Abstract
Resorbable bone cements are replaced by bone osteoclastic resorption and osteoblastic new bone formation near the periphery. However, the ideal bone cement would be replaced by new bone through processes similar to fracture repair, which occurs through a variable combination of endochondral and intramembranous ossification. In this study, nanocrystalline hydroxyapatite (nHA)-poly(thioketal urethane) (PTKUR) cements were implanted in femoral defects in New Zealand White rabbits to evaluate ossification at 4, 12, and 18 months. Four formulations were tested: an injectable, flowable cement and three moldable putties with varying ratios of calcium phosphate to sucrose granules. New bone formation and resorption of the cement by osteoclasts occurred near the periphery. Stevenel’s Blue and Safranin O staining revealed infiltration of chondrocytes into the cements and ossification of the cartilaginous intermediate. These findings suggest that nHA-PTKUR cements support combined intramembranous and endochondral ossification, resulting in enhanced osseointegration of the cement that could potentially improve patient outcomes.
Keywords: hydroxyapatite, poly(thioketal urethane), bone cement, intramembranous ossification, endochondral ossification, rabbit
Graphical Abstract
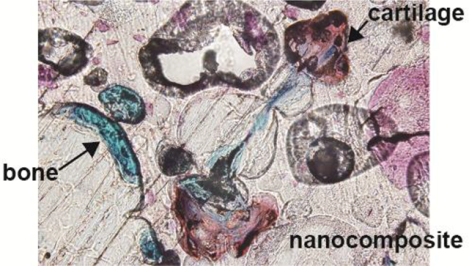
Introduction
Calcium phosphate bone cements (CPCs) are gradually replaced by bone through creeping substitution, a process characterized by new bone formation near the surface followed by osteoclast-mediated resorption of the CPC.1 Since the periphery of the CPC is relatively more vascularized, lamellar bone forms through intramembranous ossification, which is also referred to as primary bone healing.2 However, intramembranous ossification cannot occur under hypoxic conditions.2 Creeping substitution is therefore limited to the periphery, resulting in slow resorption of the implant and poor ooseointegration.3–5 Biphasic CPCs allow for bone formation throughout the interior through pores resulting from the dissolution of a soluble phase (i.e., calcium sulfate).6, 7 However, calcium phosphate bone substitutes have insufficient mechanical properties for weight-bearing applications.8
Fractures with a large osseous defect heal by the formation of a cartilage intermediate that stimulates angiogenesis and consequent bone formation, a process known as endochondral ossification or secondary bone healing.2, 9–11 Most fractures heal by a variable combination of intramembranous and endochondral ossification that is determined by the relative amounts of vascularity and strain in the fracture microenvironment.2 The ideal biomaterial for bone grafting would promote ossification similar to that observed in fractures, which is predominantly endochondral in the hypoxic interior linked to intramembranous ossification near the vascularized periphery.2 This approach is anticipated to promote new bone formation throughout the entire implant and enhance its osseointegration.12 A recent study reported a biomaterial strategy to regenerate bone through endochondral ossification by tuning pore architecture to control cellular infiltration and matrix deposition.13 However, there are currently no reports of biomaterials with bone-like strength that allow for chondrocyte infiltration and subsequent ossification of the cartilaginous intermediate.14
Nanocrystalline hydroxyapatite (nHA), which is a main component of bone15, stimulates new bone formation by enhancing osteoblastic differentiation.16, 17 nHA/poly(ε-caprolactone) (PCLUR) composites maintain osteoinductivity comparable to nHA while exhibiting enhanced mechanical properties compared with either component alone.18–20 Grafting isocyanate (NCO) groups to the hydroxyl (OH) groups on the surface of nHA particles to synthesize nHA-PCLUR nanocomposites further improves their osteoinductivity16 and mechanical properties.16, 19, 21 In a recent study, slowly degrading nHA-PCLUR cements mechanically stabilized weight-bearing tibial plateau defects in sheep and were gradually replaced by new bone via creeping substitution.22
Poly(ester urethane)s synthesized from copolymers of ε-caprolactone, D,L-lactide, and glycolide degrade significantly faster than PCLUR due to their lower Tg, which enhances water uptake, and the higher susceptibility of the ester groups in D,L-lactide and glycolide to hydrolysis.23 However, these co-polymers show accelerated late-stage degradation that promotes dramatic loss in mechanical properties23 and fibrosis.24, 25 Consequently, biomaterial degradation is unpredictable and does not align with patient biology.
Poly(thioketal urethane) (PTKURs) that degrade in response to reactive oxygen species secreted by infiltrating cells offer the advantage of cell-mediated degradation.26, 27 Reactive oxygen species (ROS) can attack the C-S bond in the thioketal (TK)26, 28 and the α-hydrogen in the lysine groups29, 30, resulting in degradation of the polymer to soluble breakdown products. In a previous study, PTKUR/ceramic composite cements were resorbed by osteoclasts, supported appositional growth of new bone, and integrated with host bone near the periphery of the implants in rabbit femoral condyle defects at 6 and 12 weeks.31 However, the presence of chondrocytes and cartilage were not reported.
In the present study, nHA-PTKUR cements were implanted in epiphyseal femoral defects in New Zealand White rabbits to assess their long-term osseointegration with host bone. We hypothesized that ROS secreted by proliferating chondrocytes32, 33 originating from the periosteum and endosteum2 would degrade and infiltrate the PTKUR26, 27, 31, leading to the formation of a cartilaginous intermediate that stimulates ossification.2 To enhance the colloidal stability, handling properties, and osteoinductivity of the nanocomposites16, needle-shaped nHA crystals (NANOSTIM™, NS) with high specific surface (106 m2/g) were reacted with lysine triisocyanate (LTI) to form an NS-g-LTI polymer prior to reaction with the TK diol. Four nanocomposites with varying concentrations of calcium phosphate granules and sucrose particles (porogen), designed to balance cellular infiltration with mechanical and handling properties, were tested. Physicochemical and mechanical properties were characterized prior to implantation in rabbits. Porosity, cellular infiltration, and formation of new cartilage and bone after implantation in rabbits were assessed by μCT, histology, and histomorphometry at 4, 12, and 18 months.
Materials and Methods
Materials.
Reagents for thioketal (TK) diol synthesis, iron (III) acetylacetonate (FeAA) catalyst, ε-caprolactone, and sucrose (200 μm) were purchased from Sigma Aldrich. FeAA was dissolved in dry ε-caprolactone (5 wt%) to prepare a catalyst solution. Lysine triisocyanate (LTI) was purchased from Jinan Haohua Industry Co., LTD (Jinan, China), dissolved in tert-butyl-methyl ether (TBME, Acros Organics), and purified by treatment with activated carbon prior to use.16, 22, 31 NANOSTIM™ Synthetic Bone Paste, a suspension of needle-shaped (20 nm ⊘ × 100 nm long, specific surface area = 106 m2/g) nanocrystalline hydroxyapatite crystals in water34, 35, and MASTERGRAFT® calcium phosphate granules (85% β-TCP/15% hydroxyapatite) were provided by Medtronic (Memphis, TN). Calcium phosphate granules were ground to 100–300 μm, washed with acetone and water, and dried under vacuum (100°C).31 Sucrose and calcium phosphate granules were γ-irradiated at 25 kGY prior to implantation in the rabbit study.
Synthesis of TK diol.
A TK diol (200 g/mol) was synthesized as described previously.31 2,2-dimethoxypropane was reacted with thioglycolic acid in acetonitrile for 24 hours at room temperature. The intermediate was filtered, dried, dissolved in tetrahydrofuran, and added to lithium aluminum hydride in diethyl ether drop-wise in an ice bath. The reaction was refluxed at 52°C for 12 hours. The product was extracted with an aqueous NaOH solution (1 M) and vacuum-dried for 48 hours.
NANOSTIM™ (NS) dewatering.
Prior to surface grafting, nanocrystalline hydroxyapatite NS crystals were isolated by dewatering the NS suspension. Approximately 5 cc NS was dispensed into a 50 mL centrifuge tube, which was filled with 40 mL 2-propanol and vortexed until the NS suspension was dispersed in the solvent. The NS crystals were separated by centrifugation and the washing process repeated 3 times. The isolated NS crystals were dried under vacuum at ambient temperature for 12 hours and then at 80°C for 24 h. The resulting dry pellet was ground using a mortar and pestle. NS crystals were vacuum-dried at 80°C again for 48 h immediately prior to surface grafting.
Synthesis of PCL-g-NS.
NS crystals were grafted with polycaprolactone (PCL-g-NS) to enhance their colloidal stability in TK diol as described previously.36 Dry ε-caprolactone was charged to a three-neck boiling flask containing dry NS crystals (3.7:1) and fitted with a condenser. The reaction was stirred for 24 h at 150°C. Grafted particles were recovered from excess ε-caprolactone by sonicating with chloroform four times. PCL-g-NS was dried under vacuum at 80°C for 12 hours. The wt% PCL was measured by thermogravimetric analysis (TGA, Instrument Specialist’s TGA-1000). Crystallinity was assessed by x-ray diffraction (XRD, Rigaku SmartLab X-Ray Diffractometer, λ = 0.154 nm).16 Average 2θ (radians) peak analysis was performed to calculate the average grain size.37
Synthesis of LTI-g-NS.
LTI was grafted to NS crystals by combining dry NS crystals with LTI (1:1) and mixing for 1 min (FlackTek SpeedMixer, DAC 150 FVZ-K). FeAA catalyst solution (5 wt% in ε-caprolactone) was added and the suspension mixed at 3500 rpm for 10 min. LTI-g-NS particles were washed with TMBE to remove excess LTI and catalyst, centrifuged, and dried under vacuum at 80°C for 12 hours. The wt% LTI was assessed by TGA and grain size by XRD.
LTI-g-NS chemical kinetics.
To determine the specific reaction rate (kNS) for NS crystals reacting with LTI, NS crystals were reacted with LTI and the %NCO measured by titration over time. Dry NS crystals were added to a 100 mL 3-neck flask and dried in situ under vacuum at 80°C for 48 h. The temperature was then reduced to 40°C, and LTI was charged to the reactor under argon and stirred with an overhead mechanical stirrer. The reaction mixture initially contained 35 wt% NS crystals and 65 wt% LTI. Samples were withdrawn from the reactor over time and %NCO measured by titration.29 The conversion of NCO groups in LTI and OH groups in NS crystals were calculated and modeled assuming second order chemical kinetics:
where t = time (h), [OH] = concentration of hydroxyl groups (mol/L), [NCO] = concentration of isocyanate groups (mol/L), and kNS is the second-order specific reaction rate (L mol−1 h−1). The subscript 0 denotes the initial concentration at t = 0.
Prepolymer synthesis.
The prepolymer component was synthesized according to methods published previously.16, 22 Dry LTI-g-NS (40 wt%) was mixed with LTI (60 wt%) in 1 min intervals using a speed mixer until homogeneous (5 min total mixing time). The %NCO was measured by titration. The prepolymer was loaded into 1-ml syringes, γ-irradiated at a dose of 25 kGY, and stored at 4°C prior to the animal study.
Polyol synthesis.
The TK diol was blended 1:1 with dry PCL-g-NS and mixed with the FeAA catalyst solution for 5 min until homogeneous using a speed mixer. This polyol component was loaded into 1-ml syringes, γ-irradiated at 25 kGY, and stored at 4°C prior to the animal study. The catalyst concentration on a total cement weight basis was 0.0625 wt% for the injectable glue formulation and 0.125 wt% for the moldable putty formulations.
Fabrication of NS-PTKUR composites.
Four NS-PTKUR composites were tested in vivo. The NS-PTKUR polymer without any solid additives was evaluated as an injectable bone glue (0C0S, Table 1). The NS-LTI prepolymer and TK polyol components were added separately to a dual-barrel syringe at a 1:1 vol/vol ratio, which corresponded to an NCO:OH index of 120 and 0.0625 wt% FeAA catalyst. A lower index and catalyst concentration were chosen for the 0C0S group to achieve approximately equal volumes of the prepolymer and polyol phases. The prepolymer and polyol components were injected through a dual-barrel syringe fitted with a static mixer and reacted to form a NS-PTKUR hybrid polymer.
Table 1.
Treatment groups evaluated in the rabbit study.
| component wt% | # of replicates | ||||||
|---|---|---|---|---|---|---|---|
| Group | FeAA | NS | C | S | 4 mos | 12 mos | 18 mos |
| 0C0S | 0.0625 | 45 | 0 | 0 | 3 | 3 | 3 |
| 45C0S | 0.125 | 24 | 45 | 0 | 3 | 3 | 3 |
| 10C35S | 0.125 | 24 | 10 | 35 | 3 | 3 | 3 |
| 0C45S | 0.125 | 24 | 0 | 45 | 3 | 3 | 3 |
Acronyms: 0 wt% C/ 0% S (0C0S), 45 wt% C/0% S (45C0S), 10 wt% C/35 wt% S (10C35S), and 0 wt% C/45 wt% S (0C45S).
Three moldable putties were prepared by adding calcium phosphate (C) and/or sucrose (S) granules to the reactive prepolymer and polyol. Three putties were tested: 45 wt% C/0% S (45C0S), 10 wt% C/35 wt% S (10C35S), and 0 wt% C/45 wt% S (0C45S). The total concentration of additives was maintained at 45 wt% to ensure comparable handling properties, while the C:S ratio was varied to investigate the effects of porosity on mechanical properties and remodeling. Putties were fabricated by mixing the NS-LTI prepolymer and TK polyol components at an NCO:OH index of 140 and 0.125 wt% FeAA catalyst by hand for 1 – 2 min until a homogeneous mixture was prepared.
Characterization of NS-PTKUR cements.
Materials were fabricated as described above, cured for 12 hours in cylindrical tubes (6 mm Ø), leached in PBS at 37°C for up to 7 days, and dried under vacuum at 80°C. The release of sucrose and consequent pore formation was evaluated by weighing the sample on days 1, 4, and 7. On day 5, composites were scanned with a voxel size of 17.2 μm using a μCT 50 (Scanco) and SEM imaging was performed on sputter-coated samples using a Zeiss Merlin SEM. Quasi-static compression testing was performed on all groups to determine the mechanical properties.31, 38 Leached cylindrical (6 mm Ø × 12 mm long) specimens were cut using an IsoMet Low Speed Saw to ensure flat, parallel edges. The samples (n=4) were compressed between circular platens at a rate of 25 mm/min and the force and displacement recorded (MTS 858 Bionix Servohydraulic Test System). The elastic modulus, maximum strength, yield strength, and yield strain were calculated for each material from the engineering stress and strain.
Implantation of NS-PTKUR cements in rabbits.
All surgical and care procedures were carried out at IBEX Preclinical Research, Inc. (Logan, UT) under aseptic conditions per the approved IACUC protocol. 18 New Zealand White rabbits were used in this study. NS-PTKUR putties (45C0S, 10C35S, and 0C45S) and the NS-PTKUR cement without solids (0C0S group) were prepared as described above. After 1 – 2 min of mixing, the three putties formed moldable pastes that were implanted in bilateral cylindrical defects (5 mm Ø x 8 mm) in the distal femoral condyles of rabbits and allowed to cure in situ. The setting time (defined as the time at which the material cures to become a tack-free solid39) was 7 ± 0.3 minutes. The injectable 0C0S cement was injected from a dual-barrel syringe through a static mixer into the defect. The setting time was slightly longer (10 minutes) due to the lower catalyst concentration. Each rabbit received a different graft in the distal femoral condyle for n=3 for each group at each time point. Animals were sacrificed at 4, 12 and 18 months. The femurs were harvested and fixed in 10% formalin.
Ex vivo analysis of implants.
While in formalin, the bones were scanned with a voxel size of 17.2 μm using a μCT 50 (Scanco). 2D images were taken at the center of the defect and the defect length and width measured using ImageJ. Calcified specimens were processed and plastic-embedded by Histion. Sections were taken from the center of the defect, ground to 30–70 μm, and stained with Stevenel’s Blue or Safranin O/Fast Green for histological analysis. Previously described methods were adapted for analysis of new bone formation by μCT and histomorphometry (Supplemental Figure 1).22, 25 The area of interest was defined as a 8 × 2 mm rectangle located 2 mm from the insertion of the cylindrical defect. The rectangle was divided into 8 smaller (1 mm width) rectangles to compare staining between the host bone, periphery of the implant, and interior of the implant. Only the distal 4 regions were included in the analysis as the marrow space of the medial regions may introduce significant error. Bone was thresholded as pink stain in the Stevenel’s Blue sections, and pores were identified as clear voids >100 μm at 4x. However, the purple color of cartilage could not be reliably thresholded from the variable non-specific blue staining of NS-PTKUR.
Statistical methods.
All data were plotted as the mean ± standard deviation unless otherwise specified. A one-way ANOVA (GraphPad Prism) with a post-hoc Tukey Test was used to test for significant differences in mechanical properties in response to material composition. A two-way ANOVA (GraphPad Prism) was performed to test for significant differences in implant diameter and length in response to material composition and time. A three-way ANOVA (R Statistical Software) was used to test for differences in BV/TV (μCT), area% bone, area% cartilage, and area% pores (histomorphometry) in response to material composition, time, and region of interest (ROI). The significance of pairwise interactions was assessed by Tukey’s multiple comparisons tests. Histomorphometry data were plotted as the mean ± standard error of the mean (SEM). Statistical significance was established as p < 0.05
Results
Characterization of NS-g-PCL and NS-g-LTI.
TGA confirmed that PCL and LTI were grafted to the surface of NS, as evidenced by higher mass loss for NS-g-PCL and NS-g-LTI compared with ungrafted NS (Figure 1A). The effect of surface grafting on the crystallinity and grain size of NS was assessed by XRD, which showed no change in the crystalline structure after grafting LTI and PCL to the surface of NS particles (Figure 1B). The average crystallite grain size calculated using the Scherrer equation was 23 nm for NS, 28 nm for NS-g-PCL, and 24 nm for NS-g-LTI. The grafting reaction rate constant (kNS) was determined to be 0.00143 L*mol−1*hr−1 by fitting the %NCO of the NS/LTI mixture measured by titration to the second-order kinetic model shown in Eq (1) (Figure 1C). After 200 h reaction time, the conversion of OH groups on NS approached 100% (Figure 1D), while the conversion of NCO groups in LTI approached 15% at steady-state (Figure 1E).
Figure 1. Characterization of NS, NS-g-PCL, and NS-g-LTI.
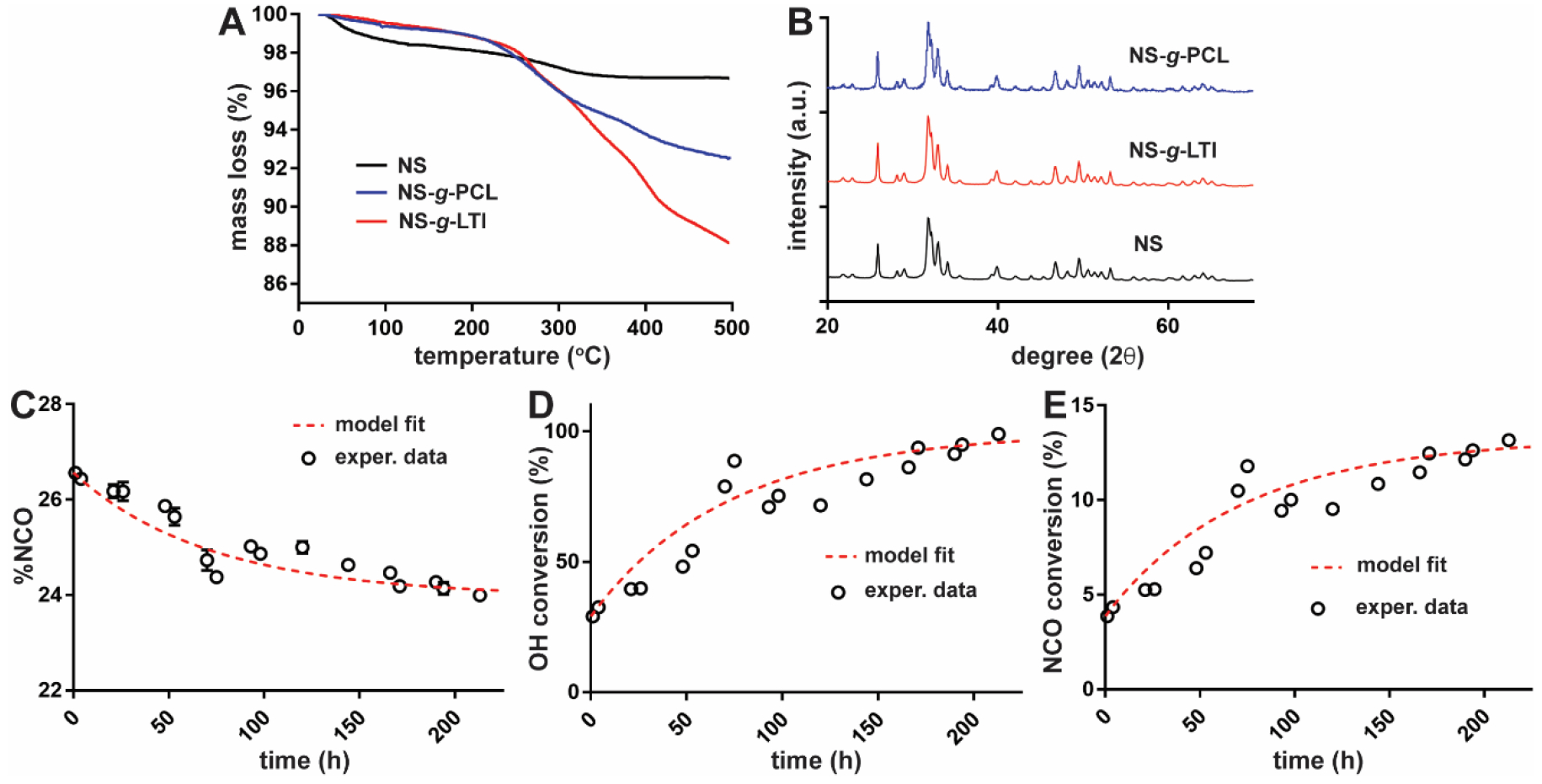
(A) Mass loss measured by TGA for NS, NS-g-PCL, and NS-g-LTI. (B) X-ray diffraction (XRD) patterns for NS, NS-g-PCL, and NS-g-LTI. (C) %NCO measured for the NS/LTI mixture at 40°C and fit to a second-order kinetic model. (D) OH and (E) NCO conversion for NS-g-LTI. Circles: experimental data, dashed line: kinetic model fit.
Characterization of NS-PTKUR composites.
2D μCT reconstructions of leached samples revealed minimal porosity for the 0C0S and 45C0S groups, while the 10C35S and 0C45S groups showed higher porosity after leaching in water for 5 days (Figure 2A). The groups containing sucrose showed ~10% mass loss on day 1 and ~1% mass loss per day for up to 7 days, indicating release of sucrose and subsequent pore formation, while groups not containing sucrose showed minimal change in mass by day 7 and thus minimal pore formation (Supplemental Figure 2). SEM images revealed the presence of calcium phosphate granules (double arrows) dispersed in the continuous NS-PTKUR phase as well as pores (single arrows, Figure 2B). Representative stress-strain curves are shown for each group in Figure 3A. The moduli of the groups without sucrose (0C0S and 45C0S) were comparable (370 MPa) and higher than the those of the groups containing sucrose (10C35S and 0C45S) (Figure 3B). The 0C0S cement exhibited significantly higher ultimate strength, yield strength, and yield strain than the other groups (Figure 3C–E). The addition of sucrose porogens (10C35S, 0C45S) did not have a significant effect on ultimate strength, yield strength, or yield strain compared with 45C0S.
Figure 2. Imaging of NS-PTKUR bone cements.
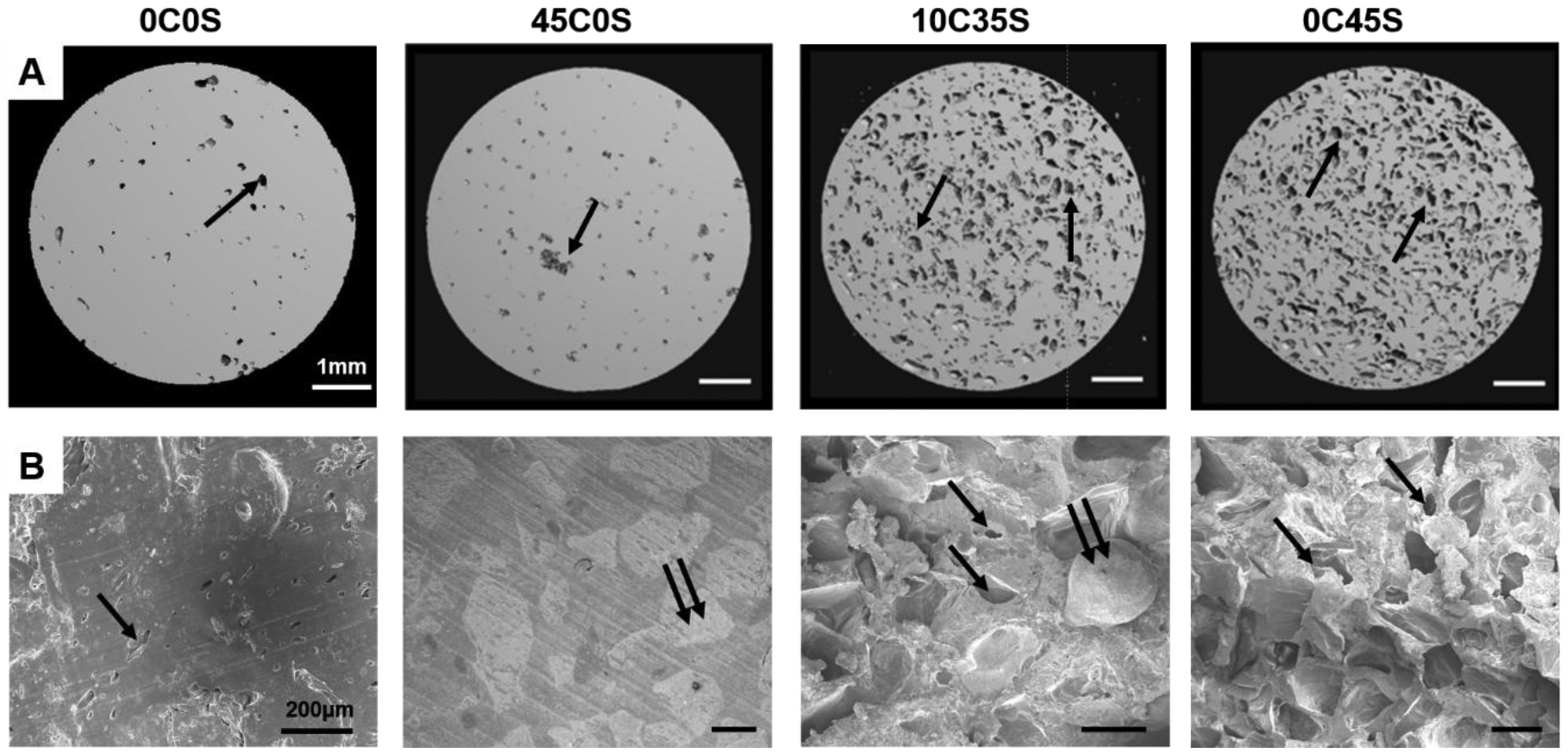
(A) Images of 2D μCT cross-sections of 0C0S, 45C0S, 10C35S, and 0C45S reveal the presence of pores (arrows) after leaching in water for 5 days. (B) SEM images of 0C0S, 45C0S, 10C35S, 0C45S show evidence of pores (single arrows) and calcium phosphate granules (double arrow).
Figure 3. Mechanical properties of NS-PTKUR bone cements.
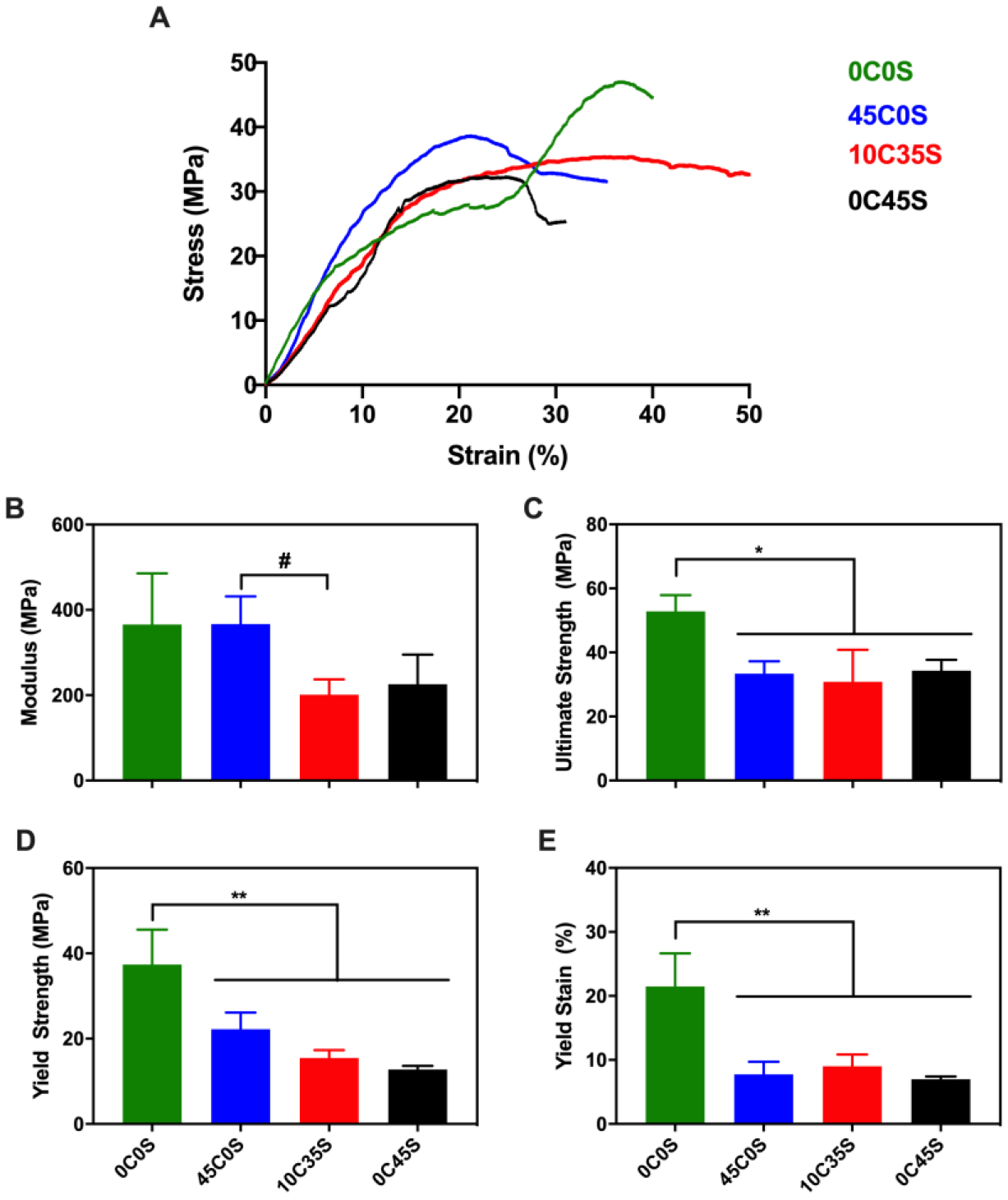
(A) Representative stress-strain curves. (B) Young’s modulus, (C) ultimate strength, (D) yield strength, and (E) yield strain measured under quasi-static compressive loading. The modulus of 45C0S was significantly higher than that of 10C35S. The ultimate strength, yield strength, and yield strain of 0C0S were significantly higher compared with any of the other groups. (#: p ≤ 0.1, *: p ≤ 0.05, **: p ≤ 0.01).
Analysis of NS-PTKUR osseointegration in vivo by ex vivo μCT evaluations.
μCT images from the transverse (Figure 4A) and coronal and sagittal (Supplemental Figure 3) planes show appositional bone formation near the periphery. μCT images were analyzed at a constant threshold (340 – 517 mg cm−3) that included bone and calcium phosphate granules but excluded NS-PTKUR (Supplemental Figure 1A). The 5 mm Ø x 8 mm defects were divided into four regions of interest (ROI): three concentric annular cylinders each with a thickness of 0.8 mm and a central core cylinder with a radius of 0.8 mm (Supplemental Figure 1B). The inner surface of ROI 4 was 2.4 mm from the centerline of the defect and consisted of residual implant and host bone. BV/TV values were calculated from the μCT reconstructions for each ROI and material composition at 4 (Figure 4B), 12 (Figure 4C), and 18 (Figure 4D) months. The three-way ANOVA showed significant effects of material composition (p < 2.2 10−16) and ROI (p < 2.2 10−16). Two-way interactions between ROI:material (p < 2.2 10−16) and time:material (p = 0.002) were also significant. For the 0C0S, 10C35S, and 0C45S groups, BV/TV was higher near the periphery (ROI 4) compared with the interior regions. However, due to the high (45 wt%) concentration of calcium phosphate granules that could not be distinguished from bone, BV/TV in the 45C0S group was higher in the interior (ROIs 1 – 3) compared with the periphery. Similarly, BV/TV in ROIs 1 – 3 of 45C0S and 10C35 was higher than that in 0C0S and 0C45S. The length and diameter of the cement within the defects were measured from 2D μCT reconstructions at 4, 12, and 18 months. The two-way ANOVA showed significant (p < 0.0005) effects of time on defect length as a result of replacement of the cement with new bone near the base. Tukey’s multiple comparisons showed a significant (p = 0.02) decrease in the length of 0C0S between 4 and 18 months (Figure 4E). The diameter of the implants did not significantly decrease over time (Supplemental Figure 4A).
Figure 4. Analysis of cement remodeling by μCT.
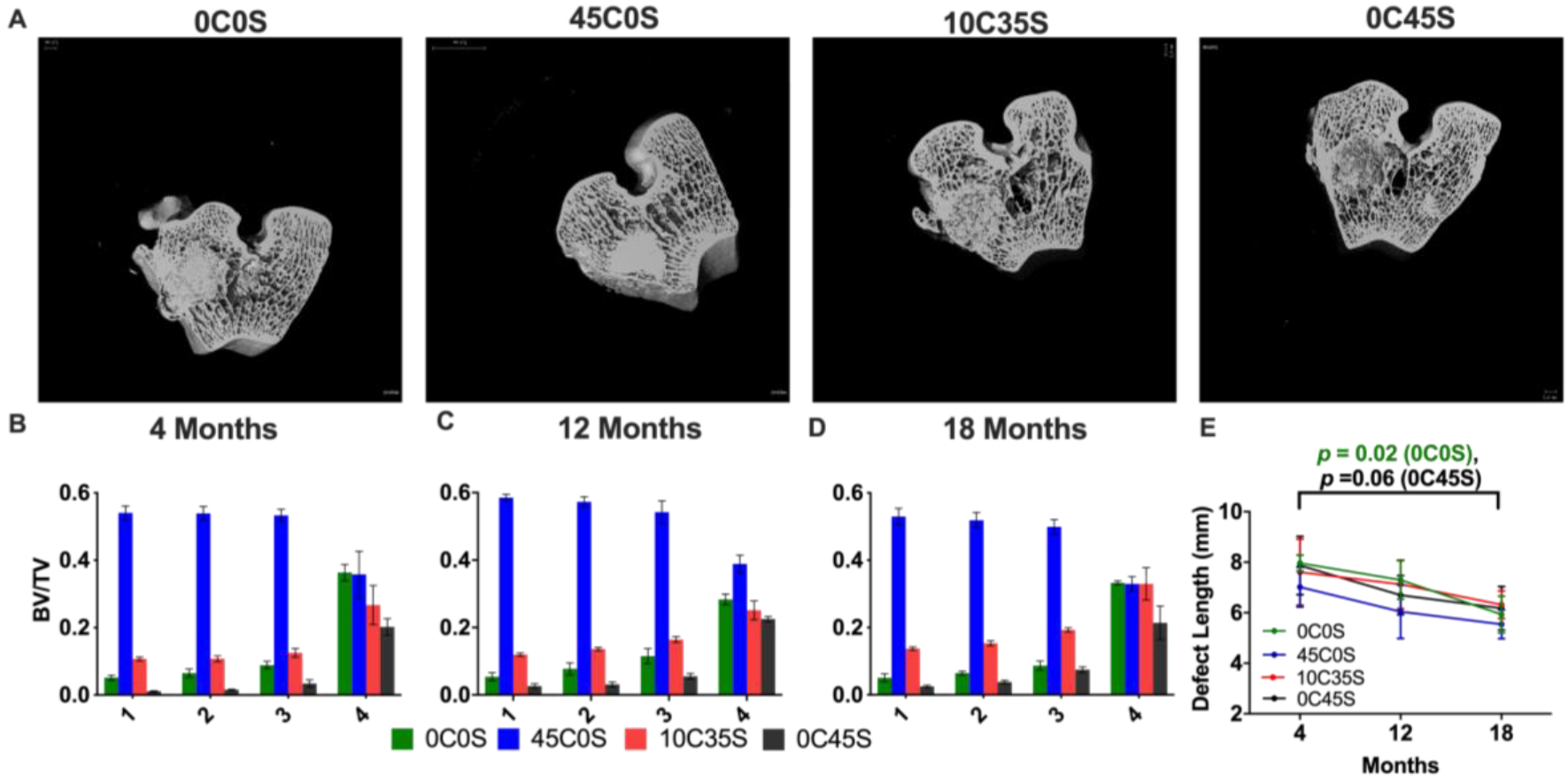
(A) Representative 2D μCT images (transverse plane) at 18 months. (B-D) BV/TV calculated from μCT reconstructions in each ROI and material at (B) 4, (C) 12,and (D) 18 months. (E) Defect length measured from 2D μCT reconstructions at 4, 12, and 18 months.
Histological assessment of NS-PTKUR osseointegration.
Histological sections stained with Stevenel’s Blue were used to evaluate new bone formation and cellular infiltration (Figure 5). Intramembranous ossification was observed near the periphery for all groups as evidenced by woven bone (double yellow arrows) transitioning into lamellar bone (single yellow arrows). Cellular infiltration within the cements was observed near the periphery of all groups, which was less extensive in the low porosity 0C0S (Figure 5A) and 45C0S (Figure 5B) cements. In contrast, the porous 10C35S (Figure 5C) and 0C45S (Figure 5D) cements exhibited more extensive cellular infiltration into the interior, and thus greater new bone formation. The histological sections indicate that NS-PTKUR cements supported new bone formation at 4, 12 and 18 months.
Figure 5. Histological characterization of NS-PTKUR bone cements.
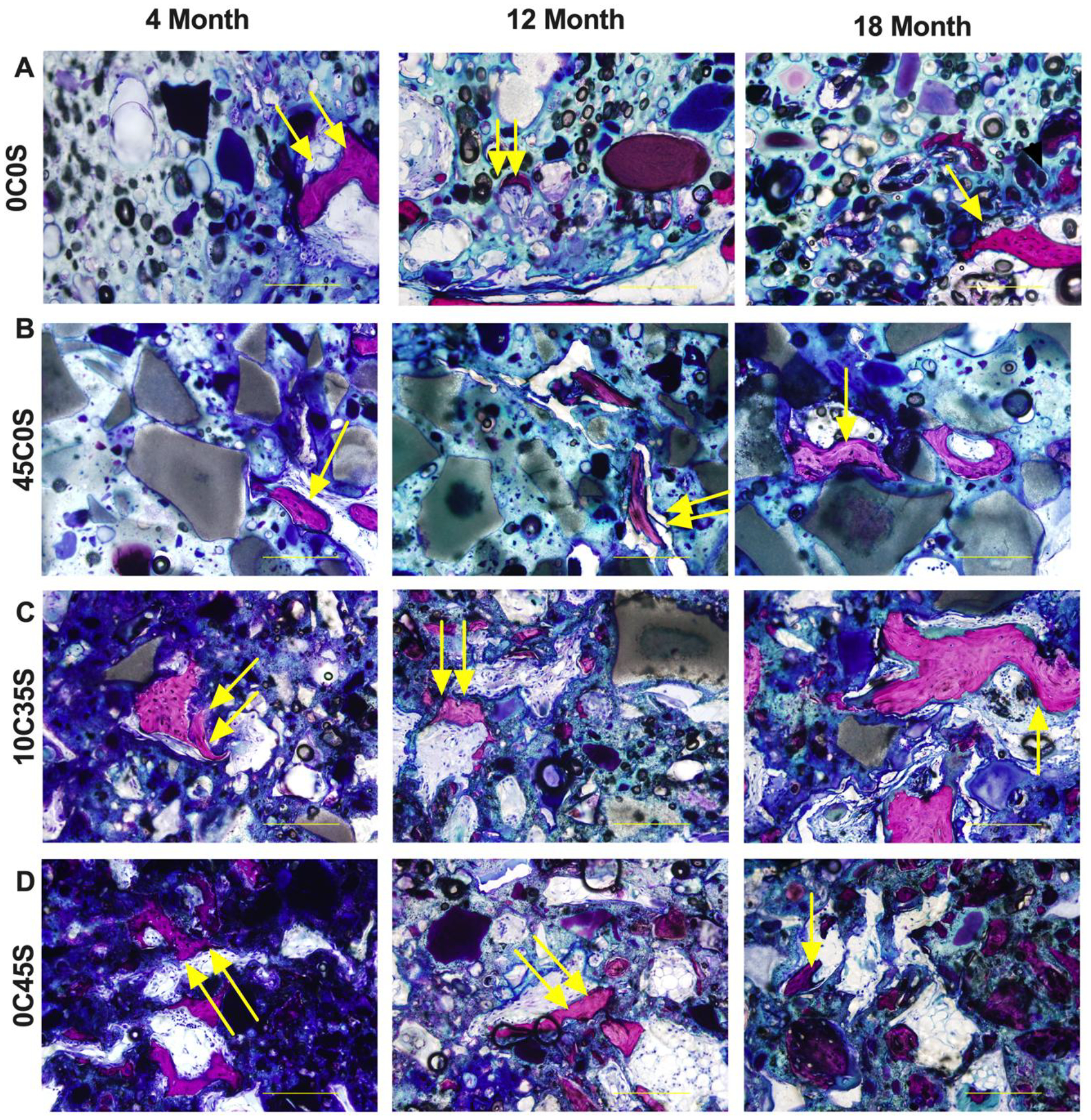
(A) High-magnification images of histological sections of (A) 0C0S, (B) 45C0S, (C) 10C35S, and (D) 0C45S at 4, 12 and 18 months stained with Stevenel’s Blue show evidence of new bone formation. Near the periphery, intramembranous ossification was observed, indicated by woven bone formation (double yellow arrows). By 18 months, mature lamellar bone formation (single yellow arrows) is seen within all samples. (Scale bar = 200 μm, single arrow: woven bone, double arrows: mature lamellar bone).
Appositional bone growth near the periphery was observed, as evidenced by the presence of osteon-like structures (single black arrows) adjacent to NS-PTKUR, which showed differential intensity of blue staining (Figure 6A). High- (40X) magnification images showed cellular activity and the presence of multi-nucleated osteoclasts (double black arrows) resorbing NS-PTKUR near the periphery of the cement (Figure 6B) and near the dispersed calcium phosphate (C) granules in 45C0S (Figure 6C). Regions of chondrocyte infiltration and cartilage formation proximal to new bone formation were also observed. Nodules of chondrocytes (purple, #) approximately 50 – 200 μm in size were observed between ceramic granules (Figure 6D) and embedded within NS-PTKUR (Figure 6E–F), suggesting the chondrocytes infiltrated the cement by oxidatively degrading NS-PTKUR. Some regions of this cartilaginous intermediate were undergoing mineralization, as evidenced by the pink-purple stain (*, Figure 6D–E).40
Figure 6. High-magnification (40X) images of histological sections stained with Stevenel’s blue at 12 months.
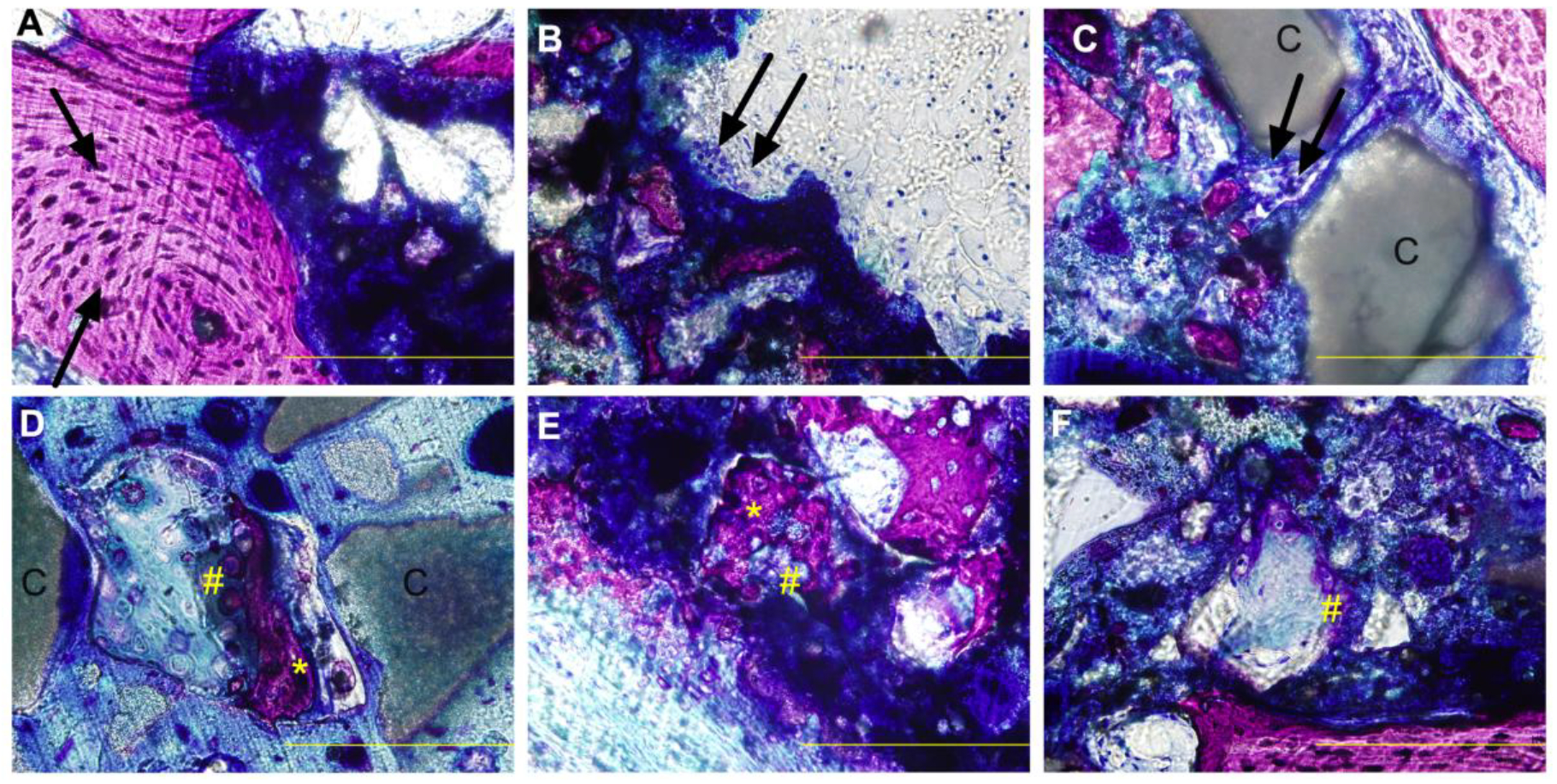
(A) Osteon-like structures (black arrow) adjacent to residual cement. (B-C) Osteoclast-mediated resorption (double arrows) of NS-PTKUR in (B) 0C45S and (C) 45C0S. (D-F) Endochondral ossification in groups (D) 45C0S, (E) 0C45S, and (F) 10C35S evidenced by nodules of cartilage/chondrocytes (purple, #) and bone (*) embedded in NS-PTKUR. (Scale bar = 200 μm, single black arrow: osteon, double black arrows: osteoclast, #: cartilage/chondrocytes, *: cartilage undergoing mineralization.)
Safranin O/Fast Green staining was performed to confirm the infiltration of chondrocytes into the cements and subsequent ossification of the cartilaginous intermediate (orange/red, single black arrows) into bone (teal, double black arrows) within the cements (Figure 7). In contrast to the Stevenel’s Blue sections, Safranin O/Fast Green sections showed minimal non-specific staining of NS-PTKUR. Regions of ongoing cartilage mineralization evidenced by co-localization of orange/red and teal staining were observed in non-porous 0C0S cements at distances >1 mm from the periphery (Figure 7B–C), suggesting that chondrocytes infiltrated the cement through ROS-mediated degradation of NS-PTKUR. Infiltration of chondrocytes and mineralization of cartilage were also observed at distances >1 mm from the periphery in porous 10C35S cements (Figure 7E–F). Thus, the Safranin O/Fast Green staining provides further evidence of ongoing endochondral ossification within the cements at 4, 12, and 18 months.
Figure 7. Safranin O/Fast Green staining of NS-PTKUR bone cements at 12 months.
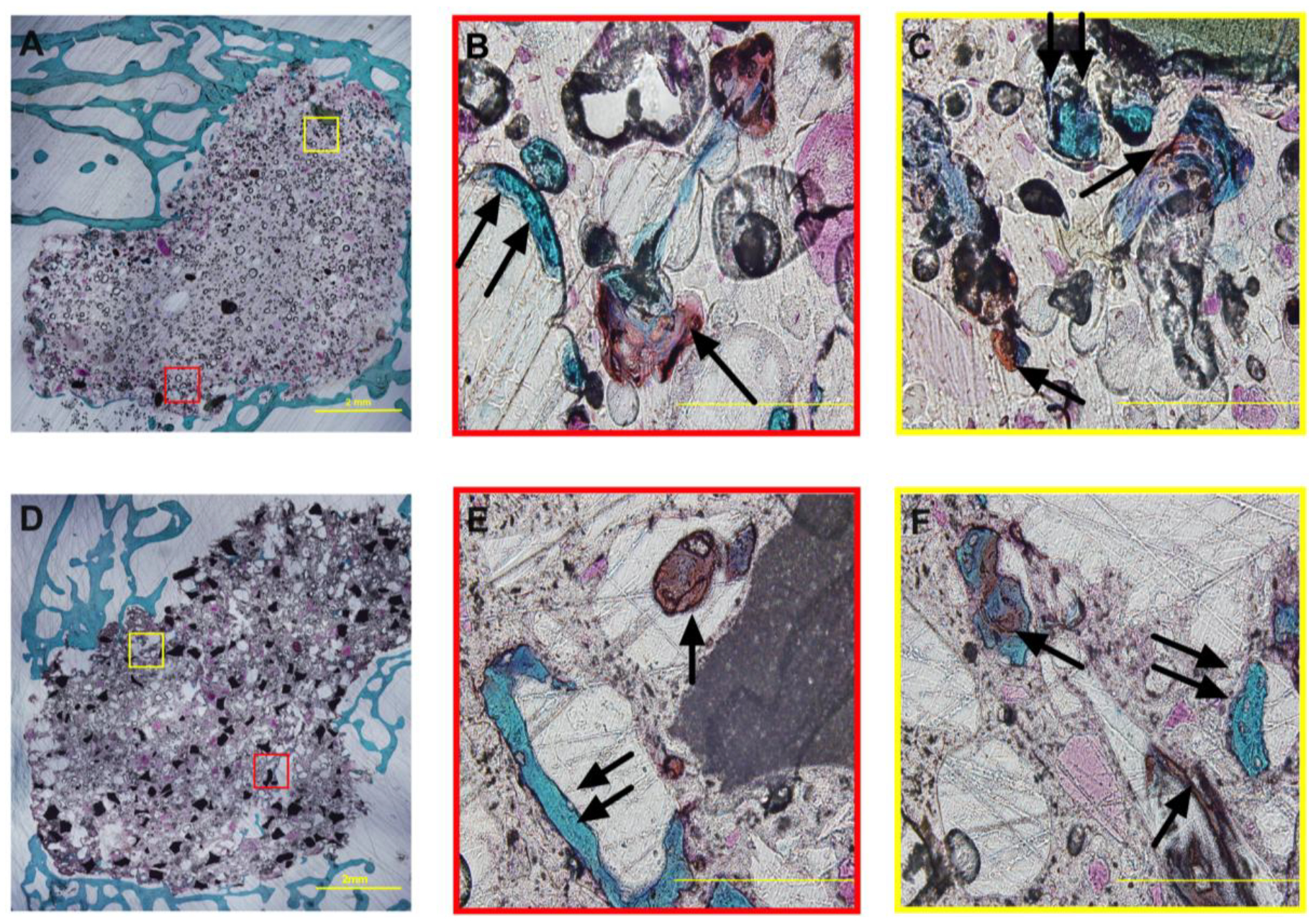
(A-C) Representative histological images of non-porous 0C0S cements at (A) low and (B-C) high magnification. Mineralization of cartilage is ongoing in the interior of 0C0S cements. (D-F) Representative histological images of porous 10C35S cements at (D) low and (E-F) high magnification. Cartilage (orange/red stain, single arrows) and bone (teal, double arrows) was evident within the cements. Scale bar = 2 mm in Panels A and D and 200 μm in Panels B-C and E-F.
Histomorphometry.
The area% bone (Figure 8A) and pores (Figure 8B) were measured in the distal half of the defects in Stevenel’s Blue-stained sections at 4, 12, and 18 months. The three-way ANOVA showed significant effects of AOI (p < 2.2 10−16). Trabecular thickening was observed in the host bone (AOI 4), which showed 35 – 50 area% bone due to appositional new bone growth near the periphery of the cements (Figure 6A). In all groups, the area% bone was higher in the outer AO Is (3 and 4) compared with the inner AOIs (1 and 2) (Figure 8A), and area% bone in the inner AOIs trended higher at 12 and 18 compared with 4 months. Surprisingly, at 4 months the 0C0S and 45C0S groups (no sucrose) showed similar porosity (12 – 22%) to the 10C35S and 0C45S groups loaded with sucrose (Figure 8B). In contrast, the SEM images (Figure 2A) and mass loss data (Supplemental Figure 2) show that the initial porosity of 0C0S and 45C0S was low (<5%). The effects of material composition (p = 8.83 10−5) and AOI (p < 2.2 10−16) were significant. The porosity of the 0C45S group trended higher than that of the 0C0S group within the cement (AOIs 1 – 3) at 12 and 18 months as anticipated. At 12 months, the porosity in AOI 1 was higher in 0C45S compared with 0C0S and 45C0S (p < 0.05).
Figure 8. Histomorphometric analysis of new bone and cartilage formation in NS-PTKUR bone cements.
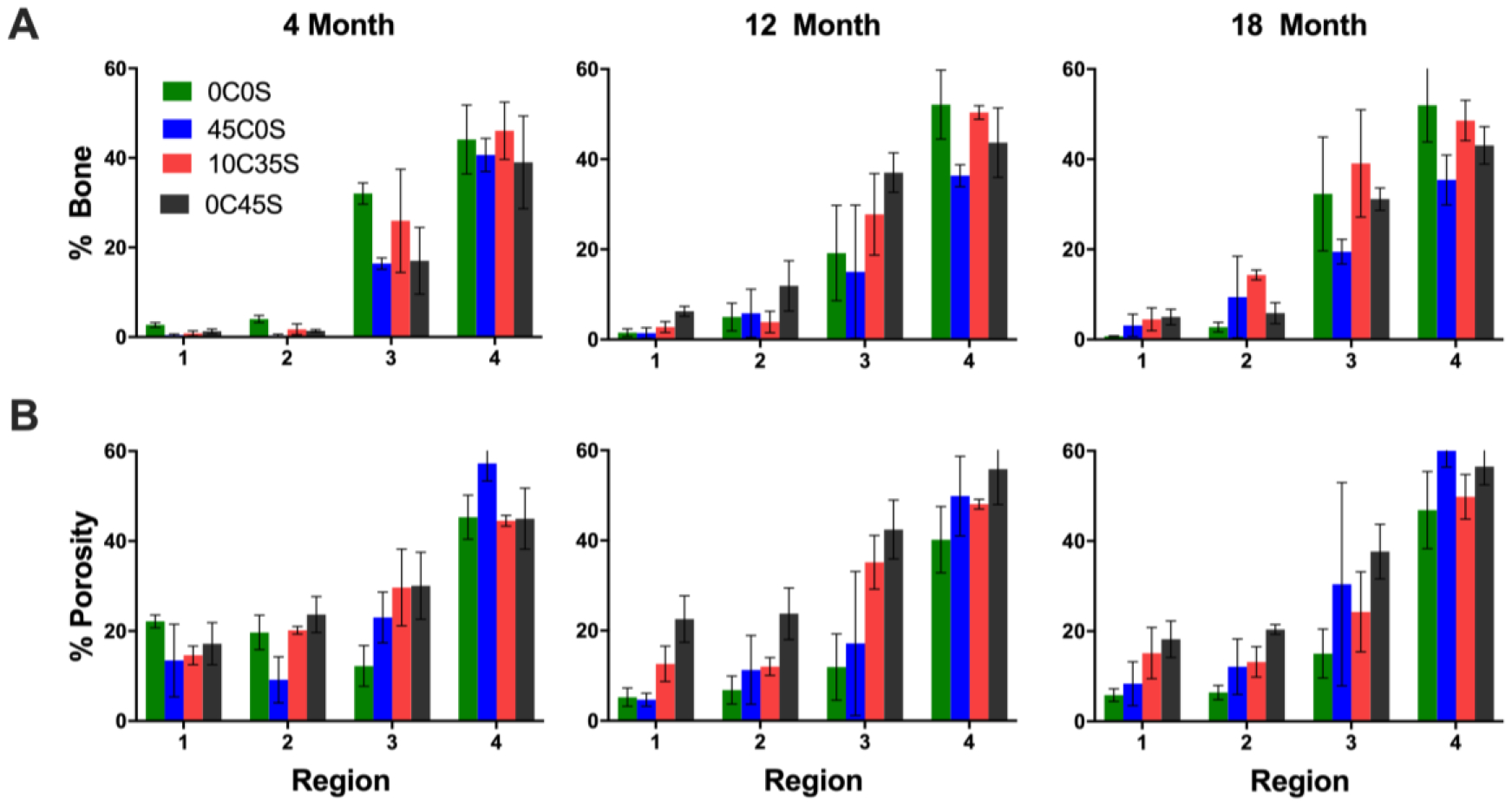
Histomorphometric analysis of (A) area% bone and (B) area% pores in sections stained with Stevenel’s Blue.
Discussion
In this study, NS-PTKUR bone cements were implanted in rabbit epiphyseal bone defects to establish how nanocomposites composed of oxidatively degradable PTKUR and osteoinductive nHA are replaced by new bone. All four cements exhibited working times comparable to those of CPCs (5 – 10 min8). The yield strength (15 – 38 MPa), yield strain (8 – 21%), and compressive modulus (200 – 360 MPa) exceeded those of trabecular bone.41, 42 Similar to fracture healing2, a combination of intramembranous ossification and endochondral ossification occurred within the cements. Ossification of the cartilaginous intermediate14 within the cements was shown by Stevenel’s Blue and Safranin O/Fast Green staining, which is anticipated to allow for a predictable osseointegration of the implant with host bone.
In a previous study, nHA (60 wt%) was mixed with LTI and TK diol prior to injection of the resulting paste into the defect.31 To facilitate direct injection of the 0C0S cement using a dual-barrel syringe fitted with a static mixer, the NS content was reduced to 45 wt% and dispersed in both the LTI (40 wt% NS) and TK diol (50 wt% NS) precursors. Preparing a colloidal dispersion of NS in LTI required grafting of LTI to NS in the absence of a catalyst to improve storage stability.16 While the kinetics of PCL polymerization to ceramic particles has been reported36, 43, the kinetics of the reaction between the NCO groups in polyisocyanates and the OH groups in nHA have not been investigated. Consistent with polyurethane reactions44, 45, the LTI-NS reaction was second-order with rate constant kNS = 0.00143 L*mol−1*h−1 at 40°C in the absence of catalyst (Figure 1). Grafting LTI to NS via this predictable second-order chemical reaction enhances the colloidal stability of NS and yields inorganic-organic hybrid polymers with improved mechanical properties compared with untreated NS.16, 46
Differences in mechanical properties between non-porous 45C0S and porous 0C45S cements were not significant (Figure 3), potentially due to weak interfacial bonding between calcium phosphate granules and PTKUR. These findings are consistent with a previous study reporting that addition of calcium phosphate granules reduced the strength of nHA-PCLUR composites.22 The yield strengths of the thioketal diol-based cements in this study (25 – 38 MPa) were less than that of non-resorbable poly(methyl methacrylate)36, 38 (PMMA, 70 – 90 MPa16), which is the only cement indicated for structural repair of bone.47 A resorbable cement based on nHA-PCLUR with 45 wt% ceramic granules had a yield strength of 75 MPa and maintained mechanical stability while integrating with host bone in weight-bearing tibial plateau defects in sheep at 16 weeks.22 The molecular weight between crosslinks of PCLUR (Mc ≅ 126 g mol−1) is twice that of PTKUR (Mc ≅ 252 g mol−1).16 Thus, the strength of NS-PTKUR cements can be increased to levels needed for weight-bearing applications by synthesizing a TK triol crosslinker to reduce Mc48 or by increasing the NS loading.16
Cancellous bone has been postulated to heal by inter-trabecular intramembranous ossification49, characterized by condensation of MSCs to form osteoid, deposition of woven bone, and subsequent remodeling to form lamellar bone in the marrow space between trabeculae.50 While intramembranous ossification can occur near the vascularized periphery of the bone cements, it does not proceed in the hypoxic interior.2 Near the periphery, NS-PTKUR cements implanted in femoral defects in rabbits showed appositional growth of new bone with osteon-like structures representative of cortical bone (Figure 6A), which is consistent with previous studies evaluating bone cements in dogs51 and sheep.22 The implants were replaced by bone via creeping substitution1, as evidenced by new bone formation and osteoclast-mediated resorption of NS-PTKUR (Figure 6B–C). Similar to CPCs and PCLUR bone cements3–5, the formation of new bone by intramembranous ossification was generally limited to the periphery of the implants.
Hypoxia mediates chondrogenesis and endochondral ossification of MSCs.52 Thus, hypoxic conditions within the cements were anticipated to promote differentiation of endogenous MSCs to chondrocytes.11, 52, 53 At distances >1 mm from the periphery, regions of proliferating chondrocytes and cartilage were observed in the interior of the cements by Stevenel’s Blue staining (Figure 6). These findings were confirmed by Safranin O/Fast Green staining, which showed ossification of the cartilaginous intermediate (Figure 7). The presence of chondrocytes, cartilage, and bone within the cements suggests that ROS produced by proliferating chondrocytes32, 33 contributed to oxidative degradation of the thioketal bonds in PTKUR26, 27, 31 and infiltration of cells into the newly created pores.
Currently available biomaterials for bone grafting are intended to be replaced by new bone via intramembranous ossification.3–5 A recent study reported a biomaterials-based strategy that recapitulates the variable combination of intramembranous and endochondral ossification observed in fracture healing.13 When the pores of a freeze-dried collagen scaffold were oriented parallel to the axis of the femur, endochondral ossification in the hypoxic interior linked to intramembranous ossification near the vascularized periphery occurred.13 However, when the pores were perpendicular to the axis of the femur, cells could not infiltrate the scaffold and only lamellar bone formation near the periphery was observed. In the present study, endochondral ossification was observed in oxidatively degradable bone cements without interconnected pores, which has not been previously reported.
The cements incorporating a sucrose porogen (10C35S and 0C45S) showed a burst release of 10% and subsequent leaching of sucrose at a rate of 1%/day in vitro (Supplemental Figure 2). The rate of sucrose leaching in vivo is anticipated to be slower since the cements are surrounded by tissue and not immersed in solution. Thus, both dissolution of sucrose and oxidative degradation of PTKUR are conjectured to result in the formation of pores and consequent cellular infiltration.
In the present study, significant increases in area% bone over time were not observed, which is consistent with the μCT data showing that changes in the diameter of the residual cement over time were not significant (Supplemental Figure 4A). Thus, the rate of replacement of the material with new bone was slow, as intended for cements designed to maintain mechanical stability while gradually being replaced by new bone.1, 22, 51 The length of the cements measured by μCT decreased to 69–79% of their original value at 18 months (Supplemental Figure 4B). In a previous study evaluating the non-weight-bearing CPC Norian® in a canine femoral defect model, the residual cement was measured by histomorphometry to be 75% at 20 months51, which is comparable to NS-PTKUR. While remodeling of CPCs by creeping substitution is limited to the periphery, endochondral ossification in NS-PTKUR extends deeper into the implant than creeping substitution. Thus, NS-PTKUR potentially offers the advantages of enhanced osseointegration resulting in strengthening of the cement/bone interface and improved mechanical stability compared with conventional bone cements.12 Synthesis of NS-PTKUR from TK triols is anticipated to increase crosslink density and mechanical properties to levels comparable to weight-bearing PMMA and nHA-PCLUR cements. Osseointegration of bone cements in large animals is faster in mechanically loaded defects compared with mechanically protected defects22, 51, 54–56, and mechanical strain enhances cartilage formation and endochondral ossification.57, 58 Thus, mechanical loading is anticipated to stimulate endochondral ossification and osseointegration of NS-PTKUR cements, which will be investigated in future studies.
Conclusion
In this study, osseointegration of oxidatively degradable NS-PTKUR nanocomposite bone cements was investigated in a rabbit epiphyseal bone defect model at 4, 12, and 18 months. New bone formation occurred via combined endochondral and intramembranous ossification. These findings highlight the potential of NS-PTKUR nanocomposites as injectable or moldable cements for repair of bone defects.
Supplementary Material
Acknowledgments:
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01 AR064304 and the National Institute of Biomedical Imaging and Bioengineering under R01 EB019409. This work was also supported by the Department of Defense (Award Numbers W81XWH-16-2-0052 and W81XWH-115-0-0001), the National Science Foundation Graduate Research Fellowship Program (Award Number 1445197), and Medtronic Spinal and Biologics. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Defense, or the National Science Foundation.
Footnotes
Supporting Information
Representative and schematic μCT thresholding images and schematic representation of histomorphometric thresholding (Figure S1), in vitro sucrose release kinetics (Figure S2), representative μCT images of sagittal and coronal planes (Figure S3), and analysis of cement remodeling (Figure S4).
References
- 1.Gisep A; Wieling R; Bohner M; Matter S; Schneider E; Rahn B, Resorption patterns of calcium-phosphate cements in bone. J Biomed Mater Res A 2003, 66 (3), 532–40. 10.1002/jbm.a.10593. [DOI] [PubMed] [Google Scholar]
- 2.Baker C; Moore-Lotridge S; Hysong A; Posey S; Robinette J; Blum D; Benvenuti M; Cole H; Egawa S; Okawa A; Saito M; McCarthy J; Nyman J; Yuasa M; Schoenecker J, Bone Fracture Acute Phase Response—A Unifying Theory of Fracture Repair: Clinical and Scientific Implications. Clinical Reviews in Bone and Mineral Metabolism 2018, 16, 142–158. doi: 10.1007/s12018-018-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan SN; Tomin E; Lane JM, Clinical applications of bone graft substitutes. Orthop Clin North Am 2000, 31 (3), 389–98. 10.1016/s0030–5898(05)70158–9. [DOI] [PubMed] [Google Scholar]
- 4.Bohner M, Physical and chemical aspects of calcium phosphates used in spinal surgery. Eur Spine J 2001, 10 Suppl 2, S114–21. 10.1007/s005860100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abramo A; Tagil M; Geijer M; Kopylov P, Osteotomy of dorsally displaced malunited fractures of the distal radius: no loss of radiographic correction during healing with a minimally invasive fixation technique and an injectable bone substitute. Acta Orthop 2008, 79 (2), 262–8. 10.1080/17453670710015085. [DOI] [PubMed] [Google Scholar]
- 6.Yeo QY; Kee Kwek EB, Use of a biphasic cement bone substitute in the management of metaphyseal fractures. J Clin Orthop Trauma 2019, 10 (4), 789–791. 10.1016/j.jcot.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horstmann PF; Hettwer WH; Kaltoft NS; Petersen MM, Early Clinical and Radiological Experience with a Ceramic Bone Graft Substitute in the Treatment of Benign and Borderline Bone Lesions. Sci Rep 2018, 8 (1), 15384 10.1038/s41598-018-33736-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohner M, Design of ceramic-based cements and putties for bone graft substitution. Eur Cell Mater 2010, 20, 1–12. 10.22203/ecm.v020a01. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert SF; Sunderland M, Osteogenesis: The Development of Bones In Developmental Biology, 6th ed.; Sinauer Associates: 2000. [Google Scholar]
- 10.Akter F; Ibanez J, Chapter 8: Bone and Cartilage Tissue Engineering In Tissue Engineering Made Easy, Akter F, Ed. Elsevier: 2016. [Google Scholar]
- 11.Dennis SC; Berkland CJ; Bonewald LF; Detamore MS, Endochondral ossification for enhancing bone regeneration: converging native extracellular matrix biomaterials and developmental engineering in vivo. Tissue Eng Part B Rev 2015, 21 (3), 247–66. 10.1089/ten.TEB.2014.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cimatti B; Santos MAD; Brassesco MS; Okano LT; Barboza WM; Nogueira-Barbosa MH; Engel EE, Safety, osseointegration, and bone ingrowth analysis of PMMA-based porous cement on animal metaphyseal bone defect model. J Biomed Mater Res B Appl Biomater 2018, 106 (2), 649–658. 10.1002/jbm.b.33870. [DOI] [PubMed] [Google Scholar]
- 13.Petersen A; Princ A; Korus G; Ellinghaus A; Leemhuis H; Herrera A; Klaumunzer A; Schreivogel S; Woloszyk A; Schmidt-Bleek K; Geissler S; Heschel I; Duda GN, A biomaterial with a channel-like pore architecture induces endochondral healing of bone defects. Nature communications 2018, 9 (1), 4430 10.1038/s41467-018-06504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlundt C; El Khassawna T; Serra A; Dienelt A; Wendler S; Schell H; van Rooijen N; Radbruch A; Lucius R; Hartmann S; Duda GN; Schmidt-Bleek K, Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018, 106, 78–89. 10.1016/j.bone.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Von Euw S; Wang Y; Laurent G; Drouet C; Babonneau F; Nassif N; Azais T, Bone mineral: new insights into its chemical composition. Sci Rep 2019, 9 (1), 8456 10.1038/s41598-019-44620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu S; McEnery M; Rogers B; Wenke J; Shimko D; Guelcher S, Resorbable Nanocomposites with Bone-Like Strength and Enhanced Cellular Activity. J Mater Chem B 2017, 5, 4198–4206. DOI: 10.1039/C7TB00657H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mi HY; Palumbo S; Jing X; Turng LS; Li WJ; Peng XF, Thermoplastic polyurethane/hydroxyapatite electrospun scaffolds for bone tissue engineering: effects of polymer properties and particle size. J Biomed Mater Res B Appl Biomater 2014, 102 (7), 1434–44. 10.1002/jbm.b.33122. [DOI] [PubMed] [Google Scholar]
- 18.Mi H-Y; Jing X; Salick MR; Cordie TM; Peng X-F; Turng L-S, Morphology, mechanical properties, and mineralization of rigid thermoplastic polyurethane/hydroxyapatite scaffolds for bone tissue applications: effects of fabrication approaches and hydroxyapatite size. Journal of Materials Science 2014, 49 (5), 2324–2337. [Google Scholar]
- 19.Tetteh G; Khan AS; Delaine-Smith RM; Reilly GC; Rehman IU, Electrospun polyurethane/hydroxyapatite bioactive scaffolds for bone tissue engineering: the role of solvent and hydroxyapatite particles. J Mech Behav Biomed Mater 2014, 39, 95–110. 10.1016/j.jmbbm.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Yang W; Both SK; Zuo Y; Birgani ZT; Habibovic P; Li Y; Jansen JA; Yang F, Biological evaluation of porous aliphatic polyurethane/hydroxyapatite composite scaffolds for bone tissue engineering. J Biomed Mater Res A 2015, 103 (7), 2251–9. 10.1002/jbm.a.35365. [DOI] [PubMed] [Google Scholar]
- 21.Cetina-Diaz SM; Chan-Chan LH; Vargas-Coronado RF; Cervantes-Uc JM; Quintana-Owen P; Paakinaho K; Kellomaki M; Di Silvio L; Deb S; Cauich-Rodriguez JV, Physicochemical characterization of segmented polyurethanes prepared with glutamine or ascorbic acid as chain extenders and their hydroxyapatite composites. J Mater Chem B 2014, 2 (14), 1966–1976. 10.1039/c3tb21500h. [DOI] [PubMed] [Google Scholar]
- 22.Lu S; McGough M; Shiels S; Zienkiewicz K; Merkel A; Vanderburgh J; Nyman J; Sterling J; Tennent D; Wenke J; Guelcher S, Settable polymer/ceramic composite bone grafts stabilize weight-bearing tibial plateau slot defects and integrate with host bone in an ovine model. Biomaterials 2018, 179, 29–45. doi: 10.1016/j.biomaterials.2018.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storey RF; Wiggins JS; Puckett AD, Hydrolyzable poly(ester-urethane) networks from L-lysine diisocyanate and D,L-lactide/e-caprolactone homo- and copolyester triols. Journal of Polymer Science, Part A: Polymer Chemistry 1994, 32 (12), 2345–2363. 10.1002/pola.1994.080321216. [DOI] [Google Scholar]
- 24.Talley AD; McEnery MA; Kalpakci KN; Zienkiewicz KJ; Shimko DA; Guelcher SA, Remodeling of injectable, low-viscosity polymer/ceramic bone grafts in a sheep femoral defect model. J Biomed Mater Res B Appl Biomater 2017, 105 (8), 2333–2343. 10.1002/jbm.b.33767. [DOI] [PubMed] [Google Scholar]
- 25.Dumas JE; Prieto EM; Zienkiewicz KJ; Guda T; Wenke JC; Bible J; Holt GE; Guelcher SA, Balancing the rates of new bone formation and polymer degradation enhances healing of weight-bearing allograft/polyurethane composites in rabbit femoral defects. Tissue Eng Part A 2014, 20 (1–2), 115–29. 10.1089/ten.TEA.2012.0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin JR; Gupta MK; Page JM; Yu F; Davidson JM; Guelcher SA; Duvall CL, A porous tissue engineering scaffold selectively degraded by cell-generated reactive oxygen species. Biomaterials 2014, 35 (12), 3766–76. 10.1016/j.biomaterials.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patil P; Martin J; Sarett S; Pollins A; Cardwell N; Davidson J; Guelcher S; Nanney L; Duvall C, Porcine Ischemic Wound Healing Model for Preclinical Testing of Degradable Biomaterials. Tissue Eng Part C Methods 2017, 23 (11), 754–762. 10.1089/ten.TEC.2017.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson DS; Dalmasso G; Wang L; Sitaraman SV; Merlin D; Murthy N, Orally delivered thioketal nanoparticles loaded with TNF-alpha-siRNA target inflammation and inhibit gene expression in the intestines. Nature materials 2010, 9 (11), 923–8. 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hafeman AE; Zienkiewicz KJ; Zachman AL; Sung HJ; Nanney LB; Davidson JM; Guelcher SA, Characterization of the degradation mechanisms of lysine-derived aliphatic poly(ester urethane) scaffolds. Biomaterials 2011, 32 (2), 419–29. 10.1016/j.biomaterials.2010.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stadtman E, Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem 1993, 62 (1), 797–821. 10.1146/annurev.bi.62.070193.004053. [DOI] [PubMed] [Google Scholar]
- 31.McEnery MA; Lu S; Gupta MK; Zienkiewicz KJ; Wenke JC; Kalpakci KN; Shimko D; Duvall CL; Guelcher SA, Oxidatively Degradable Poly(thioketal urethane)/Ceramic Composite Bone Cements with Bone-Like Strength. RSC Adv 2016, 6 (111), 109414–109424. 10.1039/c6ra24642g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolduc JA; Collins JA; Loeser RF, Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med 2019, 132, 73–82. 10.1016/j.freeradbiomed.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bai Y; Gong X; Dou C; Cao Z; Dong S, Redox control of chondrocyte differentiation and chondrogenesis. Free Radic Biol Med 2019, 132, 83–89. 10.1016/j.freeradbiomed.2018.10.443. [DOI] [PubMed] [Google Scholar]
- 34.Huber FX, First histological observations on the incorporation of a novel nanocrystalline hydroxyapatite paste in human cancellous bone. BMC musculoskeletal disorders 2006, 7 (50). 10.1186/1471-2474-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huber FX; Hillmeier J; Herzog L; McArthur N; Kock HJ; Meeder P,J, Open reduction and palmar plateosteosynthesis in combination with a nanocrystalline hydroxyapatite spacer in the treatment of comminuted fractures of the distal radius. J Hand Surg Br. 2006, 31 (3), 298–303. 10.1016/j.jhsb.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Harmata AJ; Ward CL; Zienkiewicz K; Wenke JC; Guelcher SA, Investigating the Effects of Surface-Initiated Polymerization of ε-Caprolactone to Bioactive Glass Particles on the Mechanical Properties of Settable Polymer/Ceramic Composites. J Mater Res 2014, 2014 (29), 20–30. 10.1557/jmr.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yubao L; De Groot K; De Wijn J; Klein C; Meer S, Morphology and composition of nanograde calcium phosphate needle-like crystals formed by simple hydrothermal treatment. Journal of materials science: Materials in Medicine 1994, 5 (6–7), 326–331. [Google Scholar]
- 38.Harmata AJ; Uppuganti S; Granke M; Guelcher SA; Nyman JS, Compressive fatigue and fracture toughness behavior of injectable, settable bone cements. J Mech Behav Biomed Mater 2015, 51, 345–355. 10.1016/j.jmbbm.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dumas JE; Zienkiewicz K; Tanner SA; Prieto EM; Bhattacharyya S; Guelcher S, Synthesis and Characterization of an Injectable Allograft Bone/polymer Composite Bone Void Filler with Tunable Mechanical Properties. Tissue Eng Part A 2010, 16 (8), 2505–18. 10.1089/ten.TEA.2009.0672. [DOI] [PubMed] [Google Scholar]
- 40.Cohen DJ; Cheng A; Kahn A; Aviram M; Whitehead AJ; Hyzy SL; Clohessy RM; Boyan BD; Schwartz Z, Novel Osteogenic Ti-6Al-4V Device For Restoration Of Dental Function In Patients With Large Bone Deficiencies: Design, Development And Implementation. Sci Rep 2016, 6, 20493 10.1038/srep20493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernando S; McEnery M; Guelcher SA, Polyurethanes for bone tissue engineering In Advances in Polyurethane Biomaterials, Cooper SL; Guan J, Eds. Woodhead Publishing: 2016; pp 481–501. [Google Scholar]
- 42.Karageorgiou V; Kaplan D, Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26 (27), 5474–91. 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Jiang G; Evans ME; Jones IA; Rudd CD; Scotchford CA; Walker GS, Preparation of poly(epsilon-caprolactone)/continuous bioglass fibre composite using monomer transfer moulding for bone implant. Biomaterials 2005, 26 (15), 2281–8. 10.1016/j.biomaterials.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 44.Oertel G, Polyurethane Handbook. 2nd ed.; Hanser Gardner Publications: Berlin, 1994. [Google Scholar]
- 45.Page JM; Prieto EM; Dumas JE; Zienkiewicz KJ; Wenke JC; Brown-Baer P; Guelcher SA, Biocompatibility and chemical reaction kinetics of injectable, settable polyurethane/allograft bone biocomposites. Acta Biomater 2012, 8, 4405–4416. 10.1016/j.actbio.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 46.Kickelbick G, Concepts for the incorporation of inorganic building blocks into organic polymers on a nanoscale. Progress in Polymer Science 2003, 28 (1), 83–114. 10.1016/S0079-6700(02)00019-9. [DOI] [Google Scholar]
- 47.Kuehn K-D, Bone Cements. Springer: Berlin, 2000. [Google Scholar]
- 48.Guelcher SA; Srinivasan A; Dumas JE; Didier JE; McBride S; Hollinger JO, Synthesis, mechanical properties, biocompatibility, and biodegradation of polyurethane networks from lysine polyisocyanates. Biomaterials 2008, 29, 1762–75. 10.1016/j.biomaterials.2007.12.046. [DOI] [PubMed] [Google Scholar]
- 49.Sandberg OH; Aspenberg P, Inter-trabecular bone formation: a specific mechanism for healing of cancellous bone. Acta Orthop 2016, 87 (5), 459–65. 10.1080/17453674.2016.1205172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen WT; Han da C; Zhang PX; Han N; Kou YH; Yin XF; Jiang BG, A special healing pattern in stable metaphyseal fractures. Acta Orthop 2015, 86 (2), 238–42. 10.3109/17453674.2014.1003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frankenburg E; Goldstein S; Bauer T; Harris S; Poser R, Biomechanical and histological evaluation of a calcium phosphate cement. J. Bone Joint Surg 1998, 80-A (8), 1112–1124. 10.2106/00004623-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y; Wu S; Kuss MA; Streubel PN; Duan B, Effects of Hydroxyapatite and Hypoxia on Chondrogenesis and Hypertrophy in 3D Bioprinted ADMSC Laden Constructs. ACS Biomater. Sci. Eng 2017, 3, 826–835. 10.1021/acsbiomaterials.7b00101. [DOI] [PubMed] [Google Scholar]
- 53.Bornes TD; Jomha NM; Mulet-Sierra A; Adesida AB, Hypoxic culture of bone marrow-derived mesenchymal stromal stem cells differentially enhances in vitro chondrogenesis within cell-seeded collagen and hyaluronic acid porous scaffolds. Stem Cell Res Ther 2015, 6, 84 10.1186/s13287-015-0075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ignatius A; Peraus M; Schorlemmer S; Augat P; Burger W; Leyen S; Claes L, Osseointegration of alumina with a bioactive coating under load-bearing and unloaded conditions. Biomaterials 2005, 26 (15), 2325–32. 10.1016/j.biomaterials.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 55.Lamerigts NM; Buma P; Huiskes R; Schreurs W; Gardeniers J; Slooff TJ, Incorporation of morsellized bone graft under controlled loading conditions. A new animal model in the goat. Biomaterials 2000, 21 (7), 741–7. 10.1016/s0142-9612(99)00247-1. [DOI] [PubMed] [Google Scholar]
- 56.Theiss F; Apelt D; Brand B; Kutter A; Zlinszky K; Bohner M; Matter S; Frei C; Auer JA; von Rechenberg B, Biocompatibility and resorption of a brushite calcium phosphate cement. Biomaterials 2005, 26 (21), 4383–94. 10.1016/j.biomaterials.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 57.Witt F; Petersen A; Seidel R; Vetter A; Weinkamer R; Duda GN, Combined in vivo/in silico study of mechanobiological mechanisms during endochondral ossification in bone healing. Ann Biomed Eng 2011, 39 (10), 2531–41. 10.1007/s10439-011-0338-x. [DOI] [PubMed] [Google Scholar]
- 58.Ghimire S; Miramini S; Richardson M; Mendis P; Zhang L, Role of Dynamic Loading on Early Stage of Bone Fracture Healing. Ann Biomed Eng 2018, 46 (11), 1768–1784. 10.1007/s10439-018-2083-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


