomal glycosphingolipids and cholesterol accumulate to produce a variable phenotype that generally includes progressive neurodegeneration.24,26,28 The impact on allopregnanolone synthesis by statins—which inhibit HMG-CoA reductase and thus endogenous cholesterol production—has not been thoroughly assessed. It is possible that patients with pre-treatment synthesis pathway vulnerabilities are more prone to the negative side effects of these drugs. Cortisol responses to ACTH were reduced in a small sample of individuals with familial hypercholesterolemia before and after statin treatment for two months.139 In a larger and longer study, dehydroepiandrosterone sulfate levels in children and adolescents with familial hypercholesterolemia were similar to those of their siblings before treatment with a statin, but substantially lower after 10 years of treatment.140 In a large randomized, placebo-controlled study of otherwise healthy middle-aged men with hypercholesterolemia, testosterone levels decreased, while depression, somatization, and self-reported aggression increased after two years on simvastatin.141 Investigation of the effects of statins on resting and stress-induced increases in allopregnanolone and other neuroactive steroids are therefore warranted.
Defects can occur in the trafficking of cholesterol from the OMM to the IMM. Although controversy exists over whether loss of TSPO results in a significant clinical phenotype, loss of StAR results in lipoid CAH, a condition characterized by a nearly complete absence of all circulating steroid hormones, salt-wasting due to loss of aldosterone, and feminized external genitalia in patients with an XY genotype.24,50,142 Patients with defects in 3β-HSD are also diagnosed with CAH as 3β-HSD is required for synthesis of nearly all steroidogenic end-products.63 Less severe forms of 3β-HSD deficiency may present as hyperandrogenism in women, as progesterone precursors are routed into the androgen pathways.142 Due to the accumulation of dehydroepiandrosterone precursors, they may also present with psychiatric disorders typified by anxiety, depression, aggression, and increased risk for smoking dependence and other substance abuse disorders.143
Severe mutations in 5α-R2 result in male pseudohermaphroditism wherein patients with an XY genotype are unable to convert testosterone to 5α-DHT, resulting in an inability to masculinize the external genitalia.69,144 Late masculinization of patients with mutations in 5α-R2 also may result as 5α-R1 activity increases during puberty.144 Of note, a polymorphism of 5α-R2 has been associated with increased risk for PTSD in males only.145 5α-R deficiency in males with PTSD also has been characterized by a decrease in the ratio of 5α-DHP to progesterone in CSF; the sum of CSF allopregnanolone and pregnanolone levels were, in turn, strongly and negatively correlated with PTSD symptoms.10 No mutations of the 5α-R1 gene have been recorded so far in humans, possibly because dysfunctional phenotypes have gone undetected.69 However, experimental reduction of 5α-R1 expression in male rodents results in reduced allopregnanolone levels and a behavioral phenotype characterized by anxiety, resistance to sedation by GABA receptor-active drugs, aggression, and enhanced contextual fear conditioning resistant to extinction and extinction retention,910146 phenomena consistent with PTSD in humans. 5α-R1/R2 inhibitors (e.g., dutasteride and finasteride) are commonly used in the treatment of male pattern baldness or benign prostatic hyperplasia, with a more controversial role in treating prostate cancer.69,147 One study suggested that prolonged use of finasteride reduced allopregnanolone concentrations in CSF.148 Off-target side effects of finasteride (which has submicromolar potency for 5α-R1, but sub-nanomolar potency for 5α-R2) seen in a subpopulation of males have included depression, suicidal ideation, reduced sexual interest, increased aggression, chronic pain and metabolic disturbances—conditions commonly comorbid with PTSD and major depression.149
Clinically relevant changes in 3α-HSD3 expression have not been well characterized. Studies have implicated 3α-HSD3 transcription in prostate and breast cancer.89,150,151 Reduced expression of 3α-HSD3 has been associated with hirsutism.152 It also has been associated with Niemann-Pick disease type C, as allopregnanolone treatment in a mouse model of Niemann-Pick type C delays symptom onset.12 Female-specific 3α-HSD3 deficiency, suggested by low CSF allopregnanolone levels and a low ratio of the sum of CSF allopregnanolone and pregnanolone (measured together due to technical limitations) to 5α-DHP, has been reported in premenopausal women with PTSD in association with increased negative mood and PTSD reexperiencing symptoms.125 The mechanisms by which this enzyme may become dysregulated and the range of clinical conditions resulting from such dysregulation need further investigation.
Pharmacological Implications
Pharmacological approaches to altering allopregnanolone availability or engagement with GABAA receptors have primarily targeted neuropsychiatric pathologies. Small molecule ligands for TSPO have been shown to suppress anxiety and contextual fear in animal models of PTSD in association with increasing levels of allopregnanolone.49,153–156 These ligands (PK11195, YL-IPA08, and AC-5216) were thought to increase the transfer of cholesterol from the OMM to IMM by TSPO, thereby driving allopregnanolone synthesis.49,153,155,156 A link between the administration of these ligands and GABAA receptor activity also has been demonstrated, most likely due to the effects of elevating allopregnanolone.153,155,156 However, given that TSPO may not play a significant role in steroidogenesis,55–57 the mechanism by which these ligands act to increase allopregnanolone in mouse models is uncertain. Given that TSPO is upregulated at areas of brain injury of varied etiologies and is used as a marker of neuroinflammation,156 it could be speculated that there is some commonality in the pathways of stress response leading to allopregnanolone synthesis rather than directly affecting steroidogenesis. The newer knockout mouse model studies seem to support this approach, with one study demonstrating significantly decreased capacity for cellular respiration in conditions of increased stress among TSPO knockout mice in comparison to wild type.57
Selective serotonin reuptake inhibitors increase neurosteroidogenesis at doses below those that block serotonin reuptake.158 An increase in allopregnanolone levels in the CSF of depressed patients following fluoxetine or fluvoxamine treatment correlated with decreases in depressive symptoms.129 Fluoxetine, sertraline, and paroxetine were shown to increase the affinity of 3α-HSD3 for 5α-DHP in vitro, thereby increasing the rate of allopregnanolone synthesis from 5α-DHP.158 However, another study suggested that fluoxetine acts in female, but not male, rats by inhibiting the conversion of allopregnanolone back into 5α-DHP.159 SSRIs also have shown efficacy in some subpopulations with PMDD, a disorder associated with low allopregnanolone levels in some but not all studies. However, recent studies have also demonstrated the efficacy of a GABAA receptor modulating steroid antagonist17 in treating PMDD, as well as dutasteride, which prevented luteal phase increases in plasma allopregnanolone.21 These studies thus highlight the biological heterogeneity of PMDD.
The pharmacological use of allopregnanolone itself has been under investigation for several neuropsychiatric disorders: status epilepticus, traumatic brain injury, benign essential tremor, major depression, post-partum depression, chronic pain, Alzheimer’s disease, autism, and cocaine craving.2,8,13,127,160–165 Clinical trials of ganaxolone, a 3β-methylated analog of allopregnanolone with similar GABAergic properties are underway for treatment of focal epilepsies, female pediatric epilepsy, and fragile X syndrome.166 Ganaxolone also was recently investigated in PTSD, but trough drug levels in plasma were low and prevented a conclusive assessment of its efficacy.167 In addition, it is possible that chronic administration of allopregnanolone or allopregnanolone analogs is not therapeutic in PTSD.
Indeed, support for the therapeutic effects of intermittent (rather than steady state) administration of allopregnanolone has been demonstrated in rodent models of Alzheimer’s disease14,168 as well as in preclinical models of Niemann-Pick type C disease, traumatic brain injury, diabetic neuropathy, nerve injury, multiple sclerosis, and Parkinson’s disease.14 In PTSD, too high steady-state allopregnanolone levels would be expected to suppress reactivity of the sympathetic nervous system and hypothalamic–pituitary–adrenal axis to novelty or stress,169 thereby interfering with contextual learning and extinction, processes central to PTSD recovery. Notably, in rodents with experimentally induced allopregnanolone deficiency and related PTSD-like behaviors (including enhanced conditioned contextual fear and deficits in contextual fear extinction and extinction retention), administration of a single high dose of ganaxolone after the first extinction training session markedly improved the rate of extinction and extinction retention.163
The enhancement of long-term depression (LTD) and long-term potentiation (LTP) interference by slowly rising intraneuronal levels of allopregnanolone and pregnanolone (and perhaps their sulfated metabolites) in response to NMDA receptor activation22 during extinction learning may enhance consolidation of new learning and prevent the reconsolidation of non-reinforced fear conditioned associations.120 The gradual development of LTP interference under such conditions is thought to protect newly modulated synapses from further excitation during memory consolidation.170 This may apply whether synapses are newly strengthened by LTP (e.g., as the new safe context is learned under arousing treatment conditions), or weakened by LTD (e.g., due to non-reinforcement of the previously acquired association between contextual cues and the previously experienced unconditioned stimulus). It should be noted, however, that these findings in allopregnanolone deficient rodents contrast with the studies in wild-type rodents in which acute allopregnanolone administration has been shown to negatively impact hippocampus-dependent novel object recognition, spatial learning and memory consolidation, and contextual fear conditioning.7,171
Finally, it should be noted that dramatic changes in the target receptor specificity of these GABAergic neurosteroids can result from small variations in their structure. The 3β-hydroxylated isomer of allopregnanolone (known as isopregnanolone, isoallopregnanolone, or epiallopregnanolone) has GABAA receptor subunit selective antagonist effects172,173 and thus diminishes some but not all effects of allopregnanolone.173–175 The 3β-hydroxylated isomer of pregnanolone (epipregnanolone) also negatively modulates GABAA receptor function and blocks allopregnanolone effects, while the sulfated metabolites of allopregnanolone, pregnanolone, and epipregnanolone allosterically antagonize both GABAA and NMDA receptor function.176,177 In contrast, epiallopregnanolone sulfate facilitates NMDA receptor function.178
Given the variable receptor effects of these neurosteroid isomers and metabolites, the relationship of allopregnanolone and pregnanolone to clinical phenomena of interest may vary depending on the (often unmeasured) presence of these isomers and metabolites—an important area for future research. In addition, several of these neurosteroids impact other receptor classes178 so that treatments aimed at altering their synthesis may have unanticipated off-target effects.
Conclusion
Allopregnanolone and pregnanolone play significant roles in a range of neuropsychiatric disorders. As these neurosteroids must be produced de novo in response to stress or other stimulatory demands, manipulating the pharmacokinetics of their synthesis constitutes a promising therapeutic approach and requires an understanding of enzymes involved in their synthesis and upstream molecular regulation. As noted above, much is known about the initial steps in steroidogenesis, but less is known about synthetic steps specific to allopregnanolone and pregnanolone synthesis. Further elucidation of these mechanisms and exploitation of the steps in the steroidogenic process already detailed may lead to novel therapeutics for a multitude of conditions. In addition, future pharmaceutical research should identify subpopulations of individuals for whom allopregnanolone and pregnanolone synthesis or levels should be augmented versus constrained and determine whether treatment is best delivered continuously or episodically. To harness the protective effects of these treatments and to reduce harm, it may also be necessary to both individualize drug dose and precisely time dosing relative to disorder-specific neurophysiological processes inherent to recovery.14,162,163
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Within the past three years, Dr. Rasmusson has served as a paid scientific advisor to Resilience Therapeutics and Cohen Veterans Bioscience. She has no equity in these companies.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABAA receptors: Regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacol 2013; 230(2): 151–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guennoun R, Labombarda F, Gonzalez Deniselle MC, et al. Progesterone and allopregnanolone in the central nervous system: Response to injury and implication for neuroprotection. J Steroid Biochem Mol Biol 2015; 146: 48–61. [DOI] [PubMed] [Google Scholar]
- 3.Peters JA, Kirkness EF, Callachan H, et al. Modulation of the GABAA receptor by depressant barbiturates and pregnane steroids. Brit J Pharmacol 1988; 94(4): 1257–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinna G, Uzunova V, Matsumoto K, et al. Brain allopregnanolone regulates the potency of the GABAA receptor agonist muscimol. Neuropharmacol 2000; 39(3): 440–448. [DOI] [PubMed] [Google Scholar]
- 5.Puia G, Santi M, Vicini S, et al. Neurosteroids act on recombinant human GABAA receptors. Neuron 1990; 4(5): 759–765. [DOI] [PubMed] [Google Scholar]
- 6.Hosie AM, Wilkins ME, Da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAAreceptors through two discrete transmembrane sites. Nature 2006; 444(7118): 486–489. [DOI] [PubMed] [Google Scholar]
- 7.Frye CA, Sturgis JD. Neurosteroids affect spatial/reference, working, and long-term memory of female rats. Neurobiol Learn Mem 1995; 64(1): 83–96. [DOI] [PubMed] [Google Scholar]
- 8.He J, Evans CO, Hoffman SW, et al. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp Neurol 2004; 189(2): 404–412. [DOI] [PubMed] [Google Scholar]
- 9.Rasmusson AM, Marx CE, Pineles SL, et al. Neuroactive steroids and PTSD treatment. Neurosci Lett 2017; 649: 156–163. [DOI] [PubMed] [Google Scholar]
- 10.Rasmusson AM, Pineles SL. Neurotransmitter, peptide, and steroid hormone abnormalities in PTSD: biological endophenotypes relevant to treatment. Curr Psychiatry Rep 2018; 20(7): 52. [DOI] [PubMed] [Google Scholar]
- 11.Rossetti MF, Cambiasso MJ, Holschbach MA, et al. Estrogens and progestagens: synthesis and action in the brain. J Neuroendocrinol 2016; 28: 1–11. [DOI] [PubMed] [Google Scholar]
- 12.Griffin LD, Gong W, Verot L, et al. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med 2004; 10(7): 704–711. [DOI] [PubMed] [Google Scholar]
- 13.Adeosun SO, Hou X, Jiao Y, et al. Allopregnanolone reinstates tyrosine hydroxylase immunoreactive neurons and motor performance in an MPTP-lesioned mouse model of Parkinson’s disease. PLoS One 2012; 7(11): e50040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brinton RD. Neurosteroids as regenerative agents in the brain: therapeutic implications. Nat Rev Endocrinol 2013; 9(4): 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pineles SL, Nillni YI, Pinna G, et al. PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology 2018; 93: 133–141. [DOI] [PubMed] [Google Scholar]
- 16.Pineles SL, Nillni YI, King MW, et al. Extinction retention and the menstrual cycle: Different associations for women with posttraumatic stress disorder. J Abnorm Psychol 2016; 125(3): 349–355. [DOI] [PubMed] [Google Scholar]
- 17.Bixo M, Ekberg K, Poromaa IS, et al. Treatment of premenstrual dysphoric disorder with the GABAAreceptor modulating steroid antagonist Sepranolone (UC1010)—a randomized controlled trial. Psychoneuroendocrinology 2017; 80: 46–55. [DOI] [PubMed] [Google Scholar]
- 18.Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases α4 GABAAreceptor subunit levels in association with increased anxiety in the female rat. Brain Res 2001; 910(1–2): 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulinello M. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. J Pharmacol Exp Ther 2003; 305(2): 541–548. [DOI] [PubMed] [Google Scholar]
- 20.Smith SS, Ruderman Y, Frye C, Homanics G, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3α,5β-THP: A possible model of premenstrual dysphoric disorder. Psychopharmacology 2006; 186(3): 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez PE, Rubinow DR, Nieman LK, et al. 5α-Reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology 2016; 41(4): 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izumi Y, O’dell KA, Zorumski CF. Metaplastic LTP inhibition after LTD induction in CA1 hippocampal slices involves NMDA receptor-mediated neurosteroidogenesis. Physiol Rep 2013; 1(5): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purdy RH, Morrow AL, Moore PH, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci 1991; 88(10): 4553–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller WL. Disorders in the initial steps of steroid hormone synthesis. J Steroid Biochem Mol Biol 2017; 165: 18–37. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Heikkilä P, Meng QH, Kahri AI, Tikkanen MJ, Voutilainen R. Expression of low and high density lipoprotein receptor genes in human adrenals. Eur J Endocrinol 2000; 142(6): 677–682. [DOI] [PubMed] [Google Scholar]
- 26.Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res 2011; 52(12): 2111–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol 2009; 29(4): 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller WL. Role of mitochondria in steroidogenesis. Pediatr Adrenal Dis 2010; 20(6): 1–19. [DOI] [PubMed] [Google Scholar]
- 29.Kwon HJ, Abi-Mosleh L, Wang ML, et al. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell 2009; 137(7): 1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu S, Benoff B, Liou HL, et al. Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann-Pick type C2 disease. J Biol Chem 2007; 282(32): 23525–23531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goedeke L, Fernandez-Hernando C. Regulation of cholesterol homeostasis. Cell Mol Life Sci 2012; 69(6): 915–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Illingworth DR, Kenny TA, Orwoll ES. Adrenal function in heterozygous and homozygous hypobetalipoproteinemia. J Clin Endocrinol Metab 1982; 54(1): 27–33. [DOI] [PubMed] [Google Scholar]
- 33.Clark BJ. The mammalian START domain protein family in lipid transport in health and disease. J Endocrinol 2012; 212(3): 257–275. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Agudo D, Calderon-Dominguez M, Ren S, et al. Subcellular localization and regulation of StarD4 protein in macrophages and fibroblasts. Biochim Biophys Acta - Mol Cell Biol Lipids 2011; 1811(10): 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castillo AF, Orlando U, Helfenberger KE, et al. The role of mitochondrial fusion and StAR phosphorylation in the regulation of StAR activity and steroidogenesis. Mol Cell Endocrinol 2014; 408: 73–79. [DOI] [PubMed] [Google Scholar]
- 36.de Brito OM, Scorrano L. Mitofusin-2 regulates mitochondrial and endoplasmic reticulum morphology and tethering: The role of Ras. Mitochondrion 2009; 9(3): 222–226. [DOI] [PubMed] [Google Scholar]
- 37.Soto EA, Kliman HJ, Strauss JF, et al. Gonadotropins and cyclic adenosine 3′,5′-monophosphate (cAMP) alter the morphology of cultured human granulosa cells. Biol Reprod 1986; 34(3): 559–569. [DOI] [PubMed] [Google Scholar]
- 38.Duarte A, Poderoso C, Cooke M, et al. Mitochondrial fusion is essential for steroid biosynthesis. PLoS One 2012; 7(9): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bose HS, Lingappa VR, Miller WL. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature 2002; 417(6884): 87–91. [DOI] [PubMed] [Google Scholar]
- 40.Granot Z, Melamed-Book N, Bahat A, et al. Turnover of StAR protein: roles for the proteasome and mitochondrial proteases. Mol Cell Endocrinol 2007; 265–266(Suppl.): 51–58. [DOI] [PubMed] [Google Scholar]
- 41.Miller WL, Strauss JF. Molecular pathology and mechanism of action of the steroidogenic acute regulatory protein, StAR. J Steroid Biochem Mol Biol 1999; 69(1–6): 131–141. [DOI] [PubMed] [Google Scholar]
- 42.Tsujishita Y, Hurley JH. Structure and lipid transport mechanism of a StAR-related domain. Nat Struct Biol 2000; 7(5): 408–414. [DOI] [PubMed] [Google Scholar]
- 43.Artemenko IP, Zhao D, Hales DB, et al. Mitochondrial processing of newly synthesized steroidogenic acute regulatory protein (StAR), but not total StAR, mediates cholesterol transfer to cytochrome P450 side chain cleavage enzyme in adrenal cells. J Biol Chem 2001; 276(49): 46583–46596. [DOI] [PubMed] [Google Scholar]
- 44.Bose M, Whittal RM, Miller WL, Bose HS. Steroidogenic activity of StAR requires contact with mitochondrial VDAC1 and phosphate carrier protein. J Biol Chem 2008; 283(14): 8837–8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasad M, Kaur J, Pawlak KJ, Bose M, Whittal RM, Bose HS. Mitochondria-associated endoplasmic reticulum membrane (MAM) regulates steroidogenic activity via steroidogenic acute regulatory protein (StAR)-voltage-dependent anion channel 2 (VDAC2) interaction. J Biol Chem 2015; 290(5): 2604–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marriott K-SC, Prasad M, Thapliyal V, Bose HS. Sigma-1 receptor at the mitochondrial-associated endoplasmic reticulum membrane is responsible for mitochondrial metabolic regulation. J Pharmacol Exp Ther 2012; 343(3): 578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Midzak A, Zirkin B, Papadopoulos V. Translocator protein: pharmacology and steroidogenesis. Biochem Soc Trans 2015; 43(4): 572–578. [DOI] [PubMed] [Google Scholar]
- 48.Anholt RRH, Pedersen PL, De Souza EB, Snyder SH. The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. J Biol Chem 1986; 261(2): 576–583. [PubMed] [Google Scholar]
- 49.Gatliff J, Campanella M. TSPO: kaleidoscopic 18-kDa amid biochemical pharmacology, control and targeting of mitochondria. Biochem J 2016; 473(2): 107–121. [DOI] [PubMed] [Google Scholar]
- 50.Gut P, Zweckstetter M, Banati RB. Lost in translocation: the functions of the 18-kD translocator protein. Trends Endocrinol Metab 2015; 26(7): 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li F, Liu J, Zheng Y, Garavito RM, Ferguson-Miller S. Crystal structures of translocator protein (TSPO) and mutant mimic of a human polymorphism. Science 2015; 347: 555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan J, Papadopoulos V. Evolutionary origin of the mitochondrial cholesterol transport machinery reveals a universal mechanism of steroid hormone biosynthesis in animals. PLoS One 2013; 8(10): 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez-Agudo D, Ren S, Wong E, et al. Intracellular cholesterol transporter StarD4 binds free cholesterol and increases cholesteryl ester formation. J Lipid Res 2008; 49(7): 1409–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papadopoulos V, Amri H, Boujrad N, et al. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids 1997; 62(1): 21–28. [DOI] [PubMed] [Google Scholar]
- 55.Morohaku K, Pelton SH, Daugherty DJ, Butler WR, Deng W, Selvaraj V. Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis. Endocrinology 2014; 155(1): 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tu LN, Morohaku K, Manna PR, et al. Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. J Biol Chem 2014; 289(40): 27444–27454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Banati RB, Middleton RJ, Chan R, et al. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat Commun 2014; 5: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fan J, Campioli E, Midzak A, Culty M, Papadopoulos V. Conditional steroidogenic cell-targeted deletion of TSPO unveils a crucial role in viability and hormone-dependent steroid formation. Proc Natl Acad Sci 2015; 112(23): 7261–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mukai S, Okamoto M, Yamano T, et al. Cholesterol accumulation in adrenocortical mitochondria after ACTH-stimulation. Endocrinol Jpn 1984; 31(2): 177–184. [DOI] [PubMed] [Google Scholar]
- 60.Stevens VL, Xu T, Lambeth JD. Cholesterol pools in rat adrenal mitochondria: Use of cholesterol oxidase to infer a complex pool structure. Endocrinology 1992; 130(3): 1557–1563. [DOI] [PubMed] [Google Scholar]
- 61.Tuckey RC, Cameron KJ. Catalytic properties of cytochrome P-450scc purified from the human placenta: comparison to bovine cytochrome P-450scc. Biochim Biophys Acta (BBA)/Protein Struct Mol 1993; 1163(2): 185–194. [DOI] [PubMed] [Google Scholar]
- 62.Simard J, Ricketts ML, Gingras S, et al. Molecular biology of the 3β-hydroxysteroid dehydrogenase/ Δ5-Δ4 isomerase gene family. Endocr Rev 2005; 26(4): 525–582. [DOI] [PubMed] [Google Scholar]
- 63.Thomas JL, Bose HS. Regulation of human 3-beta-hydroxysteroid dehydrogenase type-2 (3βHSD2) by molecular chaperones and the mitochondrial environment affects steroidogenesis. J Steroid Biochem Mol Biol 2015; 151: 74–84. [DOI] [PubMed] [Google Scholar]
- 64.Thomas JL, Boswell EL, Scaccia LA, Pletnev V, Umland TC. Identification of key amino acids responsible for the substantially higher affinities of human type 1 3β-hydroxysteroid dehydrogenase/isomerase (3β-HSD1) for substrates, coenzymes and inhibitors relative to human 3β-HSD2. Bioorg Chem 2005; 280(22): 21321–21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pawlak KJ, Prasad M, Thomas JL, Whittal RM, Bose HS. Inner mitochondrial translocase Tim50 interacts with 3β-hydroxysteroid dehydrogenase type 2 to regulate adrenal and gonadal steroidogenesis. J Biol Chem 2011; 286(45): 39130–39140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prasad M, Thomas JL, Whittal RM, et al. Mitochondrial 3β-hydroxysteroid dehydrogenase enzyme activity requires reversible pH-dependent conformational change at the intermembrane space. J Biol Chem 2012; 287(12): 9534–9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thomas JL, Frieden C, Nash WE, et al. An NADH-induced conformational change that mediates the sequential 3β- hydroxysteroid dehydrogenase/isomerase activities is supported by affinity labeling and the time-dependent activation of isomerase. J Biol Chem 1995; 270(36): 21003–21008. [DOI] [PubMed] [Google Scholar]
- 68.Rajapaksha M, Prasad M, Thomas JL, et al. Chaperones rejuvenate folding and activity of 3-β hydroxysteroid dehydrogenase 2. ACS Chemical Biology 2013; 8: 1000–1008. [DOI] [PubMed] [Google Scholar]
- 69.Traish AM. 5α-Reductases in human physiology: an unfolding story. Endocr Pract 2012; 1(1): 1–38. [DOI] [PubMed] [Google Scholar]
- 70.El-Awady MK, El-Garf W, El-Houssieny L. Steroid 5alpha reductase mRNA type 1 is differentially regulated by androgens and glucocorticoids in the rat liver. Endocr J 2004; 51(1): 37–46. [DOI] [PubMed] [Google Scholar]
- 71.Roselli CE, Finn TJ, Ronnekleiv-Kelly SM, Tanchuck MA, Kaufman KR, Finn DA. Localization of brain 5α-reductase messenger RNA in mice selectively bred for high chronic alcohol withdrawal severity. Alcohol 2011; 45(8): 763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Agis-Balboa RC, Pinna G, Pibiri F, et al. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc Natl Acad Sci 2007; 104(47): 18736–18741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Agís-Balboa RC, Pinna G, Zhubi A, et al. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci 2006; 103(39): 14602–14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Torres JM, Gomez-Capilla JA, Ortega E. Quantitative reverse-transcriptase polymerase chain reaction assay for mRNA levels of steroid 5α-reductase isozymes. Analytical Biochem 2002; 307(1): 177–180. [DOI] [PubMed] [Google Scholar]
- 75.Torres JM, Ortega E. Differential regulation of steroid 5α-reductase isozymes expression by androgens in the adult rat brain. FASEB J 2003; 17(11): 1428–1433. [DOI] [PubMed] [Google Scholar]
- 76.Torres JM, Ortega E. Steroid 5α-reductase isozymes in the adult female rat brain: central role of dihydrotestosterone. J Mol Endocrinol 2006; 36: 239–245. [DOI] [PubMed] [Google Scholar]
- 77.Poletti A, Coscarella A, Negri-Cesi P, Colciago A, Celotti F, Martini L. 5α-reductase isozymes in the central nervous system. Steroids 1998; 63: 246–251. [DOI] [PubMed] [Google Scholar]
- 78.Aumüller G, Eicheler W, Renneberg H, Adermann K, Vilja P, Forssmann WG. Immunocytochemical evidence for differential subcellular localization of 5 alpha-reductase isoenzymes in human tissues. Acta Anat 1996; 156(4): 241–252. [DOI] [PubMed] [Google Scholar]
- 79.Eicheler W, Tuohimaa P, Vilja P, Adermann K, Forssmann WG, Aumüller G. Immunocytochemical localization of human 5 alpha-reductase 2 with polyclonal antibodies in androgen target and non-target human tissues. J Histochem Cytochem 1994; 42: 667–675. [DOI] [PubMed] [Google Scholar]
- 80.Russell DW, Wilson JD. Steroid 5alpha-reductase: Two genes/two enzymes. Annu Rev Biochem 1994; 63: 25–61. [DOI] [PubMed] [Google Scholar]
- 81.Normington K, Russell DW. Tissue distribution and kinetic characteristics of rat steroid 5α-reductase isozyme. J Biol Chem 1992; 267(27): 19548–19554. [PubMed] [Google Scholar]
- 82.Morgan CA, Wang S, Mason J, et al. Hormone profiles in humans experiencing military survival training. Biol Psychiatry 2000; 47(10): 891–901. [DOI] [PubMed] [Google Scholar]
- 83.Bhattacharyya AK, Wang M, Rajagopalan K, et al. Analysis of the steroid binding domain of rat steroid 5α-reductase (isozyme-1) the steroid D-ring binding domain of 5α-reductase. Steroids 1999; 64(3): 197–204. [DOI] [PubMed] [Google Scholar]
- 84.Wang M, Bhattacharyya AK, Taylor MF, et al. Site-directed mutagenesis studies of the NADPH-binding domain of rat steroid 5α-reductase (isozyme-1) I: Analysis of aromatic and hydroxylated amino acid residues. Steroids 1999; 64(5): 356–362. [DOI] [PubMed] [Google Scholar]
- 85.Chen M, Jin Y, Penning TM. The rate-determining steps of aldo-keto reductases (AKRs), a study on human steroid 5β-reductase (AKR1D1). Chem Biol Interact 2015; 234: 360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Charbonneau A, The VL. Genomic organization of a human 5β-reductase and its pseudogene and substrate selectivity of the expressed enzyme. Biochim Biophys Acta - Gene Struct Expr 2001; 1517(2): 228–235. [DOI] [PubMed] [Google Scholar]
- 87.Palermo M, Marazzi MG, Hughes BA, Stewart PM, Clayton PT, Shackleton CHL. Human Δ4-3-oxosteroid 5β-reductase (AKR1D1) deficiency and steroid metabolism. Steroids 2008; 73(4): 417–423. [DOI] [PubMed] [Google Scholar]
- 88.Drury JE, Di Costanzo L, Penning TM, Christianson DW. Inhibition of human steroid 5β-reductase (AKR1D1) by finasteride and structure of the enzyme-inhibitor complex. J Biol Chem 2009; 284(30): 19786–19790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wenners A, Hartmann F, Jochens A, et al. Stromal markers AKR1C1 and AKR1C2 are prognostic factors in primary human breast cancer. Int J Clin Oncol 2015; 21(3): 548–556. [DOI] [PubMed] [Google Scholar]
- 90.Dufort I, Labrie F. Human types 1 and 3 3 alpha-hydroxysteroid dehydrogenases: differential lability and tissue distribution. J Clin Endocrinol Metab 2001; 86(2): 841–846. [DOI] [PubMed] [Google Scholar]
- 91.Penning TM, Jin Y, Steckelbroeck S, et al. Structure-function of human 3α-hydroxysteroid dehydrogenases: Genes and proteins. Mol Cell Endocrinol 2004; 215(1–2): 63–72. [DOI] [PubMed] [Google Scholar]
- 92.Jin Y, Stayrook SE, Albert RH, et al. Crystal structure of human type III 3β-hydroxysteroid dehydrogenase/bile acid binding protein complexed with NADP+ and ursodeoxycholate. Biochem 2001; 40: 10161–10168. [DOI] [PubMed] [Google Scholar]
- 93.Penning TM, Jin Y, Heredia VV, et al. Structure-function relationships in 3α-hydroxysteroid dehydrogenases: A comparison of the rat and human isoforms. J Steroid Biochem Mol Biol 2003; 85(2–5): 247–255. [DOI] [PubMed] [Google Scholar]
- 94.Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab 1981; 53(1): 58–68. [DOI] [PubMed] [Google Scholar]
- 95.Hammond GL. Plasma steroid-binding proteins: primary gatekeepers of steroid hormone action. J Endocrinol 2016; 230: R13–R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chan D, Slaunwhite WR. The binding of a synthetic progestin, R5020, to transcortin and serum albumin. J Clin Endocrinol Metab 1977; 44: 983–985. [DOI] [PubMed] [Google Scholar]
- 97.Selby C. Sex hormone binding globulin: origin, function and clinical significance. Ann Clin Biochem An Int J Biochem Lab Med 1990; 27(6): 532–541. [DOI] [PubMed] [Google Scholar]
- 98.Sigel E, Steinmann ME. Structure, function, and modulation of GABAA receptors. J Biol Chem 2012; 287(48): 40224–40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thomas P, Pang Y. Membrane progesterone receptors: evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells. Neuroendocrinology 2012; 96(2): 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frye CA, Koonce CJ, Walf A, et al. Novel receptor targets for production and action of allopregnanolone in the central nervous system: a focus on pregnane xenobiotic receptor. Front Cell Neurosci 2014; 8: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lamba V, Yasuda K, Lamba JK, et al. PXR (NR1I2): Splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol Appl Pharmacol 2004; 199(3): 251–265. [DOI] [PubMed] [Google Scholar]
- 102.Ruggiero C, Lalli E. Impact of ACTH signaling on transcriptional regulation of steroidogenic genes. Front Endocrinol 2016; 7: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lavoie HA, King SR. Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B. Exp Biol Med 2009; 234(8): 880–907. [DOI] [PubMed] [Google Scholar]
- 104.Gévry NY, Lalli E, Sassone-Corsi P, Murphy BD. Regulation of Niemann-Pick C1 gene expression by the 3′5′-cyclic adenosine monophosphate pathway in steroidogenic cells. Mol Endocrinol 2003; 17: 704–715. [DOI] [PubMed] [Google Scholar]
- 105.Lagor WR, De Groh ED, Ness GC. Diabetes alters the occupancy of the hepatic 3-hydroxy-3-methylglutaryl-CoA reductase promoter. J Biol Chem 2005; 280(44): 36601–36608. [DOI] [PubMed] [Google Scholar]
- 106.Hoivik EA, Lewis AE, Aumo L, Bakke M. Molecular aspects of steroidogenic factor 1 (SF-1). Mol Cell Endocrinol 2010; 315(1–2): 27–39. [DOI] [PubMed] [Google Scholar]
- 107.Rasmussen MK, Ekstr B, Zamaratskaia G. Regulation of 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase: A review. Int J Mol Sci 2013; 14(9): 17926–17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bassett MH, Suzuki T, Sasano H, et al. The orphan nuclear receptor NGFIB regulates transcription of 3a-hydroxysteroid dehydrogenase: Implications for the control of adrenal functional zonation. J Biol Chem 2004; 279(36): 37622–37630. [DOI] [PubMed] [Google Scholar]
- 109.Kurakula K, Koenis DS, van Tiel CM, de Vries CJM. NR4A nuclear receptors are orphans but not lonesome. Biochim Biophys Acta 2014; 1843(11): 2543–2555. [DOI] [PubMed] [Google Scholar]
- 110.Sewer MB, Dammer EB, Jagarlapudi S. Transcriptional regulation of adrenocortical steroidogenic gene expression. Drug Metab Rev 2007; 39(2–3): 371–388. [DOI] [PubMed] [Google Scholar]
- 111.Volakakis N, Kadkhodaei B, Joodmardi E, et al. NR4A orphan nuclear receptors as mediators of CREB-dependent neuroprotection. Proc Natl Acad Sci 2010; 107(27): 12317–12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Whalin ME, Boujrad N, Papadopoulos V, Krueger KE. Studies on the phosphorylation of the 18 kDa mitochondrial benzodiazepine receptor protein. J Recept Res 1994; 14: 217–228. [DOI] [PubMed] [Google Scholar]
- 113.Spanic T, Fabjan T, Majdic G. Expression levels of mRNA for neurosteroidogenic enzymes 17β-HSD, 5α-reductase, 3α-HSD and cytochrome P450 aromatase in the fetal wild type and SF-1 knockout mouse brain. Endocr Res 2015; 40(1): 44–48. [DOI] [PubMed] [Google Scholar]
- 114.Melcangi RC, Celotti F, Castano P, Martini L. Intracellular signalling systems controlling the 5 alpha-reductase in glial cell cultures. Brain Res 1992; 585(1–2): 411–415. [DOI] [PubMed] [Google Scholar]
- 115.Morita K, Arimochi H, Tsuruo Y. Adrenergic activation of steroid 5α-reductase gene expression in rat C6 glioma cells: involvement of cyclic AMP/protein kinase A-mediated signaling pathway. J Mol Neurosci 2004; 22: 205–212. [DOI] [PubMed] [Google Scholar]
- 116.Cagetti E, Pinna G, Guidotti A, Baicy K, Olsen RW. Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompanied by altered behavioral responses to neurosteroids and memory function. Neuropharmacology 2004; 46(4): 570–579. [DOI] [PubMed] [Google Scholar]
- 117.Nagaya N, Acca GM, Maren S. Allopregnanolone in the bed nucleus of the stria terminalis modulates contextual fear in rats. Front Behav Neurosci 2015; 9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proc Natl Acad Sci 2008; 105: 5567–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pinna G, Dong E, Matsumoto K, et al. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci 2003; 100: 2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pinna G, Rasmusson AM. Up-regulation of neurosteroid biosynthesis as a pharmacological strategy to improve behavioural deficits in a putative mouse model of post-traumatic stress disorder. J Neuroendocrinol 2012; 24: 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang L-M, Qiu Z-K, Zhao N, et al. Anxiolytic-like effects of YL-IPA08, a potent ligand for the translocator protein (18 kDa) in animal models of post-traumatic stress disorder. Int J Neuropsychopharmacol 2014; 17(10): 1659–1669. [DOI] [PubMed] [Google Scholar]
- 122.Locci A, Pinna G. Neurosteroid biosynthesis down-regulation and changes in GABAA receptor subunit composition: a biomarker axis in stress-induced cognitive and emotional impairment. Br J Pharmacol 2017; 74: 3226–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dichtel LE, Lawson EA, Schorr M, et al. Neuroactive steroids and affective symptoms in women across the weight spectrum. Neuropsychopharmacology 2018; 43: 1436–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Girdler SS, Lindgren M, Porcu P, Rubinow DR, Johnson JL, Morrow AL. A history of depression in women is associated with an altered GABAergic neuroactive steroid profile. Psychoneuroendocrinology 2012; 37: 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rasmusson AM, Pinna G, Paliwal P, et al. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry 2006; 60: 704–713. [DOI] [PubMed] [Google Scholar]
- 126.Romeo E, Strohle A, Spalletta G, et al. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry 1998; 155: 910–913. [DOI] [PubMed] [Google Scholar]
- 127.Scioli-Salter E, Forman DE, Tun C, et al. Potential neurobiological benefits of exercise in chronic pain and posttraumatic stress disorder: pilot study. J Rehab Research Dev 2016; 53: 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Strohle A, Romeo E, Hermann B, et al. Concentrations of 3 alpha-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol Psychiatry 1999; 45: 274–277. [DOI] [PubMed] [Google Scholar]
- 129.Uzunova V, Sheline Y, Davis JM, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci 1998; 95: 3239–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang MI, Seippel LE, Purdy RH, et al. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5 alpha-pregnane-3, 20-dione and 3 alpha-hydroxy-5 alpha-pregnan-20-one. J Clin Endocrin Metab 1996; 81: 1076–1082. [DOI] [PubMed] [Google Scholar]
- 131.Burczynski ME, Lin HK, Penning TM. Isoform-specific induction of a human aldo-keto reductase by polycyclic aromatic hydrocarbons (PAHs), electrophiles, and oxidative stress: Implications for the alternative pathway of PAH activation catalyzed by human dihydrodiol dehydrogenase. Cancer Res 1999; 59: 607–614. [PubMed] [Google Scholar]
- 132.Lou H, Du S, Ji Q, Stolz A. Induction of AKR1C2 by phase II inducers: identification of a distal consensus antioxidant response element regulated by NRF2. Mol Pharmacol 2006; 69: 1662–1672. [DOI] [PubMed] [Google Scholar]
- 133.Palackal NT, Lee SH, Harvey RG, Blair IA, Penning TM. Activation of polycyclic aromatic hydrocarbon trans-dihydrodiol proximate carcinogens by human aldo-keto reductase (AKR1C) enzymes and their functional overexpression in human lung carcinoma (A549) cells. J Biol Chem 2002; 277: 24799–24808. [DOI] [PubMed] [Google Scholar]
- 134.Gatliff J, Campanella M. TSPO is a REDOX regulator of cell mitophagy. Biochem Soc Trans 2015; 43(4): 543–552. [DOI] [PubMed] [Google Scholar]
- 135.Yahyapour R, Motevaseli E, Rezaeyan A, et al. Reduction–oxidation (redox) system in radiation-induced normal tissue injury: molecular mechanisms and implications in radiation therapeutics. Clin Transl Oncol 2018; 20(8): 975–988. [DOI] [PubMed] [Google Scholar]
- 136.Wu KC, Cui JY, Klaassen CD. Effect of graded Nrf2 activation on phase-I and -II drug metabolizing enzymes and transporters in mouse liver. PLoS One 2012; 7(7): e39006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev 2002; 23: 687–702. [DOI] [PubMed] [Google Scholar]
- 138.Frye CA, Koonce CJ, Walf AA. The pregnane xenobiotic receptor, a prominent liver factor, has actions in the midbrain for neurosteroid synthesis and behavioral/neural plasticity of female rats. Front Syst Neurosci 2014; 8: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Laue L, Hoeg JM, Barnes K, Loriaux DL, Chrousos GP. The effect of mevinolin on steroidogenesis in patients with defects in the low density lipoprotein receptor pathway. J Clin Endocrinol Metab 1987; 64: 531–535. [DOI] [PubMed] [Google Scholar]
- 140.Braamskamp MJAM, Kusters DM, Wiegman A, et al. Gonadal steroids, gonadotropins and DHEAS in young adults with familial hypercholesterolemia who had initiated statin therapy in childhood. Atherosclerosis 2015; 241(2): 427–432. [DOI] [PubMed] [Google Scholar]
- 141.Hyyppä MT, Kronholm E, Virtanen A, Leino A, Jula A. Does simvastatin affect mood and steroid hormone levels in hypercholesterolemic men? A randomized double-blind trial. Psychoneuroendocrinology 2003; 28(2): 181–194. [DOI] [PubMed] [Google Scholar]
- 142.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 2011; 32(1): 81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Simon NG, Mo Q, Hu S, Garippa C, Lu S-F. Hormonal pathways regulating intermale and interfemale aggression. Int Rev Neurobiol 2006; 73: 99–123. [DOI] [PubMed] [Google Scholar]
- 144.Imperato-McGinley J, Zhu YS. Androgens and male physiology the syndrome of 5α-reductase-2 deficiency. Mol Cell Endocrinol 2002; 198(1–2): 51–59. [DOI] [PubMed] [Google Scholar]
- 145.Gillespie CF, Almli LM, Smith AK, et al. Sex dependent influence of a functional polymorphism in steroid 5-α-reductase type 2 (SRD5A2) on post-traumatic stress symptoms. Am J Med Genet Part B Neuropsychiatr Genet 2013; 162(3): 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tanchuck-Nipper MA, Ford MM, Hertzberg A, Beadles-Bohling A, Cozzoli DK, Finn DA. Sex differences in ethanol’s anxiolytic effect and chronic ethanol withdrawal severity in mice with a null mutation of the 5α-reductase type 1 gene. Behav Genet 2015; 45(3): 354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang K, Fan D-D, Jin S, Xing N-Z, Niu Y-N. Differential expression of 5-alpha reductase isozymes in the prostate and its clinical implications. Asian J Androl 2014; 16(2): 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Caruso D, Abbiati F, Giatti S, et al. Patients treated for male pattern hair with finasteride show, after discontinuation of the drug, altered levels of neuroactive steroids in cerebrospinal fluid and plasma. J Steroid Biochem Mol Biol 2015; 146: 74–79. [DOI] [PubMed] [Google Scholar]
- 149.Melcangi RC, Santi D, Spezzano R, et al. Neuroactive steroid levels and psychiatric and andrological features in post-finasteride patients. J Steroid Biochem Mol Biol 2017; 171: 229–235. [DOI] [PubMed] [Google Scholar]
- 150.Ji Q, Aoyama C, Nien Y-D, et al. Selective loss of AKR1C1 and AKR1C2 in breast cancer and their potential effect on progesterone signaling. Cancer Res 2004; 64(20): 7610–7617. [DOI] [PubMed] [Google Scholar]
- 151.Ji Q, Chang L, VanDenBerg D, Stanczyk FZ, Stolz A. Selective reduction of AKR1C2 in prostate cancer and its role in DHT metabolism. Prostate 2003; 54(4): 275–289. [DOI] [PubMed] [Google Scholar]
- 152.Steiner AZ, Chang L, Ji Q, et al. A Novel Mechanism for Hirsutism. In Vivo 2008; 93: 1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Qiu ZK, Zhang LM, Zhao N, et al. Repeated administration of AC-5216, a ligand for the 18 kDa translocator protein, improves behavioral deficits in a mouse model of post-traumatic stress disorder. Prog Neuro-Psychopharmacology Biol Psychiatry 2013; 45: 40–46. [DOI] [PubMed] [Google Scholar]
- 154.Rupprecht R, Papadopoulos V, Rammes G, et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov 2010; 9(12): 971–988. [DOI] [PubMed] [Google Scholar]
- 155.Zhang L-M, Qiu Z-K, Chen X-F, et al. Involvement of allopregnanolone in the anti-PTSD-like effects of AC-5216. J Psychopharmacol. 2016. http://jop.sagepub.com/cgi/doi/10.1177/0269881115625115. Accessed November 26, 2018.
- 156.Zhang LM, Qiu ZK, Zhao N, et al. Anxiolytic-like effects of YL-IPA08, a potent ligand for the translocator protein (18 kDa) in animal models of post-traumatic stress disorder. Int J Neuropsychopharmacol 2014; 29(4): 1659–1669. [DOI] [PubMed] [Google Scholar]
- 157.Guilarte TR. TSPO in diverse CNS pathologies and psychiatric disease: A critical review and a way forward [published online ahead of print September 4, 2018]. Pharmacol Ther. 2018. doi:10.1016/j.pharmthera.2018.09.003. [DOI] [PMC free article] [PubMed]
- 158.Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci 1999; 96(23): 13512–13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fry JP, Li KY, Devall AJ, Cockcroft S, Honour JW, Lovick TA. Fluoxetine elevates allopregnanolone in female rat brain but inhibits a steroid microsomal dehydrogenase rather than activating an aldo-keto reductase. Br J Pharmacol 2014; 171(24): 5870–5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bäckström T, Haage D, Löfgren M, et al. Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons. Neuroscience 2011; 191: 46–54. [DOI] [PubMed] [Google Scholar]
- 161.Labombarda F, Ghoumari AM, Liere P, De Nicola AF, Schumacher M, Guennoun R. Neuroprotection by steroids after neurotrauma in organotypic spinal cord cultures: A key role for progesterone receptors and steroidal modulators of GABAA receptors. Neuropharmacology 2013; 71: 46–55. [DOI] [PubMed] [Google Scholar]
- 162.Patte-Mensah C, Meyer L, Taleb O, Mensah-Nyagan AG. Potential role of allopregnanolone for a safe and effective therapy of neuropathic pain. Prog Neurobiol 2014; 113: 70–78. [DOI] [PubMed] [Google Scholar]
- 163.Rasmusson AMPG. Ganaxolone improves behavioral deficits in a mouse model of post-traumatic stress disorder. Front Cell Neurosci 2014; 8: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kazdoba TM, Hagerman RJ, Zolkowska D, Rogawski MA, Crawley JN. Evaluation of the neuroactive steroid ganaxolone on social and repetitive behaviors in the BTBR mouse model of autism. Psychopharmacology 2016; 233(2): 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Milivojevic V, Fox HC, Sofuoglu M, Covault J, Sinha R. Effects of progesterone stimulated allopregnanolone on craving and stress response in cocaine dependent men and women. Psychoneuroendocrinology 2016; 65: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zaccara G, Schmidt D. Do traditional anti-seizure drugs have a future? A review of potential anti-seizure drugs in clinical development. Pharmacol Res 2016; 104: 38–48. [DOI] [PubMed] [Google Scholar]
- 167.Rasmusson AM, Marx CE, Jain S, et al. A randomized controlled trial of ganaxolone in posttraumatic stress disorder. Psychopharmacology 2017; 234(15): 2245–2257. [DOI] [PubMed] [Google Scholar]
- 168.Bengtsson SK, Johansson M, Bäckström T, Wang M. Chronic allopregnanolone treatment accelerates alzheimer’s disease development in AβPPSwePSEN1ΔE9mice. J Alzheimer’s Dis 2012; 31(1): 71–84. [DOI] [PubMed] [Google Scholar]
- 169.Barbaccia M, Serra M, Purdy R, Biggio G. Stress and neuroactive steroids. Int Rev Neurobiol 2001; 46: 243–272. [DOI] [PubMed] [Google Scholar]
- 170.Cantarero G, Tang B, O’Malley R, Salas R, Celnik P. Motor Learning Interference Is Proportional to Occlusion of LTP-Like Plasticity. J Neurosci 2013; 33(11): 4634–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Johansson IM, Birzniece V, Lindblad C, Olsson T, Bäckström T. Allopregnanolone inhibits learning in the Morris water maze. Brain Res 2002; 934(2): 125–131. [DOI] [PubMed] [Google Scholar]
- 172.Lundgren P, Strömberg J, Bäckström T, Wang M. Allopregnanolone-stimulated GABA-mediated chloride ion flux is inhibited by 3β-hydroxy-5α-pregnan-20-one (isoallopregnanolone). Brain Res 2003; 982(1): 45–53. [DOI] [PubMed] [Google Scholar]
- 173.Öfverman C, Strömberg J, Birzniece V, Turkmen S. The progesterone metabolite isoallopregnanolone is a subunit-selective antagonist of the GABA-A receptor. Digit Vetenskapliga Ark. 2009. http://urn.kb.se/resolve?urn=urn:nbn:se:umu:diva-27069. Accessed November 26, 2018.
- 174.Bäckström T, Wahlström G, Wahlström K, Zhu D, Wang M De. Isoallopregnanolone; an antagonist to the anaesthetic effect of allopregnanolone in male rats. Eur J Pharmacol 2005; 512(1): 15–21. [DOI] [PubMed] [Google Scholar]
- 175.Bengtsson SKS, Nyberg S, Hedström H, et al. Isoallopregnanolone antagonize allopregnanolone-induced effects on saccadic eye velocity and self-reported sedation in humans. Psychoneuroendocrinology 2015; 52(1): 22–31. [DOI] [PubMed] [Google Scholar]
- 176.Park-Chung M, Wu FS, Purdy RH, Malayev AA, Gibbs TT, Farb DH. Distinct sites for inverse modulation of N-methyl-D-aspartate receptors by sulfated steroids. Mol Pharmacol 1997; 52(6): 1113–1123. [DOI] [PubMed] [Google Scholar]
- 177.Gibbs TT, Russek SJ, Farb DH. Sulfated steroids as endogenous neuromodulators. Pharmacol Biochem Behav 2006; 84(4): 555–567. [DOI] [PubMed] [Google Scholar]
- 178.Rupprecht R, Di Michele F, Hermann B, et al. Neuroactive steroids: Molecular mechanisms of action and implications for neuropsychopharmacology. Brain Res Rev 2001; 37(1–3): 59–67. [DOI] [PubMed] [Google Scholar]