Abstract
Background:
In recent years, there has been an interest in whether environmental endocrine disruptors (EEDs) may contribute to the endocrine disorders in patients with polycystic ovary syndrome (PCOS). The clearance of EEDs from the human body is regulated by the glucuronidation of UDP-glucuronosyltransferases (UGT). This study aimed to analyze the relationship of UGT1A1, UGT2B7, and UGT2B15 polymorphisms with the metabolism of EEDs in patients with PCOS.
Methods:
A total of 357 Chinese women (119 PCOS cases and 238 controls) were genotyped for polymorphisms of UGT1A1G71R, UGT2B7H268Y, and UGT2B15D85Y. The plasma concentrations of EEDs were measured by the gas chromatography-mass spectrometry method. The association between UGT polymorphisms and the serum level of EEDs in patients with PCOS was analyzed.
Results:
The UGT2B7H268Y single nucleotide polymorphism was associated with an increased risk of PCOS. The homozygous polymorphism (TT) of UGT2B7H268Y showed higher bisphenol A and PAEs concentrations in serum. However, a single nucleotide polymorphism on UGT2B15D85Y expression was associated with a decreased risk of PCOS. Subjects homozygous for the T allele of UGT2B15D85Y had a significant effect on phthalates in the blood. In addition, our results also showed that the homozygous polymorphism (TT) of UGT2B7H268Y and UGT2B15D85Y was associated with the capacity of the excretion of androgen in patients with PCOS.
Conclusions:
Our study reported the novel associations between the UGT polymorphisms and EEDs concentrations in patients with PCOS, supporting the relevance of genetic differences in EEDs metabolism, which might be considered as an etiology of PCOS.
Keywords: environmental endocrine disruptors, glucuronosyltransferases, polycystic ovary syndrome, single nucleotide polymorphism
1. Introduction
Polycystic ovary syndrome (PCOS) is a heterogeneous disorder characterized by multiple endocrine disruptions, and its underlying causes, although uncertain, are likely to be both genetic and environmental.[1] Environmental endocrine disruptors (EEDs), such as bisphenol A (BPA), polybrominated diphenyl ethers (PBDEs), and phthalates (PAEs), are exogenous substances that interfere with hormone biosynthesis and metabolism and cause deviations from normal homeostatic control and reproduction.[2] The “environmental” contributors that have been considered are related to obesity and lifestyle, and indeed, there is extensive evidence that both play important roles in PCOS.[3] Some previous studies reported that women with PCOS had higher serum BPA concentration in comparison to normal healthy control.[1] The women who exposed to BPA had higher BPA level and higher luteinizing hormone ratio follicle-stimulating hormone ratio than healthy women without BPA contact, and these observations confirmed the potential role of BPA in PCOS pathophysiology.[4] Endocrine disruption by EEDs in humans may act via 2 main pathways: through hormone receptors (estrogenic and androgenic receptors and G protein–coupled estrogen receptor) activating or inhibiting gene expression[5] and by altering the synthesis or degradation of the hormones naturally present in the organisms (i.e., aryl hydrocarbon receptor).[6] Peretz et al[7] reported dose-dependent and time-dependent decreases in estradiol (E2), estrone, testosterone (T), androstenedione, and dehydroepiandrosterone synthesis after 12 hours of exposure to 100 and 10 μg/mL BPA. These doses of BPA also compromised follicle growth. Finally, the EEDs can disrupt ovarian function, androgen activity, and metabolic regulation, which contribute to the etiology of PCOS.
UDP-glucuronosyltransferase (UGT) is a major phase II enzyme of the detoxification system which catalyses the conjugation of nucleophiles with glucuronic acid from the cofactor UDP-glucuronic acid. After the glucuronidation reaction, the hydrophilicity of compounds increases, contributing to its excretion by the biliary or urinary route,[8–10] which are the most important pathways for the human body's elimination of endogenous and exogenous chemicals. It is known that the glucuronidation of UGT is a primary metabolizer for the clearance of EEDs from the human body. Similarly, the glucuronidation of UGT is also the major route for androgen inactivation and excretion.[11] Many previous studies showed that the polymorphisms of UGT might affect its functions. For instance, the polymorphism of UGT1A1G71R (G>A, rs4148323) is the most concerning for irinotecan-based regimens in non–small cell lung cancer, and is regarded as a predictor for severe adverse events. Nie et al[12] indicated that UGT1A1∗6 and UGT1A1∗28 polymorphisms reduced the activity of UGT1A1 by as much as 40% to 60%. A frequent polymorphism of the UGT2B7H268Y (C>T, rs7439366) has shown a variable functional impact with different substrates. Levesque et al[13,14] suggested that the TT genotype of UGT2B15D85Y (G>T, rs1902023) was twice as active as the GG genotype in the glucuronidation of dihydrotestosterone, which led to better protection against high androgen levels and decreased the risk of prostate cancer.[15] To our knowledge, the glucuronidation of BPA is mainly mediated by UGT2B15, with other UGTs (UGT1A1, UGT1A3, UGT1A9, UGT2B4, and UGT2B7) contributing to a lesser extent.[16] However, to date, the glucuronidation of PBDEs and PAEs is unclear.
Among clinical patients, most of them failed to provide an obvious exposure history to EEDs, with a few exceptions. Their EEDs concentrations in serum varied greatly, as they lived in similar environments. The great changes may have been influenced by individual internal factors, such as the differences in the functional activity of UGT caused by the polymorphism of genes. Taken together, we aimed to investigate the functional UGT1A1, UGT2B7, and UGT2B15 genetic polymorphisms in 357 Chinese women, and thus to assess the effects of UGT single nucleotide polymorphisms (SNPs) on EED metabolites in patients with PCOS.
2. Materials and methods
2.1. Study participants
Three hundred fifty-seven women (119 PCOS cases and 238 controls) from 2014 to 2016, aged 12 to 44 years, who had lived in Chengdu for at least 6 months, who had menses for at least 2 years, were included in the present study. Participants who had used hormones in the previous 3 months were excluded. Pregnant women were also excluded. The study was approved by the Human Ethics Committee of West China Second University Hospital. The diagnosis of PCOS was based on the Rotterdam criteria, with the association of at least 2 of the 3 following criteria: oligo-ovulation or anovulation evidenced by oligomenorrhea (8 or less menstrual cycles in the preceding year) or the serum progesterone level in the luteal phase (<5 ng/mL); clinical and/or biochemical signs of hyperandrogenism (HA); and polycystic ovaries.[17] Congenital adrenal hyperplasia, androgen secreting tumors, Cushing syndrome, thyroid disease, Gilbert syndrome, Crigler-Najjar syndrome, and prolactinoma were excluded. The 119 women with PCOS diagnosed according to Rotterdam recommendations were divided into 4 different phenotypes: group A, defined as having oligo-ovulation (O), HA, and polycystic ovary (PCO), was the most common phenotype seen in 64.5%; group B, defined as having O and HA, was seen in 7.5% of the phenotype; group C, defined as having HA and PCO, was seen in 14.3% and group D, defined as having O and PCO, was seen in 13.7%.
Controls matched for age were randomly chosen from the non-PCOS participants of case-control investigation with the ratio of 1:2. The authors had access to information that could identify individual participants during or after data collection.
2.2. Detection of EEDs
Fasting blood samples (15 mL) were collected from all subjects. All samples were taken during the early follicular phase of the menstrual cycle (between the third and seventh days) or after at least 3 months of amenorrhea. Blood samples were centrifuged immediately. The plasma was separated and stored at −80°C until assay. BPA, PBDEs, and PAEs were measured by the gas chromatography-mass spectrometry method, and the following instruments were used for the experiments: Trace Dynamax spectrum of quadrupole gas chromatograph-mass spectrometer (Thermo Fisher Corp), nitrogen blowing instrument (ANPEL Laboratory Technologies, Shanghai,China), Laborota 400 efficient rotary evaporator (Heidolph Corp, Germany), and SB 3200DT ultrasonic cleaner. Hexane, dichloride methane, and methanol of high-performance liquid chromatography grade were purchased from BCR Company.
2.3. DNA extraction and genotyping
Genomic DNA was extracted from peripheral blood using the phenol/chloroform method. The polymorphisms of UGT 1A1G71R (rs4148323), 2B7H268Y (rs7439366), and 2B15D85Y(rs1902023) were detected by polymerase chain reaction (PCR), and the direct sequencing PCR primers were synthesized by Tsingke (Chengdu, China). The following PCR primers and conditions were used UGT1A1G71R, 5’-AATGGATCCTGAGGTTCTGG-3’ and 5’-ATGAGCTCCTTGTTGTGCAG-3’ at 60°C annealing; UGT2B7H268Y, 5’-ATTCCTGTC AGGAAGACCCA -3’ and 5’-GTGTAAGTCAAACACTCTGAA -3’ at 58°C annealing; UGT2B15D85Y, 5’-ACCAGGATGTCTCTGAAATG -3’ and 5’-TGGTCCCA CTTCTTCAGATC -3’ at 58°C annealing. Direct sequencing was performed using a 3730xl DNA Analyzer and Sequencing Analysis 5.2 software.
2.4. Statistical analysis
We investigated associations between log10-transformed serum EEDs and UGT SNPs in PCOS patients. All the statistical analyses were completed using SPSS 18.0 software. Data were expressed as the mean (M) ± standard deviation or geometric mean ± geometric standard deviation. The χ2 test and odds ratio (OR) values were used to analyze the distribution of UGT genotypes in all subjects. One-way analysis of variance was used to investigate the association between serum concentrations of EEDs and androgen in different genotypes, an independent t test was used to investigate serum level of EEDs, and a value of P < .05 was considered statistically significant.
3. Results
3.1. The clinical characteristics of the study participants
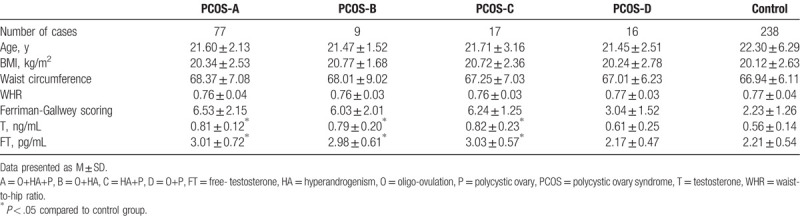
One hundred nineteen PCOS cases participated in the present study. The general baseline levels between the case and control groups were comparable (Table 1).
Table 1.
Background factors of the participants.
3.2. The genotype frequencies of UGT-related polymorphisms
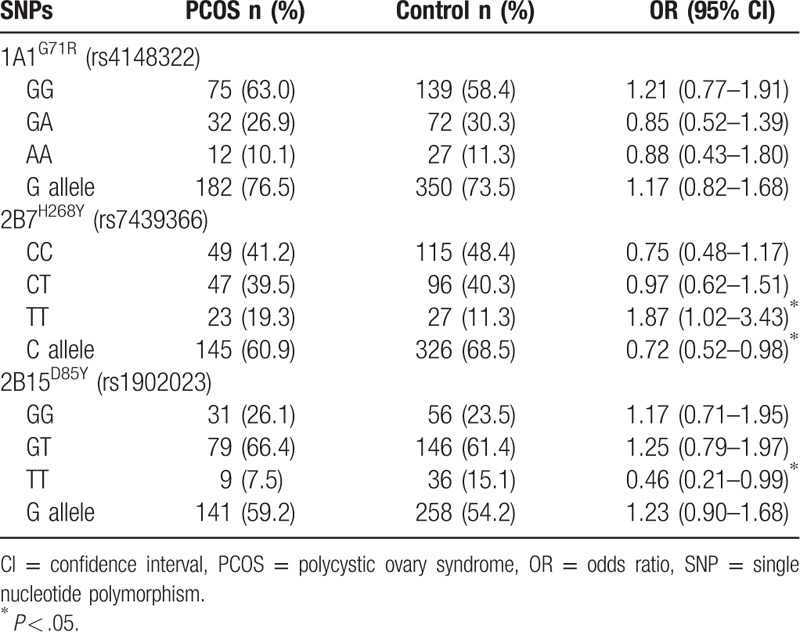
The allele and genotype frequencies of the genotyping analysis are shown in Table 2. All the genotype frequencies were in Hardy-Weinberg equilibrium. The frequency of the G allele at the 1A1G71R site, C allele at the 2B7H268Y site, and major allele G at the 2B15D85Y site was 74.5%, 70.5%, and 61.8%, respectively, which was consistent with other reports.[18–20] Comparing the genotype distribution, the results showed that the TT genotype of UGT2B7H268Y was associated with an increased risk of PCOS (OR = 1.87, 95% confidence interval = 1.02–3.43). In contrast, the TT genotype of UGT2B15D85Y was associated with a decreased risk of PCOS (OR = 0.46, 95% confidence interval = 0.21–0.98). The distribution of UGT1A1G71R showed no significant difference between the PCOS and control group (Table 2).
Table 2.
Distribution of UGT1A1, UGT2B7, and UGT2B15 genotypes.
3.3. Detection and distribution of EEDs and androgen
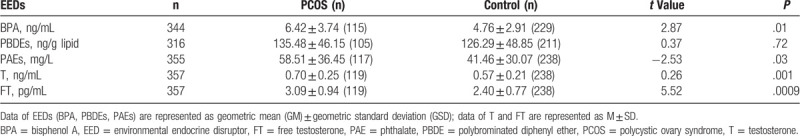
Due to the limit of detection varying by the available sample volume for each sample, some samples were not detected. We summarized the detected ratio and the range of 3 kinds of EEDs (Table 3). Our results indicated that patients with PCOS had a higher BPA concentration (6.42 ± 3.74 ng/mL) when compared to the control group (4.76 ± 2.91 ng/mL, P = .01). Consistently, PAE level was also much higher in patients with PCOS (58.51 ± 36.45 mg/L) compared to the controls (41.46 ± 30.07 mg/L, P = .03) (Table 4). The PBDEs level in PCOS group was comparable with control group.
Table 3.
Detection and distribution of environmental endocrine disruptors.
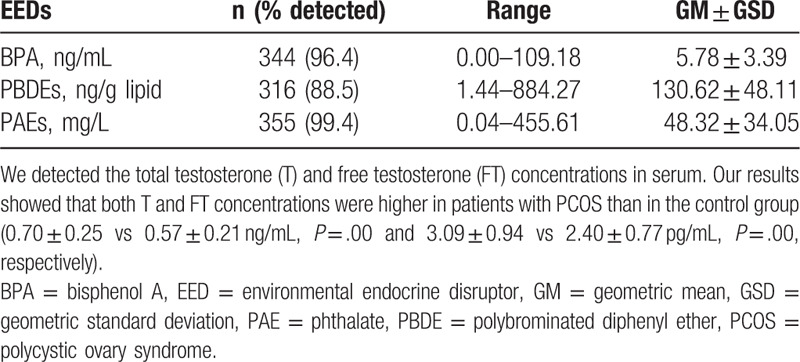
Table 4.
Distribution of environmental endocrine disruptors and androgens in polycystic ovary syndrome and control groups.
3.4. Effects of the UGT1A1 polymorphism
In this work, the distribution of UGT1A1G71R in patients with PCOS was similar to that in the control group (Table 2). Furthermore, we did not find that the polymorphism of UGT1A1G71R was associated with either EEDs (Fig. 1) or androgen concentration (Fig. 2) in serum.
Figure 1.
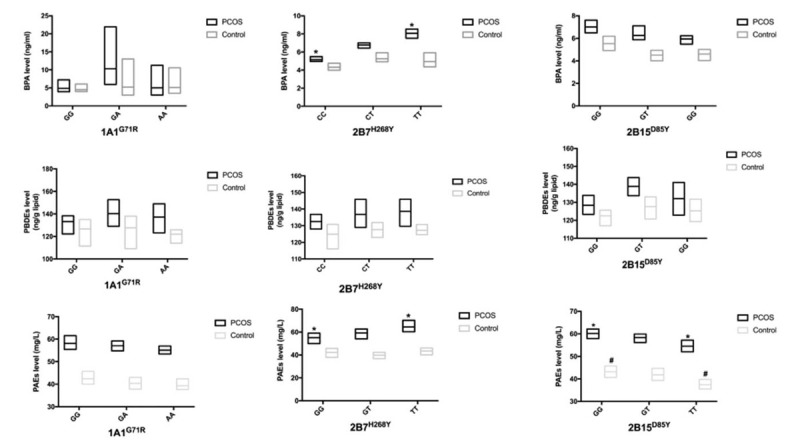
The association between glucuronosyltransferase (UGT) polymorphisms and environmental endocrine disruptors (EEDs) levels. Hyperandrogenemia (HA). ∗, #P < .05 between the 2 groups, respectively. Data are represented as geometric mean (GM) ± geometric standard deviation (GSD). BPA = bisphenol A, PAE = phthalate, PBDE = polybrominated diphenyl ether, PCOS = polycystic ovary syndrome.
Figure 2.
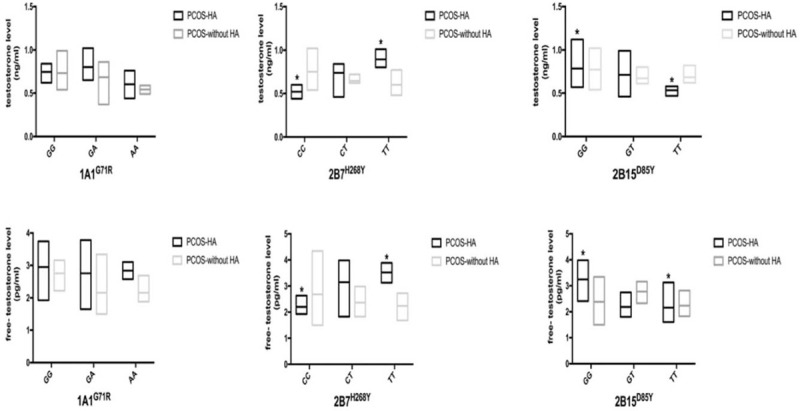
The association between glucuronosyltransferase (UGT) single nucleotide polymorphisms (SNPs) and T, FT levels. Testosterone (T), free testosterone (FT). Data are represented as M ± SD, ∗P < .05. HA = hyperandrogenemia, PCOS = polycystic ovary syndrome.
3.5. Effects of the UGT2B7 polymorphism
A previous study showed that the TT genotype was associated with decreased glucuronidation activity.[21] In the present study, the results suggested that the UGT2B7H268Y polymorphism had effects on the serum levels of EEDs and androgen. Patients with PCOS with the TT genotype had a higher BPA concentration (8.06 ± 2.01 ng/mL) when compared to the wild type (HH) (5.15 ± 2.91 ng/mL, P = .01) (Fig. 1). Moreover, in PCOS group, the PAEs concentration was also significantly higher in the TT group (64.56 ± 31.79 mg/L) when compared to the CC group (55.02 ± 33.25 mg/L, P = .01). There was no obvious association between the UGT2B7H268Y SNP and PBDEs concentration.
In addition, the TT mutation in PCOS patients with hyperandrogenemia was related to the higher testosterone (0.91 ± 0.12 ng/mL) and free testosterone concentrations (3.47 ± 0.32 pg/mL) compared to the CC individuals (0.59 ± 0.20 ng/mL, P = .03 and 2.58 ± 0.61 pg/mL, P < .05, respectively) (Fig. 2).
3.6. Effects of UGT2B15 polymorphism
The UGT2B15D85Y polymorphism had an impact on the PAEs level. Both PCOS cases and controls, the TT genotype was significantly associated with a lower PAEs concentration (54.51 ± 35.93, 37.44 ± 28.57 mg/L) compared to the GG group (60.14 ± 34.72 mg/L, P = .01; 43.16 ± 33.73 mg/L, P = .02, respectively). Serum levels of BPA and PBDEs were not associated with the UGT2B15D85Y polymorphism (Fig. 1). In addition, patients with PCOS with hyperandrogenemia also had a tendency toward lower testosterone (0.54 ± 0.18 ng/mL) and free testosterone (2.11 ± 0.60 pg/mL) levels in the TT group compared to GG group (0.75 ± 0.15 ng/mL, P < .05; 3.31 ± 0.72 pg/mL, P < .05, respectively) (Fig. 2).
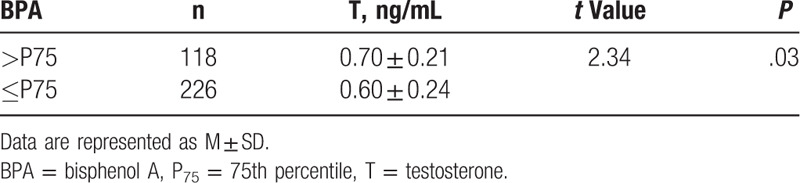
3.7. Positive relationship between BPA and T serum concentration
To date, there is not a unified reference to divide BPA into high or low levels in the human body.[4,22–24] In this work, we used the 75th percentile (P75) value to divide the subjects into either a hyper-BPA (>P75) or a hypo-BPA (≤P75) group. Our results suggested that the subjects in the hyper-BPA (>P75) group also had higher T concentration (t = 2.34, P = .03) (Table 5).
Table 5.
The relationship between bisphenol A and T serum concentration.
4. Discussion
To our knowledge, this is the first report in which the altered glucuronidation capability of EEDs has been attributed to the UGT SNPs in patients with PCOS. The frequencies of polymorphisms of UGT1A1G71R, UGT2B7H268Y, and UGT2B15D85Y from 357 subjects here were consistent with other reports in Chinese or Asian people. In the present study, the results indicated that the TT genotype of UGT2B7H268Y was associated with an increased risk of PCOS, through a decreased clearance of EEDs (BPA, PAEs) and androgen (T, FT) in patients with PCOS. In addition, the homozygous (TT) mutation of UGT2B15D85Y was associated with a decreased risk of PCOS, via promoting the removal of PAEs and androgen (T, FT) in serum. These results suggested that these SNPs of UGT might affect its functions, presumably by decreasing/increasing the efficiency of EEDs clearance in patients with PCOS.
It is known that the glucuronidation of UGT is a primary metabolizer for the clearance of EEDs from the human body. BPA undergoes hepatic first-pass metabolism to an extent of approximately 90% and is converted to phase II metabolites, which are excreted in the urine.[25] The glucuronidation of BPA is mainly mediated by UGT2B15, with other UGTs (UGT1A1, UGT1A3, UGT1A9, UGT2B4, and UGT2B7) contributing to a lesser extent.[16] Previous studies reported that a mutation in UGT2B7H268Y might significantly decrease the glucuronidation activity of the enzyme.[21,26] Consistently, our results showed that the TT mutation of UGT2B7H286Y induced a decreased clearance of BPA, resulting in a higher level of serum BPA in PCOS. Previous study suggested a positive relationship between BPA level and LH: FSH ratio in seller women, which confirmed the potential effects of BPA in PCOS etiology.[4] Many studies have reported the effects of UGT2B15 SNPs in metabolism; however, the results are inconsistent. Hanioka et al[27] reported that D85Y substitution in UGT2B15 decreased enzymatic function in vitro, which was closely associated with variations in the metabolism and toxicity of BPA. Levesque et al,[13] however, found that the TT genotype of UGT2B15D85Y increased the glucuronidation activity of the enzyme in HK293 cells, compared to the GG genotype. In this study, our results suggested that the TT genotype of UGT2B15D85Y was more active than the GG genotype in the glucuronidation of PAEs. The discrepant results may in part account for the more complicated metabolic pathway in vivo, and the different approaches to extracting and measuring EEDs in human biospecimens may be because the sensitivities, specificities, and reliabilities of analytical methods vary across laboratories.[28] It was reported that the toxic effects of PBDEs were of concern due to hydroxylated PBDEs (OH-PBDEs).[29] Our study examined the association between PBDEs, not hydroxylated PBDEs, with UGT SNPs, and there was no association between UGT polymorphisms and the serum level of PBDEs. UGT is a superfamily, and our results may imply that PBDEs are not the direct substrate for these 3 kinds of UGTs.
The hormonal anomalies characteristic of PCOS include androgen excess and insulin resistance. It is now well established that UGT2B7, UGT2B15, and UGT2B17 are the 3 major enzymes responsible for the glucuronidation of all androgen and their metabolites in humans.[30] Our results suggested that patients with PCOS had higher T and FT concentrations than those in the control group, which could be explained by the following 2 potential causes: firstly, the TT mutation of UGT2B7H268Y decreased the clearance of androgen in PCOS; secondly, although the TT mutation of UGT2B15D85Y promoted the excretion of androgen, the TT mutation frequency was much lower in patients with PCOS. In addition, Takeuchi et al[31] found that androgen level was correlated with BPA level in women. The relationships between androgen and BPA are unlikely to be simple, causal one. One possibility is that by binding to sex hormone–binding globulin, BPA displaces a proportion of the bound androgen, leading to higher free levels of androgen.[32] Another possibility is that BPA interferes with androgen catabolism. Indeed, in rat liver, BPA administration reduces the levels of enzymes needed for testosterone hydroxylation.[33] In this work, the results showed that the T concentration was also higher in the hyper-BPA (>P75) group (0.70 ± 0.21 ng/mL) compared to the hypo-BPA group (0.60 ± 0.24 ng/mL). This result showed that the clearance of androgen is negatively related concomitantly with the elevation of BPA level in serum. This result also implied a possible competitive binding between BPA, T, and UGT, which might be the other potential cause of androgen excess in patients with PCOS. Therefore, the clearance of androgen might be associated with UGT2B7 SNPs and BPA level in patients with PCOS, and whichever plays the dominant role requires further study.
In our study, the UGT1A1G71R SNP was not associated with EED metabolites in patients with PCOS, which may partly be due to the small sample size. In previous studies, UGT1A1 was mostly associated with drug glucuronidation,[34,35] and Gramec Skledar et al[9] reported that UGT1A1 influences the glucuronidation of bisphenol S. This may indicate that UGT1A1G71R is not the main enzyme for the removal of these 3 kinds of EEDs (BPA, PBDEs, PAEs).
5. Conclusions
In this case-control study of Chinese patients with PCOS, our results indicated that they were likely to be loaded with higher BPA and PAEs concentrations. It seems that the clearance of BPA and PAEs was associated with UGT2B7H268Y and UGT2B15D85Y polymorphisms. The excretion of androgen was associated with the polymorphisms of UGT2B7H268Y and UGT2B15D85Y, and the BPA level in serum. Taken together, our data suggested that the clearance of EEDs from the human body, which is related to the homozygous variants of UGT2B7H268Y and UGT2B15D85Y, might be considered as an etiology of PCOS.
Author contributions
Data curation: Yunyao Luo.
Methodology: Ying Nie.
Resources: Yunyao Luo.
Software: Lu Tang.
Supervision: Liangzhi Xu.
Writing – original draft: Yunyao Luo.
Writing – review and editing: Charles C Xu.
Footnotes
Abbreviations: BPA = bisphenol A, EED = environmental endocrine disruptor, PAE = phthalate, PBDE = polybrominated diphenyl ether, PCOS = polycystic ovary syndrome, SNP = single nucleotide polymorphism, UGT = Uridine diphosphate-glucuronosyltransferase.
How to cite this article: Luo Y, Nie Y, Tang L, Xu CC, Xu L. The correlation between UDP-glucuronosyltransferase polymorphisms and environmental endocrine disruptors (EEDs) levels in polycystic ovary syndrome patients. Medicine. 2020;99:11(e19444).
This work was supported by Scientific Research Projects of The National Natural Science Fund (41473097) and Science and Technology Project of Chengdu City (2015-HM01-00006-SF).
The authors have no conflicts of interest to disclose.
References
- [1].Barrett ES, Sobolewski M. Polycystic ovary syndrome: do endocrine-disrupting chemicals play a role? Semin Reprod Med 2014;32:166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yang H, Huang Y, Liu J, et al. Binding modes of environmental endocrine disruptors to human serum albumin: insights from STD-NMR, ITC, spectroscopic and molecular docking studies. Sci Rep 2017;7:11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Panidis D, Tziomalos K, Papadakis E, et al. Lifestyle intervention and anti-obesity therapies in the polycystic ovary syndrome: impact on metabolism and fertility. Endocrine 2013;44:583–90. [DOI] [PubMed] [Google Scholar]
- [4].Vahedi M, Saeedi A, Poorbaghi SL, et al. Metabolic and endocrine effects of bisphenol A exposure in market seller women with polycystic ovary syndrome. Environ Sci Pollut Res Int 2016;23:23546–50. [DOI] [PubMed] [Google Scholar]
- [5].Thomas P, Alyea R, Pang Y, et al. Conserved estrogen binding and signaling functions of the G protein-coupled estrogen receptor 1 (GPER) in mammals and fish. Steroids 2010;75:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sanderson JT. The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals. Toxicol Sci 2006;94:3–21. [DOI] [PubMed] [Google Scholar]
- [7].Peretz J, Gupta RK, Singh J, et al. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci 2011;119:209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bock KW. The UDP-glycosyltransferase (UGT) superfamily expressed in humans, insects and plants: Animal-plant arms-race and co-evolution. Biochem Pharmacol 2016;99:11–7. [DOI] [PubMed] [Google Scholar]
- [9].Gramec Skledar D, Troberg J, Lavdas J, et al. Differences in the glucuronidation of bisphenols F and S between two homologous human UGT enzymes, 1A9 and 1A10. Xenobiotica 2015;45:511–9. [DOI] [PubMed] [Google Scholar]
- [10].Dong G, Pang LF, Zhou HHJCJoND. Progress in the pharmacogenomics of UDP-glucuronosyltransferase. J Zhejiang Univ Sci B 2011;20:1188–93. [Google Scholar]
- [11].Barbier O, Belanger A. Inactivation of androgen by UDP-glucuronosyltransferases in the human prostate. Best Pract Res Clin Endocrinol Metab 2008;22:259–70. [DOI] [PubMed] [Google Scholar]
- [12].Nie YL, He H, Li JF, et al. Hepatic expression of transcription factors affecting developmental regulation of UGT1A1 in the Han Chinese population. Eur J Clin Pharmacol 2017;73:29–37. [DOI] [PubMed] [Google Scholar]
- [13].Levesque E, Beaulieu M, Green MD, et al. Isolation and characterization of UGT2B15(Y85): a UDP-glucuronosyltransferase encoded by a polymorphic gene. Pharmacogenetics 1997;7:317–25. [DOI] [PubMed] [Google Scholar]
- [14].MacLeod SL, Nowell S, Plaxco J, et al. An allele-specific polymerase chain reaction method for the determination of the D85Y polymorphism in the human UDP-glucuronosyltransferase 2B15 gene in a case-control study of prostate cancer. Ann Surg Oncol 2000;7:777–82. [DOI] [PubMed] [Google Scholar]
- [15].He X, Hesse LM, Hazarika S, et al. Evidence for oxazepam as an in vivo probe of UGT2B15: oxazepam clearance is reduced by UGT2B15 D85Y polymorphism but unaffected by UGT2B17 deletion. Br J Clin Pharmacol 2009;68:721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hanioka N, Naito T, Narimatsu S. Human UDP-glucuronosyltransferase isoforms involved in bisphenol A glucuronidation. Chemosphere 2008;74:33–6. [DOI] [PubMed] [Google Scholar]
- [17].Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25. [DOI] [PubMed] [Google Scholar]
- [18].Lampe JW, Bigler J, Bush AC, et al. Prevalence of polymorphisms in the human UDP-glucuronosyltransferase 2B family: UGT2B4(D458E), UGT2B7(H268Y), and UGT2B15(D85Y). Cancer Epidemiol Biomarkers Prev 2000;9:329–33. [PubMed] [Google Scholar]
- [19].Lin M, Liu J, Zhou H, et al. Effects of UDP-glucuronosyltransferase (UGT) polymorphisms on the pharmacokinetics of febuxostat in healthy Chinese volunteers. Drug Metab Pharmacokinet 2017;32:77–84. [DOI] [PubMed] [Google Scholar]
- [20].Wang T, Hsiong CH, Ho HT, et al. Genetic polymorphisms of metabolic enzymes and the pharmacokinetics of indapamide in Taiwanese subjects. AAPS J 2014;16:206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang H, Yuan L, Zeng S. Characterizing the effect of UDP-glucuronosyltransferase (UGT) 2B7 and UGT1A9 genetic polymorphisms on enantioselective glucuronidation of flurbiprofen. Biochem Pharmacol 2011;82:1757–63. [DOI] [PubMed] [Google Scholar]
- [22].Akin L, Kendirci M, Narin F, et al. The endocrine disruptor bisphenol A may play a role in the aetiopathogenesis of polycystic ovary syndrome in adolescent girls. Acta Paediatr 2015;104:e171–7. [DOI] [PubMed] [Google Scholar]
- [23].Wu XM, Bennett DH, Moran RE, et al. Polybrominated diphenyl ether serum concentrations in a Californian population of children, their parents, and older adults: an exposure assessment study. Environ Health 2015;14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Partosch F, Mielke H, Gundert-Remy U. Functional UDP-glucuronyltransferase 2B15 polymorphism and bisphenol A concentrations in blood: results from physiologically based kinetic modelling. Arch Toxicol 2013;87:1257–64. [DOI] [PubMed] [Google Scholar]
- [25].Mielke H, Gundert-Remy U. Physiologically based toxicokinetic modelling as a tool to support risk assessment: three case studies. J Toxicol 2012;2012:359471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Swanson C, Lorentzon M, Vandenput L, et al. Sex steroid levels and cortical bone size in young men are associated with a uridine diphosphate glucuronosyltransferase 2B7 polymorphism (H268Y). J Clin Endocrinol Metab 2007;92:3697–704. [DOI] [PubMed] [Google Scholar]
- [27].Hanioka N, Oka H, Nagaoka K, et al. Effect of UDP-glucuronosyltransferase 2B15 polymorphism on bisphenol A glucuronidation. Arch Toxicol 2011;85:1373–81. [DOI] [PubMed] [Google Scholar]
- [28].Vandenberg LN, Gerona RR, Kannan K, et al. A round robin approach to the analysis of bisphenol A (BPA) in human blood samples. Environ Health 2014;13:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lai Y, Lu M, Lin S, et al. Glucuronidation of hydroxylated polybrominated diphenyl ethers and their modulation of estrogen UDP-glucuronosyltransferases. Chemosphere 2012;86:727–34. [DOI] [PubMed] [Google Scholar]
- [30].Belanger A, Pelletier G, Labrie F, et al. Inactivation of androgen by UDP-glucuronosyltransferase enzymes in humans. Trends Endocrinol Metab 2003;14:473–9. [DOI] [PubMed] [Google Scholar]
- [31].Takeuchi T, Tsutsumi O, Ikezuki Y, et al. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr J 2004;51:165–9. [DOI] [PubMed] [Google Scholar]
- [32].Dechaud H, Ravard C, Claustrat F, et al. Xenoestrogen interaction with human sex hormone-binding globulin (hSHBG). Steroids 1999;64:328–34. [DOI] [PubMed] [Google Scholar]
- [33].Hanioka N, Jinno H, Nishimura T, et al. Suppression of male-specific cytochrome P450 isoforms by bisphenol A in rat liver. Arch Toxicol 1998;72:387–94. [DOI] [PubMed] [Google Scholar]
- [34].Lankisch TO, Moebius U, Wehmeier M, et al. Gilbert's disease and atazanavir: from phenotype to UDP-glucuronosyltransferase haplotype. Hepatology 2006;44:1324–32. [DOI] [PubMed] [Google Scholar]
- [35].Lankisch TO, Schulz C, Zwingers T, et al. Gilbert's Syndrome and irinotecan toxicity: combination with UDP-glucuronosyltransferase 1A7 variants increases risk. Cancer Epidemiol Biomarkers Prev 2008;17:695–701. [DOI] [PubMed] [Google Scholar]


