Abstract
Commentary on: Roddy DW, Farrell C, Doolin K, Roman E, Tozzi L, Frodl T, O'Keane V, O'Hanlon E. The Hippocampus in Depression: More Than the Sum of Its Parts? Advanced Hippocampal Substructure Segmentation in Depression. Biol Psychiatry. 2019 Mar 15;85(6):487-497. doi: 10.1016/j.biopsych.2018.08.021. Epub 2018 Sep 6. PubMed PMID: 30528746.
The hippocampus is a key cognitive hub implicated in major depressive disorder. However, major depressive disorder neuroimaging studies have used inconsistent anatomical hippocampal definitions to estimate hippocampal volumes, leading to some heterogeneity in findings. In a recent paper, we used a novel reassembly of automated hippocampal substructures (composites) to build alternative anatomical hippocampal definitions and used these to investigate differences in a well-defined cohort of major depressive disorder patients and healthy controls. We found that the most significant differences between major depressive disorder and healthy controls were localized to the core cornu ammonis (CA) regions of the hippocampus. The CA2–4 regions were smaller in first episode major depressive disorder, whereas more widespread differences were found in recurrent/chronic major depressive disorder, suggestive of a potential disease process in major depressive disorder. In this commentary, we also show how new hippocampal composites to investigate sections of the hippocampal circuitry demonstrate that differences in major depressive disorder occur across the input, middle and output circuit nodes of the hippocampal core. Hippocampal pathology localized across the core hippocampal CA circuity may account for the diverse and wide-ranging symptoms often experienced in depression.
Keywords: Hippocampus, depression, cornu ammonis, neuroimaging, neuroscience
The hippocampus is considered a key cognitive hub that has been implicated in the pathogenesis of major depressive disorder (MDD). Although hippocampal volume reductions are commonly observed in neuroimaging, such findings lack consistency in the literature due to diagnostic and other clinical differences (e.g. age, treatment states, chronicity of illness etc.).1 A further cause of heterogeneity between studies may lie with the actual definition of the hippocampus used, with over 60 different anatomical guidelines in use to estimate hippocampal volumes.2 The human hippocampus consists of interrelated yet anatomically diverse substructures within the medial temporal lobe. These substructures include both the classic hippocampal regions (i.e. dentate, cornu ammonis (CA) and subiculum) that combine organizationally to form the traditional functional unit, as well as more peripheral supporting substructures including the pre-/para-subiculum, periamygdalar and parahippocampal areas. Differing hippocampal descriptions have plagued both clinical and preclinical hippocampal research since it was first described by Arantius in the 16th century with classifications ranging from restrictive (CA only) to broad (all classic hippocampal and extra hippocampal structures).
In a recent study,3 we used advanced automated magnetic resonance hippocampal segmentation (Freesurfer 6.0) to investigate substructure differences between 80 patients with MDD and 83 healthy controls. Individual hippocampal substructure volumes were smaller, mostly on the left in the MDD group, but these were only confined to the classic hippocampal regions (dentate, CA1–4 and subiculum). By dividing our depressed group into first presentation (mean MDD duration 10 months) and recurrent (mean MDD duration 60 months), we found pathology localized to the CA2–4 regions in the first presentation group, whereas the recurrent group demonstrated much more widespread changes. Even though this study is a cross-sectional case–control sample and as such pathology progression cannot be inferred (unlike a longitudinal study), these results hint at a potential CA2–4 region locus for an initial hippocampal insult in MDD, with further disease process with extension to the other substructures. A novel finding was that the CA1 region demonstrated lateralization of pathology exclusively to the left side and this region was predictive of MDD disease duration.
The main strength of our study is the novel reassembly of the individual computed substructures into ‘composite’ groups. These composite groups were formed simply by summing the substructural volumes according to known anatomico-functional regions within the hippocampus. By creating three alternative whole hippocampal characterizations, we were able to explore the effects of differing hippocampal definitions in MDD. We found that as we restricted our definitions of hippocampus from broad (all substructures), through functional (dentate, CA1–4 and subiculum only) and finally to conservative (CA regions only), the significance of the changes in MDD increased considerably (p = 0.005 → p = 0.003 → p = 0.0002). This shows for the first time that hippocampal changes seen in MDD depend on the hippocampal definition but also that MDD pathology seems to have a greater impact on the core CA processing regions.
Additional exploratory composite measures were used to further investigate the volumes of anatomical sub-regions within the hippocampal complex. These included a more refined dentate (dentate and CA4) and CA composites (CA1–3) (with CA4 actually being the hilum of the dentate, rather than a true CA region) as well as a combined total dentate/CA composite. Using these composites to investigate these anatomico-functional sectors rather than individual substructures greatly increased the significance in our findings, with the left combined total dentate/CA composite showing the most significant volume reduction overall (p = 0.0001 [eta2 = 0.8]) with differences driven by both first presentation and recurrent groups. The dentate/CA composite corresponds to the key processing circuitry of the hippocampus.4
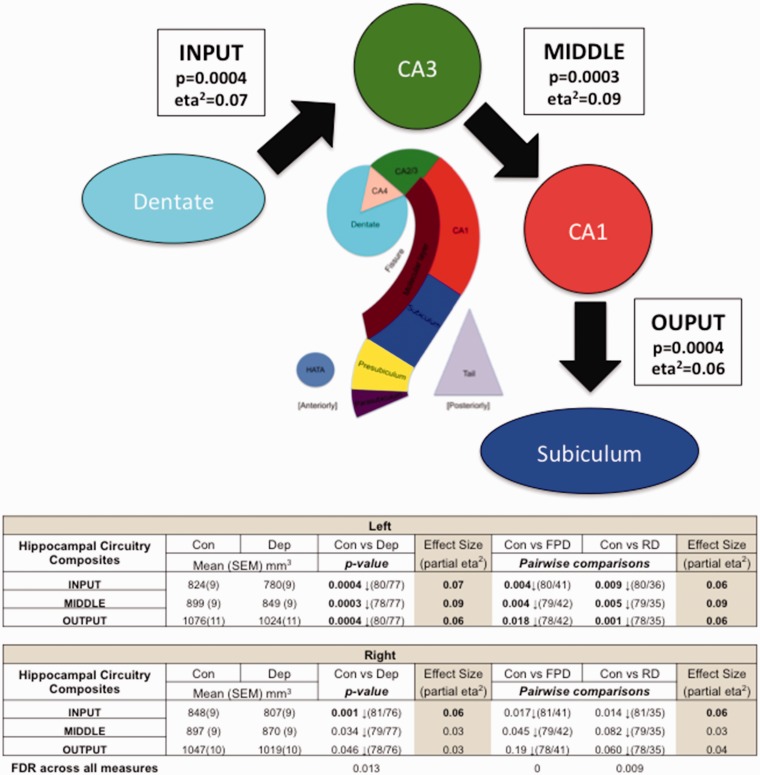
To further investigate hippocampal circuitry, we propose here, previously unpublished, three further composites to explore the course of information flow through the hippocampus. In a highly simplified form, information flows in to the hippocampus through the dentate/CA4 to the CA2/3 (mossy fibre path), then CA2/3 to the CA1 (Schaffer collaterals) and then CA1 to subiculum before exiting out from the hippocampus (Figure 1). By using novel composites to evaluate the volumes of each of these three steps (Input, Middle and Output), we may gain insight into the effects of MDD on each component of this circuit (see original paper for methods). We found within each section highly significant volume reductions in depressed compared to controls (Input p = 0.0004 (eta2 = 0.07), Middle p = 0.0002 (eta2 = 0.09) and Output p = 0.0004 (eta2 = 0.06)) suggesting that MDD impacts each part of the central circuit pathways across the hippocampus. This reinforces our hypothesis that MDD impacts the core processing activities of the hippocampus, resulting in well-documented cognitive and memory problems associated with the condition.5
Figure 1.
Information flow through the hippocampus and changes in depression. The arrows show a highly simplified account of the information flow through the hippocampus. Using computed Freesurfer 6.0 substructures, we created Input, Middle and Output composites representative of the three stages of information flow through the hippocampus. The table represents differences between controls and total depressed, controls and first presentation and controls and recurrent depressed groups. p-values denote significance between groups. Partial eta2 describes effect size (0.01 = low, 0.06 = moderate and 0.14 = large). Bold text indicates p-values that survived FDR correction or moderate to large effect size. Volumetric reductions were found across all three circuit components in depression in the left side compared to controls. CA, cornu ammonis; Con, controls; Dep, total depressed; FPD, first presentation depression; FDR, false discovery rate; RD, recurrent depression.
In summary, MDD is not a disease of the wider hippocampus, but a disease of the hippocampal core, which impacts the basic processing circuitry that is fundamental to most higher order brain activities. Pathology localized in the hippocampal core may account for the diverse and wide-ranging symptoms often experienced in depression.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Irish Health Research Board as part of the REDEEM (Research in Depression, Endocrinology, Epigenetics, and Neuroimaging) study at Trinity College Institute of Neuroscience and the Department of Psychiatry, Trinity College Dublin (Grant No. 201651.12553, to V. O).
References
- 1.MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry 2011; 16: 252–264. [DOI] [PubMed] [Google Scholar]
- 2.Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 1. Review of methodologies currently employed. Mol Psychiatry 2005; 10: 147–159. [DOI] [PubMed] [Google Scholar]
- 3.Roddy DW, Farrell C, Doolin K, et al. The hippocampus in depression: more than the sum of its parts? Advanced hippocampal substructure segmentation in depression. Biol Psychiatry 2019; 85: 487–497. [DOI] [PubMed] [Google Scholar]
- 4.Stepan J, Dine J, Eder M. Functional optical probing of the hippocampal trisynaptic circuit in vitro: network dynamics, filter properties, and polysynaptic induction of CA1 LTP. Front Neurosci 2015; 9: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohler CA, Carvalho AF, Alves GS, McIntyre RS, Hyphantis TN, Cammarota M. Autobiographical memory disturbances in depression: a novel therapeutic target? Neural Plast 2015; 2015: 759139. [DOI] [PMC free article] [PubMed] [Google Scholar]