Supplemental Digital Content is available in the text
Keywords: erythropoietin, magnetics, nanoparticles, regeneration
Abstract
The objective of this proof-of-concept study was to demonstrate the targeted delivery of erythropoietin (EPO) using magnetically guided magnetic nanoparticles (MNPs).
MNPs consisting of a ferric–ferrous mixture (FeCl3·6H2O and FeCl2·4H2O) were prepared using a co-precipitation method. The drug delivery system (DDS) was manufactured via the spray-drying technique using a nanospray-dryer. The DDS comprised 7.5 mg sodium alginate, 150 mg MNPs, and 1000 IU EPO.
Scanning electron microscopy revealed DDS particles no more than 500 nm in size. Tiny particles on the rough surfaces of the DDS particles were composed of MNPs and/or EPO, unlike the smooth surfaces of the only alginate particles. Transmission electron microscopy showed the tiny particles from 5 to 20 nm in diameter. Fourier-transform infrared spectroscopy revealed DDS peaks characteristic of MNPs as well as of alginate. Thermal gravimetric analysis presented that 50% of DDS weight was lost in a single step around 500°C. The mode size of the DDS particles was approximately 850 nm under in vivo conditions. Standard soft lithography was applied to DDS particles prepared with fluorescent beads using a microchannel fabricated to have one inlet and two outlets in a Y-shape. The fluorescent DDS particles reached only one outlet reservoir in the presence of a neodymium magnet. The neurotoxicity was evaluated by treating SH-SY5Y cells in 48-well plates (1 × 105 cells/well) with 2 μL of a solution containing sodium alginate (0.075 mg/mL), MNPs (1.5 mg/mL), or sodium alginate + MNPs. A cell viability assay kit was used to identify a 93% cell viability after MNP treatment and a 94% viability after sodium alginate + MNP treatment, compared with the control. As for the DDS particle neurotoxicity, a 95% cell viability was noticed after alginate-encapsulated MNPs treatment and a 93% cell viability after DDS treatment, compared with the control.
The DDS-EPO construct developed here can be small under in vivo conditions enough to pass through the lung capillaries with showing the high coating efficiency. It can be guided using magnetic control without displaying significant neurotoxicity in the form of solution or particles.
1. Introduction
Central nervous system (CNS) injuries, including brain and spinal cord injury, cause serious sequelae in most patients and increase the medical costs associated with individuals and nations. Among the various therapeutic strategies against CNS injuries currently under development,[1] erythropoietin (EPO) has been tested in in vitro/in vivo experiments in brain and spinal cord injury models.[2–6] Neuro-protection or -regeneration in the presence of EPO has been evaluated since early 2000s.[1,7] The widespread use of EPO in hematologic diseases suggests that it may be more readily applicable to CNS injury patients than other experimental drugs.[8] Unfortunately, the therapeutic time window within which EPO may be effective against CNS injury is very limited (no more than 6 hours after injury),[9] and few clinical trials have obtained high-quality evidence supporting the efficacy of its use. Additionally, hematopoietic and non-hematopoietic EPO receptors display significant heterogeneity and phylogenetic differences in human beings.[10] Efficient action requires EPO to escape binding at non-in situ EPO receptors. To demonstrate clinical feasibility in urgent CNS injury situations, it is essential to ensure very fast targeted delivery to an injured area.
Drug delivery systems (DDSs) have been widely investigated in recent years. Among these, magnetic-guided navigation is advantageous in its ability to guide magnetic nanoparticles (MNPs) and successfully penetrate the blood–brain barrier (BBB) in in vivo rat models.[11] Colloidal MNP solutions are stable in the presence of organic materials, such as fatty acids or polysaccharides, during delivery to targeted areas. Magnetic DDS using MNPs may be one of the most promising DDS methods identified to date.[12] Sodium alginate is a widely used, natural, non-toxic, biodegradable polymer (α-l-guluronic acid units and (1,4)-linked β-d-mannuronic acid units).[13]
This work sought to demonstrate a new therapeutic strategy against CNS injury utilizing the rapid delivery of a combination of EPO and MNPs to injured sites within a therapeutic time window and the localization of these constructs exclusively within the injured area with aid of external magnetic field guidance, to avoid competition with other EPO receptors outside of the injured sites. As proof-of-concept, the authors manufactured a DDS comprising biodegradable alginate-coated EPO-nanoparticle polymers using a nanospray drying technique. The particles were then encapsulated and characterized in terms of their size, degree of encapsulation, amenability to magnetic guidance, and neuronal cell toxicity.
2. Materials and methods
Because the research was an in vitro one, ethical approval was not necessary.
2.1. Synthesizing magnetic nanoparticles using a chemical co-precipitation method
MNPs were synthesized using a chemical co-precipitation method, the most widely used method for synthesizing MNPs.[14] Fe3O4 MNPs were prepared via the chemical co-precipitation of Fe3+ and Fe2+ ions in a molar ratio of 2:1 under basic conditions. The MNP synthesis reaction could be expressed as:
2FeCl3 + FeCl2 + 8NaOH → Fe3O4 (s) + 4H2O + 8NaCl
Ferric chloride hexahydrate (FeCl3·6H2O) and ferrous chloride tetrahydrate (FeCl2·4H2O) were purchased (Sigma–Aldrich, S. Louis, MO) and used as received. In 100 mL distilled water, 0.02 mol/L FeCl3·6H2O and 0.01 mol/L FeCl2·4H2O were dissolved to form a homogeneous solution in a 250 mL glass beaker. A NaOH (Kanto, Tokyo, Japan) solution was prepared (0.8 mol/L in distilled water) over 30 minutes. The ferric–ferrous mixture was stirred at 500 rpm, and the NaOH solution was slowly added to this solution using a syringe pump at a flow rate of 1.0 mL/min under oxygen-free conditions at room temperature. The pH value was measured continuously, and NaOH delivery was stopped once the pH reached 10.5.[15] Following completion of the NaOH reaction, the mixture was stirred continuously for 3 hours. Subsequently, MNPs (black suspended particles) in the solution were magnetically filtered using a strong permanent magnet. The supernatant containing residual chemicals was removed. The filtered MNPs were washed several times with distilled water to reach a neutral pH. The neutral MNPs were dried at 80°C overnight. Finally, the dried MNPs were annealed at 400°C for 2 hours.
2.2. Drug delivery system fabrication using a nanospray-dryer
The alginate-encapsulated EPO-MNPs were manufactured via a spray-drying technique using a nanospray-dryer B-90 (Fig. 1: Büchi Labortechnik AG, Flawil, Switzerland). The nanospray-dryer included a pulsating casing in the spray nozzle to atomize the feed, and an electrostatic particle accumulator gathered the particles.[16] This study used a small spray nozzle (4 μm). The flow rate was set to 102 to 106 L/min, and the relative spray rate was fixed at 80% with an aspirator flow rate of 27.3 m3/minute. The inlet temperature and outlet temperature were set to 120°C and 40°C, respectively with a pressure of 28 mbar.
Figure 1.

Nanospray-dryer.
The alginate solution (Chem-Supply, Gillman, Australia) was mixed with the MNPs and recombinant human erythropoietin (rhEPO: Epoetin alfa; Sigma–Aldrich, S. Louis, MO). First, 7.5 mg sodium alginate and 150 mg MNPs were added to a beaker containing 100 mL distilled water, and the solution was sonicated for 1 hour. One milliliter 1000 IU rhEPO was added to the mixture of MNPs and sodium alginate, and the mixture was stirred for 30 minutes. The final solution was filtered prior to applying the spray-drying process to avoid nozzle blockage. The solution was sprayed over 1 hour. The dried particles were collected from the particle chamber using a powder scraper, which dispersed the DDS. The dried particles comprised 7.5 mg sodium alginate, 150 mg MNPs, and 1000 IU rhEPO. The particles were stored in a desiccator at a temperature of 25°C. The neuro-protective concentrations reported previously suggested that EPO should be diluted at 10 IU/mL.[17–19] A 10 IU/mL rhEPO solution was prepared by dissolving the collected DDS particles in 1 mL distilled water and diluting the solution to 1/100. The concentrations of sodium alginate and the MNPs were also diluted to 0.075 mg/mL and 1.5 mg/mL.
2.3. Characterization of the drug delivery system particles
2.3.1. Scanning electron microscopy
The sizes, shapes, and surface aspects of the synthesized MNPs, that is, the spray-dried alginate and spray-dried DDS, were evaluated using scanning electron microscopy (SEM; SU8220, Hitachi, Tokyo, Japan). The particles were sputter-coated with platinum to enhance the surface conductivity prior to scanning and were analyzed at a voltage of 5.0 kV. The average size was calculated from a set of more than 100 particles imaged by SEM.
2.3.2. Transmission electron microscopy
The morphologic modification of the DDS following the incorporation of MNPs or EPO was examined under transmission electron microscopy (TEM; JEM – 2100F, Jeol, Tokyo, Japan). The DDS particles were diluted in alcohol and then were sonicated for 30 seconds. Small drop of the diluted DDS solution dried on a copper grid (30 μm × 30 μm) for TEM analysis.
2.3.3. Fourier-transform infrared spectroscopy
Fourier-transform infrared (FTIR) spectroscopy (Nicolet iS5, Thermo Fisher Scientific, Waltham) was conducted to confirm the formation of the DDS by measuring the characteristic peaks. The sample pellets were prepared by blending with potassium bromide (KBr) and then compressing. The transmission spectra were obtained from 400 to 4000 cm−1 with a resolution of 4 cm−1.
2.3.4. Thermal gravimetric analysis
For the strength of the alginate matrix, thermal stability with phase transitions, based on percentage of weight loss and decomposition, was analyzed using thermal gravimetric analysis (TGA; Discovery TGA 55, TA instruments, DE). The DDS particles and MNPs were analyzed by heating from 50 to 800°C in nitrogen atmosphere with a flow rate of 20 mL/minute and a ramp rate of 10°C/minute.
2.3.5. In vivo aggregation of DDS
In vivo aggregation of the DDS in the biological fluids was checked using evaluation of size distribution. 100 mg of the DDS was added into 10 mL of fetal bovine serum (FBS; S 001-01, Welgene, Seoul, Republic of Korea) for 24 hours in CO2 incubate condition, then Zetasizer Nano (Zetasizer Nano ZS90, Malvern Panalytical, Malvern, UK) was used to measure the size distribution of the DDS particles in serum.
2.4. Magnetic guidance of the drug delivery system
Standard soft lithography was used to prepare a bifurcated microchannel and demonstrate magnetic guidance of the DDS. The lithography process was used to fabricate a simple polydimethylsiloxane (PDMS) bifurcated microchannel to mimic blood vessel microchannels. The bifurcated microchannel was designed to have one inlet channel and two outlet bifurcation channels in a Y-shape. The width and height of the channel were 200 μm, and the angle between the two channel branches was 90°. A neodymium magnet was placed near the left outlet channel to guide the DDS droplets.
Fluorescence microbeads were added to the DDS. Gelation was ensured by soaking the DDS in CaCl2 for 30 minutes prior to mixing with phosphate-buffered saline (PBS). The gelated DDS were mixed with PBS at a concentration of 10 mg/mL and injected into the inlet port of the bifurcated microchannel. Fluorescence microscopy was used to monitor the movement of the DDS during magnetic guidance tests.
2.5. The neurotoxicity of the magnetic nanoparticles and the drug delivery system using SH-SY5Y cells
Prior to evaluating the neuro-protective effects of the DDS containing rhEPO, the neurotoxicity of the MNPs in the DDS was tested. The SH-SY5Y cell line, a human-derived neuronal cell line widely used in experimental neurological studies, was used for this purpose.[20] SH-SY5Y cells (Sigma–Aldrich, S. Louis, MO) were plated into 48-well plates at 1 × 105 cells/well. Cells were incubated for 24 hours in a CO2 incubator.
During incubation, three different solutions containing distilled water were prepared as followed: MNP solution (150 mg/mL), alginate solution (7.5 mg/mL), and MNP + alginate solution (150 mg/mL MNP and 7.5 mg/mL alginate). The distilled water used to prepare the solutions was sterilized by filtering through a 0.2 μm pore size membrane. All three solutions were sonicated for 1 hour at room temperature. Twenty-four hours after plating, the media of each well was replaced with 200 μL Dulbecco's Modified Eagle's medium (DMEM). As previously mentioned, the target concentrations of MNP and sodium alginate in the well were 0.075 mg/mL and 1.5 mg/mL, respectively. The 200 μL DMEM containing the incubated SH-SY5Y cells were treated with 2 μL sodium alginate solution, 2 μL MNP solution, or 2 μL sodium MNP + alginate solution.
The treated cells were incubated for 22 hours in a CO2 incubator, and 20 μL EZ-cytox reagent (DoGeNBio, Seoul, South Korea) was added to each well. The cells were then incubated for an additional 2 hours. The treated cell viability was determined using a microplate reader that measured the absorbance at 450 nm using the EZ-cytox cell viability assay kit (DoGen, Seoul, South Korea).
When measuring the neurotoxicity, DDS should be evaluated in the form of particles as well as in the form of solution. Two types of the particles (alginate-encapsulated MNPs and alginate-encapsulated combination of MNPs + EPO) were tested in 24 hours following the same treatment except CO2 incubation for 48 hours and staining with 0.4% trypan blue (Sigma–Aldrich, S. Louis, MO) in a ratio of 9:1.
2.6. Statistical analysis
Optical density and cell viability values were calculated using separate analyses using one-way analysis of variance (ANOVA). Significance was defined as <0.01 or <0.05 compared with the control group.
3. Results
3.1. Scanning electron microscopy and transmission electron microscopy
Figure 2 presents the morphologies of the synthesized MNPs, spray-dried alginate, and DDS prepared using the proposed methods. Spray-dried alginate particles were spherical in shape with smooth surfaces. SEM images indicated that the MNPs and EPO were well mixed with the alginate, and both or either component formed a rough surface on the DDS particles. The average diameter of the DDS particles was approximately 500 nm and their diameters were distributed from 100 to 875 nm.
Figure 2.

Scanning electron microscopy. SEM images showing (A) the synthesized MNPs, (B) the spray-dried alginate, and (C) the DDS composed of MNPs, alginate, and EPO. The MNPs and EPO were well mixed with alginate, and both components formed a rough surface on the DDS particles.
Figure 3 shows the spherical shape of the tiny particles composing the rough surface on the DDS of the SEM images. It reveals that the tiny particles were ranged from 5 to 20 nm in diameter (Fig. 3C). However, no morphologic difference was noticed in shape between the MNPs- and EPO-incorporated DDS.
Figure 3.
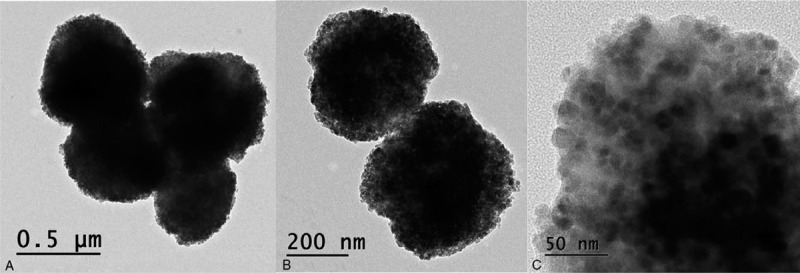
Transmission electron microscopy. TEM images presenting the DDS particles with three various magnifications (A) ×10 k, (B) ×20 k, and (C) ×100 k. The size of the spherical particles was ranged from 5 to 20 nm in diameter.
3.2. Fourier-transform infrared spectroscopy
FTIR spectroscopy was used to confirm the formation of the DDS by measuring the characteristic peaks (Fig. 4). The FTIR spectrum of the MNPs (Fig. 4A) revealed a vibrational Fe–O peak at 598 to 628 cm−1, confirming MNPs formation.[21,22] Another characteristic peak at 1632 cm−1, corresponding to O–H bending, demonstrated the presence of hydroxyl groups on the MNP surfaces. These results highlighted the presence of Fe(OH)2 on the MNP surfaces, which formed during the passivation and stabilization of the MNPs.[23]Figure 4B shows the FTIR spectra of the spray-dried alginate (i) and DDS (ii). Compared with the spray-dried alginate, the DDS included all peaks characteristic of the spray-dried alginate, as expected. The DDS, however, also included peaks characteristic of Fe–O at 589 to 630 cm−1, confirming the presence of MNPs on the DDS. A slight shift in the Fe–O peak was noted, from 598 to 628 cm−1 to 580 to 630 cm−1, attributed to the MNP size reduction during DDS formation due to prolonged sonication.[24,25] The characteristic peaks are listed in Supplement 1.[26,27]
Figure 4.
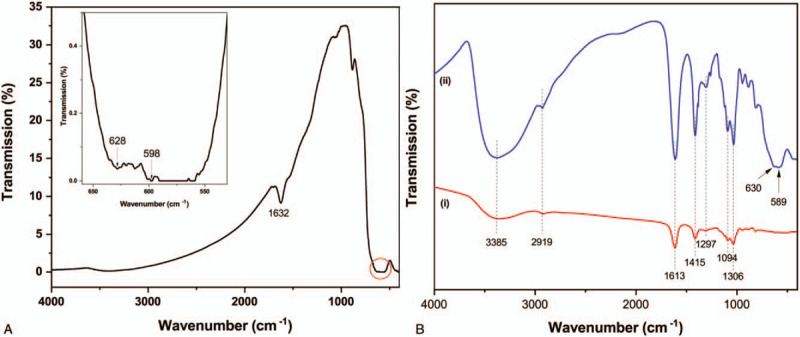
Fourier-transform infrared spectroscopy. FTIR spectra showed (A) the synthesized MNPs, (B) the spray-dried alginate (i), and the DDS composed of MNPs, alginate, and EPO (ii). The FTIR spectra of the spray-dried alginate and DDS showed that the DDS FTIR spectrum featured a peak characteristic of Fe–O at 589 to 630 cm−1, confirming the presence of MNPs and alginate on the DDS.
3.3. Thermal gravimetric analysis
Figure 5 presents the thermography of the DDS particles and MNPs. The first weight loss step, a representative of the removal of residual moisture on the surface, was noticed around 300°C in both samples. MNPs were synthesized from inorganic substances[28] such that another weight loss step was not observed in the range of 50°C to 800°C (100–96.5%). In contrast to the findings of the MNPs, the DDS particles lost the significant weight in the second step over 350°C to 500°C and 50% of the weight loss occurred at 500°C. Large amounts of weight loss during the single step might represent the decomposition of the alginate polymer chains.[29] Taken the aforementioned findings into consideration, the coating efficiency of alginate-encapsulation might be reliable.
Figure 5.
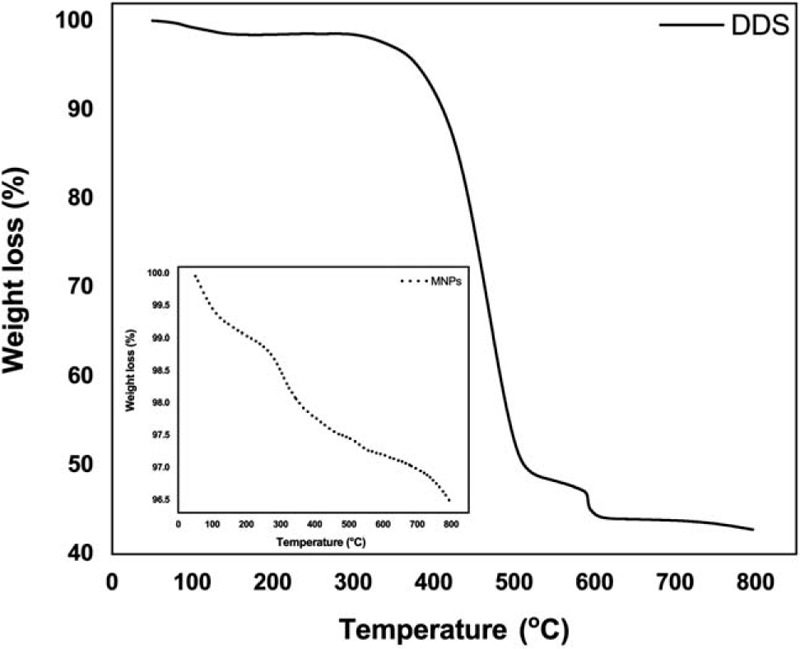
Thermal gravimetric analysis. Weight-loss curves of the MNPs and DDS particles were noticed from 50 to 800°C. The DDS particles lost the significant weight in the second step over 350°C to 500°C and 50% of the weight loss occurred at 500°C.
3.4. In vivo aggregation of the drug delivery system
Figure 6 exhibits the size distribution of the DDS particles following incubation in FBS for 24 hours. The size of the DDS particles was ranged from 450 nm to 1550 nm with showing 850 nm of mode size under in vivo conditions.
Figure 6.
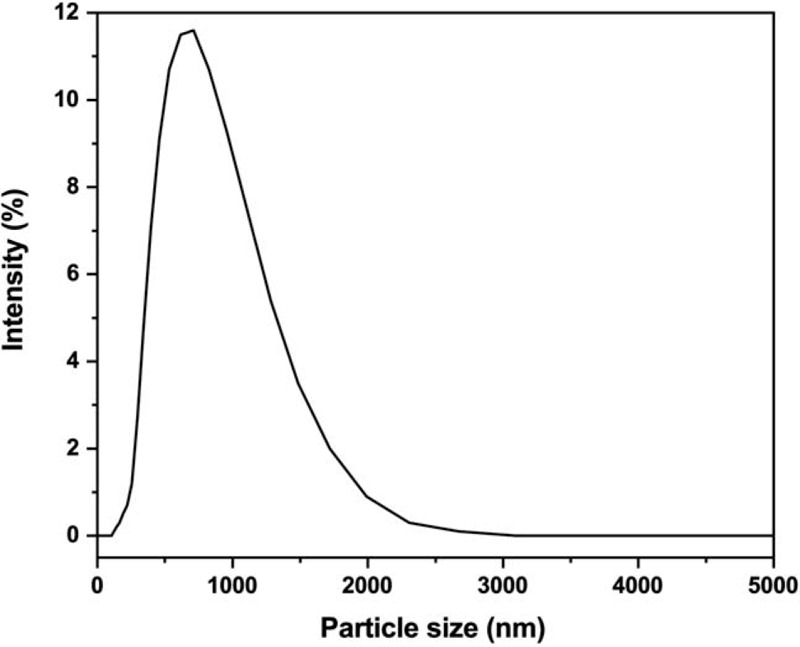
In vivo aggregation of the drug delivery system. The DDS particles under in vivo conditions were distributed in the range of 450 nm to 1550 nm with showing 850 nm of mode size.
3.5. Magnetic guidance of the drug delivery system
Figure 4 shows the results of the magnetic guidance experiments. On the left side of the bifurcated PDMS microchannels (Fig. 7A), the permanent magnet was positioned to impose a magnetic field. (The magnet is not shown in Fig. 7A.) The DDS particles prepared with fluorescent beads were injected using a syringe pump through the inlet of the PDMS microchannel (the upper branch of Fig. 7A). The injected velocity was 0.3 mL/min. The fluorescent DDS particles reached the left outlet reservoir of the PDMS microchannel (Fig. 7B). The right outlet reservoir harvested no DDS (Fig. 7C).
Figure 7.
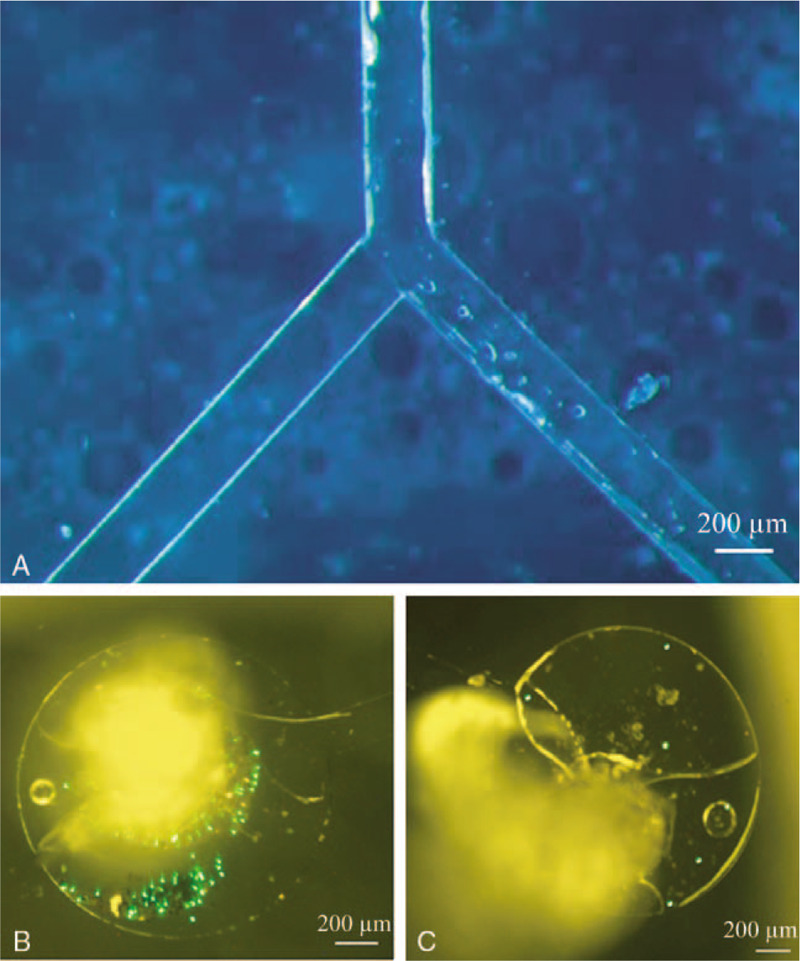
Magnetic guidance of the drug delivery system. DDS guidance experiment results. (A) Bifurcated PDMS microchannel. The magnet was positioned in the left area of the microchannel but is not shown. (B) Magnetically guided fluorescent DDS particles. (C) No DDS was harvested in the absence of guidance.
3.6. Neurotoxicity of magnetic nanoparticles and the drug delivery system, tested using SH-SY5Y cells
Figure 8A presents the SH-SY5Y neuronal cell viability test results for each solution of the three materials. The alginate-only solution did not cause cell death, as expected. Compared with the control, 1.5 mg/mL MNP solution displayed a cell viability that was approximately 7% lower than that of the control. The mixed solution of alginate and MNPs yielded a slightly higher cell viability (about 1% higher than that of the control). No significant cytotoxicity was observed for the MNP solution, compared with the alginate solution. In the form of particles, the cell viabilities of the alginate-encapsulated MNPs and DDS particles were approximately 95% and 93%, respectively, compared with the control. No significant cytotoxicity was observed for the DDS particles, compared with the alginate-encapsulated MNPs (Fig. 8B).
Figure 8.
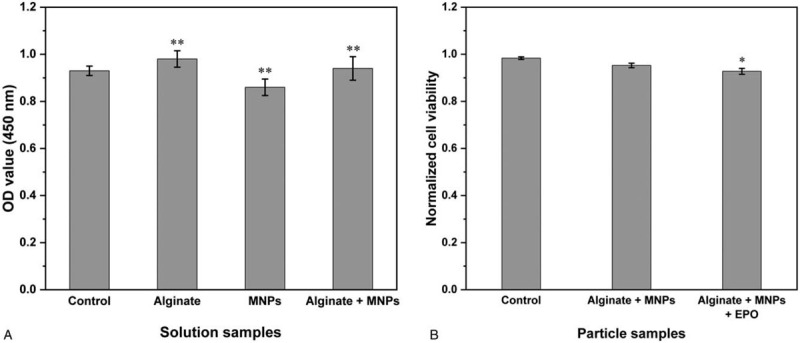
Neurotoxicity of the magnetic nanoparticles and the drug delivery system, tested using the SH-SY5Y cells. The SH-SY5Y neuronal cell viabilities of the MNPs and DDS as (A) solution and as (B) particles were compared. No significant cytotoxicity was observed for the MNP solution, compared with the alginate solution, and no significant cytotoxicity was observed for the DDS particles, compared with the alginate-encapsulated MNPs. OD, optical density; ∗∗P < .01 compared with the control, ∗P < .05 compared with the control.
4. Discussion
4.1. Significance of the method with respect to existing/alternative methods
EPO is expected to compete for binding at a heterogeneous collection of phylogenetically distinct hematopoietic and non-hematopoietic EPO receptors.[10,30] Only a limited quantity of EPO is expected to reach an injured site following conventional administration. The very short therapeutic time window for EPO (<6 hours) must be considered in in vivo CNS injury situations.[9,31,32] Systemic circulation takes only a few minutes, such that the DDS is expected to provide EPO a hundred chances to reach an injured site and remain there in a high density under magnetic control. To the best of our knowledge, the directed targeted delivery of EPO using magnetic guidance represents a departure from the approaches of most recent trials, which focused on the slow sustained release of EPO from DDS particles.[33–35]
Few prior reports have described targeted EPO delivery, and the methods that do describe such approaches are, in fact, indirect, for example, inducing BBB crossing using focused ultrasound sonication with micro-bubbles[31] or epi-cortical implantation and delivery following craniotomy.[36] By contrast, the DDS presented here is capable of targeting the delivery of EPO directly under magnetic guidance to increase the amount of EPO available for neuro-protective action and overcome the current drawbacks of EPO DDS, including in vivo competition by receptor binding after delivery.[33,34] However, authors did not demonstrate the in vivo application so that it should be concerned that the current formulation was compared with the previous formulation reported by Wang et al and Wu et al and this formulation overcome the current drawbacks of EPO DDS. Meanwhile, prior MNP-incorporated DDS trials examining the delivery of EPO did not evaluate targeted delivery; rather, they examined only the synergistic/additive effects of MNP with EPO.[35,37,38] Although nanoparticles alone can impart neuro-protective or neuro-regenerative effects by stimulating neuronal regrowth, improving neuronal survivability, or promoting neuronal differentiation,[39] the combination of nanoparticles and EPO has been shown to be 10 times as neuro-protective as rhEPO in ischemic rat models.[38] The combination of EPO and MNPs may be more promising than another magnetic mediator, magnetosomes.[40] Although the current experiment is proof of concept study, it is very important to optimize the formulation variables to obtain an optimal formulation. The process and/or formulation variables must be more optimized than in the current experiment for in vivo and clinical application in the future.
4.2. Modifications and troubleshooting
In vivo animal experiments tested both murine EPO and rhEPO. A nucleotide sequence analysis revealed that murine EPO showed only 80% homology with rhEPO.[41] rhEPO was, therefore, selected for use in DDS manufacturing. Intravenous delivery was selected as the most convenient approach to systemic circulation administration. The DDS particles must be small enough to pass through lung capillaries. The average diameter of the lung capillary is 3.0 to 13.0 μm in mice and rats and is around 6.3 μm in humans.[42,43] The ferric–ferrous MNPs were found to be 10 nm in diameter.[44] rhEPO is a small, light (30,400 Da) glycoprotein,[8] and the minimum radius of EPO is 2.1 nm, smaller than that of human fibrinogen, based on the Erickson's equation.[45] The intravenous injection of EPO has been shown in numerous rat studies to impart neuro-protective effects.[46,47] In the current experiments, the average diameter of the DDS particles prepared with alginate, EPO, and MNPs was measured to be 0.5 μm, ensuring facile passage through lung capillaries. However, the biological fluids are known to cause aggregation of the nanoparticles so that it should be established by measuring the particle size by incubating them in plasma/serum/FBS, etc. In the current experiment, the DDS particles under in vivo conditions were larger than ones under in vitro conditions; it means that DDS aggregation can occur in the biological fluids. Meanwhile, the maximal difference between two conditions in the average diameter was 350 nm and the maximum size of the DDS particles was approximately 1550 nm under in vivo conditions such that facile passage of the DDS particle through lung capillaries is still ensured. To make biologic identity alteration of nanoparticles from synthetic identify,[48] encapsulation with linear ethylene glycol polymer chains has been mostly applied to nanoparticles.[49] Similar to that, alginate-encapsulation might make the similar effect on MNPs such that in vivo aggregation of the DDS particles did not occur in a large scale. In addition, this kind of polymer encapsulation may be useful in keeping the DDS particles from sequestration by the mononuclear phagocyte system.[50]
4.3. Limitations of the method
During the spray-drying and encapsulation processing, EPO may become thermally unstable; however, carbohydrate-tagged EPO is expected to display thermal stability up to 56°C, and undergo reversible denaturation below 75°C. This form of EPO regained its conformational stability after cooling.[51] In the current experiments, the temperature of the spray nozzle was 120°C at the inlet and 40°C at the outlet. The DDS solution was sprayed very rapidly (1767 mL/second) through a very small spray nozzle (4 μm), and the DDS particles were cooled over 1 hour in a collecting chamber. The EPO subjected to this process was expected to be free from irreversible thermal denaturation. The conformational stability of EPO after processing will be confirmed in future studies, in view of the degree of in vitro/in vivo neuro-protective action.
SEM images revealed that the smooth surface of the alginate polymer was converted into a rough surface after spray-drying with the MNPs and EPO; however, it was unclear whether the MNPs or EPO formed the rough surface morphology. The ferric–ferrous MNPs were shown to be 10 nm in diameter,[44] and EPO is larger than 4.2 nm in diameter, suggesting that visual differentiation might be difficult using SEM. Moreover, TEM images presented that the tiny particles in the DDS were spherical in shape and ranged from 5 to 20 nm (×100 k). Beh et al showed the similar findings that EPO could not be differentiated visually (×20 k) from nanoparticles.[52]
High concentrations of EPO beyond a certain threshold can be cytotoxic.[5,9,53] Moreover, the degree of neuro-protection by EPO depends on the cell line and nature of the neuronal cell injury.[54] Based on several previous in vitro reports with similar study designs,[17,18,54] EPO was diluted to an optimal concentration (10 IU/mL) after DDS fabrication. As a result, the concentration of EPO, 0.000084 mg/mL (10 IU), was much lower than that of the MNPs, 1.5 mg/mL, and most of the tiny particles on the rough surfaces of the DDS particles on the SEM were likely to be MNPs, not EPO. At such low concentrations, FTIR spectroscopy was not expected to reveal peaks specific to EPO on the surface. Additionally, the characteristic FTIR spectrum of EPO (chemical formula of EPO: C815H1317N233O241S5) has not been reported,[55] and the authors do not at this time have enough information to differentiate the EPO FTIR spectrum from those of alginate and the MNPs.
The cytotoxicity of the ferric–ferrous MNPs obtained after 24 hours incubation at a density of 1500 mg/L using the methods described above (7% lethality) was comparable to the cytotoxicity obtained at much lower concentrations in the report of Mashjoor et al (5% lethality after 24 hours incubation at a density of 100 mg/L).[44] The cytotoxicity was tested here using neuronal cells instead of parasite specie cells. Among the neuronal cells of the CNS, MNP phagocytosis is more likely in PC12[56] and microglia cells.[56] Immune reactions may be precipitated by the presence of MNPs in the CNS.[56] Although neuronal cell toxicity was demonstrated here using SH-SY5Y cells, neuronal toxicity and immune reactions must be assessed in other neuronal cells because no previous reports have tested these properties in SH-SY5Y cells.
4.4. Future applications of the method and directions of this research
Because of its small size, the current DDS could be injected as a solution. After injection, the DDS is anticipated to be concentration within an injured region under a target magnetic field applied using a potentially wearable external magnet. After targeted delivery, spontaneous separation of EPO from the DDS is critical for receptor binding. Alginate maintained its cross-linking stability with the aid of other molecules, such as gelatin or calcium.[57,58] The spray-drying technique has been used previously to fabricate alginate-atenolol microparticles without aiding particles[59] and was, therefore, chosen for use in the current studies. The stability in the human body of the three individual components of the DDS as well as of their composite particles prepared without aiding particles must be evaluated. It may be necessary to introduce additional materials that stabilize the DDS in vivo and provide an EPO half-life comparable to that of conventional rhEPO (6–8 hours)[60] with a peak rhEPO presence in the cerebrospinal fluid (CSF) 2 hours after intravenous injection.[46] Whereas DDS particles have been reported to spontaneously break down, the maximum long-term stability of an EPO-polymer (EPO coated with biodegradable gelatin microspheres) was reported to be 8 weeks in an in vivo ischemic limb mouse model.[61]
An alternating magnetic field could potentially induce motion among the MNPs inside a DDS. Rapid movement of MNPs under an external high-frequency magnetic field could create heat inside a DDS via piezo-electric effects. This heat could increase the alginate degradation rate. Controlling the alginate degradation rate could potentially control the EPO release rate. In this way, an alternating magnetic field applied to the target area could control the EPO dose.
Hematopoietic complications, such as teratogenicity, increased viscosity, and thrombosis have been reported.[62] Human studies should seek to avoid these adverse events. Carbamylation has been shown to prevent EPO binding to hematopoietic EPO receptors and preclude hematopoietic activity while still providing neuro-protection in a focal ischemia rat model.[47] Moreover, CNS neuro-protection and -regeneration would require BBB penetration. EPO could be detected in the cisterna magna of rats up to 8 hours after intravenous injection and was found to be present in concentrations comparable to those of simultaneously injected mannitol.[46] Carbamylated non-hematopoietic EPO was also found in the CSF 4 to 24 hours after intravenous administration in rats.[47]
5. Conclusions
The DDS developed here, an alginate-encapsulated EPO-MNP polymer fabricated using the nanospray drying technique, was characterized by the high coating efficiency and by an in vivo size that was small enough to permit passage through lung capillaries while also being susceptible to magnetic guidance (Fig. 9). No significant neuronal cytotoxicity was observed in the form of solution or particles. This work lays a foundation for in vivo direct methods of targeting the delivery of various types of drugs.
Figure 9.
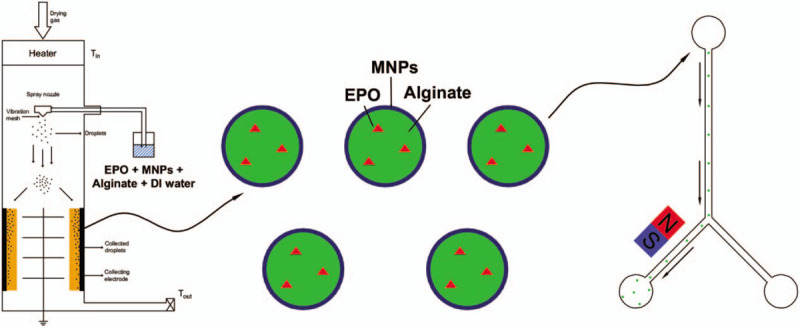
Schematic diagram of the magnetically guided targeted delivery of erythropoietin.
Acknowledgments
Authors give our special thanks for FT-IR consulting to Professor Chaenyung Cha, UNIST, Ulsan, Republic of Korea. We give our thanks to Seul Ah Mun for conducting in vitro experiment in part.
Author contributions
Conceptualization: Kyo-in Koo, Chang Ho Hwang.
Data curation: Chanh Trung Nguyen, Chung Reen Kim, Chang Ho Hwang.
Formal analysis: Chanh Trung Nguyen, Thi Huong Le, Kyo-in Koo, Chang Ho Hwang.
Funding acquisition: Chung Reen Kim, Kyo-in Koo, Chang Ho Hwang.
Investigation: Chanh Trung Nguyen, Thi Huong Le, Kyo-in Koo, Chang Ho Hwang.
Methodology: Chung Reen Kim, Kyo-in Koo, Chang Ho Hwang.
Project administration: Chanh Trung Nguyen, Thi Huong Le, Kyo-in Koo, Chang Ho Hwang.
Resources: Chung Reen Kim, Kyo-in Koo, Chang Ho Hwang.
Software: Chanh Trung Nguyen, Thi Huong Le, Chang Ho Hwang.
Supervision: Chung Reen Kim, Thi Huong Le, Kyo-in Koo, Chang Ho Hwang.
Validation: Chanh Trung Nguyen, Thi Huong Le, Kyo-in Koo, Chang Ho Hwang.
Visualization: Chung Reen Kim, Kyo-in Koo, Chang Ho Hwang.
Writing – original draft: Kyo-in Koo, Chang Ho Hwang.
Writing – review & editing: Chung Reen Kim, Kyo-in Koo, Chang Ho Hwang.
Supplementary Material
Footnotes
Abbreviations: ANOVA = analysis of variance, BBBs = blood–brain barriers, CNS = central nervous system, DDS = drug delivery system, DMEM = Dulbecco's Modified Eagle's medium, EPO = erythropoietin, FBS = fetal bovine serum, FTIR = Fourier-transform infrared, MNPs = magnetic nanoparticles, PBS = phosphate-buffered saline, PDMS = polydimethylsiloxane, rhEPO = recombinant human erythropoietin, SEM = scanning electron microscopy, TEM = transmission electron microscopy, TGA = thermal gravimetric analysis.
How to cite this article: Nguyen CT, Kim CR, Le TH, Koo Ki, Hwang CH. Magnetically guided targeted delivery of erythropoietin using magnetic nanoparticles: proof of concept. Medicine. 2020;99:19(e19972).
CTN and CRK contributed equally as the first authors.
The research project of Basic Science Research Program, through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future, Planning (NRF-2017R1D1A1B03034982, NRF-2017M3A9E2062707, and NRF-2017R1A2B4011478).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Pearse D, Jarnagin K. Abating progressive tissue injury and preserving function after CNS trauma: the role of inflammation modulatory therapies. Curr Opin Investig Drugs 2010;11:1207–10. [PubMed] [Google Scholar]
- [2].Juul S. Erythropoietin in the central nervous system, and its use to prevent hypoxic-ischemic brain damage. Acta Paediatr Suppl 2002;91:36–42. [DOI] [PubMed] [Google Scholar]
- [3].Matis GK, Birbilis TA. Erythropoietin in spinal cord injury. Eur Spine J 2009;18:314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vitellaro-Zuccarello L, Mazzetti S, Madaschi L, et al. Erythropoietin-mediated preservation of the white matter in rat spinal cord injury. Neuroscience 2007;144:865–77. [DOI] [PubMed] [Google Scholar]
- [5].Morishita E, Masuda S, Nagao M, et al. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience 1997;76:105–16. [DOI] [PubMed] [Google Scholar]
- [6].Cho YK, Kim G, Park S, et al. Erythropoietin promotes oligodendrogenesis and myelin repair following lysolecithin-induced injury in spinal cord slice culture. Biochem Biophys Res Commun 2012;417:753–9. [DOI] [PubMed] [Google Scholar]
- [7].Eid T, Brines M. Recombinant human erythropoietin for neuroprotection: what is the evidence? Clin Breast Cancer 2002;3: Suppl 3: S109–15. [DOI] [PubMed] [Google Scholar]
- [8].Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med (Maywood, NJ) 2003;228:1–4. [DOI] [PubMed] [Google Scholar]
- [9].Hong HN, Shim JH, Won YJ, et al. Therapeutic time window for the effects of erythropoietin on astrogliosis and neurite outgrowth in an in vitro model of spinal cord injury. Medicine 2018;97:e9913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dumont F, Bischoff P. Non-erythropoietic tissue-protective peptides derived from erythropoietin: WO2009094172. Expert Opin Ther Pat 2010;20:715–23. [DOI] [PubMed] [Google Scholar]
- [11].Do TD, Ul Amin F, Noh Y, et al. Guidance of magnetic nanocontainers for treating Alzheimer's disease using an electromagnetic, targeted drug-delivery actuator. J Biomed Nanotechnol 2016;12:569–74. [DOI] [PubMed] [Google Scholar]
- [12].Tietze R, Zaloga J, Unterweger H, et al. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem Biophys Res Commun 2015;468:463–70. [DOI] [PubMed] [Google Scholar]
- [13].Huq T, Salmieri S, Khan A, et al. Nanocrystalline cellulose (NCC) reinforced alginate based biodegradable nanocomposite film. Carbohydr Polym 2012;90:1757–63. [DOI] [PubMed] [Google Scholar]
- [14].Majidi S, Sehrig FZ, Farkhani SM, et al. Current methods for synthesis of magnetic nanoparticles. Artif Cells Nanomed Biotechnol 2016;44:722–34. [DOI] [PubMed] [Google Scholar]
- [15].Mascolo MC, Pei Y, Ring TA. Room temperature co-precipitation synthesis of magnetite nanoparticles in a large pH window with different bases. Materials (Basel) 2013;6:5549–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Harsha SN, Aldhubiab BE, Nair AB, et al. Nanoparticle formulation by Buchi B-90 Nano Spray Dryer for oral mucoadhesion. Drug Des Devel Ther 2015;9:273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li G, Ma R, Huang C, et al. Protective effect of erythropoietin on beta-amyloid-induced PC12 cell death through antioxidant mechanisms. Neurosci Lett 2008;442:143–7. [DOI] [PubMed] [Google Scholar]
- [18].Zhi-Kun S, Hong-Qi Y, Zhi-Quan W, et al. Erythropoietin prevents PC12 cells from beta-amyloid-induced apoptosis via PI3KAkt pathway. Transl Neurodegener 2012;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ma R, Xiong N, Huang C, et al. Erythropoietin protects PC12 cells from beta-amyloid(25-35)-induced apoptosis via PI3K/Akt signaling pathway. Neuropharmacology 2009;56:1027–34. [DOI] [PubMed] [Google Scholar]
- [20].Xicoy H, Wieringa B, Martens GJ. The SH-SY5Y cell line in Parkinson's disease research: a systematic review. Mol Neurodegener 2017;12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bordbar AK, Rastegari AA, Amiri R, et al. Characterization of modified magnetite nanoparticles for albumin immobilization. Biotechnol Res Int 2014;2014:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Saranya T, Parasuraman K, Anbarasu M, et al. XRD, FT-IR and SEM study of magnetite (Fe3O4) nanoparticles prepared by hydrothermal method. Nano Vision 2015;5:149–54. [Google Scholar]
- [23].Mahdavi M, Ahmad MB, Haron MJ, et al. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 2013;18:7533–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sompech S, Srion A, Nuntiya A. Proc Eng 2012;32:1012–8. [Google Scholar]
- [25].Khmara I, Kubovcikova M, Koneracka M, et al. Preparation and characterization of magnetic nanoparticles. Acta Phys Pol A 2018;133:704–6. [Google Scholar]
- [26].Deepa B, Abraham E, Pothan AL, et al. Biodegradable nanocomposite films based on sodium alginate and cellulose nanofibrils. Materials 2016;9: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li D, Li P, Zang J, et al. Enhanced hemostatic performance of tranexamic acid-loaded chitosan/alginate composite microparticles. J Biomed Biotechnol 2012;2012:981321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yen FS, Chen WC, Yang JM, et al. Crystallite size variations of nanosized Fe2O3 powders during γ- to α-phase transformation. Nano Lett 2002;2:245–52. [Google Scholar]
- [29].Guadarrama-Acevedo MC, Mendoza-Flores RA, Del Prado-Audelo ML, et al. Development and evaluation of alginate membranes with curcumin-loaded nanoparticles for potential wound-healing applications. Pharmaceutics 2019;11:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jelkmann W. The enigma of the metabolic fate of circulating erythropoietin (Epo) in view of the pharmacokinetics of the recombinant drugs rhEpo and NESP. Eur J Haematol 2002;69:265–74. [DOI] [PubMed] [Google Scholar]
- [31].Wu SK, Yang MT, Kang KH, et al. Targeted delivery of erythropoietin by transcranial focused ultrasound for neuroprotection against ischemia/reperfusion-induced neuronal injury: a long-term and short-term study. PLoS One 2014;9:e90107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ishii T, Asai T, Urakami T, et al. Accumulation of macromolecules in brain parenchyma in acute phase of cerebral infarction/reperfusion. Brain Res 2010;1321:164–8. [DOI] [PubMed] [Google Scholar]
- [33].Zhang W, Zhou G, Gao Y, et al. A sequential delivery system employing the synergism of EPO and NGF promotes sciatic nerve repair. Colloids Surf B Biointerfaces 2017;159:327–36. [DOI] [PubMed] [Google Scholar]
- [34].Zhang W, Gao Y, Zhou Y, et al. Localized and sustained delivery of erythropoietin from PLGA microspheres promotes functional recovery and nerve regeneration in peripheral nerve injury. BioMed Res Int 2015;2015:478103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fayed BE, Tawfik AF, Yassin AE. Novel erythropoietin-loaded nanoparticles with prolonged in vivo response. J Microencapsul 2012;29:650–6. [DOI] [PubMed] [Google Scholar]
- [36].Wang Y, Cooke MJ, Morshead CM, et al. Hydrogel delivery of erythropoietin to the brain for endogenous stem cell stimulation after stroke injury. Biomaterials 2012;33:2681–92. [DOI] [PubMed] [Google Scholar]
- [37].He N, Wang T, Jiang L, et al. Therapy for cerebral ischemic injury with erythropoietin-containing nanoparticles. J Nanosci Nanotechnol 2010;10:5320–3. [DOI] [PubMed] [Google Scholar]
- [38].Chen H, Spagnoli F, Burris M, et al. Nanoerythropoietin is 10-times more effective than regular erythropoietin in neuroprotection in a neonatal rat model of hypoxia and ischemia. Stroke 2012;43:884–7. [DOI] [PubMed] [Google Scholar]
- [39].Khan FA, Almohazey D, Alomari M, et al. Impact of nanoparticles on neuron biology: current research trends. Int J Nanomed 2018;13:2767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Uebe R, Schuler D. Magnetosome biogenesis in magnetotactic bacteria. Nat Rev Microbiol 2016;14:621–37. [DOI] [PubMed] [Google Scholar]
- [41].Shoemaker CB, Mitsock LD. Murine erythropoietin gene: cloning, expression, and human gene homology. Mol Cell Biol 1986;6:849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Townsley MI. Structure and composition of pulmonary arteries, capillaries, and veins. Compr Physiol 2012;2:675–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hafeli UO, Saatchi K, Elischer P, et al. Lung perfusion imaging with monosized biodegradable microspheres. Biomacromolecules 2010;11:561–7. [DOI] [PubMed] [Google Scholar]
- [44].Mashjoor S, Yousefzadi M, Zolgharnain H, et al. Organic and inorganic nano-Fe3O4: Alga Ulva flexuosa-based synthesis, antimicrobial effects and acute toxicity to briny water rotifer Brachionus rotundiformis. Environ Pollut (Barking, Essex: 1987) 2018;237:50–64. [DOI] [PubMed] [Google Scholar]
- [45].Erickson HP. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol Proc online 2009;11:32–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ceaglio N, Orozco G, Etcheverrigaray M, et al. High performance collection of cerebrospinal fluid in rats: evaluation of erythropoietin penetration after osmotic opening of the blood-brain barrier. J Neurosci Methods 2013;219:70–5. [DOI] [PubMed] [Google Scholar]
- [47].Leist M, Ghezzi P, Grasso G, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science 2004;305:239–42. [DOI] [PubMed] [Google Scholar]
- [48].Cedervall T, Lynch I, Lindman S, et al. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci U S A 2007;104:2050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Vonarbourg A, Passirani C, Saulnier P, et al. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials 2006;27:4356–73. [DOI] [PubMed] [Google Scholar]
- [50].Owens DE3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm 2006;307:93–102. [DOI] [PubMed] [Google Scholar]
- [51].Narhi LO, Arakawa T, Aoki KH, et al. The effect of carbohydrate on the structure and stability of erythropoietin. J Biol Chem 1991;266:23022–6. [PubMed] [Google Scholar]
- [52].Beh CY, How CW, Foo JB, et al. Development of erythropoietin receptor-targeted drug delivery system against breast cancer using tamoxifen-loaded nanostructured lipid carriers. Drug Des Devel Ther 2017;11:771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yoo JY, Won YJ, Lee JH, et al. Neuroprotective effects of erythropoietin posttreatment against kainate-induced excitotoxicity in mixed spinal cultures. J Neurosci Res 2009;87:150–63. [DOI] [PubMed] [Google Scholar]
- [54].Wu Y, Shang Y, Sun S, et al. Antioxidant effect of erythropoietin on 1-methyl-4-phenylpyridinium-induced neurotoxicity in PC12 cells. Eur J Pharmacol 2007;564:47–56. [DOI] [PubMed] [Google Scholar]
- [55].Storring PL, Tiplady RJ, Gaines Das RE, et al. Epoetin alfa and beta differ in their erythropoietin isoform compositions and biological properties. Br J Haematol 1998;100:79–89. [DOI] [PubMed] [Google Scholar]
- [56].Pinkernelle J, Calatayud P, Goya GF, et al. Magnetic nanoparticles in primary neural cell cultures are mainly taken up by microglia. BMC Neurosci 2012;13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Balakrishnan B, Joshi N, Jayakrishnan A, et al. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater 2014;10:3650–63. [DOI] [PubMed] [Google Scholar]
- [58].Esser E, Tessmar JK. Preparation of well-defined calcium cross-linked alginate films for the prevention of surgical adhesions, Journal of biomedical materials research Part B. Appl Biomater 2013;101:826–39. [DOI] [PubMed] [Google Scholar]
- [59].Ceschan NE, Bucala V, Ramirez-Rigo MV, et al. Impact of feed counterion addition and cyclone type on aerodynamic behavior of alginic-atenolol microparticles produced by spray drying. Eur J Pharm Biopharm: Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV 2016;109:72–80. [DOI] [PubMed] [Google Scholar]
- [60].Jelkmann W. Molecular biology of erythropoietin. Int Med (Tokyo, Japan) 2004;43:649–59. [DOI] [PubMed] [Google Scholar]
- [61].Li L, Okada H, Takemura G, et al. Sustained release of erythropoietin using biodegradable gelatin hydrogel microspheres persistently improves lower leg ischemia. J Am Coll Cardiol 2009;53:2378–88. [DOI] [PubMed] [Google Scholar]
- [62].Lippi G, Franchini M, Favaloro EJ. Thrombotic complications of erythropoiesis-stimulating agents. Semin Thromb Hemost 2010;36:537–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


