Abstract
To conduct a STARD-compliant validity that the contrast-enhanced ultrasound (CEUS) evaluation of prostate for the improvement of positive rate of biopsy and diagnostic efficiency of prostate carcinoma (PCa).
Data of 137 patients with suspected PCa who underwent relevant examinations and treatment were reviewed, and 82 of 137 patients were finally included. The patients consisted of Group 1 (26 patients) and Group 2 (42 patients) according to which they underwent transrectal ultrasound (TRUS) biopsy selected from CEUS evaluation of the prostate and who underwent TRUS-guided biopsy directly. A systematic 12-core biopsy was performed at first, and additional 1 to 2 cores biopsy was made in the suspected target area where CEUS had showed abnormal enhancement. The assumed diagnoses were compared with pathological findings.
There were 37 patients with PCa and 31 patients with benign lesions; and 14 patients without biopsy after CEUS did not find PCa emerging in follow-up (18–47 months). The positive rates of biopsy-malignant lesions were 73.1% and 42.8% in Group 1 and Group 2, respectively. The positive rate of biopsy in Group 1 was significantly higher than that in Group 2 (P = .024). The sensitivity and accuracy of TRUS biopsy and a combination of TRUS biopsy after transrectal CEUS for the evaluation of prostate benign and malignant lesion were 60% and 66.7% (P=0.0139), and 94.4% and 88.5% (P=0.0453), respectively.
CEUS evaluation of the prostate of PSA-elevated patient before biopsy can help select target patient with high risk of PCa, reduce unnecessary biopsy, increase detection rate of PCa, and improve diagnostic sensitivity and accuracy.
Keywords: biopsy, contrast-enhanced ultrasound, prostate carcinoma, transrectal ultrasound
1. Introduction
Prostate carcinoma (PCa) is a common malignant tumor in elder males in western world, with high morbidity and mortality.[1–3] In the early stage, PCa is insidious and asymptomatic; when clinical manifestations present, most patients have entered the late stage. Therefore, early diagnosis is very important for the prognosis and management. At present, clinical diagnostic methods for PCa include digital rectal examination (DRE), determination of serum prostate specific antigen (PSA), transrectal ultrasound (TRUS), magnetic resonance imaging (MRI), ultrasound-guided prostate biopsy, etc. Biopsy and pathological analysis is the gold standard for PCa diagnosis. However, in spite of prostate biopsy protocols have become more extensive over the last decades, missed diagnosis has been a problem.[1,2,4] Ultrasound-guided prostate biopsy is much better than blind biopsy, but it is still difficult to undergo biopsy in each case under direct visualization, for some small focal lesions and diffuse PCa in the prostate are almost invisible. Improving the quality of biopsy specimens is the key to enhance the diagnostic sensitivity and accuracy. Contrast enhanced ultrasound (CEUS) is a relatively new technique, which can characterize some lesions that are difficult to be shown on gray-scale ultrasound and color Doppler flow imaging, and can increase visualization of focal lesions in organs.[2–11] Currently, the novel ultrasonic elastography-based prostate biopsy has not been used routinely because of limitations; fusion of MRI and TRUS images for targeted biopsy of prostate is regarded as a state of the art, but it is costly and inconvenient.[12–14] A combination of CEUS and biopsy for the diagnosis of PCa is still used widely. Previous studies have shown that combining CEUS and biopsy guided by TRUS may reduce biopsy puncture, improve the sampling quality of prostatic lesions, and trace missed lesions in the previous procedure on the basis of visualizing puncture, but the actual methods and results are various and inconsistent; and further study may add more information to the literature.[2–11,15–17] The aim of this study was to investigate the validity that a combination of TRUS biopsy after transrectal CEUS evaluation of prostate for the improvement of positive rate of biopsy and diagnostic efficiency of PCa.
2. Patients and methods
2.1. Study population
Archives of 137 patients clinically suspected of PCa who underwent relevant examinations and treatment from January 2015 to July 2019 were reviewed. The inclusion criteria were that who meet the following 3 or 4 indicators:
-
(1)
Prostate abnormality was found in DRE;
-
(2)
Persistent elevated levels of PSA (PSA > 4.0 ng/mL);
-
(3)
Suspicious PCa was found by TRUS;
-
(4)
Suspicious PCa was found by transrectal CEUS.
The exclusion criteria were:
-
(1)
Those with active prostatitis or serious diseases were not suitable for biopsy;
-
(2)
Those with bleeding tendency and abnormal coagulation function;
-
(3)
CEUS showed benign prostatic nodules without local vascular distortion and asymmetrical distribution;
-
(4)
Those who were reluctant to do biopsy.
At last, 82 patients were included, and 55 patients were excluded (2 patients occurred systemic metastasis; 19 patients with high age and other serious diseases were waived due to reluctance; 2 patients with active prostatitis; 20 patients showed possible benign prostatic nodules; 12 patients selected MRI evaluation and follow-up). All patients who underwent biopsy had signed informed consent form. The ages of the patients ranged from 50 to 87 years, and the median age was 67.2 years. The latest PSA levels of the patients were 4.2 to 25.3 ng/mL (median 7.2 ng/mL). The prostate volumes of the patients were 20.9 to 64.2 mL (median 28.4 mL). The patients who underwent TRUS-guided biopsy came from two groups. The patients in Group 1 (26 patients) were those who had performed TRUS, contrast-enhanced TRUS evaluation of the prostate, and other related examinations and assumed PCa, and patients in the Group 2 (42 patients) were those who had performed TRUS evaluation of the prostate and other related examinations and assumed PCa.
2.2. Examination methods
(1) TRUS examination: GE Logiq E9 ultrasound system was used, with a transrectal transducer with frequency of 5 to 9 MHz. After proper preparation, a transrectal transducer was slowly inserted into the anorectal canal of the patient. The prostate was scrutinizingly scanned, the morphology, size, internal echogenicity, capsule integrity, internal and peripheral blood flow were observed, and the location of focal lesions and suspected lesions were recorded.
(2) CEUS evaluation: Contrast agent of sulfur hexafluoride microbubbles for injection (SonoVue) (Shanghai Bracco Sine Pharmaceutical Corp. Ltd., Shanghai, China) was used. During the examination, the patient took the left lateral decubitus position, the suspicious lesions found on the basis of two-dimensional gray-scale ultrasound and color Doppler flow imaging were reviewed, then 2.4 mL of SonoVue suspension was injected through median cubital vein, followed by 5 mL of 0.9% sodium chloride solution for injection, the enhancement patterns of the prostate were observed, including enhancement time, intensity, pattern, and dynamic changes. The observation time was 4 minutes, and dynamic images were saved in the ultrasound system. Locations of focal lesion, suspicious lesion, and other anomalies were recorded. Manifestations of PCa on ultrasonography include:
-
(1)
Irregularly shaped focal hypoechoic or isoechoic nodules located in the peripheral zone;
-
(2)
Distorted blood vessels distribution in a focal area.
Manifestations of PCa on CEUS include:
-
(1)
Focal hyper-enhancement or rapid (10–20 s) high enhancement nodules or areas;
-
(2)
Rapid hypo-enhancement (wash out) (10–25 s);
-
(3)
Blood vessels in prostate gland distributed asymmetry.
The following manifestations are considered as benign lesions: isoechoic or slightly hypoechoic nodules with normal vessels distribution; blood vessels in prostate gland or nodule distributed evenly; nodules with iso-enhancement or hypo-enhancement but no “wash out”; areas without focal abnormal echogenicity.
(3) TRUS-guided prostate biopsy:
-
(1)
Preoperative preparation: Routine assessment of health status; red blood cell, white blood cell, and platelet counts; and prothrombin time, and thrombin time determination. The use of anticoagulant drugs was suspended one week before the procedure. Oral antibacterial ciprofloxacin was given 3 days before biopsy. Enema cleaning was done 3 hours before biopsy.
-
(2)
Biopsy method: The patient took a decubitus position with the right leg flexion, the anus was fully exposed, and the perineum, anus, and anorectal canal were disinfected using iodophor. The transducer top was spread with a proper amount of coupling gel and covered with a sterilization condom, and was slowly placed into the anorectal canal. Biopsy was performed with an 18-gauge biopsy needle. A systematic 12-core biopsy was performed at first, including 6 biopsies from base, middle, and apex of bilateral peripheral zone, 4 biopsies from the margin of bilateral peripheral zone, and 2 biopsies from bilateral transition zone. Additional 1 to 2 cores biopsy was made in the suspected target area where CEUS had shown abnormal enhancement. Samples of the biopsies were labeled and fixed with a 10% formaldehyde solution, histopathological analysis and Gleason score were executed by a senior pathologist of prostatic glands with reference to diagnostic criteria of prostatic lesions, and Gleason score of 7 to 10 was considered as high malignancy.
2.3. Statistical analysis
The assumed diagnoses of benign and malignant lesions based on TRUS and TRUS after CEUS were compared with biopsy pathological findings, and their sensitivity, specificity, and accuracy were calculated. Chi-square test was used to compare the difference between the two groups, with P < .05 as the difference having statistical significance. SPSS 20.0 software (SPSS, IBM, Armonk, NY) was used for statistical analysis.
2.4. Ethic statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent of patient was not necessary for the retrospective study. Ethical approval was approved by the ethic committee of the first affiliated hospital of Hainan Medical University, and patient informed consent was not necessary because of the retrospective study.
3. Results
PCa and prostate benign lesions confirmed by pathology were 37 and 31 patients, respectively, and 14 patients without biopsy after CEUS showed no PCa emerging during follow-up (18–47 months). Of the 26 suspected PCa patients in Group 1, 19 patients had PCa by biopsy pathology (Gleason score of 7, 8, and 9 accounted for 8, 5, and 6 patients, respectively) and 7 patients had benign prostate hyperplasia (BPH). The positive rate of biopsy malignant lesions was 73.1%. Of the 42 suspected PCa patients in Group 2, 18 patients were confirmed as PCa by biopsy pathology (Gleason score of 7, 8, and 9 accounted for 6, 7, and 5 patients, respectively), 22 patients had BPHs, 2 patients had chronic prostatitis, and the positive rate of biopsy malignant lesions was 42.8%. The positive rate of biopsy in Group 1 was significantly higher than that in Group 2 (Pearson chi-square value was 5.912, P = .024). The sensitivity and accuracy of TRUS and transrectal CEUS for the evaluation of prostate benign and malignant lesion were 60% (95% confidence interval (CI) 58.2%–63.4%) and 66.7% (95% CI 62.6%–73.3%) (P = 0.0139), and 94.4% (95% CI 90.1%–97.2%) and 88.5% (95% CI 84.7%–93.2%) (P = 0.0453) respectively. The results of prostate biopsy in Group 1 and Group 2 and the comparison are shown in Table 1. Figures 1 and 2 illustrate some CEUS characteristics of PCa.
Table 1.
Results and comparison of prostate biopsy between Group 1 and Group 2.

Figure 1.
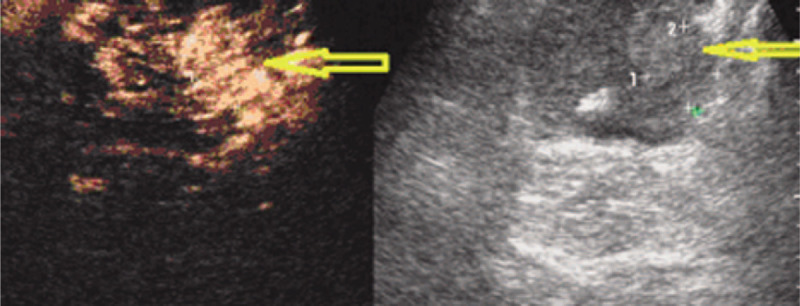
A 64-year-old patient with a PSA level of 9.8 ng/ml. Sonography shows an ill-defined heterogeneous slight high echogenic nodular area (right plot, arrow). It presents rapid heterogeneous hyper-enhancement 16 second after administration of 2.4 mL SonoVue (left plot, arrow); it presents slight hypoechoic in 30th second (not shown). Biopsy targeted to this suspicious area revealed Gleason 6 prostate carcinoma. PSA = serum prostate specific antigen.
Figure 2.
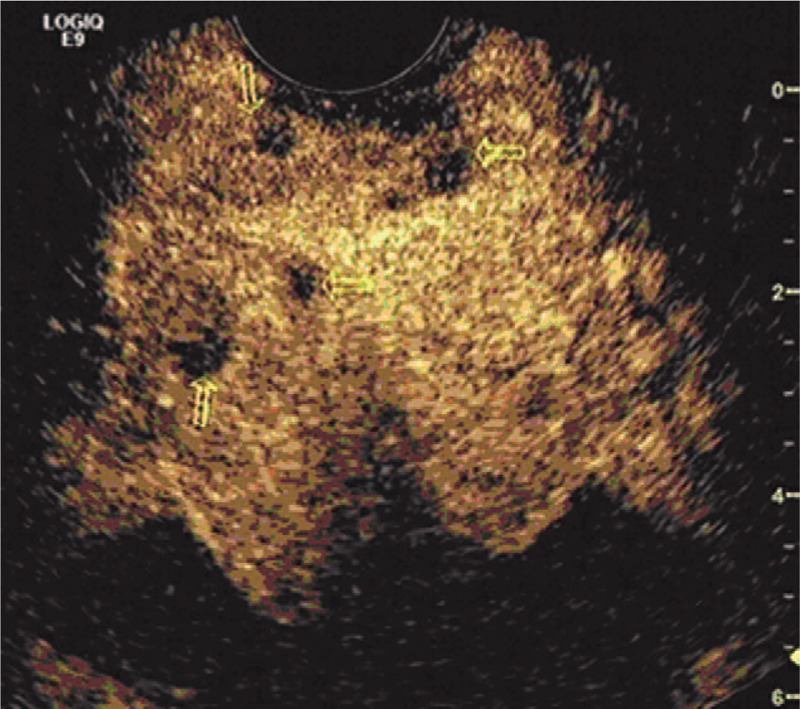
A 71-year-old patient with a PSA level of 21.2 ng/ml. Sonography does not reveal any distinct nodular lesions in the prostate. CEUS shows heterogeneous slight high enhancement (13–16 second) after administration of 2.4 mL SonoVue, and three nodular rapid hypo-enhancement areas in 17th second (arrows). Biopsy targeted to this suspicious area revealed Gleason 8 prostate carcinoma. CEUS = contrast-enhanced ultrasound, PSA = serum prostate specific antigen.
4. Discussion
Ultrasound has been used for diseases evaluation in many fields of medicine for decades, with the merit of noninvasive, convenient, widely accessible, and reasonable cost.[16,18,19] The sensitivity, specificity, and accuracy of TRUS-guided prostate biopsy diagnosis of PCa relate closely to the sample quality of biopsy. Currently, to reduce and avoid missed diagnosis, most biopsy adopts 10 or 12 core punctures.[1,4,6–9] However, excessive and repeat puncture increases patient's suffering and complications. Therefore, it is of great significance to improve biopsy. Compared with BPH and normal tissues, the number of microvessels in PCa is significantly increased in many patients, so detecting the changes of microvessel density in the prostate gland can reveal clue of PCa.[2–11] However, except for a few large blood vessels or vessel with higher velocity blood flow, color Doppler ultrasound cannot show small blood vessels in PCa.[11] This problem may be solved by CEUS, which can exhibit microcirculation perfusion and demonstrate related characteristics, and improve the sensitivity and specificity of PCa diagnosis.[2–11] The results of this study validate the previous studies that CEUS can significantly improve sensitivity and accuracy, but fail to enforce the diagnostic specificity. In this study, TRUS-guided biopsy after CEUS can significantly improve the positive rate of biopsy (P = .024), which was consistent with some previous studies.[6–8,11] Our results showed that biopsy after CEUS reduces the uncertainty of blind puncture and unnecessary puncture of benign nodules. Diffuse PCa has no characteristics on CEUS, which is a cause of miss sampling at biopsy.[9] Therefore, to a negative prostate after CEUS evaluation, diffuse PCa should be vigilant against, and a combination of biopsy with PSA test follow-up is necessary and reasonable. Some prostatic hyperplasia and chronic inflammation in the transition zone show hypervascularity and hyper-enhancement on CEUS, which mimick malignancy, and it is a cause of misdiagnosis.[11] Microcirculation display is not ideal for diffuse lesions; some lesions with small volume or unclear boundary or lesions locate at the margin of peripheral zone.[2,5,7–11] On these conditions, the diagnostic efficiency is compromised. In this study, two patients were assumed had BPH after CEUS, but the biopsy and prostatectomy pathological results showed they were PCas, and the PCa lesions were at the margin of the compressed thinner peripheral zone. Meanwhile, 14 patients on CEUS showed no features of malignancy in the prostate, biopsy was not performed, and no malignancy was found during follow-up. This suggested that performance of transrectal CEUS before TRUS biopsy can reduce the number of punctures and suspected patients according to the results, that is, the conventional puncture layout can be spared for those parts of the prostate that nodules without perfusion and enhancement, and nodules or regions have no abnormal findings on CEUS.[6] Our results that the positive rate of systematic plus targeted biopsy was 72.1% in 26 suspected patients and 42.8% in Group 2 (P = .024) supported that the sensitivity and cancer detection rate of systematic plus targeted biopsy after CEUS were significantly higher than those of systematic biopsy reported by Seitz et al, Zhao et al, and Cornelis et al.[4,5,8] In this study, of the included 82 patients with elevated PSA level, 31 patients were confirmed with PCa, 37 patients were confirmed with benign lesions, and 14 patients were not found to have cancer during follow-up. This is consistent with reports that PSA level is an important indicator for PCa, but its specificity is not high.[20]
The potential limitations of this study were that some small focal lesions do not locate at the same plane, some contrast enhancement characteristics may be missed at CEUS, because some lesions show hyper-enhancement rapidly, and some small focal lesions at distal field could not be displayed clearly at CEUS; and the sample is relative small.
In conclusion, CEUS evaluation of the prostate of PSA-elevated patient can help select target patient with high PCa risk, a combination of TRUS biopsy after transrectal CEUS can reduce unnecessary biopsy, increase detection rate of PCa, and improve diagnostic sensitivity and accuracy.
Author contributions
Conceptualization: Guangqing Liu.
Data curation: Guangqing Liu, Size Wu.
Formal analysis: Size Wu.
Investigation: Guangqing Liu, Li Huang.
Methodology: Guangqing Liu, Size Wu, Li Huang.
Supervision: Guangqing Liu.
Validation: Li Huang.
Visualization: Size Wu, Li Huang.
Writing – original draft: Size Wu.
Writing – review & editing: Size Wu.
Footnotes
Abbreviations: BPH = benign prostate hyperplasia, CEUS = contrast-enhanced ultrasound, DRE = digital rectal examination, MRI = magnetic resonance imaging, PCa = prostate carcinoma, PSA = serum prostate specific antigen, SPSS = Statistical Product and Service Solutions, TRUS = transrectal ultrasound.
How to cite this article: Liu G, Wu S, Huang L. Contrast-enhanced ultrasound evaluation of the prostate before transrectal ultrasound-guided biopsy can improve diagnostic sensitivity: A STARD-compliant article. Medicine. 2020;99:19(e19946).
The authors have no conflicts of interest to disclose.
References
- [1].Bostanci Y, Kazzazi A, Djavan B. Optimizing prostate biopsy. Minerva Urol Nefrol 2012;64:233–43. [PubMed] [Google Scholar]
- [2].Postema AW, Frinking PJ, Smeenge M, et al. Dynamic contrast-enhanced ultrasound parametric imaging for the detection of prostate cancer. BJU Int 2016;117:598–603. [DOI] [PubMed] [Google Scholar]
- [3].Sidhu PS, Cantisani V, Dietrich CF, et al. The EFSUMB Guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (Short Version). Ultraschall Med 2018;39:154–80. [DOI] [PubMed] [Google Scholar]
- [4].Cornelis F, Rigou G, Le Bras Y, et al. Real-time contrast-enhanced transrectal US-guided prostate biopsy: diagnostic accuracy in men with previously negative biopsy results and positive MR imaging findings. Radiology 2013;269:159–66. [DOI] [PubMed] [Google Scholar]
- [5].Seitz M, Gratzke C, Schlenker B, et al. Contrast-enhanced transrectal ultrasound (CE-TRUS) with cadence-contrast pulse sequence (CPS) technology for the identification of prostate cancer. Urol Oncol 2011;29:295–301. [DOI] [PubMed] [Google Scholar]
- [6].Mitterberger M, Horninger W, Aigner F, et al. Contrast-enhanced colour Doppler-targeted vs a 10-core systematic repeat biopsy strategy in patients with previous high-grade prostatic intraepithelial neoplasia. BJU Int 2010;105:1660–2. [DOI] [PubMed] [Google Scholar]
- [7].Li H, Xia J, Xie S, et al. Prostate cancer: a comparison of the diagnostic performance of transrectal ultrasound versus contrast enhanced transrectal ultrasound in different clinical characteristics. Int J Clin Exp Med 2015;8:21428–34. [PMC free article] [PubMed] [Google Scholar]
- [8].Zhao HX, Xia CX, Yin HX, et al. The value and limitations of contrast-enhanced transrectal ultrasonography for the detection of prostate cancer. Eur J Radiol 2013;82:e641–7. [DOI] [PubMed] [Google Scholar]
- [9].Gao Y, Liao XH, Lu L, et al. Contrast-enhanced transrectal ultrasonography for the detection of diffuse prostate cancer. Clin Radiol 2016;71:258–64. [DOI] [PubMed] [Google Scholar]
- [10].Qi T, Chen Y, Zhu Y, et al. Contrast-enhanced transrectal ultrasonography for detection and localization of prostate index tumor: correlation with radical prostatectomy findings. Urology 2014;84:138–43. [DOI] [PubMed] [Google Scholar]
- [11].Xie SW, Li HL, Du J, et al. Contrast-enhanced ultrasonography with contrast-tuned imaging technology for the detection of prostate cancer: comparison with conventional ultrasonography. BJU Int 2012;109:1620–6. [DOI] [PubMed] [Google Scholar]
- [12].Schiffmann J, Grindei M, Tian Z, et al. Limitations of elastography based prostate biopsy. J Urol 2016;195:1731–6. [DOI] [PubMed] [Google Scholar]
- [13].Filson CP, Natarajan S, Margolis DJ, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: the role of systematic and targeted biopsies. Cancer 2016;122:884–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815–22. [DOI] [PubMed] [Google Scholar]
- [15].Yi A, Kim JK, Park SH, et al. Contrast-enhanced sonography for prostate cancer detection in patients with indeterminate clinical findings. AJR Am J Roentgenol 2006;186:1431–5. [DOI] [PubMed] [Google Scholar]
- [16].Gao Y, Liao XH, Ma Y, et al. Prostate ultrasound imaging: evaluation of a two-step scoring system in the diagnosis of prostate cancer. Discov Med 2017;24:295–303. [PubMed] [Google Scholar]
- [17].Yunkai Z, Yaqing C, Jun J, et al. Comparison of contrast-enhanced ultrasound targeted biopsy versus standard systematic biopsy for clinically significant prostate cancer detection: results of a prospective cohort study with 1024 patients. World J Urol 2019;37:805–11. [DOI] [PubMed] [Google Scholar]
- [18].Wu WT, Chang KV, Mezian K, et al. Basis of shoulder nerve entrapment syndrome: an ultrasonographic study exploring factors influencing cross-sectional area of the suprascapular nerve. Front Neurol 2018;9:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chang KV, Yang KC, Wu WT, et al. Association between metabolic syndrome and limb muscle quantity and quality in older adults: a pilot ultrasound study. Diabetes Metab Syndr Obes 2019;12:1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Janbaziroudsari H, Mirzaei A, Maleki N. Association of serum prostate-specific antigen levels with the results of the prostate needle biopsy. Bull Cancer 2016;103:730–4. [DOI] [PubMed] [Google Scholar]


