Abstract
This review discusses the current state of knowledge surrounding the role of commensal bacteria in supporting intestinal mucosal barrier protection. We focus on two aspects of physical barrier function: Tight junction maintenance and mucus production.
Keywords: Commensal microbiota, Intestinal mucosa, Tight junctions, Mucus layers
INTRODUCTION
Throughout the small and large intestine, a protective mucosal barrier lines exchange surfaces. This barrier is made up of intestinal epithelial cells (IECs) coated with two mucus layers. IECs function as the first line of defense against invading pathogens and external toxins [1]. IECs are connected by tight junctions to prevent entry of pathogens and allow for selective absorption of nutrients. Mucus layers permit regulation of barrier homeostasis and provide an interface for immune molecules to initiate immune responses [2].
The intestinal mucosal barrier also contains a diverse population of harmless commensal bacteria, known as the gut microbiota (about 1014 bacteria). Commensal bacteria help to maintain gut homeostasis in a variety of ways, including: 1) allowing for the digestion of dietary fiber; 2) supporting host immune system function; 3) preventing colonization of enteric pathogens [2]. Notably, commensal bacteria have been shown to engage in complex interactions with IECs and to modulate intestinal barrier function [1].
TIGHT JUNCTIONS
Intestinal epithelial cells (IECs) are held together by tight junctions (TJs). TJs are integral membrane proteins. TJs encircle IECs on the apical edges and limit the permeability of the epithelial lining in order to prevent entrance of pathogens into the body tissue and bloodstream. Modifying TJs has been associated with changes in gut permeability, as a “leaky” gut epithelium exasperates many gastrointestinal disorders [3].
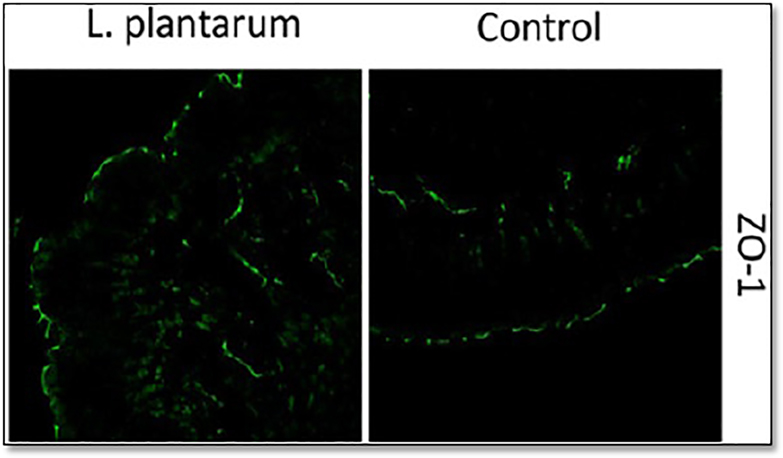
Several recent studies have investigated how commensal microbiota may influence TJ presence. In one study, Karczewski et al. [3] investigated how the commensal bacterium Lactobacillus plantarum enhanced intestinal barrier protection. They administered L. plantarum to human subjects for a 6 hour time periods, collected duodenum biopsies, and performed immunostaining with fluorescent antibodies targeting tight junction proteins ZO-1 (zonula occluden-1) and occludin. They quantified the fluorescence intensity of these two proteins and found that both ZO-1 and occludin had increased staining in IECs (Figure 1). Their results suggested that L. plantarum strengthens TJs by decreasing gut permeability and by enhancing barrier protection.
Figure 1.
Increased ZO-1 levels observed in plantarum-treated epithelia. Human duodenal tissue was immunostained for zonula occludents (ZO, green) and viewed by conflocal microscopy. Values from 5 human biopsy samples were process following feeding of a control or Lactobacillus plantarum (L. plantarum). Colocalization of Occludin with ZO-1 is detected within tight junctions (TJs) on the apical side of the epithelium. Modified and reproduced with permission from Karczewski et al. [3].
A second study by Shimada et al. [4] inquired how indole, a quorum-sensing molecule present in commensal bacteria communities, might positively influence intestinal barrier function. They demonstrated that Germ-Free (GF) mice, which contain no microbiota, have reduced expression of TJ-associated molecules. Further, when treated with capsules containing indole, GF mice recovered increased mRNA expression of the same TJ molecules [4]. This result directly demonstrates that a product of commensal bacteria-indole- increases gut epithelium integrity by upregulating TJ molecules.
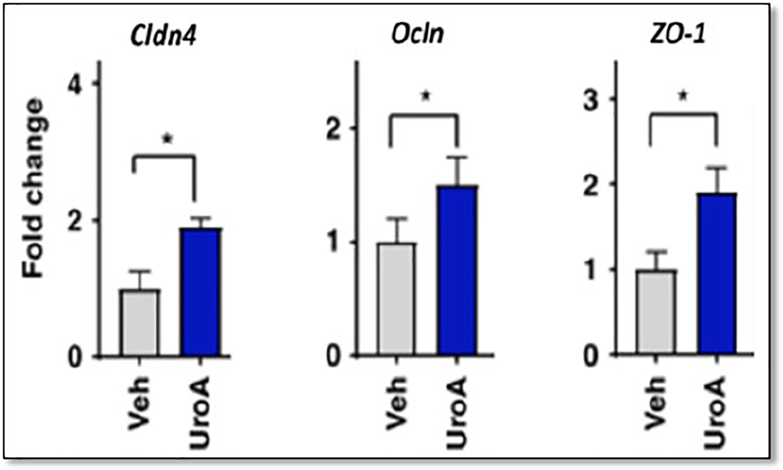
A third study provided further support for the positive role of commensal bacteria in strengthening barrier protection. Singh et al. [5] observed that TJ proteins ZO-1, Cldn4 and Ocln are significantly upregulated in an epithelial cell line after being exposed to the microbial metabolite UroA (Figure 2).
Figure 2.
TJ proteins Zona occludens (ZO-1), claudin 4 (Cldn4) and Occludin (OcIn) are significantly upregulated in an epithelial cell line following exposure to UroA. mRNA fold levels were determined by RT-PCR of each. P values were determined by unpaired t-tests). Modified and reproduced with permission from Singh et al. [5].
These three studies together demonstrate that commensal bacteria, as well as products and metabolites of commensal bacteria, cause increased expression of proteins that make up TJs in IECs. These studies provide strong evidence that commensal bacteria play beneficial roles in maintaining the intestinal mucosal barrier by decreasing permeability of the epithelium.
MUCUS LAYERS
The second major component of intestinal barrier function involves the mucus layers which coat the epithelial lining. These mucus layers are secreted by IECs, termed goblet cells, are essential for gut homeostasis. For example, goblet cells provide a functional barrier between host IECs and all pathogens and toxins within the gut. They create a sheltering environment for commensal bacteria and they provide an interface for immune responses [6].
Recently Martín et al. [7] investigated how the commensal bacterium Lactobacillus rhamnosus CNCM I-3690 protects the intestinal barrier. To accomplish this, they treated mice with CNCM I-3690 bacteria and performed cell staining of intestinal epithelium samples. This approach allowed quantitative determination of the number of goblet cells in bacteria-treated mice. Additionally, mucus layer thickness was measured by immunohistochemistry. They found that the untreated group had a significantly thinner mucus lining than the group treated with CNCM I-3690 [7].
These results suggest that the commensal bacterium Lactobacillus rhamnosus CNCM I-3690 is able to maintain mucus-secreting goblet cells and the mucus layer, which in turn benefits barrier protection.
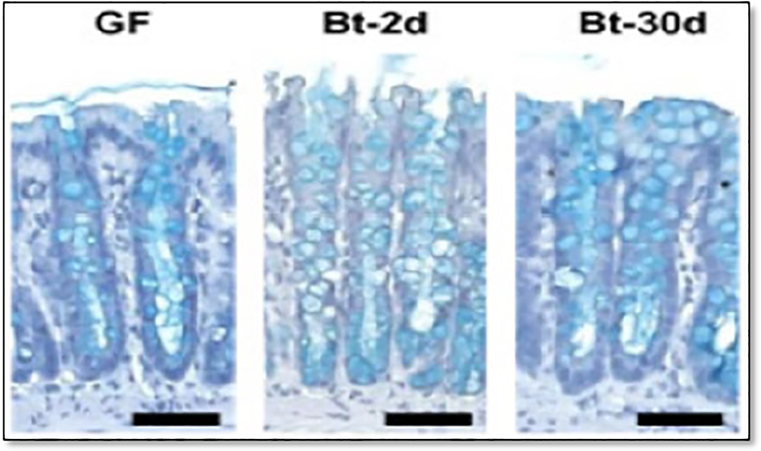
In another study, Wrzosek et al. [8] queried how the commensal bacteria Bacteroides thetaiotaomicron impacts the intestinal mucus layer. They observed that mice inoculated with B. thetaiotaomicron contain significantly more goblet cells than Germ-Free (GF) mice, have more KLF4 protein (important for goblet cell differentiation) and express higher levels of genes involved in mucus synthesis [8] (Figure 3). This study provided further evidence that commensal bacteria promote mucus production, and thereby, gut homeostasis.
Figure 3.
Goblet cells in Germ Free (GF) mice and in GF mice treated with Beta-Thetaliotaomicron (BT) for 2 days (Gt-2d) or for 30 days (Dt-30) days. Goblet cells were visualized by staining with alcian blue. Scale bars (horizontal lines), 50 μm. Modified and reproduced with permission from Wrzosek et al. [8].
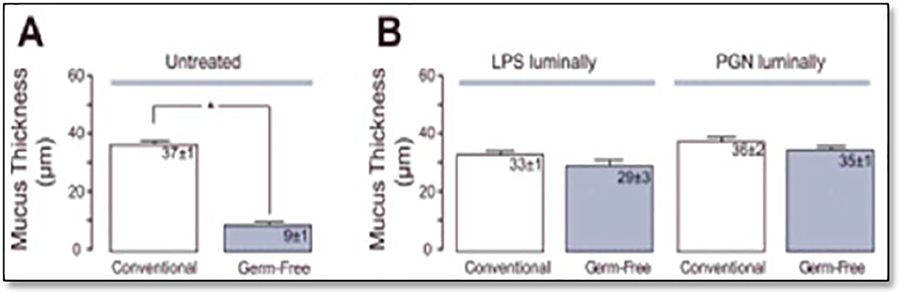
Finally, a third study by Petersson et al. [6] investigated whether bacterial products can positively impact the mucosal barrier. To assess this question, the group measured the thickness of the intestinal mucus layer in GF and normal mice. They found that GF mice had a much thinner layer of mucus on the epithelium. Then, they treated GF mice with commensal bacterial products such as peptidoglycan (PGN) and lipopolysaccharides (LPS). Within 40 min posttreatment, the mucus layer had restored itself to normal levels [6] (Figure 4).
Figure 4.
(A). Untreated conventional or GF mice show low mucus thickness. (B). Conventional and GF mice treated with bacterial LPS or peptidoglycan (PGN) show restored, normal mucus thickness (luminally). Histological samples were stained with Periodic Acid-Schiff (PAS solution). Mucus thickness (width) was quantified in μm; *p<0.05. Modified and reproduced with permission from Petersson et al. [6].
This result is remarkable as it suggests a direct link between mucus production and the products of commensal bacteria. It further strengthens a model in which commensal bacteria and their products promote intestinal barrier function specifically through maintenance of the mucosal layer [9].
CONCLUDING REMARKS
For some time now, commensal gut microbiotas have been associated with positive health outcomes for a variety of systemic diseases. But only recently have the mechanisms by which commensal bacteria maintain homeostasis begun to be elucidated [6]. The studies reported in this review provide strong evidence that commensal bacteria play an essential role in protecting the intestinal epithelial lining— the body’s first defense against any ingested pathogen or toxin. The experiments described above report that upon treatment with commensal bacteria or their products, tight junction integrity is enhanced among IECs and goblet cell differentiation as well as mucus production is increased overall.
Tight junction and mucus layer presence are the two major physical barriers of the intestinal mucosal lining that provide protection from enteric pathogens and a variety of inflammatory bowel diseases. Therefore, not only do these studies advance the current state of knowledge surrounding gut microbiota, but they will be invaluable in generating treatment plans for individuals with gastrointestinal disorders for years to come.
ACKNOWLEDGEMENT
We thank members of our laboratory for suggestions. HOT is supported by NIH grant NIH Grant R01CA31534, Cancer Prevention Research Institute of Texas (CPRIT) Grants RP120348 and RP120459 and the Marie Betzner Morrow Centennial Endowment.
REFERENCES
- 1.Martens EC, Neumann M, Desai MS (2018) Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol 16: 457–470. [DOI] [PubMed] [Google Scholar]
- 2.Goto Y, Ivanov I (2013) Intestinal epithelial cells as mediators of the commensal-host immune cross-talk. Immunol Cell Biol 91: 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezum M, et al. (2010) Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol 298: G851–G859. [DOI] [PubMed] [Google Scholar]
- 4.Shimada Y, Kinoshita M, Harada K, Mizutani M, Masahata K, et al. (2013) Commensal bacteriadependent indole production enhances epithelial barrier function in the colon. PLoS One 8: e80604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh R, Chandrashekharappa S, Bodduluri SR, Baby BV, Hegde B, et al. (2019) Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Comm 10: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersson J, Schreiber O, Hansson GC, Gendler SJ, Velcich A, et al. (2011) Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol 300: G327–G333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martín R, Chamignon C, Mhedbi-Hajri N, Chain F, Derrien M, et al. (2019) The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci Rep 9: 5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrzosek L, Miquel S, Noodrine ML, Bouet S, Joncquel Chevalier-Curt M, et al. (2013) Bacteroides thetaiotaomicron and Fecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol 11: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Littman DR, Pamer EG (2012) Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe 10: 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]