Abstract
Background:
Research suggests adults with autism spectrum disorder (ASD) may use executive functions to compensate for social difficulties. Given hallmark age-related declines in executive functioning and the executive brain network in normal aging, there is concern that older adults with ASD may experience further declines in social functioning as they age. In a male-only sample, we hypothesized: 1) older adults with ASD would demonstrate greater ASD-related social behavior than young adults with ASD, 2) adults with ASD would demonstrate a greater age group reduction in connectivity of the executive brain network than neurotypical (NT) adults, and 3) that behavioral and neural mechanisms of executive functioning would predict ASD-related social difficulties in adults with ASD.
Methods:
Participants were a cross-sectional sample of non-intellectually disabled young (ages 18–25) and middle-aged (ages 40–70) adult men with ASD and NT development (young adult ASD: n=24; middle-age ASD: n=25; young adult NT: n=15; middle-age NT: n=21). We assessed ASD-related social behavior via the self-report Social Responsiveness Scale-2 (SRS-2) Total Score, with exploratory analyses of the Social Cognition Subscale. We assessed neural executive function via connectivity of the resting-state executive network (EN) as measured by independent component analysis. Correlations were investigated between SRS-2 Total Scores (with exploratory analyses of the Social Cognition Subscale), EN functional connectivity of the dorsolateral prefrontal cortex (dlPFC), and a behavioral measure of executive function, Tower of London (ToL) Total Moves.
Results:
We did not confirm a significant age group difference for adults with ASD on the SRS-2 Total Score; however, exploratory analysis revealed middle-age men with ASD had higher scores on the SRS-2 Social Cognition Subscale than young adult men with ASD. Exacerbated age group reductions in EN functional connectivity were confirmed (left dlPFC) in men with ASD compared to NT, such that older adults with ASD demonstrated the greatest levels of hypoconnectivity. A significant correlation was confirmed between dlPFC connectivity and the SRS-2 Total Score in middle-age men with ASD, but not young adult men with ASD. Furthermore, exploratory analysis revealed a significant correlation with the SRS-2 Social Cognition Subscale for young and middle-aged ASD groups and ToL Total Moves.
Conclusions:
Our findings suggest that ASD-related difficulties in social cognition and EN hypoconnectivity may get worse with age in men with ASD and is related to executive functioning. Further, exacerbated EN hypoconnectivity associated with older age in ASD may be a mechanism of increased ASD-related social cognition difficulties in older adults with ASD. Given the cross-sectional nature of this sample, longitudinal replication is needed.
Keywords: Aging, Autism Spectrum Disorder, fMRI, Social Behavior, Executive Network, Executive Functions
Introduction
Much research has focused on the developmental course of autism spectrum disorder (ASD) in children, with less attention given to changes that occur across the adult lifespan. Given the rise in ASD diagnoses over the past 30 years, the population of older adults with ASD is projected to increase rapidly over the next decade (Piven & Rabins, 2011). Furthermore, the care needs for adults with ASD will likely widen as family caregivers enter advanced aging and no longer have the ability to care for their aging adult children (Piven & Rabins, 2011). Research on aging in adults with ASD is emerging; however, further studies are needed in order to better understand the needs of this population and inform intervention.
Social symptom severity is a strong predictor of positive aging outcomes in ASD (Howlin, Moss, Savage, & Rutter, 2013), and there is emerging evidence that this domain may be vulnerable to aging. Three recent cross-sectional studies have reported greater overall ASD traits and ASD-related social behavior associated with older age (Abbott, Happé, & Charlton, 2018; Happé et al., 2016), with one study demonstrating a peak in middle adulthood (Lever & Geurts, 2018). However, these findings have not been consistently observed across studies, with some evidence of improved social functioning during early middle adulthood that is related to greater inferior frontal lobe recruitment to social stimuli (Bastiaansen et al., 2011). Still other studies showing no associations between age and ASD-related social behavior (Bishop & Seltzer, 2012; Howlin et al., 2013; Shattuck et al., 2007). These findings underscore the importance of further describing age group differences in ASD social symptoms to inform intervention and care plans. Furthermore, describing the neural substrates underlying these changes may provide further insight into the mechanisms underlying age group differences in ASD symptoms.
Cognitive Mechanisms Underlying ASD-Related Social Behavior
The cognitive mechanisms underlying ASD-related social behavior are not well established. ASD-related social behaviors are characterized by difficulties in social-emotional reciprocity, poor nonverbal communication, and difficulties establishing and maintaining social relationships (American Psychiatric Association, 2013). For example, children and adults with ASD may exhibit difficulty understanding expectations for social behavior, and they may have difficulty empathizing and sharing interests with others. Various theories have attempted to explain the cognitive mechanisms underlying ASD-related social behavior [e.g., Theory of Mind Hypothesis, Weak Central Coherence Hypothesis, etc.; see Frith (2012) for a review of cognitive theories of ASD]. However, each falls short of accounting for the heterogeneous symptom presentation, perhaps because they fail to describe compensatory mechanisms contributing to development and change over time (Livingston & Happé, 2017).
Recently, it has been suggested that developmental and lifespan changes in ASD-related social behavior may be partially dependent on executive functions (Livingston & Happé, 2017). Although many studies have documented executive functioning difficulties in younger persons with ASD (Lai et al., 2017), these are not universal (Brunsdon et al., 2015). Given variability of executive functioning abilities in individuals with ASD, executive functioning may be a candidate mechanism for social compensation (Livingston & Happé, 2017). For example, some individuals with ASD may capitalize on relatively intact executive functions to determine when and how to implement explicitly learned social rules. Recently, metacognitive aspects of executive functioning (e.g., initiation, working memory, planning, organization, monitoring) have been found to uniquely predict ASD-related social behavior in ASD but not neurotypical (NT) children and adolescents (Leung, Vogan, Powell, Anagnostou, & Taylor, 2015). On the other hand, behavioral regulation aspects of executive functioning (e.g., inhibition, shifting, and emotional control) predict social behavior similarly in both groups (Leung, Vogan, Powell, Anagnostou, & Taylor, 2015). The hypothesis that persons with ASD capitalize on metacognitive aspects of executive functions to compensate for social challenges may account for the observed improvement in social functioning in some adolescents and young adults with ASD, although with persistent residual deficits (Howlin & Magiati, 2017). Importantly, this hypothesis has negative implications for aging adults with ASD, given that declines in executive functioning are a hallmark of the normal aging process.
Metacognitive Executive Functions in Aging Adults with ASD
Metacognitive aspects of executive functioning show some preliminary evidence of exacerbated age-related differences in ASD, and this cognitive domain may be particularly relevant to ASD social symptomology. In an exploratory cross-sectional analysis, Abbott et al. (2018) reported steeper trajectories of age-related differences in planning and reasoning in ASD such that older age was associated with worse performance relative to normative expectations for NT adults. However, these age associations have not been observed in smaller samples (Davids, Groen, Berg, Tucha, & van Balkom, 2016; Geurts & Vissers, 2012). Furthermore, trajectories of “exacerbated aging” have not been observed in other metacognitive domains including initiation, working memory, organization, or monitoring in cross-sectional samples (Davids et al., 2016; Geurts & Vissers, 2012; Lever, Werkle-Bergner, Brandmaier, & Ridderinkhof, 2015; Powell, Klinger, & Klinger, 2017). Importantly, older adults with ASD relative to matched NT adults show reduced abilities in metacognitive aspects of executive functioning based on self-and informant-reports (Davids et al., 2016), indicating persistent difficulties in this domain into older adulthood. It should be noted that Davids et al. (2016) reported inconsistent convergence between self-report and objective measures of executive functioning in older adults with ASD (Davids et al., 2016), with the exception of the Tower of London (ToL) planning task. Given the relevance of metacognitive aspects of executive functioning to compensation for social challenges (Leung et al., 2015), potentially exacerbated age group differences in this domain are particularly concerning.
Role of the Executive Network in ASD-Related Social Behavior and Aging
There is emerging evidence that older age is associated with exacerbated profiles of hypoconnectivity in adults with ASD relative to NT adults, although findings are inconsistent. Brain aging in older adults with ASD have been examined in two cross-sectional structural neuroimaging studies, with one study showing evidence of exacerbated age-related differences in white matter integrity (Koolschijn, Caan, Teeuw, Olabarriaga, & Geurts, 2016) and another reporting parallel age-related differences in gray matter volume and thickness (Koolschijn & Geurts, 2016). Furthermore, aberrant use of a functionally connected brain network implicated in executive functioning has also been observed in older men with ASD (Braden et al., 2017). Functional brain connectivity analyses are used to infer functional networks from fMRI data, often derived while a participant is at rest in the scanner (resting-state functional connectivity), by examining correlations in voxel time courses distributed throughout the brain (Bressler & Menon, 2010). Task-based and resting-state paradigms show similar preservation of individual connectivity differences (Shah, Cramer, Ferguson, Birn, & Anderson, 2016), with resting-state paradigms creating fewer constraints for participants’ abilities. Aberrant functional connectivity of higher level cognitive brain networks are suggested to underlie core symptoms in ASD (Elton, Di Martino, Hazlett, & Gao, 2016), as well as normal age-related cognitive decline (Damoiseaux, 2017). Therefore, the integrity of functional brain networks underlying higher level cognitive processing, including executive functioning, may be vulnerable to exacerbated age-related decline in ASD. Moreover, measures of functional brain connectivity may be particularly sensitive to early age-related differences in older adults with ASD.
The Executive Network (EN) is a canonical functional brain network mediating executive control, which contains key nodes in the dorsolateral prefrontal cortices (dlPFC) and posterior parietal cortices (PPC; Bressler & Menon, 2010). Recent theories have ascribed a broader, domain general role for the EN in selecting and inhibiting relevant external stimuli and internal conceptual representations in a goal-directed manner (Lindquist & Barrett, 2012). Beyond its canonical role in executive functioning, the EN has been suggested to play a significant role in socio-affective processing (Lindquist & Barrett, 2012). Further, a recent meta-analysis of activation patterns associated with a metacognitive planning task, the ToL, demonstrate consistency with the EN, in particular with nodes of activation in the bilateral dlPFC (Nitschke, Köstering, Finkel, Weiller, & Kaller, 2017). This makes the EN an ideal candidate to explore a neural basis for metacognitive planning and associations with social behavior without the constraint of in-scanner task demand.
The EN has been implicated as a core network demonstrating aberrant functional connectivity in younger persons with ASD, which has been linked to both ASD-related social behavior (Elton et al., 2016) and cognitive control difficulties in younger persons with ASD (Solomon, Hogeveen, Libero, & Nordahl, 2017). While certain patterns of connectivity in this network predict social behavior in both younger persons with ASD and NT in a congruent manner, other connectivity patterns within the EN uniquely predict social behavior in ASD but not NT participants (Elton et al., 2016). This suggests that the EN may play an additional role in social behavior in ASD beyond its role for NT adults, and age-related differences in connectivity may have a more dramatic impact on social behavior in persons with ASD.
The Current Study
In the present study, we examined age group differences in ASD-related social behavior and EN resting-state functional connectivity in a cross-sectional sample of young adult and middle-age men with and without ASD. Further, we investigated the relationship between ASD-related social behavior and behavioral (ToL performance) and neural (EN connectivity within the bilateral dlPFC) indicators of executive function in young adult and middle-age men with ASD. Since there are sex differences in many of the cognitive and brain measure we investigated, a male only sample was chosen for this initial study due to the difficulty of recruiting sufficient middle-age women with ASD to explore sex interactions. We hypothesized that older men with ASD would demonstrate increased ASD-related social behavior relative to young adult men with ASD. In line with our hypothesis of exacerbated age-related reductions in EN functional connectivity in ASD, we expected that age group differences in hypoconnectivity would be greater in magnitude for men with ASD relative to NT men, with older men with ASD demonstrating the greatest level of hypoconnectivity in the EN. Furthermore, we hypothesized a significant relationship between ASD-related social behavior and behavioral and neural indicators of executive functioning in men with ASD, such that worse performance on the ToL and reduced connectivity of the bilateral dlPFC within the EN would correlate with increased ASD-related social behavior. Due to its specific relevance to metacognitive aspects of social functioning, we also investigated exploratory analyses of a Social Cognition subscale measure.
Methods
Participants and Materials
Eighty-five adult men with and without ASD were recruited from the greater Phoenix area for this cross-sectional study, in either young adult (18–25 years) or middle-age (40–70 years) cohorts [24 young-adults with ASD (mean age = 21.1 years); 25 middle-age adults with ASD (mean age = 53.0 years); 15 young-adults NT adults (mean age = 21.0 years); and 21 middle-age NT adults (mean age = 50.0 years)]. The middle-age range was chosen based on the only other study of brain connectivity in older adults with ASD; this group demonstrated structural connectivity differences beginning around age 40 and extended to early 70s (Koolschijn et al., 2016). Further, others have found this middle-age range to be relatively stable in ASD traits (Lever and Geurts, 2018). We chose our young adult group with 15 years of separation from our middle-age group in order to reduce variance and gain power for age group comparisons. See Table 1 for demographic information across discrete young adult and middle-aged groups. There were no significant differences in age between diagnosis group cohorts for the young adult (t37=−0.20, p=0.85) and middle-aged groups (t44=−1.42, p=0.16). Participants with ASD were recruited via the Southwest Autism Research and Resource Center (SARRC) lifetime database, a voluntarily enrolled database that includes information from all clients who participated in a clinical or research program at SARRC. Other participants with ASD were recruited via grassroots community groups and flyers posted at ASD community events, and their diagnosis was confirmed by SARRC upon enrollment. NT participants were recruited via word of mouth and flyers posted throughout the community.
Table 1.
Demographic and behavioral data for young and middle-aged groups with and without ASD
| YA-ASD (n=24) | MA-ASD (n=25) | YA-NT (n=15) | MA-NT (n=21) | Statistics | |
|---|---|---|---|---|---|
| Age (years) | 21.1 (2.3) | 53.0 (8.8) | 20.9 (2.4) | 49.7 (6.9) | YA: t37=−0.20, p=.85, ηp2=0.00 |
| Range | 18 – 25 | 40 – 70 | 18 – 25 | 40 – 64 | MA: t44=−1.42, p=.16, ηp2=0.05 |
| IQ | 104.6 (14.4) | 109.6 (15.0) | 111.8 (13.1) | 111.0 (13.5) | F(3, 81)=1.11, p=.35, ηp2=0.04 |
| Range | 85 – 139 | 70 – 131 | 88 – 135 | 89 – 141 | |
| Education | 12.7 (1.3) | 15.5 (2.7) | 14.7 (2.0) | 16.0 (2.4) | F(3, 81)=10.47, p=.00*, ηp2=0.28 |
| Range | 9 – 15 | 11 – 20 | 12 – 18 | 9 – 20 | |
| ADOS-2 Total | 10.3 (3.6) | 10.6 (3.0) | - | - | t47=−0.33, p=.75, ηp2=0.00 |
| Range | 2 – 17a | 7 – 19 | |||
| SRS-2 Totalb | 69.7 (11.0) | 73.2 (10.0) | 46.6 (7.3) | 45.7 (6.3) | ASD: F(1, 42)=1.72, p=.20, ηp2=0.04f |
| Range | 50 – 84 | 56 – 89 | 39 – 66 | 37 – 60 | |
| SRS-2 SC | 65.8 (11.1) | 71.3 (11.1) | 45.8 (6.8) | 45.5 (7.8) | ASD: F(1, 42)=4.10,p=.05^ ηp2=0.09f |
| Range | 46 – 83 | 48 – 90 | 37 – 60 | 37 – 72 | (Exploratory) |
| Tol Moves | 27.7 (19.8) | 20.5 (16.2) | 15.3 (14.0) | 19.6 (8.7) | F(3, 80)=1.74, p=.17, ηp2=0.06f |
| Range | 5 – 76 | 0 – 52 | 0 – 45 | 2 – 36 | |
| Depressionc (% of sample) | 25.00% | 40.00% | 0.00% | 0.00% | |
| Anxietyd (% of sample) | 29.00% | 56.00% | 0.00% | 0.00% | |
| ADHD/ADDe (% of sample) | 12.50% | 24.00% | 0.00% | 4.76% |
p≤0.05;
p≤0.05 explorato; Middle-age (MA); Young Adult (YA); Tol (Tower of London); SRS-2 (Social Responsiveness Scale-2n Edition); SC (Social Cognition subscale).
Two YA-ASD did not meet ADOS-2 criteria, but cut-off was overruled based on clinician judgment.
Three YA-ASD and one MA-ASD were missing SRS-2 scores.
Measured by the Beck Depression Inventory-II in ASD groups and self-report in NT groups.
Measured by the State Trait AnxieV Inventory in ASD groups and self-report in NT groups; One YA ASD missing scores.
Based on self-report;
IQ included as a covariate
All participants with ASD either reported a clinical or suspected diagnosis of ASD. A research reliable psychometrist with 10 years of experience assessing individuals with ASD for research purposes conducted the Autism Diagnostic Observation Schedule-2, module 4 (ADOS-2; Lord et al., 2012) and a brief self-reported psychiatric history interview. Results of these assessments were reviewed by a psychologist with 25 years of experience (CJS) diagnosing ASD for research purposes, who completed a DSM-5 checklist (American Psychiatric Association, 2013) based on current presentation and history. Two young adult participants in this sample did not meet ADOS-2 criteria for ASD diagnosis. In these two cases, the ADOS-2 was overruled based on clinician judgment and known sensitivity limitations of the standard cut-off criteria for non-intellectually disabled adults with ASD (similar to Bastiaansen et al., 2011). Young-adult and middle-age ASD groups did not differ in their overall symptom severity based on the ADOS-2 Total Score (t47=−0.33, p=0.75; Table 1). When asked the date of the original diagnosis, a moderate response rate of 50% young adults and 68% of middle-age adults was achieved. Of these respondents, there was a wide age range for the first ASD diagnosis (2–64 years). More specifically, the young vs. middle-age groups endorsed (respectively), 16% vs. 56% adult diagnosis (18 years of age or older), 25% vs. 4% late childhood diagnosis (8–17 years of age), 8% vs. 8% early childhood diagnosis (before 8 years of age).
NT participants reported no suspected or confirmed diagnosis of ASD. NT participants were then administered the Social Responsiveness Scale-2 Adult Self-Report (SRS-2; Constantino, 2012). Due to the relatively low level of specificity (0.60; Mandell et al., 2012) the cutoff for NT participants was set at the moderate range (T-score = 66), in order to accommodate normal variation in social behavior that is unrelated to ASD. Two young adults inquiring about participation as a NT were screened and excluded for SRS-2 scores exceeding this threshold. To further increase confidence in NT status, having a first-degree relative with an ASD diagnosis was an exclusionary criteria for NT participants.
Other inclusion criteria for all participants were score >70 on the Kaufman Brief Intelligence Test – 2nd Edition (KBIT – 2; Kaufman & Kaufman, 2004) and score ≥26 on the Mini Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975). There were no significant differences across age or diagnostic groups in IQ (Table 1; F(3, 81)=1.11; p=0.35). Participants also self-reported years of education attained. There were significant group differences in educational attainment (Table 1; F(3, 81), =10.47; p<0.001), with post-hoc tests showing middle aged adults with ASD were significantly more educated than young adults with ASD (p<0.001), and young NT adults were significantly more educated than young adults with ASD (p=0.01). The former was expected due to the targeted “college age” of the young-adult group.
General medical history was self-reported by all participants. No participants reported a history of neurological illness such as stroke or dementia, head injury with loss of consciousness, or any known genetic disorders. As common with ASD, a small percentage of our participants had experienced a single childhood seizure, but none continued to experience seizures in adulthood nor took seizure medication. NT participants were free from a history of psychiatric mood disorders based on self-report. We did not, however, exclude history of depression or anxiety in participants with ASD since these conditions are very common comorbidities (Lever & Geurts, 2016). For ASD participants, depression was measured by the Beck Depression Inventory-II (Beck, Steer, Ball, & Ranieri, 1996) and anxiety was measured by the State Trait Anxiety Inventory (Spielberger, 1983; Table 1). Based on these self-report assessments, 40% and 56% of the middle-aged sample with ASD and 25% and 29% of the young adult sample with ASD reported clinically significant levels of depression and anxiety, respectively (Table 1). Self-reported history of ADD/ADHD was allowed and reported in six middle-aged adults with ASD (24% of group), three young adults with ASD (12.5% of group), and one middle-aged NT adult (4.8% of group; Table 1). One young adult participant with ASD also reported bipolar disorder, and results were unchanged when they were excluded from the analyses. This study was conducted in compliance with Arizona State University’s ethical standards for research and the Declaration of Helsinki 2000 revision. All participants provided written consent approved by the Institutional Review Board.
Behavioral Dependent Measures
The SRS-2 (Constantino, 2012), a reliable and valid 65-item a self-report questionnaire measuring social behavior associated with ASD, was administered to all participants. However, only participants with ASD were included in SRS-2 analyses, as high SRS-2 scores were exclusionary for NT participants. The scale yields a total T-score used for screening purposes that summarizes social behaviors associated with ASD. A score 59 or below indicates the individual likely does not present with significant social behaviors associated with ASD, with higher scores indicating mild, moderate, or severe presence of social behaviors associated with ASD. This measure contains subscales measuring Social Awareness (8 items), Social Cognition (12 items), Social Communication (22 items), Social Motivation (11 items), and Restricted Interests/Repetitive Behaviors (12 items). The measure also provides a total score summarizing ASD-related social behavior. The SRS-2 Total Score was used as a primary dependent measure. Exploratory analyses investigated the Social Cognition subscale, due to its specific relevance to metacognitive aspects of social functioning.
In order to assess executive functioning, the ToL (Shallice, 1982) was administered to all participants, as done previously in individuals with ASD (Hill, 2004). Briefly, this test evaluates planning aspects of executive functioning by requiring participants to match a target design by rearranging beads on a pegboard from a set starting position in as few moves as possible. This measure, specifically the Total Moves score, predicts performance on self and proxy reported metacognitive aspects of executive functioning in older adults with ASD (Davids et al., 2016), which have be shown to uniquely predict social functioning in ASD (Leung, Vogan, Powell, Anagnostou, & Taylor, 2015). Thus, the dependent measure we used in statistical analyses was the Total Moves score. The task was administered by highly trained graduate student researchers.
MRI Parameters
Images were collected using a 3-Tesla Philips Ingenia MRI scanner with a maximum gradient strength of 45 mT/m. All participants underwent high-resolution, T1-weighted scans (3D magnetization prepared rapid acquisition gradient echo [MPRAGE]; 170 axial slices, 1.2 mm slice thickness, field of view=240 mm, 256×256 acquisition matrix). Functional blood-oxygen-level dependent (BOLD) signal images were collected via a gradient-echo echo-planar series with whole brain coverage (repetition time=3000ms, echo time=25ms, flip angle=80°, 3mm slice thickness, 24mm field of view, 64×64 acquisition matrix). Resting-state scans were six minutes in duration, during which 120 brain volumes were collected while the participant had their eyes closed. Prior to MRI data acquisition, the option to visit the imaging center and experience the MRI environment was provided to all participants to minimize anxiety-related motion during fMRI acquisition. Padding/headphones were also used to minimize head motion in the scanner.
Resting-State MRI
FMRI Preprocessing.
Resting-state fMRI was selected for connectivity analysis as it has shown good reproducibility (Shah et al., 2016), and potential for identification of neurophenotypes in psychiatric disorders (Van Essen & Ugurbil, 2012). Resting-state data were preprocessed using Statistical Parametric Mapping software (SPM-12; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK) in Matlab (Mathworks, Natick, MA). The first two volumes were removed to account for time needed for scanner magnetization to reach a steady state. Wavelet Despiking using the BrainWavelet toolkit (Patel et al., 2014) was conducted on raw image data to reduce secondary motion artifacts. Slice-time correction and realignment were then performed to correct for differences in slice acquisition timing and motion during scanning, respectively. The structural images were segmented into gray matter and white matter tissue maps and then skull-stripped to improve co-registration. Each participant’s functional image series was co-registered to their skull-stripped T1 image. Using DARTEL Tools implemented in SPM-12, a common template that maximizes inter-participant alignment was generated based on participants’ gray and white matter segmented images using an iterative registration process. The common template was then transformed to MNI space, and each participant’s DARTEL flow field and MNI transformation parameters were applied to their functional image series. During this step, the data underwent smoothing using a 6-mm full-width half-maximum Gaussian kernel to reduce spatial noise. Images were visually inspected after each step in the preprocessing pipeline. Using the Artifact Detection Tools toolbox, smoothed and normalized images were inspected for high-motion volumes to be censored. However, no volumes exceeded the moderately conservative thresholding criteria (0.9mm) of relative scan-to-scan displacement. Using the maximum threshold recommended for resting-state functional connectivity analyses (0.2mm) identified only 7% of the sample exceeding this level of motion. However, this stringent level of scrubbing was not deemed appropriate because our method of independent component analysis (ICA; describe below) has been found to outperform motion scrubbing in a study comparing denoising methods (Middlebrooks et al., 2017). A recent review outlines the number one benefit of ICA is its robustness to artifacts (Calhoun & Lacy, 2017). Furthermore, motion was not included as a nuisance variable because ICA captures motion as discrete components and removes the variance from other, cognitive-based components.
Functional Connectivity.
ICA was performed on resting-state data to decompose the complex spatial-temporal signal into independent component networks and parse out noise components (Calhoun & Lacy, 2017; Middlebrooks et al., 2017). For this reason, traditional band-pass filtering was not applied during preprocessing as frequency information valuable for ICA can be lost (Pignat et al., 2013). Preprocessed images were entered into the Group ICA for fMRI Toolbox (GIFT) implemented in MatLab (GIFT; http://icatb.sourceforge.net/). Prior to ICA, images underwent intensity normalization and then principle components analysis (PCA) to estimate the optimal number of components using the minimum description length criteria. Subsequently, ICA was performed using the Infomax algorithm for the estimated number of networks determined during PCA (Li, Adali, & Calhoun, 2007). Back-reconstruction was performed on each component to generate individual subject time courses and networks (V. Calhoun, Adali, Pearlson, & Pekar, 2001). ICA output constitutes spatial z-maps for each network in which z-scores denote the relative contribution of each voxel (e.g. connectivity) to the estimated overall time course of the network (Beckmann et al., 2005).
Components were first spatially sorted to standard white matter and cerebral spinal fluid masks and disregarded from analysis if r2>.02 or r2>.05, respectively (Kim, Mathalon, Ford, & Mannell, 2009). The GIFT Component Labeler Utility and visual inspection were then used to identify group-level spatial component maps. The Component Labeler Utility matches observed group-level spatial component maps to canonical resting-state network templates, and was used first to empirically identify components most likely to be associated with a given network. Three components were identified by the component labeler as having significant spatial overlap with the EN. As is considered best practice, final component selection was done through visual inspection of the selected spatial component maps by an expert neuroscientist (BBB; see Assaf et al., 2010 and von dem Hagen, Stoyanova, Baron-Cohen, & Calder, 2013 for similar methods). Two of the identified components were head motion-related. Visual inspection was done to discard these motion-related components (Mckeown et al., 1998). The final component was confirmed as the EN based on the presence of key bilateral network nodes in the dlPFC and PPC (Figure 1; Seeley et al., 2007).
Figure 1.
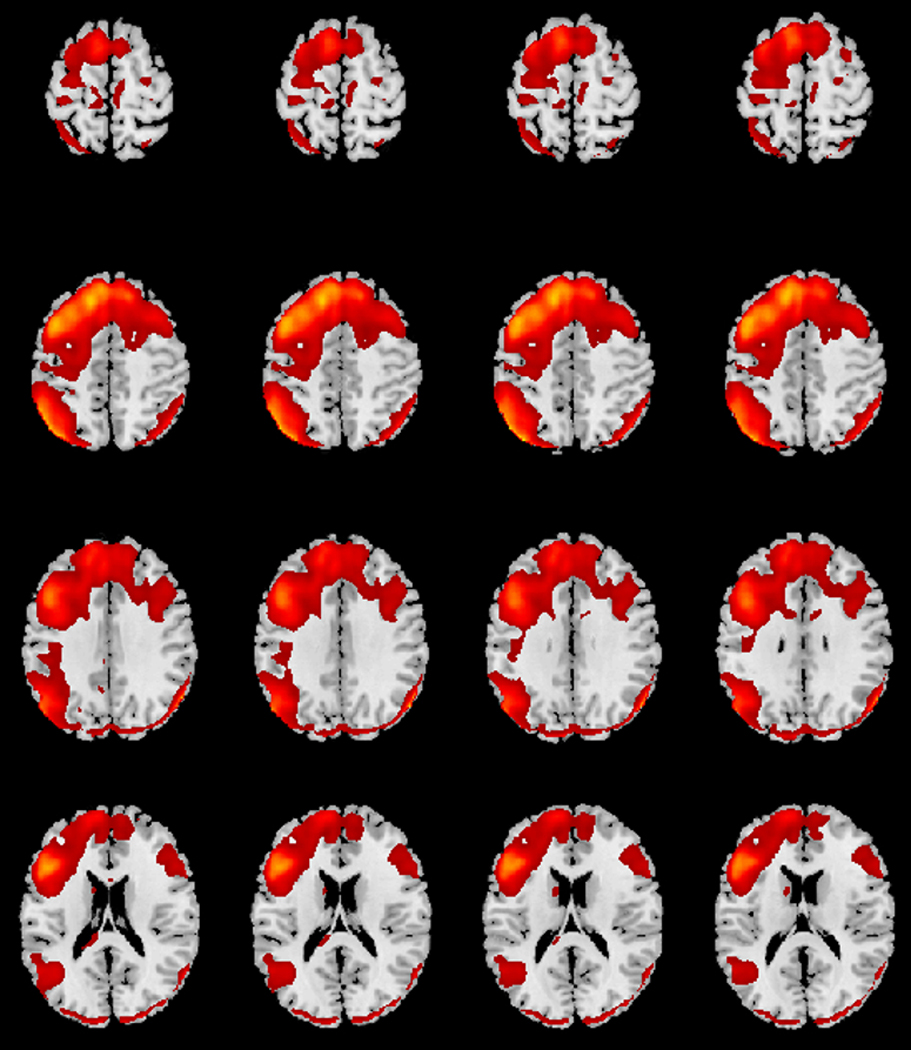
Spatial map for the executive network (EN) across all participants, which contain key nodes in the bilateral dorsolateral prefrontal cortex (dlPFC) and posterior parietal cortex (PPC), with moderate left lateralization.
Analyses
Behavior Comparisons and Correlations.
All analyses were conducted in SPSS (IBM SPSS Statistics for Windows, Version 25.0, Armonk, NY). In order to test the confirmatory hypothesis that older adults with ASD demonstrate greater ASD-related social behavior than young adults, a one-way ANCOVA was run on SRS-2 Total Scores between young adult and middle-aged adults with ASD. For the behavioral measure of executive function, a one-way ANCOVA was performed on the ToL measure of interest (Total Move Count) with group as four-level factor. Least Significant Difference post-hoc comparisons were conducted when significant omnibus effects were present. For analyses of executive functioning and ASD-related social behavior, IQ was included as a covariate given that its influence on cognitive and social functioning may mask associations with age and other variables (Bora & Pantelis, 2016; Steinberg, Bieliauskas, Smith, Ivnik, & Malec, 2005). To confirm the relationship between ASD-related social behavior and metacognitive aspects of executive functioning in young and middle-aged adults with ASD, correlations were evaluated between the SRS-2 Total Score and the ToL Total Move Count. Alpha was set at 0.025 for family-wise error correction of the correlation run separately in the two groups. Exploratory analyses were run on the SRS-2 Social Cognition Subscale, due to its specific relevance to cognitive interpretation of social behavior, with no correction for multiple comparisons.
Group ICN Comparisons.
A group-level random effects factorial analysis was conducted in SPM-12 on the EN network (Figure 1) with age group (young adult, middle-aged) and diagnosis group (NT, ASD) as factors and IQ as a covariate given its potential to mask age effects (Adleman et al., 2002). The whole brain analysis was overlayed with an inclusive neurosynth.org generated mask using the search term “executive,” which yields 786 studies informing brain regions relevant to executive functioning. The remaining voxels were analyzed with a threshold of p<.05, False Discovery Rate corrected. The a priori contrast of interest modeled “exacerbated aging” in ASD (henceforth referred to as the “exacerbated aging in ASD” contrast) to detect areas where functional connectivity within the EN would be greater for young-adult NT participants than young adult participants with ASD (e.g. Just, Cherkassky, Keller, Kana, & Minshew, 2007), with an increased magnitude of hypoconnectivity between young versus middle-aged adults with ASD compared to NT adults. Normal age-related hypoconnectivity of the EN is established in the literature (Damoiseaux, 2017; Zhang et al., 2014), and this contrast tests the hypothesis that adults with ASD experience exacerbated age-related hypoconnectivity. Main effects of age group and diagnosis group were also examined, but not expected given the hypothesis that age group would affect diagnosis groups differently.
EN Correlations with ASD-Related Social Behavior.
Mean region of interest z-scores were extracted for each participant using the marsbar tool in SPM-12 (Brett, Anton, Valabregue, 2002) for 5mm spherical regions of interest (ROIs) based on Seeley and colleagues’ (2007) analysis of the EN. In a confirmatory analysis, correlations were examined for both middle-age and young adult ASD participants between the SRS-2 Total Score and functional connectivity in bilateral dlPFC. These two ROIs were selected based on Nitschke et al.’s (2017) meta-analysis results implicating dlPFC in planning performance during the ToL. Alpha was set at 0.0125 for family-wise error correction of the two correlations run in each of the two groups. Exploratory correlations were run between the SRS-2 Total Score and the remaining EN ROIs from Seeley and Colleagues (2007) as well as the Social Cognition Subscale and all EN ROIs, with no correction for multiple comparisons. No correlations were investigated between the ToL and EN connectivity, as a detailed examination of relationships between the EN and behavioral measures of executive function were beyond the scope of this study and have previously been explored extensively (Niendam et al., 2012).
Results
Behavioral Comparisons and Correlations
There were no significant differences between young and middle-age men with ASD on SRS-2 Total Scores (F(1, 42)=1.72, p=0.20, ηp2=0.04; Table 1). However, exploratory analyses revealed middle-age men with ASD had higher scores on the SRS-2 Social Cognition Subscale compared to young adult men (F(1, 42)=4.10, p=0.05, ηp2=0.09; Table 1). There were no significant differences between groups (young and middle-age ASD and NT) for the ToL Total Move score (F(3, 80)=1.74, p=0.17, ηp2=0.06; Table 1).
The correlation between SRS-2 Total Scores and ToL Total Moves was not significant for middle-age men with ASD (r21=0.39, p=0.07) or young-adult men with ASD (r18=0.10, p=0.68; Figure 2a, Table 2). However, for the SRS-2 Social Cognition Subscale exploratory analyses, there were significant correlations with ToL Total Moves for middle-age (r21=0.49, p=0.02) and young adult men with ASD (r18=0.49, p=0.03; Figure 2b, Table 2).
Figure 2.
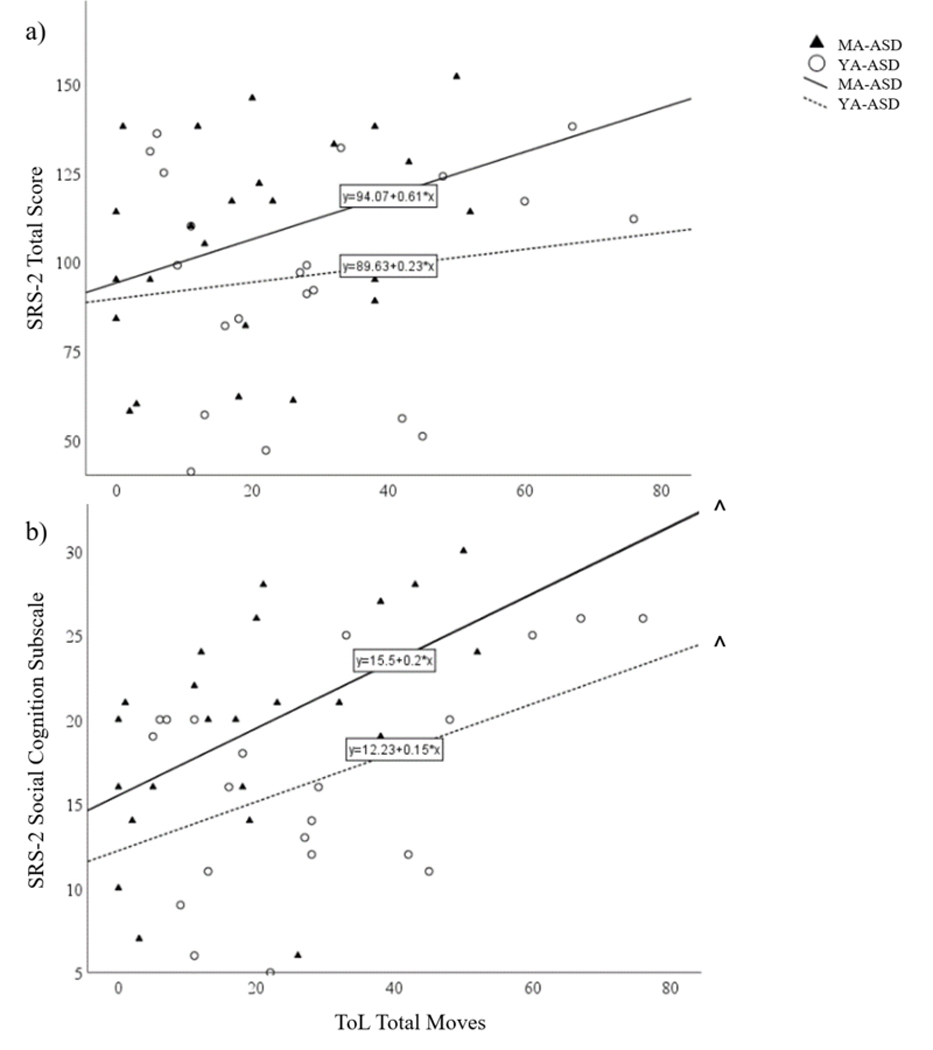
Correlation between Tower of London Total Moves and SRS-2 (a) Total and (b) Social Cognition Subscale Raw scores. *p<0.05, corrected; ^p≤0.05 exploratory; Middle-Age (MA); Young-Adult (YA)
Table 2.
Correlations between executive functioning measures and SRS-2 Scores
| Behavioral Executive Functioning (ToL) | |||
|---|---|---|---|
| SRS-2 Correlations |
|||
| Age Group | Total Score | Social Cognition Subscale (Exploratory) | |
| YA-ASD | r18=. 10 | r18=.49 | |
| Total Moves | p=.68 | p=.03^ | |
| MA-ASD | r21=.39 | r21=.49 | |
| p=.07 | p=.02^ | ||
| Neural Executive Functioning (EN Connectivity) | |||
| SRS-2 Correlations |
|||
| ROI Coordinate | Age Group | Total Score | Social Cognition Subscale (Exploratory) |
|
4Right dIPFC 46 36 18 |
YA-ASD 4MA-ASD |
r18=.36 p=.11 r21=−.52 p=.01* |
r18=.13 p=.58 r21=−.47 p=.02^ |
|
4Left dIPFC −42 34 20 |
YA-ASD 4MA-ASD |
r18=.28 p=.22 r21=.25 p=.25 |
r18=.04 p=.87 r21=.05 p=.82 |
p<.05, corrected;
p<0.05 exploratory; Middle-age (MA); Young Adult (YA); Social Responsiveness Scale-2 (SRS); Tower of London (ToL); Executive Network (EN); Region of Interest (ROI); IQ included as a covariate in all analyses
EN Comparisons and Correlations
Using the previously described “exacerbated aging in ASD” contrast, whole-brain analyses using small-volume correction with an inclusive EN mask revealed two bilateral clusters in the left and right dlPFC (Figure 3). However, only the left dlPFC survived statistical correction at the cluster level (p=0.025, FDR-corrected), while both the right and left dlPFC peak values approached significance (Table 3). No significant main effects were observed for age group or diagnosis group within the EN (Supp. Table 1).
Figure 3.
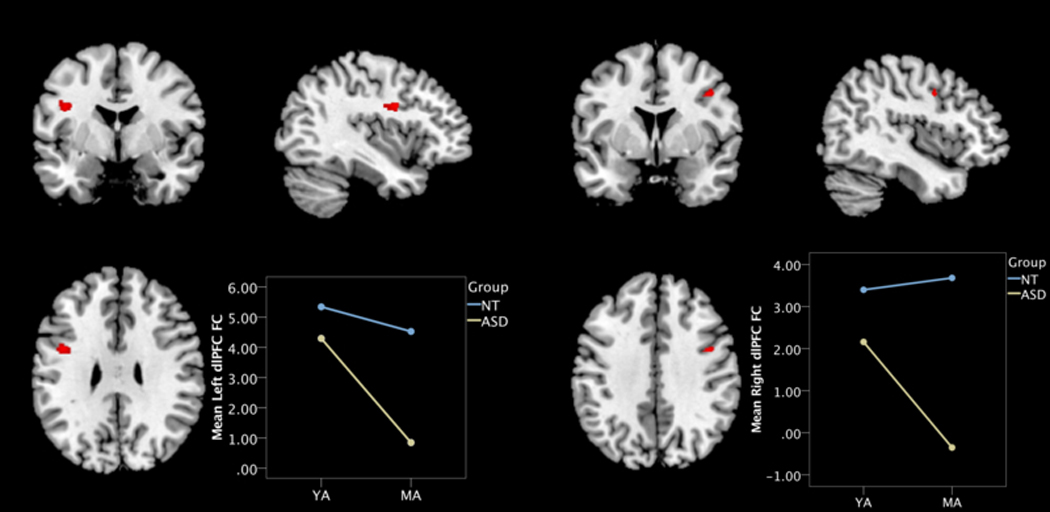
EN connectivity regions of age group by diagnosis group “exacerbated aging” interaction contrast in (a) left and (b) right hemispheres. Insets depict mean FC values from cluster peak to the rest of the EN for each group: Neurotypical (NT; blue); autism spectrum disorder (ASD; yellow); Middle-Age (MA); Young-Adult (YA); dorsolateral prefrontal cortex (dlPFC)
Table 3.
EN connectivity regions of age group by diagnosis group “exacerbated aging” interaction contrast
| Brodmann Area | Peak Voxel Coordinate | Cluster Size (KE) | Peak Significance (FDR-corrected, threshold p=.05) | Z-Score | T-Score | Cluster-Level (FDR-corrected, threshold p=.05) | |
|---|---|---|---|---|---|---|---|
| L dIPFC | 6, 9 | −40 0 28 | 115 | p=. 072 | 4.01 | 4.23 | p=.025* |
| R dIPFC | 6, 9 | 42 2 38 | 18 | p=. 072 | 3.99 | 4.21 | p=.281 |
Threshold: p<0.001, uncorrected; Extent Threshold: 10 voxels; Small volume, false discovery rate correction applied using an Executive Network mask;
p<0.05; dorsolateral prefrontal cortex (dIPFC); Left (L); Right (R)
Confirmatory analyses examined associations between bilateral dlPFC ROIs and SRS-2 Total Scores. Indeed, a significant correlation was found between the right dlPFC and SRS-2 Total Scores for middle-aged men with ASD (r21= −0.52, p=.01), but not young adult men with ASD (r18=0.36, p=0.11; Figure 4a; Table 2). Correlations with the left dlPFC were not significant for either group (young adult: r18=0.28, p=0.22; middle-age: r21=0.25, p=0.25; Table 2). Further exploratory analyses examining correlations with the SRS-2 Social Cognition Subscale identified a weaker correlation with the right dlPFC in middle-age men with ASD (r21= −0.47, p=0.02), but not young adult men with ASD (r18=0.13, p=0.58; Figure 4b; Table 3). There were no significant relationships between left dlPFC and SRS-2 scores in either group (young adult: r18=0.04, p=0.87; middle-age: r21=0.05, p=0.82), or for any of the additional exploratory EN ROIs (Supp. Table 2).
Figure 4.
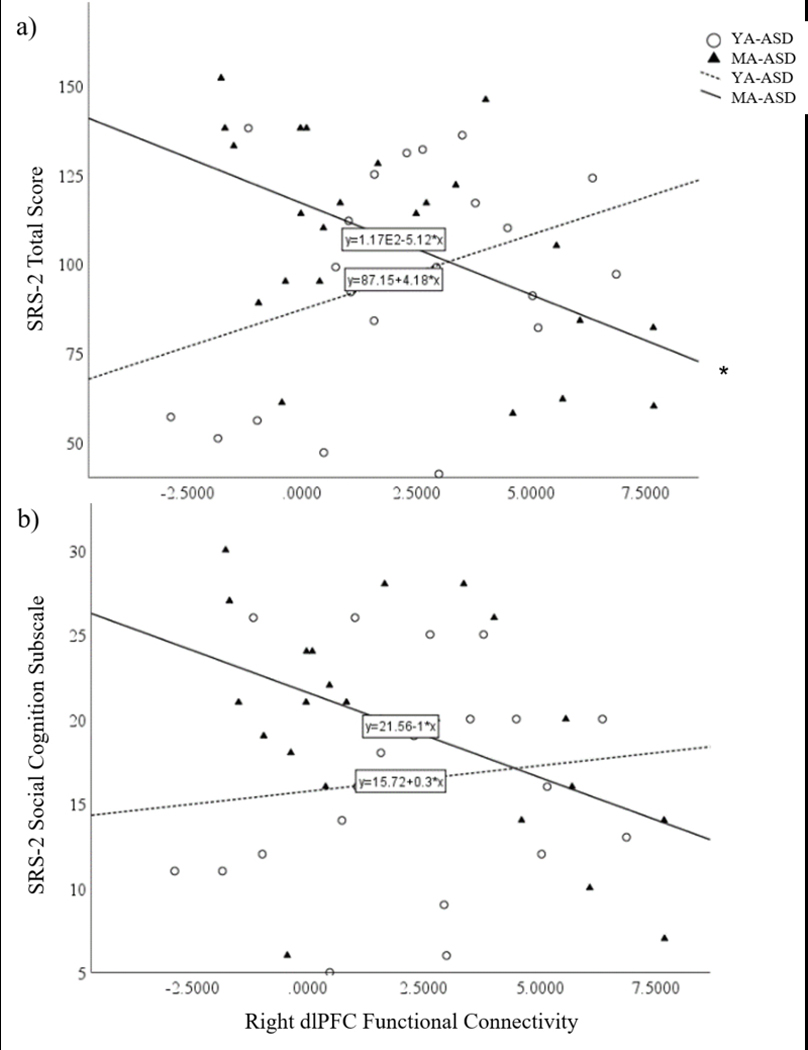
Correlation between functional connectivity of the right dlPFC ROI and SRS-2 (a) Total and (b) Social Cognition Subscale Raw scores. *p<0.05, corrected; ^p≤0.05 exploratory; Middle-Age (MA); Young-Adult (YA)
Discussion
The present study examined age group differences in ASD-related social behavior and underlying neural mechanisms in a cross-sectional sample of men with and without ASD. Our hypothesis of age group differences in ASD-related social behavior based on the SRS-2 Total Score was not supported; however, an exploratory analysis revealed older men with ASD demonstrated greater ASD-related social cognition difficulties that was related to decreased executive function performance. These findings may suggest increased presence of ASD-related social cognition difficulties in older men with ASD, and that social cognition difficulties are underpinned by executive function abilities across the adult range. However, this should be interpreted with caution due to the exploratory nature of analyses involving the SRS-2 Social Cognition subscale. We confirmed that men with ASD demonstrate a pattern of hypoconnectivity in the left dlPFC of the EN, as compared to NT men, and that the magnitude of hypoconnectivity is greater in older men with ASD. This suggests older age group membership may have a larger effect on EN hypoconnectivity in adults with ASD than NT adults. Further, we confirmed that older men with the least connectivity of the right dlPFC to the EN endorsed the greatest levels ASD-related social difficulties. These findings provide preliminary evidence that age group differences in dlPFC connectivity may increase social difficulties in older adults with ASD.
Age Group Differences in ASD-Related Social Behavior
In the present study, exploratory analysis of the SRS-2 Social Cognition Subscale suggests that, compared to young adults, older men with ASD show poorer performance in the interpretation of social behavior. However, previous literature is mixed on age-related symptom exacerbation in ASD. In cross-sectional samples, three studies have reported that older age is associated with increased overall self-reported ASD symptoms and/or ASD-related social behavior in non-intellectually disabled adults with ASD (Abbott et al., 2018; Happé et al., 2016; Lever & Geurts, 2018). All of these studies utilized the Autism Spectrum Quotient (AQ; Baron-Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001), a screener intended to identify traits associated with ASD in the general population. Lever and Geurts (2018) reported a quadratic association between age and overall AQ scores, suggesting that symptoms peak during middle-age. However, AQ social subscale scores followed a linear trend indicating that this domain may continue to be vulnerable to aging, although the cross-sectional nature of these studies cannot control for cohort effects. On the other hand, there are several studies that did not find associations between age and ASD-related social behavior. Bishop and Seltzer (2007) did not find changes associated with age in a cross-sectional sample using the AQ; however; their sample included a large proportion of intellectually disabled participants. There is some evidence that higher intellectual ability is associated with more endorsement of ASD symptoms on self-report measures (Bishop & Seltzer, 2012). Bastiaansen et al. (2011) found older age in adults with ASD was associated with better scores on the Social Functioning Scale, a self-report and informant-report measure developed to describe social adjustment in schizophrenia (Birchwood, Smith, Cochrane, Wetton, & Copestake, 1990), but not on the ADOS-2 Social and Reciprocal Interactions subscale. It is unclear how sensitive the Social Functioning Scale is at detecting ASD-related social behavior. Additionally, two studies have utilized the Autism Diagnostic Interview – Revised (ADI-R; Lord et al., 1994) to probe the question of age effects on ASD symptoms. In these longitudinal studies, Howlin and colleagues (2013) found improvements on the ADI-R social domain between young and middle adulthood; while Shattuck and colleagues (2007) found no changes after a four-year follow up period in adults over 30. Unfortunately, the single follow-up in the study by Howlin and colleagues (2013) leaves open to question the trajectory of symptoms within that 20-year follow up period; similarly, the 4 year follow-up period leaves to question the possibility of changes at later life stages. It has been suggested that older adults with ASD experience symptom amelioration during adolescence and young adulthood (Magiati, Tay, & Howlin, 2014) and symptom exacerbation during middle-age (Lever & Geurts, 2018), and therefore multiple longitudinal follow-ups would be required to describe such patterns.
Three studies have examined constructs related to social behavior (e.g. empathy, perspective-taking, and personal distress in response to others’ distress) in cross-sectional samples with ASD. While Lever and Geurts (2016) found that middle age was related to worse theory of mind scores on a performance-based measure; a subsequent study by Lever and Geurts (2016) found no age-related differences in perspective-taking, empathy, or personal distress in response to others’ distress using a self-report measure. Similarly, Happé et al. (2016) and Abbott et al. (2018) found no changes in self-reported empathy. However, the lack of age effects on affective empathy measures in some of these studies may suggest that aging specifically impacts cognitive rather than affective aspects of social functioning. Given this literature and the current findings, it is difficult to form decisive conclusions regarding the trajectory of ASD-related social difficulties into later adulthood in ASD. Further research is warranted in longitudinal samples to disentangle cohort effects.
Age Group Differences in EN Functional Connectivity
Our findings provide preliminary evidence that older men with ASD experience a larger age group reduction in prefrontal EN connectivity than NT men. Research on brain aging in ASD remains limited and inconclusive. One cross-sectional study demonstrated exacerbated brain aging trajectories in ASD in white matter integrity (Koolschijn et al., 2016). However, Koolschijn and Geurts (2016) reported that declines in gray matter volume and thickness were parallel to declines observed in NT adults. While no studies have examined age-related differences in functional brain connectivity in ASD, studies examining young and older adults separately indicate hypoconnectivity within the EN and related executive control networks. For example, older adults with ASD have demonstrated reduced task-based engagement of a working memory network compared to NT adults, suggesting potential hypoconnectivity in executive control systems (Braden et al., 2017). In younger persons with ASD, connectivity abnormalities within the EN are characterized by reduced connectivity within prefrontal regions, including the bilateral dlPFC in both resting-state and task-based fMRI (Elton et al., 2016; Just et al., 2007; Solomon et al., 2017). It has been suggested that frontal-posterior underconnectivity is a hallmark of ASD and may underlie symptoms (Just, Keller, Malave, Kana, & Varma, 2012); this study provides preliminary evidence that older adults show reduced network integration in a key dlPFC node of the EN compared to young adults. In literature on NT aging, findings of reduced integration in prefrontal and other regions of the EN with age have also been reported (Sala-Llonch, Bartrés-Faz, & Junqué, 2015). Furthermore, these declines are more dramatic relative to three other resting-state canonical cognitive networks, showing predominantly right lateralized reductions in connectivity in the EN (Zhang et al., 2014). Thus, our findings specific to the PFC show convergence with other findings in younger persons with ASD and normal aging. However, our effects were strongest in the left prefrontal region of the EN, perhaps suggesting that the left hemisphere may be more affected in ASD or effects are more bilateral than in normal aging. Lastly, one group found an age-related association of increased inferior frontal lobe recruitment in early middle-age adults with ASD in response to social stimuli which was correlated with improved ASD-related social behavior (Bastiaansen et al., 2011). This may reflect important age-related compensatory mechanisms in ASD. Given the limited scope of literature on functional brain aging in ASD, further research is necessary to verify these findings in a longitudinal sample.
Relationships between Planning and ASD-Related Social Behavior
No significant associations were observed between ToL and SRS-2 Total Scores; however, exploratory correlations found that in both older and young adult men with ASD, those who had the poorest executive function (i.e. planning via ToL) endorsed more ASD-related social cognition difficulties. One study has previously examined the relationship between executive functioning and ASD traits in older adults with ASD. In contrast to our findings, the study found executive functioning and ASD traits were correlated in females but not males in a sample of adults ranging in ages from 18–75 (Abbott et al., 2018). Interestingly, Abbott and colleagues (2018) demonstrated that planning measures were the most significant executive functioning predictors of AQ scores when accounting for anxiety, empathy, and ASD-related systematizing behavior in both male and female groups. However, similar to some of our analyses, Abbott and colleagues (2018) was exploratory and did not correct for multiple comparisons, so replication is warranted. In younger persons with ASD, the relationship between executive functioning and ASD-related social behavior has been documented in several studies. Across development, better executive functioning scores predict improved social behavior (Pugliese et al., 2016, 2015). Metacognitive aspects of executive functioning (which include planning abilities) uniquely predict ASD-related social behavior in children and adolescents with ASD, while behavior regulation aspects of executive functioning predict social behavior in both ASD and NT groups (Leung et al., 2015). Furthermore, there is emerging evidence that good executive functioning is a feature of a subset of individuals with ASD who demonstrate effective symptom “masking” (Livingston, Colvert, Bolton, & Happé, 2018). Given the limited research on relationships between executive functioning and social behavior in older adults with ASD, further research is warranted.
Behaviorally, this study did not observe significant age group differences in ToL performance for either ASD or NT groups. This lack of effect may be due to the middle-age bracket of this study (relative to an elderly group), where age-related cognitive differences may not yet be detectable using behavioral measures. Alternatively, it may be the case that age-related planning differences in ASD parallel NT aging or remain relatively preserved. Importantly, it should be noted that variance in executive functioning abilities is observed in younger persons with ASD (Lai et al., 2017), suggesting that this cross-sectional sample may lack sufficient power to detect subtle cognitive changes associated with early stages of aging in ASD. Further research is necessary to confirm the trajectory of age-related planning differences in ASD using older age brackets and longitudinal observations.
Relationship between EN Functional Connectivity and ASD-Related Social Behavior
In this study, older men with the least connectivity of the right dlPFC to the EN endorsed the greatest levels ASD-related social behavior. The left and right mid-dorsolateral prefrontal cortices (BA 46) are heavily implicated in planning and planning complexity in NT adults (Nitschke et al., 2017). Thus, findings that better self-reported social functioning is associated to higher connectivity in a region of right BA 46 linked to planning provides corroborating neural evidence for behavioral associations between worse ToL performance and more ASD-related difficulties with social cognition. More specifically with reference to the right dlPFC, this region has been implicated in social norm compliance when emotions motivate individuals to take action. Specifically, increased connectivity between the lateral orbitofrontal cortex and right dorsolateral prefrontal cortex is implicated in increased social norm compliance (Spitzer, Fischbacher, Herrnberger, Grön, & Fehr, 2007). It is unclear if connectivity between these regions is driving the observed association with social behavior, given that the current analysis examined regional connectivity with the whole EN. Thus, it remains unclear which specific EN connections with the right dlPFC underlie the association with ASD-related social behavior. Future work investigating this is warranted.
Although no studies have examined relationships between EN functional connectivity and symptoms in older adults with ASD, there is evidence in younger persons suggesting that connectivity within the EN is linked to ASD-related social behavior. Interestingly, EN connectivity was not related to social behavior in our group of young-adult men with ASD. Although there appears to be a positive relationship between these two variables (Figure 4a), this was not significant, thus cannot be interpreted. Elton and colleagues (2016) found that patterns of increased connectivity within the EN and between the EN and other networks (increased segregation) was related to increased ASD-related social behavior. Two task-based studies provide evidence that increased network integration in the EN is linked to reduced ASD traits and ASD-related difficulties in theory of mind, respectively. One study found that reduced mean connectivity within the EN was linked to increased ADOS scores (Just et al., 2007). Another study reported reduced prefrontal connectivity in the EN during a theory of mind task relative to NT participants (Kana, Keller, Cherkassky, Minshew, & Just, 2009). These studies provide general evidence that improved integration in the EN that may extend beyond the dlPFC supports social behavior. It has been suggested that reduced synchronization of prefrontal with more posterior brain regions underlies ASD traits (Just et al., 2012), in line with our observation of reduced dlPFC synchronization within the EN. We conducted exploratory correlations with ASD-related social behavior and other non-prefrontal EN nodes based on Seeley and Colleagues (2007; dorsomedial PFC, right and left PPC, and left frontal operculum) and found no significant effects. Given the exploratory nature of these additional nodes, further research is needed to describe specific connectivity patterns associated with executive functioning and their relationship to social behavior in adults with ASD.
Limitations
Despite significant contributions to the understanding of ASD-related social behavior in advanced age groups and its relationship with behavioral and brain measures of executive function, some limitations remain that warrant caution in interpretation of findings. First, this is a small cross-sectional sample containing moderately unequal distributions across groups and cohort differences (e.g. age of diagnosis, treatment history, etc.). We chose our young adult group with 15 years of separation from our middle-age group in order to reduce variance and gain power for age group comparisons. Although our middle-age range was chosen based on the only other study of brain connectivity in older adults with ASD (Koolschijn et al., 2016), it is relatively large, and groups would be very small if divided into age bins (i.e. 10 year increments). Importantly, others have found this range to be relatively stable in ASD traits (Lever and Geurts, 2018) and global cognitive function in NT adults (MMSE; Hoogendam, Hofman, van der Geest, van der Lugt, & Ikram, 2014), suggesting it is reasonable to group this wide range. Although our sample size is in line with other fMRI studies in ASD (e.g. Assaf et al., 2010; Just et al., 2006), future larger studies are needed that can assess age as a continuous variable across adults with longitudinal follow-up to parse out cohort effects. Second, this is a male-only sample. Since there are sex differences in many of the cognitive and brain measure we investigated, a male only sample was chosen for this initial study due to the difficulty of recruiting sufficient middle-age women with ASD to explore sex interactions. Future research on cognitive and brain aging in women with ASD is sorely needed. Third, psychiatric diagnoses of depression and anxiety were not considered exclusionary criteria for the ASD group due to high co-morbidity in the population. Additionally, it was not possible to match groups on education and achieve a reasonable age distance between young-adult and middle-age groups, thus there were significant differences on this measure across groups. Longitudinal examinations will be important for parsing out potentially confounding cohort effects of individual participant differences such as education, intervention history, and psychiatric comorbidities. Lastly, this sample was limited to non-intellectually disabled men in order to ensure task compliance. Future research is warranted to examine a broader range of intellectual functioning with appropriate methods.
Motion often presents as a limitation for MRI research in ASD populations, and has been found to confound resting-state functional connectivity results (Power, Schlaggar, & Petersen, 2015). However, motion in the sample was very limited, with only 7% of the sample demonstrating >0.2mm translational movement, which has been recommended as a maximum threshold for resting-state functional connectivity analyses. However, no participants exceeded the moderate threshold of 0.9mm translational movement. Motion was further mitigated by the use of independent component analysis to identify and remove motion-related components, a method that has been found to be effective in the absence of motion scrubbing even in high-motion cohorts (Starck et al., 2013). In fact, ICA has been found to outperform motion scrubbing in a study comparing denoising methods (Middlebrooks et al., 2017). A recent review outlines the number one benefit of ICA is its robustness to artifacts (Calhoun & Lacy, 2017). However, we cannot be certain motion did not add minor noise to our data.
Supplementary Material
Implications.
This study furthers understanding of age group differences in ASD-related social behavior and executive functioning from both a behavioral and a neural perspective. Results provide exploratory evidence for increased levels of ASD traits, specifically difficulties with social cognition (e.g., interpretation of social cues and actions) in older adults vs. young adults with ASD. Furthermore, this study observed a pattern of hypoconnectivity within the dlPFC of the EN in middle-age vs. young adults with ASD relative to NT groups consistent with a pattern of “exacerbated aging” in ASD. Poorer planning performance was associated with increased ASD-related social cognition difficulties in older men with ASD; this finding was validated at a neural level between dlPFC hypoconnectivity and greater ASD-related social behavior. The findings of this study raise concerns that aging may increase ASD-related social difficulties. This study also provides preliminary evidence that a mechanism for age-related increases in ASD-related social behavior may be declining executive functioning, a cognitive domain that may enable symptom masking/compensation. Longitudinal replications are warranted to confirm findings of age-related differences in the absence of cohort differences. Ultimately, characterizations of age-related changes in ASD traits and functional brain connectivity will inform identification and development of individualized, neurobiologically-based interventions for adults with ASD across the lifespan.
Acknowledgements
This work was funded by the Department of Defense (AR140105) and the Arizona Alzheimer’s Consortium (State of Arizona). Neither sponsor was involved in study design; the collection, analysis or interpretation of data; the writing of the report; and the decision to submit the article for publication. We are grateful to our participants who made this study possible, and to Sharmeen Maze for her dedication to pristine MRI data collection.
Footnotes
Conflict of Interest
The authors of this study have no financial, personal, or other relationships that may pose a potential conflicts of interest related to this research.
References
- Abbott P, Happé FG, & Charlton RA (2018). Exploratory Study of Executive Function Abilities Across the Adult Lifespan in Individuals Receiving an ASD Diagnosis in Adulthood. Journal of Autism and Developmental Disorders, 0(0), 0 10.1007/s10803-018-3675-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, & Reiss AL (2002). A developmental fMRI study of the Stroop color-word task. NeuroImage, 16(1), 61–75. 10.1006/nimg.2001.1046 [DOI] [PubMed] [Google Scholar]
- American Pychological Association. (2013). Diagnostic and statistical manual of mental disorders (5th editio). Washington, DC. [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, … Pearlson GD (2010). Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. NeuroImage, 53(1), 247–256. 10.1016/j.neuroimage.2010.05.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, & Clubley E. (2001). The Autism Spectrum Quotient : Evidence from Asperger syndrome/high functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31(1), 5–17. 10.1023/A:1005653411471 [DOI] [PubMed] [Google Scholar]
- Bastiaansen JA, Thioux M, Nanetti L, Van Der Gaag C, Ketelaars C, Minderaa R, & Keysers C. (2011). Age-related increase in inferior frontal gyrus activity and social functioning in autism spectrum disorder. Biological Psychiatry, 69(9), 832–838. 10.1016/j.biopsych.2010.11.007 [DOI] [PubMed] [Google Scholar]
- Bastiaansen J, Meffert H, Hein S, Huizinga P, Ketelaars C, Pijnenborg M, … De Bildt A. (2011). Diagnosing autism spectrum disorders in adults: The use of Autism Diagnostic Observation Schedule (ADOS) module 4. Journal of Autism and Developmental Disorders, 41(9), 1256–1266. 10.1007/s10803-010-1157-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, & Ranieri W. (1996). Comparison of beck depression inventories-IA and-II in psychiatric outpatients. Journal of Personality Assessment, 67(3), 588–597. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Deluca M, Devlin JT, Smith SM, Hospital JR, & Ox O. (2005). Investigations into resting-state connectivity using independent component analysis, 360, 1001–1013. 10.1098/rstb.2005.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchwood M, Smith J, Cochrane R, Wetton S, & Copestake S. (1990). The social functioning scale: The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. The British Journal of Psychiatry, 157(6), 853–859. [DOI] [PubMed] [Google Scholar]
- Bishop SL, & Seltzer MM (2012). Self-reported autism symptoms in adults with autism spectrum disorders. Journal of Autism and Developmental Disorders, 42(11), 2354–2363. 10.1007/s10803-012-1483-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, & Pantelis C. (2016). Meta-analysis of social cognition in attention-deficit/hyperactivity disorder (ADHD): Comparison with healthy controls and autistic spectrum disorder. Psychological Medicine, 46(4), 699–716. 10.1017/S0033291715002573 [DOI] [PubMed] [Google Scholar]
- Braden BB, Smith CJ, Thompson A, Glaspy TK, Wood E, Vatsa D, … Baxter LC (2017). Executive function and functional and structural brain differences in middle-age adults with autism spectrum disorder. Autism Research, 1–15. 10.1002/aur.1842 [DOI] [PubMed] [Google Scholar]
- Bressler SL, & Menon V. (2010). Large-scale brain networks in cognition: emerging methods and principles. Trends in Cognitive Sciences, 14(6), 277–290. 10.1016/j.tics.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Brunsdon VEA, Colvert E, Ames C, Garnett T, Gillan N, Hallett V, … Happé F. (2015). Exploring the cognitive features in children with autism spectrum disorder, their co-twins, and typically developing children within a population-based sample. Journal of Child Psychology and Psychiatry and Allied Disciplines, 56(8), 893–902. 10.1111/jcpp.12362 [DOI] [PubMed] [Google Scholar]
- Calhoun V, Adali T, Pearlson G, & Pekar J. (2001). Group ICA of functional MRI data: Separability, stationarity, and inference. In Proc. Int. Conf. on ICA and BSS San Diego, CA, 155, 155–160. [Google Scholar]
- Calhoun VD, & Lacy ND (2017). Ten key observations on the analysis of resting-state functional MR imaging data using independent component analysis. Neuroimaging Clinical N Am, 27(4), 561–579. 10.1016/j.nic.2017.06.012.Ten [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN (2012). Social Responsiveness Scale-2nd Edition Los Angeles: Western Psychological Services. [Google Scholar]
- Damoiseaux JS (2017). Effects of Aging on Functional and Structural Brain Connectivity. NeuroImage, (January), 1–9. 10.1016/j.neuroimage.2017.01.077 [DOI] [PubMed] [Google Scholar]
- Davids RCD, Groen Y, Berg IJ, Tucha OM, & van Balkom IDC (2016). Executive Functions in Older Adults With Autism Spectrum Disorder: Objective Performance and Subjective Complaints. Journal of Autism and Developmental Disorders, 46(9), 2859–2873. 10.1007/s10803-016-2831-4 [DOI] [PubMed] [Google Scholar]
- Elton A, Di Martino A, Hazlett HC, & Gao W. (2016). Neural Connectivity Evidence for a Categorical-Dimensional Hybrid Model of Autism Spectrum Disorder. Biological Psychiatry, 80(2), 120–128. 10.1016/j.biopsych.2015.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Frith U. (2012). Why we need cognitive explanations of autism. The Quarterly Journal of Experimental Psychology, 65(11), 2073–2092. 10.1080/17470218.2012.697178 [DOI] [PubMed] [Google Scholar]
- Geurts HM, & Vissers ME (2012). Elderly with autism: Executive functions and memory. Journal of Autism and Developmental Disorders, 42(5), 665–675. 10.1007/s10803-011-1291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé FG, Mansour H, Barrett P, Brown T, Abbott P, & Charlton RA (2016). Demographic and Cognitive Profile of Individuals Seeking a Diagnosis of Autism Spectrum Disorder in Adulthood. Journal of Autism and Developmental Disorders, 46(11), 3469–3480. 10.1007/s10803-016-2886-2 [DOI] [PubMed] [Google Scholar]
- Hill EL (2004). Executive dysfunction in autism. Trends in Cognitive Sciences, 8(1), 26–32. 10.1016/j.tics.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Hoogendam YY, Hofman A, van der Geest JN, van der Lugt A, & Ikram MA (2014). Patterns of cognitive function in aging : the Rotterdam Study. European Journal of Epidemiology, 29, 133–140. 10.1007/s10654-014-9885-4 [DOI] [PubMed] [Google Scholar]
- Howlin P, & Magiati I. (2017). Autism spectrum disorder: Outcomes in adulthood. Current Opinion in Psychiatry, 30(2), 69–76. 10.1097/YCO.0000000000000308 [DOI] [PubMed] [Google Scholar]
- Howlin P, Moss P, Savage S, & Rutter M. (2013). Social Outcomes in Mid-to Later Adulthood. Journal of the American Academy of Child & Adolescent Psychiatry, 52(6), 572–581. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, & Minshew NJ (2007). Functional and anatomical cortical underconnectivity in autism: Evidence from an fmri study of an executive function task and corpus callosum morphometry. Cerebral Cortex, 17(4), 951–961. 10.1093/cercor/bhl006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, & Varma S. (2012). Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neuroscience and Biobehavioral Reviews, 36(4), 1292–1313. 10.1016/j.neubiorev.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, & Just MA (2009). Regions in Autism During Mental State Attribution. Social Neuroscience, 4(2), 135–152. 10.1080/17470910802198510.Atypical [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS KN (2004). KBIT-2: Kaufman Brief Intelligence Test (2nd ed.). Upper Saddle River, NJ: Pearson Education, Inc. [Google Scholar]
- Kim D, Mathalon DH, Ford JM, & Mannell M. (2009). Auditory Oddball Deficits in Schizophrenia : An Independent Component Analysis of the fMRI Multisite Function BIRN Study, 35(1), 67–81. 10.1093/schbul/sbn133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PCMP, Caan MWA, Teeuw J, Olabarriaga SD, & Geurts HM (2016). Age-related differences in autism: The case of white matter microstructure. Human Brain Mapping. 10.1002/hbm.23345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PCMP, & Geurts HM (2016). Gray Matter Characteristics in Mid and Old Aged Adults with ASD. Journal of Autism and Developmental Disorders, 46(8), 2666–2678. 10.1007/s10803-016-2810-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CLE, Lau Z, Lui SSY, Lok E, Tam V, Chan Q, … Cheung EFC (2017). Meta-analysis of neuropsychological measures of executive functioning in children and adolescents with high-functioning autism spectrum disorder. Autism Research, 10(5), 911–939. 10.1002/aur.1723 [DOI] [PubMed] [Google Scholar]
- Leung RC, Vogan VM, Powell TL, Anagnostou E, & Taylor MJ (2015). The role of executive functions in social impairment in Autism Spectrum Disorder. Child Neuropsychology, 7049(March), 1–9. 10.1080/09297049.2015.1005066 [DOI] [PubMed] [Google Scholar]
- Lever AG, & Geurts HM (2016). Psychiatric Co-occurring Symptoms and Disorders in Young, Middle-Aged, and Older Adults with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 46(6), 1916–1930. 10.1007/s10803-016-2722-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever AG, & Geurts HM (2018). Is Older Age Associated with Higher Self-and Other-Rated ASD Characteristics? Journal of Autism and Developmental Disorders, 48(6), 2038–2051. 10.1007/s10803-017-3444-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever AG, Werkle-Bergner M, Brandmaier AM, & Ridderinkhof RK (2015). Atypical working memory decline across the adult lifespan in autism spectrum disorder? Journal of Abnormal Psychology, 124(4), 1014–1026. Retrieved from [DOI] [PubMed] [Google Scholar]
- Li Y, Adali T, & Calhoun VD (2007). Estimating the Number of Independent Components for Functional Magnetic Resonance Imaging Data. Human Brain Mapping, 28, 1251–1266. 10.1002/hbm.20359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, & Barrett LF (2012). A functional architecture of the human brain: Insights from emotion. Trends in Cognitive Sciences, 16(11), 533–554. 10.1016/j.tics.2012.09.005.A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston LA, Colvert E, Bolton P, & Happé F. (2018). Good social skills despite poor theory of mind: exploring compensation in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 10.1111/jcpp.12886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston LA, & Happé F. (2017). Conceptualising Compensation in Neurodevelopmental Disorders: Reflections from Autism Spectrum Disorder Conceptualising Compensation in Neurodevelopmental Disorders: Reflections from Autism Spectrum Disorder. Neuroscience and Biobehavioral Reviews. 10.1016/j.neubiorev.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop S. (2012). Autism diagnostic observation schedule: ADOS-2. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, & Le Couteur A. (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 10.1007/BF02172145 [DOI] [PubMed] [Google Scholar]
- Magiati I, Tay XW, & Howlin P. (2014). Cognitive, language, social and behavioural outcomes in adults with autism spectrum disorders: A systematic review of longitudinal follow-up studies in adulthood. Clinical Psychology Review, 34(1), 78–86. 10.1016/j.cpr.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Mandell DS, Lawer LJ, Branch K, Brodkin ES, Healey K, Witalec R, … Gur RE (2012). Prevalence and correlates of autism in a state psychiatric hospital. Autism, 16(6), 557–567. 10.1177/1362361311412058 [DOI] [PubMed] [Google Scholar]
- Brett Matthew, Anton Jean-Luc, Valabregue Romain, P J-B (2002). Region of interest analysis using an SPM toolbox. [Abstract] Presented at the 8th International Conference on Functional Mapping of the Human Brain Sendai, Japan: Available on CD-ROM in NeuroImage. [Google Scholar]
- Mckeown MJ, Makeig S, Brown GG, Jung T, Kindermann SS, Bell AJ, & Sejnowski TJ (1998). Analysis of fMRI Data by Blind Separation Into Independent Spatial Components, 188(January), 160–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks EH, Frost CJ, Tuna IS, Schmalfuss IM, Rahman M, & Old Crow A. (2017). Reduction of motion artifacts and noise using independent component analysis in task-based functional MRI for preoperative planning in patients with brain tumor. American Journal of Neuroradiology, 38(2), 336–342. 10.3174/ajnr.A4996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, & Carter CS (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions, 241–268. 10.3758/s13415-011-0083-5 [DOI] [PMC free article] [PubMed]
- Nitschke K, Köstering L, Finkel L, Weiller C, & Kaller CP (2017). A Meta-analysis on the neural basis of planning: Activation likelihood estimation of functional brain imaging results in the Tower of London task. Human Brain Mapping, 38(1), 396–413. 10.1002/hbm.23368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AX, Kundu P, Rubinov M, Jones PS, Vértes PE, Ersche KD, … Bullmore ET (2014). A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. NeuroImage, 95, 287–304. 10.1016/j.neuroimage.2014.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignat JM, Koval O, Van De Ville D, Voloshynovskiy S, Michel C, & Pun T. (2013). The impact of denoising on independent component analysis of functional magnetic resonance imaging data. Journal of Neuroscience Methods, 213(1), 105–122. 10.1016/j.jneumeth.2012.10.011 [DOI] [PubMed] [Google Scholar]
- Piven J, & Rabins P. (2011). Autism spectrum disorders in older adults: Toward defining a research agenda. Journal of the American Geriatrics Society, 59(11), 2151–2155. 10.1111/j.1532-5415.2011.03632.x [DOI] [PubMed] [Google Scholar]
- Powell PS, Klinger LG, & Klinger MR (2017). Patterns of Age-Related Cognitive Differences in Adults with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 47(10), 3204–3219. 10.1007/s10803-017-3238-6 [DOI] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, & Petersen SE (2015). Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage, 105, 536–551. 10.1016/j.neuroimage.2014.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese CE, Anthony LG, Strang JF, Dudley K, Wallace GL, Naiman DQ, & Kenworthy L. (2016). Longitudinal Examination of Adaptive Behavior in Autism Spectrum Disorders: Influence of Executive Function. Journal of Autism and Developmental Disorders, 46(2), 467–477. 10.1007/s10803-015-2584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese CE, Anthony L, Strang JF, Dudley K, Wallace GL, & Kenworthy L. (2015). Increasing Adaptive Behavior Skill Deficits From Childhood to Adolescence in Autism Spectrum Disorder: Role of Executive Function. Journal of Autism and Developmental Disorders, 45(6), 1579–1587. 10.1007/s10803-014-2309-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Llonch R, Bartrés-Faz D, & Junqué C. (2015). Reorganization of brain networks in aging: a review of functional connectivity studies. Frontiers in Psychology, 6(May), 1–11. 10.3389/fpsyg.2015.00663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, … Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci, 27(9), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah LM, Cramer JA, Ferguson MA, Birn RM, & Anderson JS (2016). Reliability and reproducibility of individual differences in functional connectivity acquired during task and resting state. Brain and Behavior, 6(5), 1–15. 10.1002/brb3.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. (1982). Specific impairments in planning. Phil. Trans. R. Soc. Lond, 298, 199–209. [DOI] [PubMed] [Google Scholar]
- Shattuck PT, Seltzer MM, Greenberg JS, Orsmond GI, Bolt D, Kring S, … Lord C. (2007). Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. Journal of Autism and Developmental Disorders, 37(9), 1735–1747. 10.1007/s10803-006-0307-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Hogeveen J, Libero LE, & Nordahl CW (2017). An Altered Scaffold for Information Processing: Cognitive Control Development in Adolescents With Autism. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(6), 464–475. 10.1016/j.bpsc.2017.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD (1983). State-trait anxiety inventory for adults. Palo Alto, CA: Mind Garden. [Google Scholar]
- Spitzer M, Fischbacher U, Herrnberger B, Grön G, & Fehr E. (2007). The Neural Signature of Social Norm Compliance. Neuron, 56(1), 185–196. 10.1016/j.neuron.2007.09.011 [DOI] [PubMed] [Google Scholar]
- Starck T, Nikkinen J, Rahko J, Remes J, Hurtig T, Haapsamo H, … Kiviniemi VJ (2013). Resting state fMRI reveals a default mode dissociation between retrosplenial and medial prefrontal subnetworks in ASD despite motion scrubbing. Frontiers in Human Neuroscience, 7, 802 10.3389/fnhum.2013.00802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg BA, Bieliauskas LA, Smith GE, Ivnik RJ, & Malec JF (2005). Mayo’s older Americans normative studies: Age-and IQ-adjusted norms for the Auditory Verbal Learning Testing and the Visual Spatial Learning Test. The Clinical Neuropsychologist, 19(3–4). [DOI] [PubMed] [Google Scholar]
- Van Essen DC, & Ugurbil K. (2012). The future of the Human Connectome. Neuroimage, 62(2), 1299–1310. 10.1215/10407391-2009-001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von dem Hagen EAH, Stoyanova RS, Baron-Cohen S, & Calder AJ (2013). Reduced functional connectivity within and between “social” resting state networks in autism spectrum conditions. Social Cognitive and Affective Neuroscience, 8(6), 694–701. 10.1093/scan/nss053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Chen W, Jiao Y, Xu Y, Zhang X, & Wu J. (2014). Selective Vulnerability Related to Aging in Large-Scale Resting Brain Networks, 9(10), 1–8. 10.1371/journal.pone.0108807 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


