Adaptive radiotherapy has been an advancing topic of research since the late 1990s when Yan et al published the first article discussing the mathematical concept and potential benefits of this approach.1 Nine years ago, adaptive radiotherapy was the topic of Seminars in Radiation Oncology. In his introduction, Dr. Yan, the special editor of the issue, described the potential improvements in clinical treatment outcomes reported in the issue to be “extremely encouraging and greatly promote adaptive radiotherapy.”2 It is timely to evaluate, nearly a decade later, how far we have come in advancing adaptive radiotherapy in the clinic and what new emerging technology is opening new doors. The goal of this issue of Seminars in Radiation Oncology is to discuss the current status and future directions in adaptive radiotherapy. The issue specifically focuses on advances in the tools needed for adaptive radiotherapy, including autosegmentation, deformable registration, and automated planning, advances in workflows, including offline, real-time, functional, and anatomical, and evidence of benefit in clinical sites, including head and neck, lung, and cervix.
At its inception, adaptive radiotherapy was often implemented to refine the planning target volumes to account for additional information gained about the patient’s setup characteristics. With the advancement of daily soft tissue visualization via in-room imaging, adaptive radiotherapy has advanced to enable the modification of the treatment plan to improve the therapeutic ratio of the treatment using anatomical and functional information acquired over the course of treatment.3 This may mean correcting the treatment plan to account for the new representation of the patient (ie, accounting for weight loss and tumor response4–10) or it may mean changing the clinical goals to be more or less aggressive in the treatment plan based on information learned about the patient over the treatment (ie, functional changes obtained from PET images11–14).
Adaptive radiotherapy represents a continuum of increasing sophistication, as illustrated in Figure 1. In its basic form, adaptive radiotherapy enables the treatment to be changed, or adapted, to respond to a signal that additional information is known about the patient or that the patient has changed from the original state at the time of planning. This may be as simple as creating a treatment plan, performing periodic imaging and deciding to create a new treatment plan when deemed needed by the clinical team, Figure 1A. This can be performed without any sophisticated tools, such as deformable image registration (DIR), automated planning, dose accumulation or decision-making. The new treatment plan can be generated using the same clinical criteria as the original plan. However this process is typically ad hoc and does not allow us to gain knowledge about the delivered dose, the toxicity rates, and the benefit of adaptation. It also risks taxing clinical resources for little or no benefit or missing the opportunity to improve the therapeutic ratio if the time to adapt is missed In addition, little information is available to feed into an outcomes database to improve normal tissue complication models, tumor control probabilities, clinical trial design and protocol development.
Figure 1.
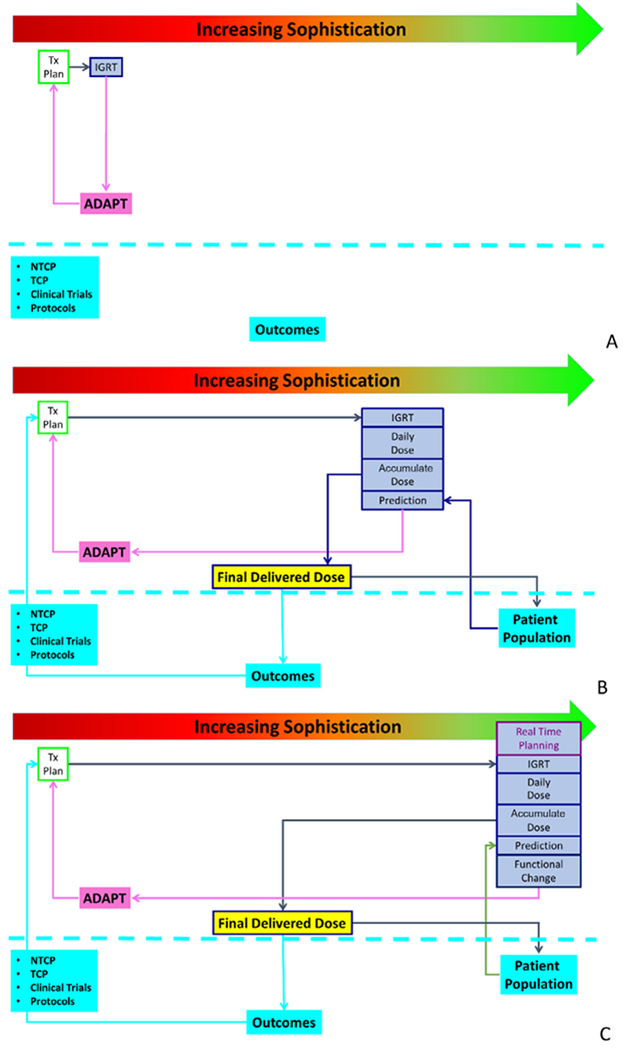
Increasing levels of sophistication in adaptive radiotherapy, ranging from simple IGRT-based replanning (A) to DIR-based dose accumulation and the development of predictive models tied to an outcomes database (B) to online replanning and functional guidance (C). DIR, deformable image registration; IGRT, adaptive image-guided radiotherapy.
As technology increases, the sophistication of adaptive therapy can increase as well. Volumetric imaging and autosegmentation enable the daily dose to be calculated, allowing decisions on adaptation to be made based on dosimetric information rather than geometric information alone Data have shown that cone beam CT (CBCT) can be calibrated to enable dose calculations directly on the image.15 Auto-segmentation of the CBCT, via DIR or an independent algorithm, allows the assessment of the daily dose.16 Further sophistication, including DIR, allows for the accumulation of dose over the course of treatment, adaptation based on the accumulated dose rather than independent snapshots, and the recording of the final estimated delivered dose, including adaptations.17 The calculation of the final delivered dose can then be linked to an outcomes database, supporting the refinement of normal tissue complication models, tumor control probabilities, clinical trials, and protocol development.18 Once the delivered dose is quantified for a population of patients, decision support tools can be developed empowering the clinical team with evidence-driven metrics to guide the decision to replan, Figure 1B.
Finally, the next layer of sophistication in adaptive therapy is the additional evaluation of functional changes of the patient and online replanning. These are emerging areas of adaptive planning that the field is starting to see in prospective clinical trials and simulation studies, especially with the emergence of in-room MR imaging, dedicated MR imaging within radiation oncology departments, and automated planning capabilities, Figure 1C.
Several factors are contributing to the continued advancement of adaptive radiotherapy, most notably computational advances, specifically artificial intelligence (AI), advanced in-room imaging, specifically MR-guided delivery, and the increasing amount of data acquired on clinical trials to drive evidence-based assessment of the benefit of adaptive radiotherapy.
Impact of AI
AI, specifically deep learning applications, has become a prominent topic throughout society and radiation oncology is no exception. Rapid advancement has led to hype, skepticism, confusion, and excitement. Fundamentally, AI is a computer algorithm, Radiation Oncology has been relying on computers for decades, and it is one of the fundamental advances that enabled the clinical application of adaptive radiotherapy. The exciting advances described in this edition of Seminars in Radiation Oncology on adaptive radiotherapy have leveraged the computational advances in plan optimization, both in speed and sophistication in developing highly sculpted dose distributions, anatomical modeling, enabling accurate correlations of anatomy over time, and advanced image reconstruction, allowing us to obtain quality images in the treatment room. The future advancement of adaptive radiotherapy hinges on our ability to handle the rapidly increasing amount of data that we are obtaining as we see more images, contouring, dosimetric constraints, complexity of treatment plans, and handoffs between systems to integrate the process. All of this data leads to more decisions to be made in the adaptive radiotherapy process in tighter time constraints. All of this, combined with the economic demands to keep healthcare costs down and show evidence of improved outcomes, creates both incredible demands on the system as well as opportunities for innovation and advancement.
The impact of AI on adaptive radiotherapy has the potential to be very significant, as one of the largest hindrances to the widespread clinical implementation is workload. The efficiency and effectiveness that AI has demonstrated since its surge into the radiotherapy environment have shown great promise. All of the main adaptive radiotherapy components: contouring, registration, planning, quality assurance (QA), and decision-making, may benefit from AI-based algorithms.19–22 AI-based auto-segmentation algorithms are demonstrating improved accuracy compared to previous algorithms, such as model-based and atlas-based segmentation. Even this AI “low hanging fruit” has the potential to make ideas previously deemed “impossible” into probable, such as real-time targeting of tissue through auto-segmentation, rapid replanning of patients, and real-time dose calculations. In addition, with this technology, we now have the ability to develop adaptive radiotherapy strategies never previously considered.
Changing Landscape of In-room Imaging
At the advent of adaptive radiotherapy, in-room imaging consisted primarily of planar MV imaging, enabling the visualization of bony anatomy, and the idea of a dedicated MR within the radiation oncology department was a dream, let alone an MRI in the treatment room! Today, advances in imaging technology in the treatment room enable the ability to visualize, at each treatment fraction, anatomical, and in some cases functional, the response of the tumor and normal tissue to treatment.23–31 This has dramatically changed the scope of adaptive radiotherapy. In addition to the increasing use of MR imaging, advances in image reconstruction for CBCT imaging, enabling 4D CBCT, breath-hold CT, and artifact reduction, has advanced the use of CBCT for adaptive radiotherapy, enabling dose calculations, detection of anatomical response, and improved understanding of physiological motion. Novel imaging for tumor targeting in the treatment room continues to be developed, including the integration of PET with treatment delivery machines.
Where Do We Go From Here?
The 3 papers in this issue focused on adaptive radiotherapy for cervix, head and neck, and lung, represent some of the most active areas of clinical trials. Each of these representative anatomical sites demonstrate the translation of adaptive radiotherapy from retrospective studies on small cohorts of patients into large scale, multi-institutional trials using adaptive techniques. Each site leverages the advances in imaging technology, image processing, and workflow advances investigate the potential clinical benefits for cancer patients. These trials begin to show the clinical impact of adaptive radiotherapy, in terms of improved local control and reduced toxicities, however much more work needs to be done. We, as a collaborative field of radiation oncologists, medical physicists, and industry partners, must continue to work together to improve the workflow, technical accuracy, and decision-making in adaptive radiotherapy. We must strive to ensure that the studies and data are analyzed to determine, at a fundamental evidence-based level, the impact of adaptive radiotherapy on survival and quality of life for the patients. In addition, we, as a field, must work to share data to ensure the rapid, safe, and effective advancement of the field of adaptive radiotherapy.32
Acknowledgement
This work was supported by a grants from the NIH, NCI R01CA221971, and RaySearch Laboratories.
Conflicts of Interest: Dr. Brock has a licensing agreement with RaySearch Laboratories for deformable registration technology. She received funding from NIH 1R01CA221971 and from RaySearch Laboratories.
References
- 1.Yan D, Vicini F, Wong J, et al. : Adaptive radiation therapy. Phys Med Biol 42:123–132, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Yan D: Adaptive radiotherapy: Merging principle into clinical practice. Semin Radiat Oncol 20:79–83, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Jaffray DA: Image-guided radiotherapy: From current concept to future perspectives. Nat Rev Clin Oncol 9:688–699, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Oh S, Stewart J, Moseley J, et al. : Hybrid adaptive radiotherapy with online MRI in cervix cancer IMRT. Radiother Oncol 110:323–328, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Lim K, Stewart J, Kelly V, et al. : Dosimetrically triggered adaptive intensity modulated radiation therapy for cervical cancer. Int J Radiat Oncol BiolPhys 90:147–154, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Yan D, Liang J: Expected treatment dose construction and adaptive inverse planning optimization: Implementation for offline head and neck cancer adaptive radiotherapy. Med Phys 40:021719. [DOI] [PubMed] [Google Scholar]
- 7.Koay EJ, Lege D, Mohan R, et al. : Adaptive/nonadaptive proton radiation planning and outcomes in a phase II trial for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 84:1093–1100, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park SS, Yan D, McGrath S, et al. : Adaptive image-guided radiotherapy (IGRT) eliminates the risk of biochemical failure caused by the bias of rectal distension in prostate cancer treatment planning: Clinical evidence. Int J Radiat Oncol Biol Phys 83:947–952, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Hugo GD, Yan D, Liang J: Population and patient-specific target margins for 4D adaptive radiotherapy to account for intra- and inter-fraction variation in lung tumour position. Phys Med Biol 52:257–274, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Brabbins D, Martinez A, Yan D, et al. : A dose-escalation trial with the adaptive radiotherapy process as a delivery system in localized prostate cancer: Analysis of chronic toxicity. Int J Radiat Oncol Biol Phys 61:400–408,2005 [DOI] [PubMed] [Google Scholar]
- 11.Yan D, Chen S, Krauss DJ, et al. : Tumor voxel dose response matrix and dose prescription function derived using (18)F-FDG PET/CT images for adaptive dose painting by number. Int J Radiat Oncol Biol Phys pii: S0360-3016(19)30159-2, 2019. http://dx.doi:10.1016/j.ijrobp.2019.01.077 [DOI] [PubMed] [Google Scholar]
- 12.Urwin R, Barrington SF, Mikhaeel NG: Role of PET imaging in adaptive radiotherapy for lymphoma. Q J Nucl Med Mol Imaging 62:411–419, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Peyraga G, Robaine N, Khalifa J, et al. : Molecular PET imaging in adaptive radiotherapy: Brain. QJ Nucl Med Mol Imaging 62:337–348, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Hamming-Vrieze O, Navran A, Al-Mamgani A, et al. : Biological PET-guided adaptive radiotherapy for dose escalation in head and neck cancer: A systematic review. Q J Nucl Med Mol Imaging 62:349–368, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Giacometti V, King RB, Agnew CE, et al. : An evaluation of techniques for dose calculation on cone beam computed tomography. Br J Radiol 2018:20180383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang T, Chi Y, Meldolesi E, Yan D: Automatic delineation of on-line head-and-neck computed tomography images: Toward on-line adaptive radiotherapy. Int J Radiat Oncol Biol Phys 68:522–530, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Velec M, Moseley JL, Craig T, et al. : Accumulated dose in liver stereotactic body radiotherapy: Positioning, breathing, and deformation effects. Int J Radiat Oncol Biol Phys 83:1132–1140, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCulloch MM, Muenz DG, Schipper MJ, et al. : A simulation study to assess the potential impact of developing normal tissue complication probability models with accumulated dose. Adv Radiat Oncol 3:662–672, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson P, Hardcastle N, Dawe N, et al. : Deep learning renal segmentation for fully automated radiation dose estimation in unsealed source therapy. Front Oncol 8:215, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang S, Tang F, Huang X, et al. : Deep-learning-based detection and segmentation of organs at risk in nasopharyngeal carcinoma computed tomographic images for radiotherapy planning. Eur Radiol 2018 [DOI] [PubMed] [Google Scholar]
- 21.Men K, Zhang T, Chen X, et al. : Fully automatic and robust segmentation of the clinical target volume for radiotherapy of breast cancer using big data and deep learning. Phys Med 50:13–19, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Kisling K, Zhang L, Simonds H, et al. : Fully automatic treatment planning for external-beam radiation therapy of locally advanced cervical cancer: A tool for low-resource clinics. J Glob Oncol 2019: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohamed ASR, Bahig H, Aristophanous M, et al. : Prospective in silico study of the feasibility and dosimetric advantages of MRI-guided dose adaptation for human papillomavirus positive oropharyngeal cancer patients compared with standard IMRT. Clin Transl Radiat Oncol 11:11–18, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van de Lindt T, Sonke JJ, Nowee M, et al. : A self-sorting coronal 4D-MRI method for daily image guidance of liver lesions on an MR-LINAC. Int J Radiat Oncol Biol Phys 102:875–884, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Winkel D, Bol GH, Kiekebosch IH, et al. : Evaluation of online plan adaptation strategies for the 1.5T MR-linac based on “First-In-Man” treatments. Cureus 10:e2431, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chuter RW, Pollitt A, Whitehurst P, et al. : Assessing MR-linac radiotherapy robustness for anatomical changes in head and neck cancer. Phys Med Biol 63:125020. [DOI] [PubMed] [Google Scholar]
- 27.Ramey SJ, Padgett KR, Lamichhane N, et al. : Dosimetric analysis of stereotactic body radiation therapy for pancreatic cancer using MR-guided Tri-(60)Co unit, MR-guided LINAC, and conventional LINAC-based plans. Pract Radiat Oncol 8:e312–e321, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Kadoya N: Current status of MR-Linac system. Igaku Butsuri 36:229–235, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Yun J, Yip E, Gabos Z, et al. : Neural-network based autocontouring algorithm for intrafractional lung-tumor tracking using Linac-MR. Med Phys 42:2296–2310, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Kontaxis C, Bol GH, Lagendijk JJ, et al. : Towards adaptive IMRT sequencing for the MR-linac. Phys Med Biol 60:2493–2509, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Stanescu T, Wachowicz K, Jaffray DA: Characterization of tissue magnetic susceptibility-induced distortions for MRIgRT. Med Phys 39:7185–7193, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Jaffray DA, Lindsay PE, Brock KK, et al. : Accurate accumulation of dose for improved understanding of radiation effects in normal tissue. Int J Radiat Oncol Biol Phys 76(3 Suppl):S135–S139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]


