Abstract
Antiretroviral therapy (ART) inhibits HIV replication but is not curative. During ART, the integrated HIV genome persists indefinitely within CD4+ T cells and perhaps other cells. Here, we describe the mechanisms thought to contribute to its persistence during treatment and highlight findings from numerous recent studies describing the importance of cell proliferation in that process. Continued progress elucidating the biology will enhance our ability to develop effective curative interventions.
Introduction
Although antiretroviral therapy (ART) can durably suppress HIV replication indefinitely, it is not curative and must be taken for life. Due to various challenges, many people are unable to achieve long-term viral suppression (Ndung’u et al., 2019). Despite a massive global investment to provide ART, only about half of the world’s HIV-positive population is now on effective ART. Curing HIV is now a global priority.
As a retrovirus, HIV-1 integrates its proviral genome into the host genome of its target cells. In the absence of ART, HIV-1 preferentially infects activated CD4+ T cells, most of which die quickly. A small proportion of these infected cells exist in a long-term resting state in which the integrated replication-competent viral genome persists indefinitely. These cells, called the latent reservoir, decay very slowly, with a half-life of approximately 44 months, implying that treatment will never be curative (Crooks et al., 2015; Finzi et al., 1999).
This conceptual model for HIV-1 persistence during ART was established in the mid-1990s (Chun et al., 1997; Finzi et al., 1997; Wong et al., 1997). Until recently, it was assumed that quiescent cells harboring an intact genome persisted indefinitely, presumably maintained by their slow turnover. With the emergence of advanced single-cell methodologies and next-generation sequencing capacities, it is now clear that the reservoir is far more dynamic, with multiple factors contributing to its maintenance.
In this review, we discuss how the reservoir is maintained during ART, where the virus resides during treatment, how gender, age and other parameters affect the reservoir, and finally how knowledge of these factors might lead to effective interventions.
Clonal expansion of infected cells
When the latent reservoir was first described, most attributed its apparently stability to the long lifespan of non-dividing resting memory CD4+ T cells endowed with pro-survival capacities. Recent technological advances demonstrate that the persistence of the reservoir is ensured through massive and sustained clonal expansion of cells infected with both intact and defective proviruses. This cell proliferation is thought to maintain the majority of infected cells during ART and shapes the location and disposition of the provirus population (Figure 1).
Figure 1. HIV-1 persistence through clonal proliferation.
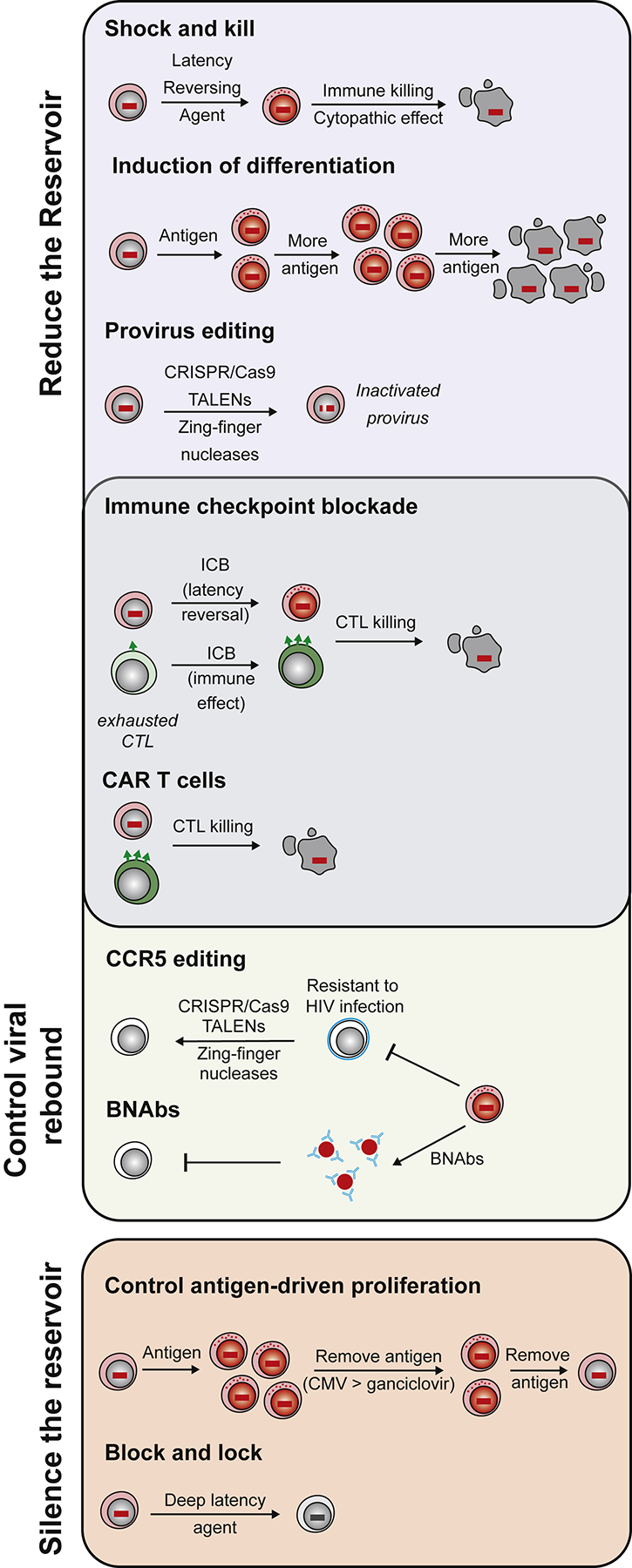
Three independent mechanisms are thought to drive proliferation of latently infected cells. First, the viral integration site may provide a survival advantage allowing preferential proliferation of the infected clone. Second, homeostatic cytokines, such as IL-7, may signal latently infected cells to divide. Finally, latently infected CD4+ T cells with antigen specific T cell receptors may divide in response to recurrent antigen exposure.
Three mechanisms might contribute to the clonal expansion of infected cells: integration in or near genes associated with cell growth, homeostatic proliferation and antigen-driven proliferation. These are not mutually exclusive and is likely that all mechanisms apply to varying degrees across individuals and perhaps time.
It has been proposed that proviral integration near genes that control cell division, including genes involved in cancer, promotes cellular proliferation(Maldarelli et al., 2014; Wagner et al., 2014). HIV-1 preferentially integrates into highly transcribed genes, many of which are actively involved in cell growth. Thus, it has been difficult to definitively determine whether preferential integration in such regions is a cause or consequence of cell activation and proliferation. Unlike transforming retroviruses that integrate into cancer genes and cause unrestricted cell growth, HIV-1 is not known to cause T cell cancers by integration. Nevertheless, altered gene expression induced via the introduction of a viral promoter is one possible mechanism to explain infected cell expansion.
In normal T cell homeostasis, memory T cell clones are maintained in response to cytokines such as IL-7. These same factors contribute to the maintenance of the reservoir (Chomont et al., 2009). This homeostatic proliferation occurs in the absence of virus reactivation (Bosque et al., 2011; Vandergeeten et al., 2013), indicating that the low levels of proliferation required for normal T cell homeostasis allows the reservoir to be maintained while remaining invisible to the immune system and many immunotherapies.
Antigenic stimulation due to chronic exposure of microbial peptides may also drive expansion and maintenance of the latent reservoir. Early studies argued that the virus may be enriched in HIV-1 specific CD4+ T cells (Douek et al., 2002), perhaps because such cells are more likely to be present and activated at sites of virus replication. More recent studies suggested that if there is enrichment, the effect is modest (Hey-Nguyen et al., 2019). Co-infection with viruses such as cytomegalovirus (CMV) and Epstein-Barr virus (EBV) are widespread and could also lead to antigen-driven proliferation of latently infected cells. Indeed, Henrich and colleagues identified HIV-1 DNA enrichment in CMV and EBV-specific CD4+ T cells after CD4+ T cell reconstitution following chemotherapy (Henrich et al., 2017b). Recently, clones of HIV-1 and CMV responsive CD4+ T cells containing defective or intact latent proviruses were found in ART suppressed individuals (Mendoza et al., 2020). Thus, chronic or repeated exposure to antigen likely contributes to the longevity of the HIV-1 reservoir by stimulating the clonal expansion of latently infected CD4+ T cells, resulting in sequential episodes of expansion and contraction in the reservoir (Wang et al., 2018)(Figure 2).
Figure 2. Antigen driven viral persistence.
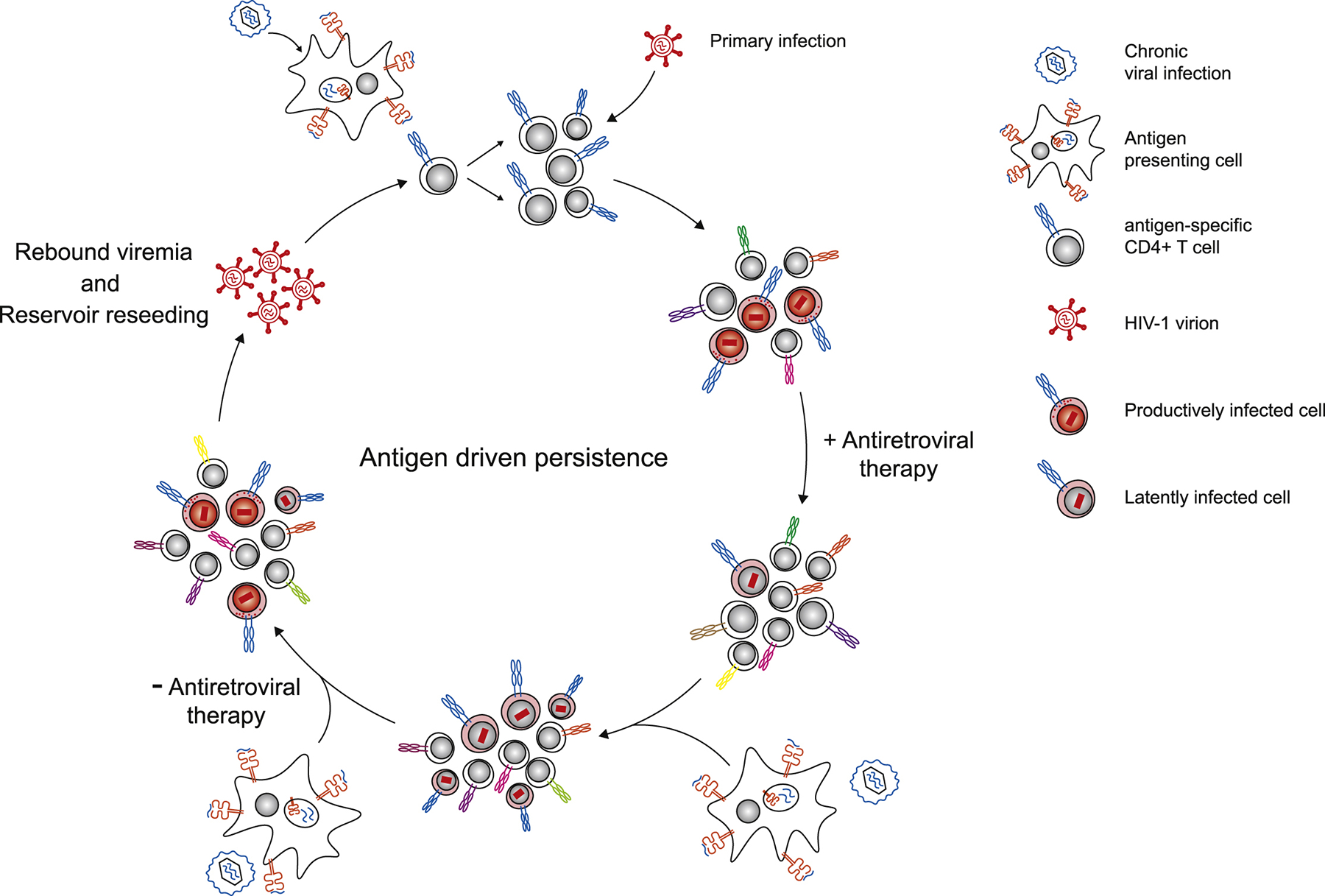
The presence of chronic viral infection (in blue) leads to specific activation of antigen responsive CD4+ T cells. These activated T cells are targets for primary HIV-1 infection. Upon initiation of antiretroviral therapy, the majority of productively infected cells die rapidly, leaving behind latently infected cells. During repeated exposure to chronic virus, the latently infected, antigen specific cells divide, and the clones wax and wane in response to antigen exposure. If therapy is ceased, chronic viral antigen can be presented to latently infected cells which may trigger HIV-1 transcription and virus production, resulting in viral rebound and latent reservoir reseeding.
The active reservoir
During effective ART, a minority of HIV-1-infected cells are transcriptionally active, producing elongated HIV-1 RNA, HIV-1 proteins and intact virions. The reservoir is hence heterogenous, with a continuum from “deep latency” (no or very little RNA produced) through a state of active virion production. Using a method to quantify unspliced HIV-1 RNA production on a per cell basis, one group estimated that approximately 10% (with wide variability) of circulating infected cells expressed detectable levels of HIV-1 RNA (Wiegand et al., 2017). Higher levels of transcriptional activity may be evident if the early viral transcript TAR is measured (Yukl et al., 2018). The frequency of cells expressing RNA are similar in blood and lymph nodes(McManus et al., 2019), but may be relatively lower in the gut (Telwatte et al., 2018). Notably, although the frequencies of infected cells expressing viral RNA were similar in those on or off ART, the level of production was consistently very low during ART(Wiegand et al., 2017), while occasional cells from untreated individuals expressed high levels of RNA.
Simply transcribing HIV may not be sufficient to make a cell productive. In one provocative study, the main mechanisms responsible for HIV latency were not transcriptional interference or block to initiation but rather blocks to proximal elongation, polyadenylation, and splicing(Yukl et al., 2018). Clarity these issues will require characterizing the transcriptional profile of infected cells on a single cell basis using emerging technologies.
The active reservoir may be enriched in cells that are phenotypically distinct cells from those harboring the more quiescent latent reservoir. For example, one group found that within B cell follicles, most HIV-1 RNA-expressing cells also expressed CD32a, while cells expressing only HIV-1 RNA or CD32a were rare (Abdel-Mohsen et al., 2018). Similarly, CD30 was found to be a marker of the active (HIV-1 RNA-expressing) reservoir in blood and tissues (Hogan et al., 2018).
Most of the integrated viral genomes are defective(Bruner et al., 2016; Cohn et al., 2015; Ho et al., 2013). In one intensively studied individual, the frequency of cells expressing RNA was similar in cells harboring intact and defective genomes(Musick et al., 2019). Defective genomes can also produce viral proteins that are immunogenic (Imamichi et al., 2020; Pollack et al., 2017) and might prove to be detrimental to health, as they likely contribute to persistent inflammation during ART.
Dynamics of infected cells during ART
When and how the latent reservoir is established is not fully known. In SIV/SHIV infection, it is possible to initiate ART before the detection of circulating viral particles in the plasma(Whitney et al., 2014). In one study, ART started 4 to 5 days post-infection prevented the establishment of a permanent latent reservoir (Okoye et al., 2018). This may occur in humans receiving post-exposure prophylaxis (PEP) but once infection is clinically evident in people, a small but persistent reservoir is unavoidably established (Colby et al., 2018; Henrich et al., 2017a). A long-lived reservoir is also established during maternal-to-fetal transmission and/or at birth (Garcia-Broncano et al., 2019; Persaud et al., 2013).
Although viruses are deposited into longer lived memory cells early during infection(Leyre et al., 2020; Puertas et al., 2014), the reservoir during the untreated state is not stable. Most of the reservoir that persists during ART appears to be have formed just prior to treatment initiation (Abrahams et al., 2019; Brodin et al., 2016; Pankau et al., 2020). The pool of cells harboring potentially replication competent HIV-1 decays more rapidly during early compared to late ART (Laanani et al., 2015; Peluso et al., 2020), presumably due to the rapid clearance of a pool of relatively short-lived infected cells(Leyre et al., 2020). The frequency of cells actively producing virus within lymphoid tissue also decays more rapidly during the first few years of ART(Banga et al., 2016).
Over a period of years to decades, the circulating reservoir becomes increasingly clonal (Cohn et al., 2015) and is often found in cells that are proliferating (Wagner et al., 2014) and more differentiated (Hiener et al., 2017). As the modern ART era is now over 25 years old, studies of individuals with decades of viral suppression are now possible. The reservoir may be qualitatively different in people treated for short compared to long period of times, and indeed defective and intact proviruses have been shown to decay at different rates during therapy(Peluso et al., 2020).
Characteristics of infected cells in blood and tissue
Cellular reservoirs.
HIV-1 persists in all subsets of memory CD4+ T cells, including the classic memory subsets (memory stem cells, central memory cells, transitional memory cells, and effector memory cells), and a variety of functional subsets (particularly T follicular helper cells, T regulatory cells, Th1 cells and Th17 cells). Although memory cells harbor the bulk of HIV proviral DNA during ART, naïve cells can also contribute to HIV persistence (Roche et al., 2019; Venanzi Rullo et al., 2019). Cells that have intrinsic self-renewing capacity might prove to be the most recalcitrant source of virus during long-term ART. T memory stem and central memory CD4+ T cells (defined by expression of CCR7 and CD27) are generally accepted to be important reservoirs that can differentiate into effector cells (Buzon et al., 2014; Chomont et al., 2009; Jaafoura et al., 2014), the latter of which can expand dramatically. Bone marrow-derived CD34+ hematopoietic stem cells were found to harbor HIV-1 provirus in some but not all studies(Carter et al., 2010; Durand et al., 2012).
Infected memory CD4+ T cells within distinct memory subsets display different HIV transcriptional activity, proviral inducibility and contribution to the pool of cells harboring genetically intact genomes(Grau-Exposito et al., 2019; Kulpa et al., 2019; Kwon et al., 2020; Pardons et al., 2019b). Specifically, more differentiated effector memory cells have been shown to be enriched in intact genomes compared to less differentiated central memory cells (Hiener et al 207). The less quiescent status of effector memory cells has also been associated with greater inducibility in most (Pardons et al., 2019b Kulpa et al. 2019) but not all (Kwon et al 2020) studies. Person-to-person variability in the size and distribution of the viral reservoir is substantial (Chomont et al., 2009; Eriksson et al., 2013), which complicates efforts to design a “one size fit all” curative strategy.
A major focus of ongoing research is the precise characterization of cells harboring latent infection. If a signature phenotype can be identified and validated, then therapies can be developed to eliminate the precise cells harboring the latent reservoir. Due to the rarity of infected cells, this has proven difficult, though significant progress has been made by many independent groups. As might be expected considering the role of cell activation and proliferation in maintaining the reservoir, HIV proviruses are enriched in cells that express the canonical markers of activation HLA-DR (MHC class II)(Horsburgh et al., 2020; Lee et al., 2019a), CD25 (the alpha chain of the IL-2 receptor and a constitutive marker of regulatory T cells)(Tran et al., 2008) and CD69 (a marker of tissue-resident memory T cells)(Cantero-Perez et al., 2019). Therefore, subsets of cells that express HLA-DR, CD25 or CD69, and hence do not fulfill the classical definition of resting CD4+ T cells, contribute to the long-term persistence of HIV during ART. These studies highlight the importance of studying total CD4+ T cells rather than resting CD4+ T cells to comprehensively address the issues of HIV persistence. In addition, HIV-1 proviral DNA has in most (but not all) studies found to be modestly enriched in cells expressing immune checkpoint receptors, programmed cell death-1 (PD-1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), T cell immunoglobulin, and ITIM domain (TIGIT), lymphocyte activation gene 3 (LAG-3), T cell immunoglobulin and mucin 3 (TIM-3) and CD160 (Chew et al., 2016; Fromentin et al., 2016; Pardons et al., 2019a). Importantly, the level of enrichment increased as more receptors were expressed (Chew et al., 2016; Fromentin et al., 2016).
Several CD4+ T cell subsets endowed with specific immune functions are enriched in persistent HIV. In tissues, the B cell follicle is a major viral reservoir, particularly during the first few years of ART. HIV-1 proviruses are highly enriched in CD4+ T follicular helper (Tfh) cells. When these cells circulate, they upregulate expression of the chemokine receptor CXCR3. As expected, such cells tend to harbor more replication-competent HIV-1 provirus (Banga et al., 2018). Several groups have argued that tissue-based Th17 cells are enriched for HIV-1 DNA, presumably because they largely reside in the gut, a preferred site for high levels of virus replication (Pardons et al., 2019a; Wacleche et al., 2016). In another study, clonal expansions of intact genomes were preferentially observed in cells displaying Th1 functions(Lee et al., 2017), a subset that encompasses most virus-specific cells, including HIV specific and CMV-specific CD4+ T cells. Tissue resident memory CD4+ T cells can harbor HIV, as expected, and may be difficult to characterize given that they primarily reside in difficult-to-access tissues (Cantero-Perez et al., 2019).
The role of non-CD4+ T cells as a reservoir is controversial. Although HIV-1 can infect macrophages and may be important in the establishment of chronic infection at the site of viral exposure, it is unclear whether infected macrophages persist during ART (Calantone et al., 2014). In a humanized myeloid-only mouse (MoM) model, macrophages harbored replication-competent HIV-1, but these cells had a limited half-life in vivo and hence would not be expected to be a major reservoir during long-term ART (Honeycutt et al., 2017). As most macrophages exist in hard-to-access tissues, their relevance as a reservoir during ART remains largely undefined.
Tissue reservoirs.
The geographic distribution of HIV-1 within the body is now being mapped by multiple groups. Tissues that are highly enriched with lymphoid structures, particularly lymph nodes and the gut mucosa, contain the largest amount of virus and highest frequency of infected cells (Banga et al., 2016; Chun et al., 2008; Yukl et al., 2010). Precise mapping of the tissue reservoir in living people is not possible. Non-human primate studies and more recently human autopsy studies are better suited to address this issue. In a comprehensive assessment of the reservoir in SIV-infected macaques on effective ART, the vast majority (> 98 %) of the total body reservoir was found in the gut (Estes et al., 2017). The gut is also likely the largest reservoir of HIV-1 in people (Chun et al., 2008; Estes et al., 2017). In a recent prospectively-designed rapid autopsy study (the Last Gift cohort), HIV proviruses were detected in all 28 tissues analyzed (Chaillon et al., 2020). Importantly, lineage phylogenetic analyses revealed that the blood and lymphoid tissue act as the main vehicle for virus dissemination throughout the body. The degree to which low-levels of intact HIV-1 persists in the brain remains controversial, though all 6 donors from the above study had detectable HIV DNA in this organ.
The B cell follicle within lymphoid structures has unique properties that make it ideal to support HIV-1 replication and persistence. In states of inflammation – directly and indirectly induced by HIV-1 infection – germinal centers containing activated Tfh cells develop. To maintain normal B cell function, CD8+ T cells and other effector cells are actively excluded from the follicle, thus providing the virus with an immune sanctuary (Connick et al., 2014; Fukazawa et al., 2015). It has also been argued that antiretroviral drug penetration into the lymph nodes is suboptimal (Fletcher et al., 2014), although the mechanisms for this effect is unknown and the data are inconsistent (Burgunder et al., 2019).
The reproductive tracts are important and poorly understood tissue reservoirs. These tissues tend to be rich in macrophages and might be potential immune sanctuaries. Antiretroviral drug penetration into these tissues may be suboptimal. Recent findings suggest that macrophages within the male genital tract may be an important reservoir (Ganor et al., 2019). Additionally, tissue resident memory CD4+ T cells in the female genital tract (particularly the cervix) are highly enriched with HIV-1 DNA (Cantero-Perez et al., 2019).
Population-specific characteristics of the reservoir
Most studies of HIV-1 persistence have been performed in middle-aged and older men who live in resource rich areas, were infected with subtype-B virus and who started ART during chronic infection. Data from such studies are not fully generalizable as age, sex, HIV subtype, duration of ART and timing of ART initiation are now known to impact the size, distribution and/or activity of the reservoir.
Biological sex.
In the absence of therapy, women generally have lower plasma viral loads than men, particularly in early-stage disease(Gandhi et al., 2002). In the presence of ART, reproductive-aged women have similar levels of cell-associated HIV-1 DNA to well-matched men, but lower levels of cell-associated RNA (in isolated CD4+ T cells) and residual plasma HIV-1 RNA levels (Scully et al., 2019). In Uganda, the size of the replication-competent reservoir that could be activated ex vivo was lower in women than men. These sex-dependent effects may be due to the activity of estrogen and estrogen resceptor-1, which inhibit HIV-1 transcription and blunt the activity of latency reversal agents (LRAs) in vitro (Das et al., 2018).
Age.
The biology of HIV-1 replication is also age dependent. The neonatal and infant immune system are characterized by an abundance of naïve CD4+ T cells and a limited capacity to generate antigen-specific memory cells; it is hence to be expected that the distribution and activity of the reservoir will be unique, but such studies are difficult to perform. When ART is initiated during this period, the reservoir is exceedingly small and the immune system apparently protected(Garcia-Broncano et al., 2019). The latent reservoir in perinatal infection may be slower to reactivate and of lower magnitude compared to adult infection, independent of proviral load(Dhummakupt et al., 2020). Whether this difference in the inducibility of the latent reservoir between adults and children is attributed to differential epigenetic regulations of the provirus or to different location of the reservoir in subsets that are differentially prone to reactivate HIV remains to be determined. In children, the reservoir size is highly dependent on when ART was initiated, with a dose response towards higher levels if therapy was delayed until after the age of one and even more so after the age of five years(Persaud et al., 2014).
The impact of advanced aging on the reservoir is not known. As advance age is associated with profound changes in T cell dynamics (Thome et al., 2014), it is reasonable to assume that the latent reservoir will also evolve over time. In blood, the immune system in the elderly is characterized by lower levels of naïve cells and massive expansion of effector cells, many of which are likely driven by prevalent antigens, particularly CMV. As people and their immune system age, the reservoir is likely to become increasingly clonal and concentrated in these cells. Data on this issue are sparse, but a recent study showed the a clonal virus population can be readily detected in CMV-specific cells from most people (Mendoza et al., 2020). Notably, older age has been associated with slower decays in the reservoir size, as estimated using HIV-1 DNA measurements (Golob et al., 2018)
HIV subtype.
Defining the impact of HIV-1 subtype would require a careful comparison of the latent reservoir in people infected with subtype B (the focus of most studies) and non-B (more prevalent globally). Such studies might prove impossible to perform carefully, as multiple confounders might affect such a comparison (people living in resource rich areas vs. resource poor regions in terms of ART exposure, co-infections and genetic backgrounds). In one comparative study, the latent reservoir was approximately three-fold lower in people infected with HIV-1 living in Uganda compared to those living in Baltimore, MD (Prodger et al., 2017), which may have been due to differences in viral subtype, although multiple other factors differed between these two groups. A higher reservoir in subtype B versus other subtypes was noted in a separate cohort, and mechanistically attributed to a greater activity of the HIV-1 Nef protein, particularly with regard to the down-regulation of HLA class I (which in turn prevents immune clearance) (Omondi et al., 2019).
Timing of ART initiation.
The impact of duration of ART on the reservoir differs among those treated early versus late. A more rapid and sustained decay in the reservoir size has been observed among individuals treated during acute compared to chronic infection (Chun et al., 2007; Hocqueloux et al., 2013). The mechanism for this potential more rapid decay in those treated very early is not known, but might include the preservation of more effective HIV-specific immunity and/or prevention of rapid escape mutations(Lee et al., 2019b; Leyre et al., 2020; Ndhlovu et al., 2019; Takata et al., 2017). It has also been suggested that longer lived cells are less activated during acute infection and hence less likely to become infected, resulting in a reservoir that is enriched in shorter-lived cells(Cheret et al., 2015; Leyre et al., 2020).
Co-infections and inflammation.
HIV-1 induces a chronic inflammatory state that persists during ART. HIV-1-associated harm to the gut mucosa results in chronic systemic exposure to microbial products and more inflammation (Brenchley et al., 2006). HIV-1-related immunodeficiency increases the burden of CMV and other viruses, also contributing to chronic inflammation (Hunt et al., 2011). Although there are exceptions (Gandhi et al., 2017), most studies to date suggest that the inflammatory environment shapes the distribution and reservoir in a complex manner, with more activation leading to higher reservoir sizes (Banga et al., 2016; Chomont et al., 2009; Fromentin et al., 2019). HIV/SIV DNA has been found to be enriched in cells that express markers related to immune activation (e.g., HLA-DR, CCR5, PD-1, CD30) (Lee et al., 2019a; McGary et al., 2017; Pardons et al., 2019a; Thornhill et al., 2019). The inflammatory environment of the lymphoid tissues is widely assumed to cause immune dysfunction and reduced immunity to HIV-1.
These observations collectively argue that any chronic inflammatory state will alter the relationship between the host immune system and the latent reservoir. Therefore, regional differences driven by prevalent co-infections or microbial products may have important implications for any future cure strategy. Intensive investigations of these issues are needed.
Therapeutic versus immunologic control.
There are ample data indicating that sustained immunologic control of HIV/SIV is possible. A small proportion of individuals naturally control HIV replication in the absence of therapy (“elite controllers”). A recently appreciated subset of elite controllers have particularly low reservoir sizes and a normal immune system; cohorts of these “exceptional controllers” are now being assembled as they may prove useful as a model for an ideal remission or cure (Canoui et al., 2017; Casado et al., 2020; Mendoza et al., 2012). A small proportion of individuals present with high levels of viremia, go on effective ART for years, interrupt therapy and subsequently maintain durable virus control (“post-treatment controllers”) (Namazi et al., 2018; Saez-Cirion et al., 2013). Finally, in the modern era of cure studies, a number of promising immune therapies have generated sustained control of SIV in non-human primates (Borducchi et al., 2016; Borducchi et al., 2018; Hansen et al., 2013) and perhaps in people (Mendoza et al., 2018; Niessl et al., 2020). In all four of these unique phenotypes, control is likely mediated in large part by virus-specific T cells that are functional and target vulnerable and conserved regions, although other likely pathways are involved.
The circulating reservoir in those controlling HIV via a sustained host-response is often much smaller than in typical person on ART(Hatano et al., 2009; Saez-Cirion et al., 2013; Sharaf et al., 2018). This small reservoir may be both a cause and a consequence of sustained control(Conway and Perelson, 2015). In contrast to those on ART, the virus population in most elite controllers replicates and evolves (Mens et al., 2010). Some (Miura et al., 2009) but not all (Salgado et al., 2014) studies have found that the virus population in controllers is less fit than that in non-controllers. Finally, even though the replicating population in blood is low in controllers, it may remain high in tissues, particularly in the B cell follicle, an immune-privileged region lacking in CD8+ T cells (Boritz et al., 2016; Fukazawa et al., 2015). As many immunotherapies aim to recapitulate exceptional and/or post-treatment control, more studies detailing the mechanisms associated with these unique clinical phenotypes are warranted.
Reservoir eradication and control strategies in the clinic
Knowledge regarding how the reservoir is maintained, how it evolves and where it resides has direct implications for the HIV-1 cure agenda (Figure 3).
Figure 3. Clinical strategies for eradication.
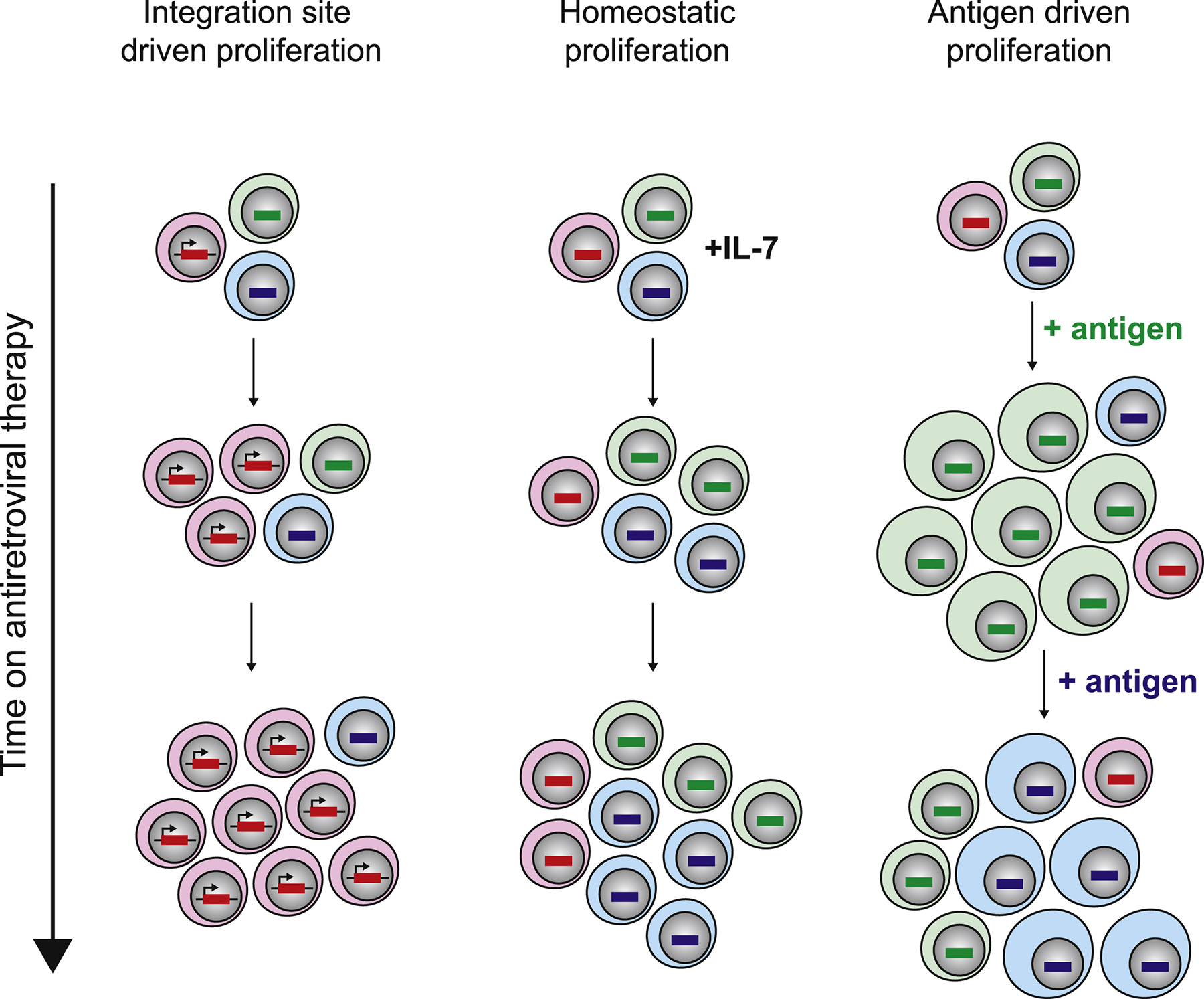
Strategies are divided into those which aim to reduce the size of the reservoir, control viral rebound, or silence the reservoir.
Antiretroviral therapy.
Given the slow rate of virus decay during ART, current therapies are unlikely to be curative. As some people have unusually low reservoirs and others have rapid decay rates(Leyre et al., 2020; Peluso et al., 2020), it remains possible that some will be cured after decades of ART. If, as proposed by some (Buzon et al., 2010; Fletcher et al., 2014; Lorenzo-Redondo et al., 2016), ongoing virus spread and reseeding of the reservoir continues during otherwise effective ART, then more potent drugs with the capacity to penetrate all tissue reservoirs might accelerate that decay for the virus, in some leading eventually to a cure. As any residual virus spread is assumed to be local, definitive studies on this issue will be challenging, even as more potent drugs emerge.
The transition from the untreated to the treated state provides unique opportunities for curative interventions. During long-term ART, much of the reservoir apparently derives from cells that were infected just prior to treatment initiation, arguing that in untreated disease the putative reservoir is unstable (Abrahams et al., 2019; Brodin et al., 2016). This implies that during early ART, the rapidly changing immune environment shifts the balance toward a state in which latency can be achieved. Presumably, the massive reductions in HIV-1-associated inflammation and T cell activation reduces the turnover of the reservoir, leading to generation of longer-lived cells harboring intact genomes. Immune stimulation under the cover of ART (preferably with co-administration of a therapy that induces killing of infected cells) might work best during this window of opportunity.
Latency reversal.
The molecular mechanisms maintaining latency are now being actively targeted with the goal of reversing transcriptional silencing (“latency reversal”), thus inducing cells to produce viral RNA and proteins, which in turn makes the cell recognizable to the immune system or immunotherapies (“shock and kill” strategy). The first generation of latency reversal agents (LRAs) successfully induced RNA production, but only some drugs (and in only some studies) induced the production of proteins and viral particles. None of these interventions caused a reduction in the reservoir size, presumably because the degree of latency reversal was limited and/or the immune system was not primed to clear antigen-expressing cells(Kim et al., 2018).
The next generations of LRAs are more promising. Pre-clinical data suggest that small-molecule inhibitor of apoptosis antagonists, particularly the SMAC mimetic compounds, induces reversal of latency (Pache et al., 2015). Importantly, these drugs have demonstrated potent and consistent activities in multiple animal models and by independent teams(Nixon et al., 2020). Studies in people have not yet started.
Immune stimulating approaches that activate CD4+ T cells might also prove useful. TLR-7 agonists have demonstrated direct latency reversing activity in non-human primates (Lim et al., 2018) but the effect was not confirmed in subsequent studies (Del Prete et al., 2019). When used in combination with other immunotherapies in non-human primates, TLR-7 agonists induced a state of remission and perhaps even a complete cure (Borducchi et al., 2016; Borducchi et al., 2018); the mechanism for this effect may have been via their immune-enhancing activities rather than as latency reversing agents. Other TLR agonists and cytokines (particularly IL-15) are being actively studied.
Latency silencing.
If the virus cannot be fully induced and cleared, can it be permanently silenced? Deep and irreversible latency of HIV-1 would not be unprecedented. A substantial proportion of the human genome consists of ancient retrovirus DNA that is not transcribed, except under rare circumstances. These human endogenous retroviruses (HERVs) are silenced through epigenetic mechanisms including DNA methylation(Schulz et al., 2006). Although not definitely proven, it is likely that latent HIV proviruses are regulated similarly to HERVs. Indeed, there are emerging data that through multiple mechanisms, over time the HIV genome becomes preferentially enriched in intergenic regions and/or become hypermethylated, resulting in less expression (Einkauf et al., 2019). This evolution of proviral distribution likely reflects the survival of cells with defective viruses and viruses in regions of the genome that allow for “deep latency.”
Identifying therapeutics that accelerate this process is a high priority. Proof-of-concept studies involved didehydro-cortistatin A (dCA), an inhibitor of the viral transcriptional activator Tat (Mousseau et al., 2015). In humanized mice, dCA administration during ART reduced reservoir activation post-treatment, although the effect was modest (Kessing et al., 2017). In a large screen of other potential latency silencing approaches, inhibitors of mTOR were identified as promising candidates for block and lock strategies (Besnard et al., 2016). This approach is being studied in the clinic.
Immunotherapy.
There is a robust and rapidly expanding pre-clinical and early clinical research program aimed at inducing a sustained HIV-1-specific immune response that can effectively clear infected cells during ART and/or control any persistently infected cells post-ART cessation.
Immunotherapies that directly target cells expressing the envelop protein for killing are particularly attractive. A number of broadly neutralizing antibodies (bNAbs) that target the envelope glycoprotein suppress virus replication in vivo (Bar et al., 2016; Mendoza et al., 2018), presumably due to their ability to block virus entry and virus spread. Theoretically, bNAbs might also bind envelope proteins expressed on the cell surface and trigger host-mediated cytotoxicity, which could reduce the active reservoir. Whether cell killing happens in vivo remains unproven, although indirect evidence suggests it may have some effect (Lu et al., 2016). Similar to bNAbs, emerging HIV-specific CAR-T cells target envelop on the cell surface, which might lead to reservoir reduction during ART or virus-control post-ART. Several approaches are moving toward the clinic.
Antibodies and CAR-T cells share the same potential limitations: both require that the epitope be expressed on the cell surface at high densities and both target the highly variable envelope glycoprotein. In a recent pre-clinical series of studies involving a novel HIV-1-specific CAR-T cell approach, efficacy was largely dependent of the levels of envelope expression in the targeted cells (Herzig et al., 2019). These therapies may only work when used in combination with latency reversing agents or during a treatment interruption.
A number of strategies aimed at enhancing control are undergoing evaluation in early proof-of-concept studies. Even if bNAbs are unable to clear reservoir cells directly, they hae been postulated to for highly immunogenic antibody/antigen responses, which in might stimulate potent HIV-specific immune responses and post-ART control (Niessl et al., 2020). Leveraging advances made in the management of cancer, several groups are studying immune checkpoint blockade. This effect is justified in part by the enrichment for HIV proviruses in CD4+ T cells expressing immune checkpoint molecules(Chew et al., 2016; Fromentin et al., 2016), by the ability of antibodies against PD-1 and CTLA-4 to enhance latency reversal(Evans et al., 2018; Fromentin et al., 2019), and by the well-known capacity of these approaches to boost antigen-specific T cell responses. Although immune checkpoint blockade led to a marked decrease in markers of HIV persistence in case report studies(Fromentin et al., 2019; Guihot et al., 2018), two NIH-sponsored clinical trials were prematurely terminated due to unacceptable toxicity(Gay et al., 2017), indicating that the dose of immune checkpoint blockers will need to be adjusted if additional studies are to be conducted.
Clonal proliferation.
The reservoir is largely maintained by the proliferation and expansion of memory CD4+ T cells. Treatment with IL-7 – a key cytokine driving T cell homeostasis – increases the proliferation and expansion of memory T cells, and the reservoir size increases accordingly (Vandergeeten et al., 2013). Any cytoreductive intervention that reduces the total CD4+ T cell count would have the opposite effect, as demonstrated in the most extreme cases with ablative chemotherapy followed by allogenic stem transplants, but ultimately a small reservoir of infected progenitor cells persists, providing a self-renewing source for continued clonal expansions as the immune system is reconstituted (Henrich et al., 2017b). If proliferation is to be targeted, the ideal intervention would selectively block infected over uninfected cells from expanding, thus allowing the potential for the total memory population to be slowly enriched for uninfected cells. Alternatively, should some chronic antigen be found to be driving the continued proliferation of memory cells harboring intact HIV-1 then targeting this antigen might reduce the reservoir. CMV, gut microbes and other persistent antigens that are enhanced by HIV-1 might be targeted (Figure 3).
Irrespective of the strategy to be used to either induce or limit T cell expansions in the reservoir, the core of the reservoir is likely to lie in stem and central memory cells (Buzon et al., 2014; Jaafoura et al., 2014) which have a unique capacity to self-renew upon antigen stimulation. Even if most of the “visible” reservoir of intact genomes is seen in cells displaying a more differentiated phenotype following clonal expansions, a small frequency of poorly differentiated parent cells with high survival capacities may represent long-lived sources of infected cells during ART.
Gene editing.
Once the cellular and tissue reservoirs of HIV are carefully characterized, it may be possible to design therapies that can deliver gene editing technologies (e.g., Zinc-finger nucleases or CRISPR-Cas9) that disrupt the provirus(Dash et al., 2019). To be curative, all infected cells need to be treated, which will be challenging due to the rarity and the diversity of integrated proviruses even within a single individual. Disruption of the virus co-receptor CCR5 and other pathways needed for virus replication might be easier to achieve; in these cases, the gene edits might not need to be 100% effective, as simply reducing the number of susceptible targets blunt the massive amplification of the virus population that occurs when a systemic infection is established (Davenport et al., 2019; Hataye et al., 2019).
Conclusions
Despite progress in both the prevention and treatment, developing an effective and scalable cure for HIV-1 remains is now a global public health priority (Deeks et al., 2016). Antiretroviral therapy fails to cure individuals due a long-lived latent reservoir of infected cells. Since its initial description in 1997, advances in technology have enabled immense progress in understanding the biological characteristics of this reservoir. Continued progress elucidating the biology will enhance our ability to develop effective curative interventions. Further study is required to uncover the contribution of infected cells in the periphery to viral rebound, and a method to assess the whole-body virus burden in people living with HIV-1 is urgently needed. It is also possible that a sterilizing cure may not be achievable. In this case, additional insight into immune control of HIV-1, possibly through the study of individuals who spontaneously control HIV-1 infection, will prove to be important. Finally, as new therapies emerge, it is critically important that funding, existing networks and infrastructure, and education provide access to all communities across the globe.
Acknowledgements
This work was supported by the Chan Zuckerberg Biohub, the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health (UM1AI126611), the Canadian HIV Cure Enterprise (CanCURE) Team Grant HIG-133050 from the CIHR in partnership with CANFAR. N.C. is supported by Research Scholar Career Awards of the FRQ-S (#253292). S.G.D. is supported by the amfAR Institute for HIV Cure Research (amfAR 109301) and the Delaney AIDS Research Enterprise (DARE; A127966).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
In this review, Cohn et al. examine the mechanisms that contribute to HIV persistence during antiretroviral therapy. Topics include discussions on latent versus active reservoir, the size and distribution of the reservoir in different populations, and emerging therapeutic strategies aimed at curing the infection.
References
- Abdel-Mohsen M, Kuri-Cervantes L, Grau-Exposito J, Spivak AM, Nell RA, Tomescu C, Vadrevu SK, Giron LB, Serra-Peinado C, Genesca M, et al. (2018). CD32 is expressed on cells with transcriptionally active HIV but does not enrich for HIV DNA in resting T cells. Sci Transl Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahams MR, Joseph SB, Garrett N, Tyers L, Moeser M, Archin N, Council OD, Matten D, Zhou S, Doolabh D, et al. (2019). The replication-competent HIV-1 latent reservoir is primarily established near the time of therapy initiation. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga R, Procopio FA, Noto A, Pollakis G, Cavassini M, Ohmiti K, Corpataux JM, de Leval L, Pantaleo G, and Perreau M (2016). PD-1 and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med. [DOI] [PubMed] [Google Scholar]
- Banga R, Procopio FA, Ruggiero A, Noto A, Ohmiti K, Cavassini M, Corpataux JM, Paxton WA, Pollakis G, and Perreau M (2018). Blood CXCR3(+) CD4 T Cells Are Enriched in Inducible Replication Competent HIV in Aviremic Antiretroviral Therapy-Treated Individuals. Front Immunol 9, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, Petrone ME, Salantes DB, Seamon CA, Scheinfeld B, Kwan RW, et al. (2016). Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. N Engl J Med 375, 2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard E, Hakre S, Kampmann M, Lim HW, Hosmane NN, Martin A, Bassik MC, Verschueren E, Battivelli E, Chan J, et al. (2016). The mTOR Complex Controls HIV Latency. Cell Host Microbe 20, 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borducchi EN, Cabral C, Stephenson KE, Liu J, Abbink P, Ng’ang’a D, Nkolola JP, Brinkman AL, Peter L, Lee BC, et al. (2016). Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature 540, 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borducchi EN, Liu J, Nkolola JP, Cadena AM, Yu WH, Fischinger S, Broge T, Abbink P, Mercado NB, Chandrashekar A, et al. (2018). Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 563, 360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boritz EA, Darko S, Swaszek L, Wolf G, Wells D, Wu X, Henry AR, Laboune F, Hu J, Ambrozak D, et al. (2016). Multiple Origins of Virus Persistence during Natural Control of HIV Infection. Cell 166, 1004–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosque A, Famiglietti M, Weyrich AS, Goulston C, and Planelles V (2011). Homeostatic proliferation fails to efficiently reactivate HIV-1 latently infected central memory CD4+ T cells. PLoS Pathog 7, e1002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. (2006). Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12, 1365–1371. [DOI] [PubMed] [Google Scholar]
- Brodin J, Zanini F, Thebo L, Lanz C, Bratt G, Neher RA, and Albert J (2016). Establishment and stability of the latent HIV-1 DNA reservoir. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner KM, Murray AJ, Pollack RA, Soliman MG, Laskey SB, Capoferri AA, Lai J, Strain MC, Lada SM, Hoh R, et al. (2016). Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgunder E, Fallon JK, White N, Schauer AP, Sykes C, Remling-Mulder L, Kovarova M, Adamson L, Luciw P, Garcia JV, et al. (2019). Antiretroviral Drug Concentrations in Lymph Nodes: A Cross-Species Comparison of the Effect of Drug Transporter Expression, Viral Infection, and Sex in Humanized Mice, Nonhuman Primates, and Humans. J Pharmacol Exp Ther 370, 360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzon MJ, Massanella M, Llibre JM, Esteve A, Dahl V, Puertas MC, Gatell JM, Domingo P, Paredes R, Sharkey M, et al. (2010). HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med 16, 460–465. [DOI] [PubMed] [Google Scholar]
- Buzon MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, Martin-Gayo E, Leng J, Henrich TJ, Li JZ, et al. (2014). HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med 20, 139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calantone N, Wu F, Klase Z, Deleage C, Perkins M, Matsuda K, Thompson EA, Ortiz AM, Vinton CL, Ourmanov I, et al. (2014). Tissue myeloid cells in SIV-infected primates acquire viral DNA through phagocytosis of infected T cells. Immunity 41, 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canoui E, Lecuroux C, Avettand-Fenoel V, Gousset M, Rouzioux C, Saez-Cirion A, Meyer L, Boufassa F, Lambotte O, Noel N, et al. (2017). A Subset of Extreme Human Immunodeficiency Virus (HIV) Controllers Is Characterized by a Small HIV Blood Reservoir and a Weak T-Cell Activation Level. Open Forum Infect Dis 4, ofx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero-Perez J, Grau-Exposito J, Serra-Peinado C, Rosero DA, Luque-Ballesteros L, Astorga-Gamaza A, Castellvi J, Sanhueza T, Tapia G, Lloveras B, et al. (2019). Resident memory T cells are a cellular reservoir for HIV in the cervical mucosa. Nat Commun 10, 4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CC, Onafuwa-Nuga A, McNamara LA, Riddell J.t., Bixby D, Savona MR, and Collins KL (2010). HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med 16, 446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado C, Galvez C, Pernas M, Tarancon-Diez L, Rodriguez C, Sanchez-Merino V, Vera M, Olivares I, De Pablo-Bernal R, Merino-Mansilla A, et al. (2020). Permanent control of HIV-1 pathogenesis in exceptional elite controllers: a model of spontaneous cure. Sci Rep 10, 1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaillon A, Gianella S, Dellicour S, Rawlings SA, Schlub TE, De Oliveira MF, Ignacio C, Porrachia M, Vrancken B, and Smith DM (2020). HIV persists throughout deep tissues with repopulation from multiple anatomical sources. J Clin Invest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheret A, Bacchus-Souffan C, Avettand-Fenoel V, Melard A, Nembot G, Blanc C, Samri A, Saez-Cirion A, Hocqueloux L, Lascoux-Combe C, et al. (2015). Combined ART started during acute HIV infection protects central memory CD4+ T cells and can induce remission. J Antimicrob Chemother 70, 2108–2120. [DOI] [PubMed] [Google Scholar]
- Chew GM, Fujita T, Webb GM, Burwitz BJ, Wu HL, Reed JS, Hammond KB, Clayton KL, Ishii N, Abdel-Mohsen M, et al. (2016). TIGIT Marks Exhausted T Cells, Correlates with Disease Progression, and Serves as a Target for Immune Restoration in HIV and SIV Infection. PLoS Pathog 12, e1005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, et al. (2009). HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 15, 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Justement JS, Moir S, Hallahan CW, Maenza J, Mullins JI, Collier AC, Corey L, and Fauci AS (2007). Decay of the HIV Reservoir in Patients Receiving Antiretroviral Therapy for Extended Periods: Implications for Eradication of Virus. J Infect Dis 195, 1762–1764. [DOI] [PubMed] [Google Scholar]
- Chun TW, Nickle DC, Justement JS, Meyers JH, Roby G, Hallahan CW, Kottilil S, Moir S, Mican JM, Mullins JI, et al. (2008). Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis 197, 714–720. [DOI] [PubMed] [Google Scholar]
- Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, and Fauci AS (1997). Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A 94, 13193–13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn LB, Silva IT, Oliveira TY, Rosales RA, Parrish EH, Learn GH, Hahn BH, Czartoski JL, McElrath MJ, Lehmann C, et al. (2015). HIV-1 integration landscape during latent and active infection. Cell 160, 420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby DJ, Trautmann L, Pinyakorn S, Leyre L, Pagliuzza A, Kroon E, Rolland M, Takata H, Buranapraditkun S, Intasan J, et al. (2018). Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med 24, 923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connick E, Folkvord JM, Lind KT, Rakasz EG, Miles B, Wilson NA, Santiago ML, Schmitt K, Stephens EB, Kim HO, et al. (2014). Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL. J Immunol 193, 5613–5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway JM, and Perelson AS (2015). Post-treatment control of HIV infection. Proc Natl Acad Sci U S A 112, 5467–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks AM, Bateson R, Cope AB, Dahl NP, Griggs MK, Kuruc JD, Gay CL, Eron JJ, Margolis DM, Bosch RJ, et al. (2015). Precise Quantitation of the Latent HIV-1 Reservoir: Implications for Eradication Strategies. J Infect Dis 212, 1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Dobrowolski C, Luttge B, Valadkhan S, Chomont N, Johnston R, Bacchetti P, Hoh R, Gandhi M, Deeks SG, et al. (2018). Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc Natl Acad Sci U S A 115, E7795–E7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Kaminski R, Bella R, Su H, Mathews S, Ahooyi TM, Chen C, Mancuso P, Sariyer R, Ferrante P, et al. (2019). Sequential LASER ART and CRISPR Treatments Eliminate HIV-1 in a Subset of Infected Humanized Mice. Nat Commun 10, 2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MP, Khoury DS, Cromer D, Lewin SR, Kelleher AD, and Kent SJ (2019). Functional cure of HIV: the scale of the challenge. Nat Rev Immunol 19, 45–54. [DOI] [PubMed] [Google Scholar]
- Deeks SG, Lewin SR, Ross AL, Ananworanich J, Benkirane M, Cannon P, Chomont N, Douek D, Lifson JD, Lo YR, et al. (2016). International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med 22, 839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete GQ, Alvord WG, Li Y, Deleage C, Nag M, Oswald K, Thomas JA, Pyle C, Bosche WJ, Coalter V, et al. (2019). TLR7 agonist administration to SIV-infected macaques receiving early initiated cART does not induce plasma viremia. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhummakupt A, Rubens JH, Anderson T, Powell L, Nonyane BA, Siems LV, Collinson-Streng A, Nilles T, Jones RB, Tepper V, et al. (2020). Differences in inducibility of the latent HIV reservoir in perinatal and adult infection. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douek DC, Betts MR, Brenchley JM, Hill BJ, Ambrozak DR, Ngai KL, Karandikar NJ, Casazza JP, and Koup RA (2002). A novel approach to the analysis of specificity, clonality, and frequency of HIV-specific T cell responses reveals a potential mechanism for control of viral escape. J Immunol 168, 3099–3104. [DOI] [PubMed] [Google Scholar]
- Durand CM, Ghiaur G, Siliciano JD, Rabi SA, Eisele EE, Salgado M, Shan L, Lai JF, Zhang H, Margolick J, et al. (2012). HIV-1 DNA is detected in bone marrow populations containing CD4+ T cells but is not found in purified CD34+ hematopoietic progenitor cells in most patients on antiretroviral therapy. J Infect Dis 205, 1014–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einkauf KB, Lee GQ, Gao C, Sharaf R, Sun X, Hua S, Chen SM, Jiang C, Lian X, Chowdhury FZ, et al. (2019). Intact HIV-1 proviruses accumulate at distinct chromosomal positions during prolonged antiretroviral therapy. J Clin Invest 129, 988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, Lysenko ES, Bosch RJ, Lai J, Chioma S, Emad F, et al. (2013). Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog 9, e1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes JD, Kityo C, Ssali F, Swainson L, Makamdop KN, Del Prete GQ, Deeks SG, Luciw PA, Chipman JG, Beilman GJ, et al. (2017). Defining total-body AIDS-virus burden with implications for curative strategies. Nat Med 23, 1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans VA, van der Sluis RM, Solomon A, Dantanarayana A, McNeil C, Garsia R, Palmer S, Fromentin R, Chomont N, Sekaly RP, et al. (2018). Programmed cell death-1 contributes to the establishment and maintenance of HIV-1 latency. Aids 32, 1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, et al. (1999). Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 5, 512–517. [DOI] [PubMed] [Google Scholar]
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, et al. (1997). Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300. [DOI] [PubMed] [Google Scholar]
- Fletcher CV, Staskus K, Wietgrefe SW, Rothenberger M, Reilly C, Chipman JG, Beilman GJ, Khoruts A, Thorkelson A, Schmidt TE, et al. (2014). Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromentin R, Bakeman W, Lawani MB, Khoury G, Hartogensis W, DaFonseca S, Killian M, Epling L, Hoh R, Sinclair E, et al. (2016). CD4+ T Cells Expressing PD-1, TIGIT and LAG-3 Contribute to HIV Persistence during ART. PLoS Pathog 12, e1005761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromentin R, DaFonseca S, Costiniuk CT, El-Far M, Procopio FA, Hecht FM, Hoh R, Deeks SG, Hazuda DJ, Lewin SR, et al. (2019). PD-1 blockade potentiates HIV latency reversal ex vivo in CD4(+) T cells from ART-suppressed individuals. Nat Commun 10, 814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, Hagen SI, Shoemaker R, Deleage C, Lucero C, et al. (2015). B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 21, 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, and Greenblatt RM (2002). Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis 35, 313–322. [DOI] [PubMed] [Google Scholar]
- Gandhi RT, McMahon DK, Bosch RJ, Lalama CM, Cyktor JC, Macatangay BJ, Rinaldo CR, Riddler SA, Hogg E, Godfrey C, et al. (2017). Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog 13, e1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganor Y, Real F, Sennepin A, Dutertre CA, Prevedel L, Xu L, Tudor D, Charmeteau B, Couedel-Courteille A, Marion S, et al. (2019). HIV-1 reservoirs in urethral macrophages of patients under suppressive antiretroviral therapy. Nat Microbiol 4, 633–644. [DOI] [PubMed] [Google Scholar]
- Garcia-Broncano P, Maddali S, Einkauf KB, Jiang C, Gao C, Chevalier J, Chowdhury FZ, Maswabi K, Ajibola G, Moyo S, et al. (2019). Early antiretroviral therapy in neonates with HIV-1 infection restricts viral reservoir size and induces a distinct innate immune profile. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay CL, Bosch RJ, Ritz J, Hataye JM, Aga E, Tressler RL, Mason SW, Hwang CK, Grasela DM, Ray N, et al. (2017). Clinical Trial of the Anti-PD-L1 Antibody BMS-936559 in HIV-1 Infected Participants on Suppressive Antiretroviral Therapy. J Infect Dis 215, 1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golob JL, Stern J, Holte S, Kitahata MM, Crane HM, Coombs RW, Goecker E, Woolfrey AE, and Harrington RD (2018). HIV DNA levels and decay in a cohort of 111 long-term virally suppressed patients. Aids 32, 2113–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau-Exposito J, Luque-Ballesteros L, Navarro J, Curran A, Burgos J, Ribera E, Torrella A, Planas B, Badia R, Martin-Castillo M, et al. (2019). Latency reversal agents affect differently the latent reservoir present in distinct CD4+ T subpopulations. PLoS Pathog 15, e1007991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guihot A, Marcelin AG, Massiani MA, Samri A, Soulie C, Autran B, and Spano JP (2018). Drastic decrease of the HIV reservoir in a patient treated with nivolumab for lung cancer. Ann Oncol 29, 517–518. [DOI] [PubMed] [Google Scholar]
- Hansen SG, Jr MP, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, et al. (2013). Immune clearance of highly pathogenic SIV infection. Nature 502, 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano H, Delwart EL, Norris PJ, Lee TH, Dunn-Williams J, Hunt PW, Hoh R, Stramer SL, Linnen JM, McCune JM, et al. (2009). Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol 83, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hataye JM, Casazza JP, Best K, Liang CJ, Immonen TT, Ambrozak DR, Darko S, Henry AR, Laboune F, Maldarelli F, et al. (2019). Principles Governing Establishment versus Collapse of HIV-1 Cellular Spread. Cell Host Microbe 26, 748–763 e720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich TJ, Hatano H, Bacon O, Hogan LE, Rutishauser R, Hill A, Kearney MF, Anderson EM, Buchbinder SP, Cohen SE, et al. (2017a). HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study. PLoS Med 14, e1002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich TJ, Hobbs KS, Hanhauser E, Scully E, Hogan LE, Robles YP, Leadabrand KS, Marty FM, Palmer CD, Jost S, et al. (2017b). Human Immunodeficiency Virus Type 1 Persistence Following Systemic Chemotherapy for Malignancy. J Infect Dis 216, 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig E, Kim KC, Packard TA, Vardi N, Schwarzer R, Gramatica A, Deeks SG, Williams SR, Landgraf K, Killeen N, et al. (2019). Attacking Latent HIV with convertibleCAR-T Cells, a Highly Adaptable Killing Platform. Cell 179, 880–894 e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hey-Nguyen WJ, Bailey M, Xu Y, Suzuki K, Van Bockel D, Finlayson R, Leigh Brown A, Carr A, Cooper DA, Kelleher AD, et al. (2019). HIV-1 DNA Is Maintained in Antigen-Specific CD4+ T Cell Subsets in Patients on Long-Term Antiretroviral Therapy Regardless of Recurrent Antigen Exposure. AIDS Res Hum Retroviruses 35, 112–120. [DOI] [PubMed] [Google Scholar]
- Hiener B, Horsburgh BA, Eden JS, Barton K, Schlub TE, Lee E, von Stockenstrom S, Odevall L, Milush JM, Liegler T, et al. (2017). Identification of Genetically Intact HIV-1 Proviruses in Specific CD4(+) T Cells from Effectively Treated Participants. Cell Rep 21, 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, Lai J, Blankson JN, Siliciano JD, and Siliciano RF (2013). Replication-Competent Noninduced Proviruses in the Latent Reservoir Increase Barrier to HIV-1 Cure. Cell 155, 540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocqueloux L, Avettand-Fenoel V, Jacquot S, Prazuck T, Legac E, Melard A, Niang M, Mille C, Le Moal G, Viard JP, et al. (2013). Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother 68, 1169–1178. [DOI] [PubMed] [Google Scholar]
- Hogan LE, Vasquez J, Hobbs KS, Hanhauser E, Aguilar-Rodriguez B, Hussien R, Thanh C, Gibson EA, Carvidi AB, Smith LCB, et al. (2018). Increased HIV-1 transcriptional activity and infectious burden in peripheral blood and gut-associated CD4+ T cells expressing CD30. PLoS Pathog 14, e1006856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt JB, Thayer WO, Baker CE, Ribeiro RM, Lada SM, Cao Y, Cleary RA, Hudgens MG, Richman DD, and Garcia JV (2017). HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nat Med 23, 638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh BA, Lee E, Hiener B, Eden JS, Schlub TE, von Stockenstrom S, Odevall L, Milush JM, Liegler T, Sinclair E, et al. (2020). High levels of genetically-intact HIV in HLA-DR+ memory T-cells indicates their value for reservoir studies. Aids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, Tracy RP, Corey L, and Deeks SG (2011). Valganciclovir Reduces T Cell Activation in HIV-infected Individuals With Incomplete CD4+ T Cell Recovery on Antiretroviral Therapy. J Infect Dis 203, 1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamichi H, Smith M, Adelsberger JW, Izumi T, Scrimieri F, Sherman BT, Rehm CA, Imamichi T, Pau A, Catalfamo M, et al. (2020). Defective HIV-1 proviruses produce viral proteins. Proc Natl Acad Sci U S A 117, 3704–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaafoura S, de Goer de Herve MG, Hernandez-Vargas EA, Hendel-Chavez H, Abdoh M, Mateo MC, Krzysiek R, Merad M, Seng R, Tardieu M, et al. (2014). Progressive contraction of the latent HIV reservoir around a core of less-differentiated CD4(+) memory T Cells. Nat Commun 5, 5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessing CF, Nixon CC, Li C, Tsai P, Takata H, Mousseau G, Ho PT, Honeycutt JB, Fallahi M, Trautmann L, et al. (2017). In Vivo Suppression of HIV Rebound by Didehydro-Cortistatin A, a “Block-and-Lock” Strategy for HIV-1 Treatment. Cell Rep 21, 600–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Anderson JL, and Lewin SR (2018). Getting the “Kill” into “Shock and Kill”: Strategies to Eliminate Latent HIV. Cell Host Microbe 23, 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulpa DA, Talla A, Brehm JH, Ribeiro SP, Yuan S, Bebin-Blackwell AG, Miller M, Barnard R, Deeks SG, Hazuda D, et al. (2019). Differentiation into an Effector Memory Phenotype Potentiates HIV-1 Latency Reversal in CD4(+) T Cells. J Virol 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KJ, Timmons AE, Sengupta S, Simonetti FR, Zhang H, Hoh R, Deeks SG, Siliciano JD, and Siliciano RF (2020). Different human resting memory CD4(+) T cell subsets show similar low inducibility of latent HIV-1 proviruses. Sci Transl Med 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laanani M, Ghosn J, Essat A, Melard A, Seng R, Gousset M, Panjo H, Mortier E, Girard PM, Goujard C, et al. (2015). Impact of the Timing of Initiation of Antiretroviral Therapy During Primary HIV-1 Infection on the Decay of Cell-Associated HIV-DNA. Clin Infect Dis 60, 1715–1721. [DOI] [PubMed] [Google Scholar]
- Lee E, Bacchetti P, Milush J, Shao W, Boritz E, Douek D, Fromentin R, Liegler T, Hoh R, Deeks SG, et al. (2019a). Memory CD4 + T-Cells Expressing HLA-DR Contribute to HIV Persistence During Prolonged Antiretroviral Therapy. Front Microbiol 10, 2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GQ, Orlova-Fink N, Einkauf K, Chowdhury FZ, Sun X, Harrington S, Kuo HH, Hua S, Chen HR, Ouyang Z, et al. (2017). Clonal expansion of genome-intact HIV-1 in functionally polarized Th1 CD4+ T cells. J Clin Invest 127, 2689–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GQ, Reddy K, Einkauf KB, Gounder K, Chevalier JM, Dong KL, Walker BD, Yu XG, Ndung’u T, and Lichterfeld M (2019b). HIV-1 DNA sequence diversity and evolution during acute subtype C infection. Nat Commun 10, 2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyre L, Kroon E, Vandergeeten C, Sacdalan C, Colby DJ, Buranapraditkun S, Schuetz A, Chomchey N, de Souza M, Bakeman W, et al. (2020). Abundant HIV-infected cells in blood and tissues are rapidly cleared upon ART initiation during acute HIV infection. Sci Transl Med 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, Osuna CE, Hraber PT, Hesselgesser J, Gerold JM, Barnes TL, Sanisetty S, Seaman MS, Lewis MG, Geleziunas R, et al. (2018). TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Sci Transl Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo-Redondo R, Fryer HR, Bedford T, Kim EY, Archer J, Kosakovsky Pond SL, Chung YS, Penugonda S, Chipman JG, Fletcher CV, et al. (2016). Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 530, 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CL, Murakowski DK, Bournazos S, Schoofs T, Sarkar D, Halper-Stromberg A, Horwitz JA, Nogueira L, Golijanin J, Gazumyan A, et al. (2016). Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science 352, 1001–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldarelli F, Wu X, Su L, Simonetti FR, Shao W, Hill S, Spindler J, Ferris AL, Mellors JW, Kearney MF, et al. (2014). HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345, 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGary CS, Deleage C, Harper J, Micci L, Ribeiro SP, Paganini S, Kuri-Cervantes L, Benne C, Ryan ES, Balderas R, et al. (2017). CTLA-4(+)PD-1(−) Memory CD4(+) T Cells Critically Contribute to Viral Persistence in Antiretroviral Therapy-Suppressed, SIV-Infected Rhesus Macaques. Immunity 47, 776–788 e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus WR, Bale MJ, Spindler J, Wiegand A, Musick A, Patro SC, Sobolewski MD, Musick VK, Anderson EM, Cyktor JC, et al. (2019). HIV-1 in lymph nodes is maintained by cellular proliferation during antiretroviral therapy. J Clin Invest 130, 4629–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza D, Johnson SA, Peterson BA, Natarajan V, Salgado M, Dewar RL, Burbelo PD, Doria-Rose NA, Graf EH, Greenwald JH, et al. (2012). Comprehensive analysis of unique cases with extraordinary control over HIV replication. Blood 119, 4645–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, Millard K, Lehmann C, Suarez I, Oliveira TY, Lorenzi JCC, et al. (2018). Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561, 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza P, Jackson JR, Oliveira T, Gaebler C, Ramos V, Caskey M, Jankovic M, Nussenzweig MC, and Cohn LB (2020). Antigen responsive CD4+ T cell clones contribute to the HIV-1 latent reservoir. bioRvix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mens H, Kearney M, Wiegand A, Shao W, Schonning K, Gerstoft J, Obel N, Maldarelli F, Mellors JW, Benfield T, et al. (2010). HIV-1 Continues to Replicate and Evolve in Patients with Natural Control of HIV infection. J Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T, Brockman MA, Schneidewind A, Lobritz M, Pereyra F, Rathod A, Block BL, Brumme ZL, Brumme CJ, Baker B, et al. (2009). HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte [corrected] recognition. J Virol 83, 2743–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousseau G, Kessing CF, Fromentin R, Trautmann L, Chomont N, and Valente ST (2015). The Tat Inhibitor Didehydro-Cortistatin A Prevents HIV-1 Reactivation from Latency. MBio 6, e00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musick A, Spindler J, Boritz E, Perez L, Crespo-Velez D, Patro SC, Sobolewski MD, Bale MJ, Reid C, Keele BF, et al. (2019). HIV Infected T Cells Can Proliferate in vivo Without Inducing Expression of the Integrated Provirus. Front Microbiol 10, 2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namazi G, Fajnzylber JM, Aga E, Bosch RJ, Acosta EP, Sharaf R, Hartogensis W, Jacobson JM, Connick E, Volberding P, et al. (2018). The Control of HIV After Antiretroviral Medication Pause (CHAMP) Study: Posttreatment Controllers Identified From 14 Clinical Studies. J Infect Dis 218, 1954–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndhlovu ZM, Kazer SW, Nkosi T, Ogunshola F, Muema DM, Anmole G, Swann SA, Moodley A, Dong K, Reddy T, et al. (2019). Augmentation of HIV-specific T cell function by immediate treatment of hyperacute HIV-1 infection. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndung’u T, McCune JM, and Deeks SG (2019). Why and where an HIV cure is needed and how it might be achieved. Nature 576, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessl J, Baxter AE, Mendoza P, Jankovic M, Cohen YZ, Butler AL, Lu CL, Dube M, Shimeliovich I, Gruell H, et al. (2020). Combination anti-HIV-1 antibody therapy is associated with increased virus-specific T cell immunity. Nat Med 26, 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon CC, Mavigner M, Sampey GC, Brooks AD, Spagnuolo RA, Irlbeck DM, Mattingly C, Ho PT, Schoof N, Cammon CG, et al. (2020). Systemic HIV and SIV latency reversal via non-canonical NF-kappaB signalling in vivo. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoye AA, Hansen SG, Vaidya M, Fukazawa Y, Park H, Duell DM, Lum R, Hughes CM, Ventura AB, Ainslie E, et al. (2018). Early antiretroviral therapy limits SIV reservoir establishment to delay or prevent post-treatment viral rebound. Nat Med 24, 1430–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omondi FH, Chandrarathna S, Mujib S, Brumme CJ, Jin SW, Sudderuddin H, Miller RL, Rahimi A, Laeyendecker O, Bonner P, et al. (2019). HIV Subtype and Nef-Mediated Immune Evasion Function Correlate with Viral Reservoir Size in Early-Treated Individuals. J Virol 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pache L, Dutra MS, Spivak AM, Marlett JM, Murry JP, Hwang Y, Maestre AM, Manganaro L, Vamos M, Teriete P, et al. (2015). BIRC2/cIAP1 Is a Negative Regulator of HIV-1 Transcription and Can Be Targeted by Smac Mimetics to Promote Reversal of Viral Latency. Cell Host Microbe 18, 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankau MD, Reeves DB, Harkins E, Ronen K, Jaoko W, Mandaliya K, Graham SM, McClelland RS, Matsen Iv FA, Schiffer JT, et al. (2020). Dynamics of HIV DNA reservoir seeding in a cohort of superinfected Kenyan women. PLoS Pathog 16, e1008286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardons M, Baxter AE, Massanella M, Pagliuzza A, Fromentin R, Dufour C, Leyre L, Routy JP, Kaufmann DE, and Chomont N (2019a). Single-cell characterization and quantification of translation-competent viral reservoirs in treated and untreated HIV infection. PLoS Pathog 15, e1007619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardons M, Fromentin R, Pagliuzza A, Routy JP, and Chomont N (2019b). Latency-Reversing Agents Induce Differential Responses in Distinct Memory CD4 T Cell Subsets in Individuals on Antiretroviral Therapy. Cell Rep 29, 2783–2795 e2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso MJ, Bacchetti P, Ritter KD, Beg S, Lai J, Martin JN, Hunt PW, Henrich TJ, Siliciano JD, Siliciano RF, et al. (2020). Differential decay of intact and defective proviral DNA in HIV-1-infected individuals on suppressive antiretroviral therapy. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M Jr., Chun TW, Strain M, Richman D, and Luzuriaga K (2013). Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med 369, 1828–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud D, Patel K, Karalius B, Rainwater-Lovett K, Ziemniak C, Ellis A, Chen YH, Richman D, Siberry GK, Van Dyke RB, et al. (2014). Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA Pediatr 168, 1138–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack RA, Jones RB, Pertea M, Bruner KM, Martin AR, Thomas AS, Capoferri AA, Beg SA, Huang SH, Karandish S, et al. (2017). Defective HIV-1 Proviruses Are Expressed and Can Be Recognized by Cytotoxic T Lymphocytes, which Shape the Proviral Landscape. Cell Host Microbe 21, 494–506 e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodger JL, Lai J, Reynolds SJ, Keruly JC, Moore RD, Kasule J, Kityamuweesi T, Buule P, Serwadda D, Nason M, et al. (2017). Reduced Frequency of Cells Latently Infected With Replication-Competent Human Immunodeficiency Virus-1 in Virally Suppressed Individuals Living in Rakai, Uganda. Clin Infect Dis 65, 1308–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertas MC, Massanella M, Llibre JM, Ballestero M, Buzon MJ, Ouchi D, Esteve A, Boix J, Manzardo C, Miro JM, et al. (2014). Intensification of a raltegravir-based regimen with maraviroc in early HIV-1 infection. Aids 28, 325–334. [DOI] [PubMed] [Google Scholar]
- Roche M, Tumpach C, Symons J, Gartner M, Anderson JL, Khoury G, Cashin K, Cameron PU, Churchill MJ, Deeks SG, et al. (2019). CXCR4-using HIV strains predominate in naive and central memory CD4+ T cells in people living with HIV on antiretroviral therapy: implications for how latency is established and maintained. J Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, et al. (2013). Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 9, e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado M, Swanson MD, Pohlmeyer CW, Buckheit RW 3rd, Wu J, Archin NM, Williams TM, Margolis DM, Siliciano RF, Garcia JV, et al. (2014). HLA-B*57 elite suppressor and chronic progressor HIV-1 isolates replicate vigorously and cause CD4+ T cell depletion in humanized BLT mice. J Virol 88, 3340–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz WA, Steinhoff C, and Florl AR (2006). Methylation of endogenous human retroelements in health and disease. Curr Top Microbiol Immunol 310, 211–250. [DOI] [PubMed] [Google Scholar]
- Scully EP, Gandhi M, Johnston R, Hoh R, Lockhart A, Dobrowolski C, Pagliuzza A, Milush JM, Baker CA, Girling V, et al. (2019). Sex-Based Differences in Human Immunodeficiency Virus Type 1 Reservoir Activity and Residual Immune Activation. J Infect Dis 219, 1084–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharaf R, Lee GQ, Sun X, Etemad B, Aboukhater LM, Hu Z, Brumme ZL, Aga E, Bosch RJ, Wen Y, et al. (2018). HIV-1 proviral landscapes distinguish posttreatment controllers from noncontrollers. J Clin Invest 128, 4074–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata H, Buranapraditkun S, Kessing C, Fletcher JL, Muir R, Tardif V, Cartwright P, Vandergeeten C, Bakeman W, Nichols CN, et al. (2017). Delayed differentiation of potent effector CD8+ T cells reducing viremia and reservoir seeding in acute HIV infection. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telwatte S, Lee S, Somsouk M, Hatano H, Baker C, Kaiser P, Kim P, Chen TH, Milush J, Hunt PW, et al. (2018). Gut and blood differ in constitutive blocks to HIV transcription, suggesting tissue-specific differences in the mechanisms that govern HIV latency. PLoS Pathog 14, e1007357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, Kato T, Lerner H, Shen Y, and Farber DL (2014). Spatial Map of Human T Cell Compartmentalization and Maintenance over Decades of Life. Cell 159, 814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornhill JP, Pace M, Martin GE, Hoare J, Peake S, Herrera C, Phetsouphanh C, Meyerowitz J, Hopkins E, Brown H, et al. (2019). CD32 expressing doublets in HIV-infected gut-associated lymphoid tissue are associated with a T follicular helper cell phenotype. Mucosal Immunol 12, 1212–1219. [DOI] [PubMed] [Google Scholar]
- Tran TA, de Goer de Herve MG, Hendel-Chavez H, Dembele B, Le Nevot E, Abbed K, Pallier C, Goujard C, Gasnault J, Delfraissy JF, et al. (2008). Resting regulatory CD4 T cells: a site of HIV persistence in patients on long-term effective antiretroviral therapy. PLoS One 3, e3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandergeeten C, Fromentin R, DaFonseca S, Lawani MB, Sereti I, Lederman MM, Ramgopal M, Routy JP, Sekaly RP, and Chomont N (2013). Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood 121, 4321–4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venanzi Rullo E, Cannon L, Pinzone MR, Ceccarelli M, Nunnari G, and O’Doherty U (2019). Genetic Evidence That Naive T Cells Can Contribute Significantly to the Human Immunodeficiency Virus Intact Reservoir: Time to Re-evaluate Their Role. Clin Infect Dis 69, 2236–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacleche VS, Goulet JP, Gosselin A, Monteiro P, Soudeyns H, Fromentin R, Jenabian MA, Vartanian S, Deeks SG, Chomont N, et al. (2016). New insights into the heterogeneity of Th17 subsets contributing to HIV-1 persistence during antiretroviral therapy. Retrovirology 13, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner TA, McLaughlin S, Garg K, Cheung CY, Larsen BB, Styrchak S, Huang HC, Edlefsen PT, Mullins JI, and Frenkel LM (2014). HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 345, 570–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gurule EE, Brennan TP, Gerold JM, Kwon KJ, Hosmane NN, Kumar MR, Beg SA, Capoferri AA, Ray SC, et al. (2018). Expanded cellular clones carrying replication-competent HIV-1 persist, wax, and wane. Proc Natl Acad Sci U S A 115, E2575–E2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, et al. (2014). Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 512, 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand A, Spindler J, Hong FF, Shao W, Cyktor JC, Cillo AR, Halvas EK, Coffin JM, Mellors JW, and Kearney MF (2017). Single-cell analysis of HIV-1 transcriptional activity reveals expression of proviruses in expanded clones during ART. Proc Natl Acad Sci U S A 114, E3659–E3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, and Richman DD (1997). Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278, 1291–1295. [DOI] [PubMed] [Google Scholar]
- Yukl SA, Gianella S, Sinclair E, Epling L, Li Q, Duan L, Choi AL, Girling V, Ho T, Li P, et al. (2010). Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis 202, 1553–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukl SA, Kaiser P, Kim P, Telwatte S, Joshi SK, Vu M, Lampiris H, and Wong JK (2018). HIV latency in isolated patient CD4(+) T cells may be due to blocks in HIV transcriptional elongation, completion, and splicing. Sci Transl Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]


