Supplemental Digital Content is available in the text
Keywords: breast cancer, breast cancer survival, elderly women, surgery
Abstract
Elderly women with early-stage, nonmetastatic breast cancer do not always receive recommendations for definitive surgical treatment. The reasons vary and include patient and provider-related reasons.
We queried the surveillance, epidemiology, and end results database from 2010 to 2013 for women age 60 and older with stage I/II/III invasive breast cancer for whom local treatment was known. We divided the patients into 3 groups: patients for whom surgery was performed; patients for whom surgery was recommended but not performed; patients for whom surgery was not recommended and not performed. We used Kaplan–Meier method to generate OS curves and the Cox proportional hazard test to compare survival outcomes.
A total of 119,404 patients were eligible for study with a median age between 70 and 74 years old. Compared with patients who received breast surgery, patients who did not receive surgery had a worse overall survival (OS) (hazard ratio [HR], 7.39; 95% confidence interval [CI], 6.98–7.83, P < .001). Patients who were recommended but ultimately did not undergo surgery had better OS than those who were recommended against surgery (adjusted HR, 0.60; 95% CI, 0.53–0.69). However, their survival was significantly inferior to patients who underwent surgery (adjusted HR, 2.81; 95% CI 2.48–3.19). Similar results were found regardless of age, tumor stage, estrogen receptor, or human epidermal growth factor receptor 2 status and were recapitulated in analyses of cancer-specific survival.
Upfront definitive breast surgery should be performed in medically-fit elderly patients with early-stage, nonmetastatic breast cancer given significant survival benefit.
1. Introduction
The United States (US) population is aging. The number of Americans aged 65 years or older is estimated to reach 88.5 million by 2050, more than doubling the number of 40.2 million from 2010.[1] The aging of the population is coupled with a dramatic increase in cancer incidence with an approximate 67% increase for older adults.[2] Breast cancer is the most common cancer among women, and the second leading cause of cancer-related death after lung cancer in the US.[3] Although national guidelines for cancer treatment do not differentiate treatment strategies based on patient age, elderly patients often receive less aggressive treatment modalities. Reasons include significant medical comorbidities, lack of social support, patient preference and potential treatment-related complications. A few small studies found that when age alone was used to determine the type of treatment, this resulted in suboptimal outcomes in the elderly breast cancer patients.[4,5]
Definitive surgery (lumpectomy or mastectomy) with or without radiation is the cornerstone of curative treatment for localized breast cancer. However, a significant proportion of elderly patients (especially >70 years) are denied surgery due to concerns over operative mortality and other surgical complications. We utilized the surveillance, epidemiology, and end results (SEER) database to explore the impact of surgery or lack thereof on elderly patients with early-stage, localized breast cancer in the US.
2. Methods
2.1. Data source
The SEER database is an authoritative source of national cancer incidence and survival. It collects patient-level data from 18 cancer registries across the US and captures 28% of the US population. For this analysis, we used the SEER 18 dataset and identified breast epithelial cancer using The International Classification of Diseases for Oncology, 3rd revision codes. Variables collected included tumor stage, estrogen receptor/progesterone receptor (ER/PR) status, human epidermal growth factor receptor 2 (HER2) status, surgical or nonsurgical treatments, and individual characteristics such as age, race, and marital status. Institutional review board approval was waived for this study because SEER database is a public anonymized database.
2.2. Study population
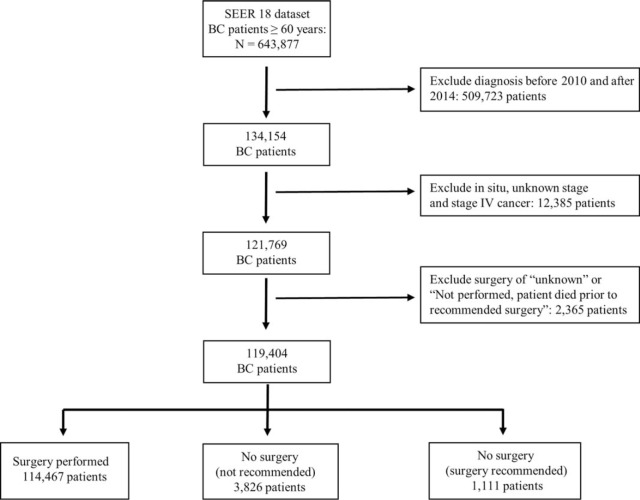
Figure 1 showed the method of patient selection. We included female patients age 60 and older who at the time of diagnosis had American Joint Committee on Cancer stages I, II, or III invasive ductal or invasive lobular breast carcinoma.[6] All other cancer histologies were excluded. Although SEER database includes data dating back to the 1970s, we included patients diagnosed between 2010 and 2013 to provide contemporary treatment strategies and adequate follow-up time. The coding of surgery for primary site by SEER can be divided into 5 categories:
Figure 1.
Flow chart of patient selection.
-
(1)
surgery was performed;
-
(2)
surgery was recommended but was not performed;
-
(3)
surgery was not recommended and was not performed;
-
(4)
it is unknown if surgery was performed; and
-
(5)
surgery was not performed, and patient died before recommended surgery.
We excluded patients in category (4) and (5) because of the uncertain relationship between surgical decision and clinical outcomes.
2.3. Statistical analysis
The primary outcome evaluated was overall survival (OS) and cancer-specific survival (CSS). Univariable Cox regression analysis was performed to calculate crude hazard ratio (HR) and 95% confidence interval (CI) for death risk, and screen for confounding factors. Multivariable Cox regression was used to assess the relation of pathological factors and treatment methods on survival, while controlling confounding factors, including age, ethnicity, tumor stage, tumor grade, ER/PR, and HER2 status as well as marital status. Missing values were coded separately for analysis purpose. Kaplan-Meier curve was used for cumulative probabilities. A Chi-square test was used to determine differences in frequency of distribution. Statistical analysis was performed using SAS 9.1 software (SAS Inc, Chicago, IL). A P-value of .05 or less was considered statistically significant.
3. Results
3.1. Patient characteristics
Clinical and pathological characteristics are shown in Table 1. The SEER database reports patient age in 5-year intervals (eg, 60–64 years). A total of 119,404 patients were eligible for final analysis with a median age between 70 and 74 years old. The majority of patients were Caucasian (83.2%), followed by African American (9.1%) and other ethnicities (7.7%). 71,638 patients were diagnosed at stage I; 37,524, at stage II; and 10,245, at stage III. Among the included patients, 85.2% were ER/PR +, and 12.4% were HER2 +. Surgery was performed in 95.9% of women. There was a trend to withhold surgery in the more advanced age population. Only 0.5% patients of 60 to 69 years old did not have recommended surgery, while the number increased to 0.7% in patients of 70 to 79 years old, 1.5% in patients of 80 to 84 years old, and 3.8% in patients of 85+ years old (Table 1).
Table 1.
Patient characteristics.
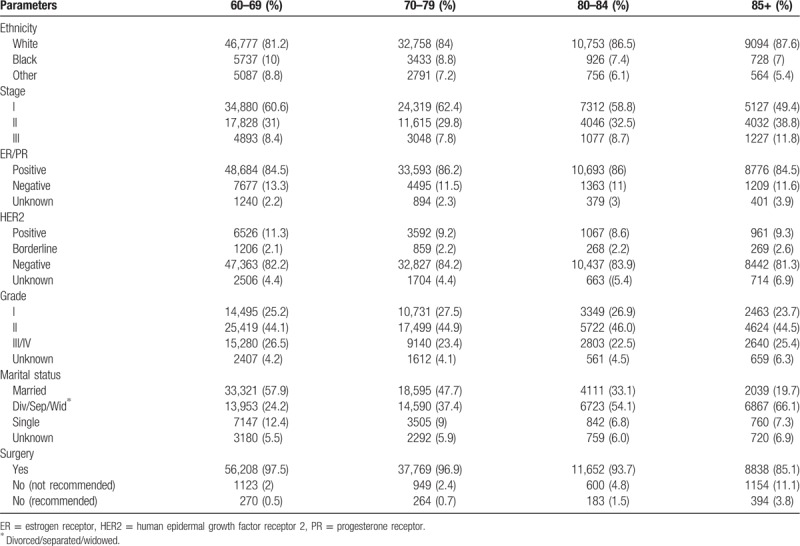
3.2. Survival outcomes and clinicopathological features
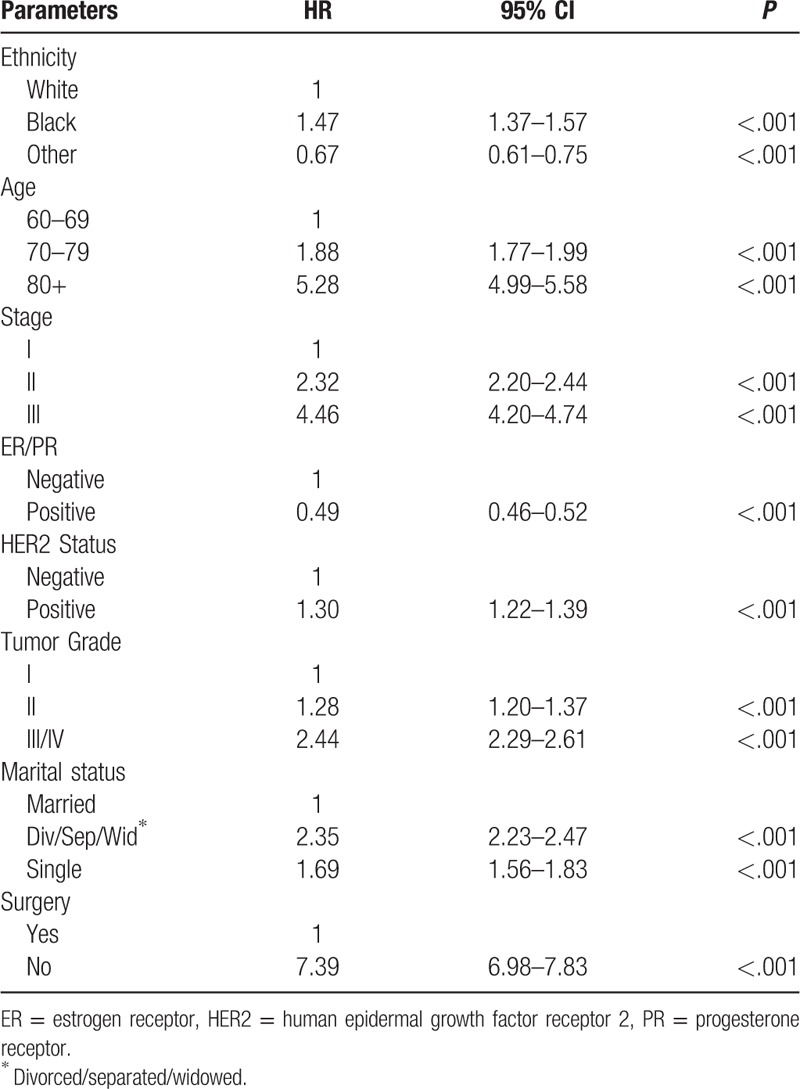
The associations between OS and clinicopathological characteristics were examined by univariate analyses to identify confounding factors. All the parameters shown in Table 2 (ethnicity, age, stage, ER/PR status, HER2 status, tumor grade, marital status, and surgery) seemed to have a significant impact on survival outcomes. Compared with Caucasians, African Americans had a higher risk of death, while ethnicities other than Caucasians and African Americans had a lower risk of death. Older age, advanced stage, negative ER/PR status, positive HER2 status, high tumor grade, and “nonmarried” status were associated with a worse OS. For all parameters, surgery was the single most important factor significantly associated with OS, with a greater than 7-fold increase in risk of death in patients who did not undergo surgery (HR, 7.39; 95% CI, 6.98–7.83; P < .001). Similar results were obtained in the analyses of CSS (Supplementary Table 1).
Table 2.
Association of clinicopathological factors with OS.
3.3. Factors associated with surgical decision
Since multiple factors may influence the decision to perform surgery, we decided to divide patients into 3 groups to compare survival outcomes. Group 1: surgery was performed; Group 2: surgery was recommended but was not performed; and Group 3: surgery was not recommended and was not performed. We then investigated how the clinicopathological factors impacted surgical decisions, particularly in patients of Group 2 and 3. As shown in Table 3, ethnicity, tumor stage, HER2 status, tumor grade, and marital status all showed association with surgical decision, but the ER/PR status did not. Patients of African American origin, advanced stage or tumor grade, positive HER2, and marital status of divorce/widowed/single were more likely to be found in Group 2 and 3 (Fig. 2).
Table 3.
Factors associated with surgical decision.
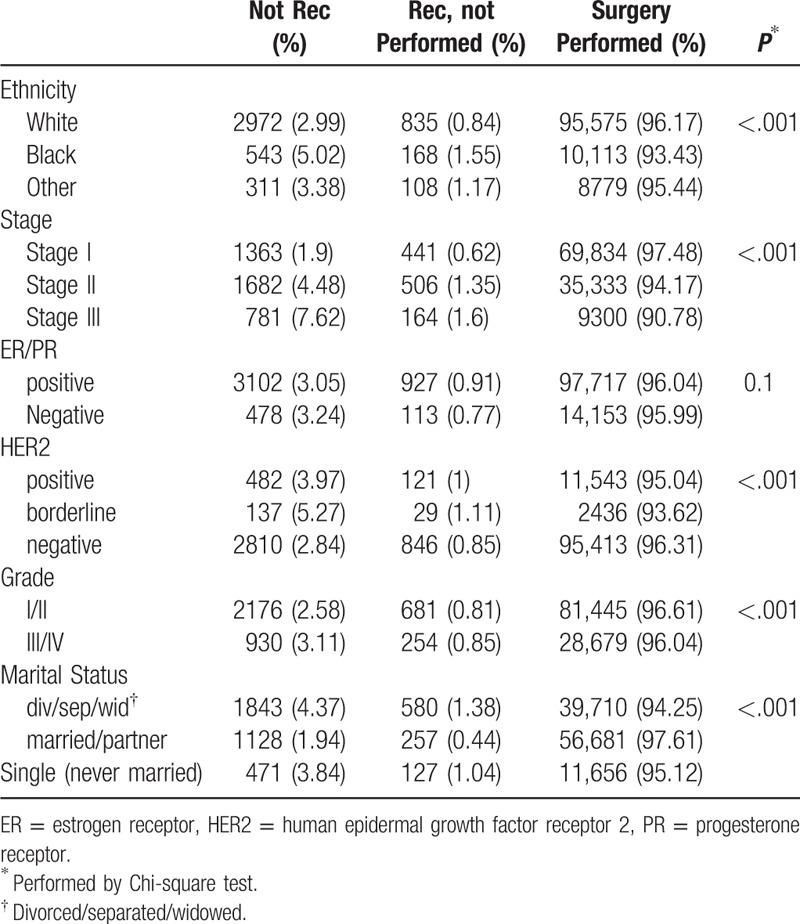
Figure 2.
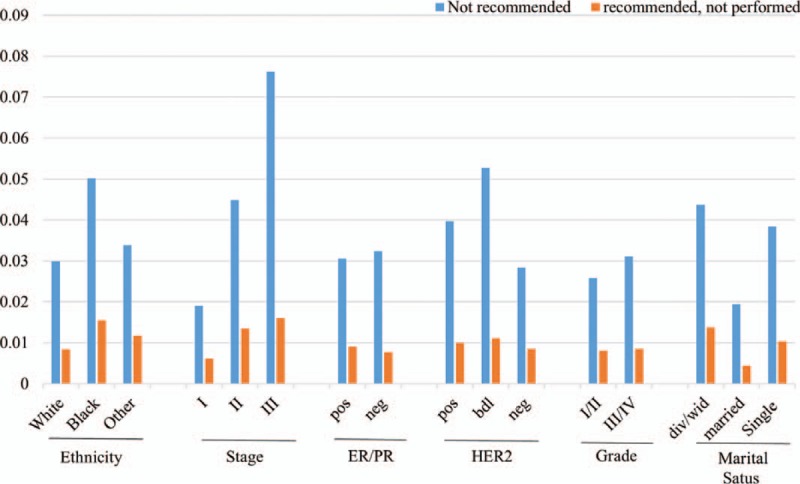
Bar graph comparing “surgery not recommended” and “surgery recommended but not performed” in patients with different characteristics.
3.4. Impact of surgery decision on survival outcomes
Patients in whom breast surgery was performed (Group 1) had the best survival outcomes, followed by patients in Group 2 (HR, 5.08; 95% CI, 4.48–5.76, P < .001; adjusted HR, 2.81; 95% CI 2.48–3.19, P < .001), and patients in Group 3 (HR, 8.21; 95% CI, 7.71–8.74, P < .001; adjusted HR, 4.57; 95% CI 4.27–4.88, P < .001) (Fig. 3). To determine if our findings apply to breast cancer patients with different clinicopathological features, we performed subgroup analyses by age, tumor stage, tumor grade, ER/PR and HER2 status. As shown in Table 4 and Figure 4A–E, patients of Group 1 had superior survival compared to patients in Group 2, regardless of the parameters used for stratification. Patients who were not considered medically fit for surgery (Group 3) had the worse survival outcomes. The survival benefit associated with surgery was most pronounced in patients of early-stage and younger age (stage I and age 60–69) and diminished in patients with advanced stages or older age (adjusted HR of Group 2: 3.64 vs 2.72 vs. 2.19 for stage I-III; 4.17 vs 3.61 vs 2.34 for age of 60s, 70s, and 80+). Surgery seemed especially important in patients with triple-negative breast cancer (TNBC) as Group 2 and Group 3 in TNBC patients had a median survival of 39 versus 34 months (Fig. 4E). The above findings were recapitulated in the analyses of CSS (Supplementary Table 2).
Figure 3.
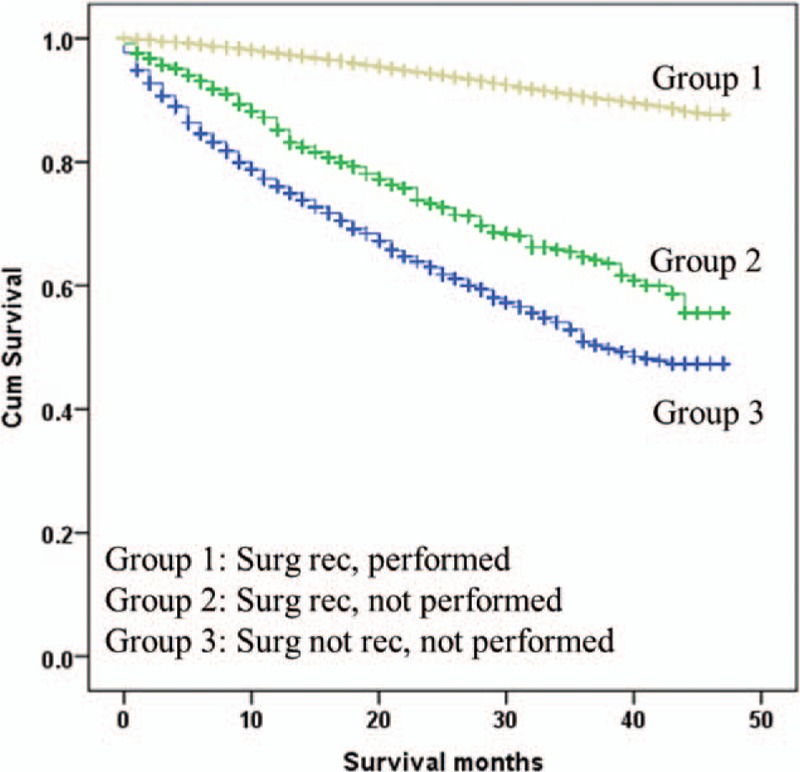
Kaplan–Meier curves for overall survival by different treatment groups in the whole patient population.
Table 4.
Impact of surgery on OS by selected parameters.
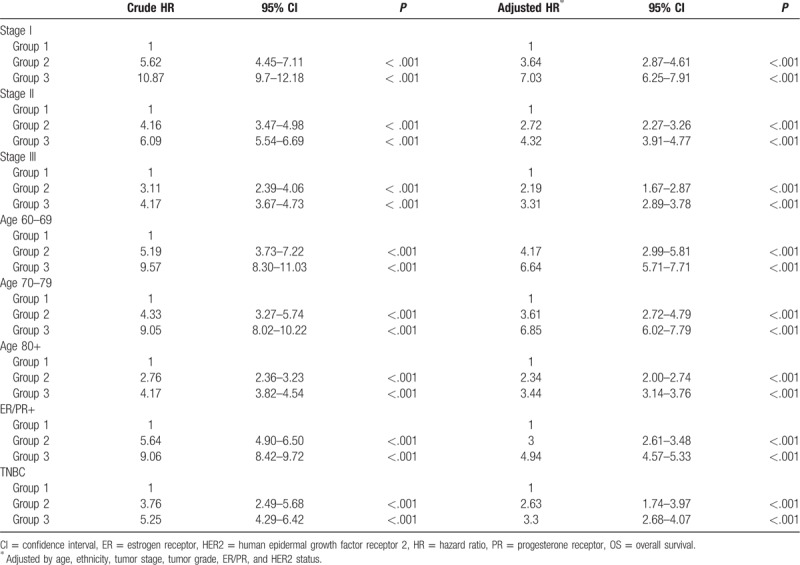
Figure 4.
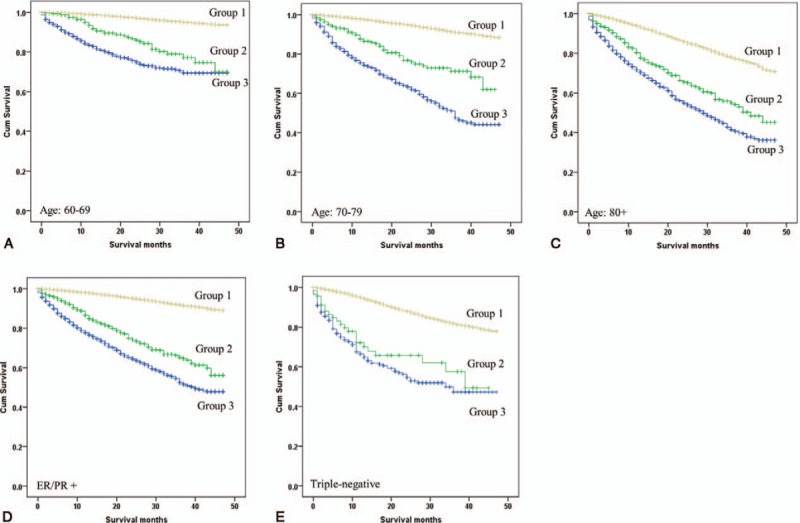
Kaplan–Meier survival curves of different treatment groups stratified by patient age (A, B, and C), ER/PR (D) and triple negative status (E). ER = estrogen receptor, PR = progesterone receptor.
4. Discussion
The decision to perform surgery in elderly patients with breast cancer can pose significant clinical challenges because of their under-representation in clinical trials, reduced life expectancy, and significant competing risks from other comorbid diseases. Our present study of 119,404 female breast cancer patients represents the largest reported series to date, aiming to determine if surgery with curative intent impacts survival outcomes in older patients with localized breast cancer. We show a superior OS and CSS in patients who received surgery, both for the overall patient population and in subgroup analyses, regardless of age, tumor stage, ER/PR, and HER2 status. Surgery was the single most important factor significantly associated with a better survival.
Because the US population is aging, the impact of surgery for breast cancer needs to be better understood. Breast cancer patients with age of 70 or beyond are generally considered elderly, while we included patients aged 60 years or older in this study because
-
(1)
the median age of diagnosis of breast cancer for women is 62 in the US[7]; and
-
(2)
the patient group of 60 to 69 age can serve as a “control” group to explore if the same treatment principles apply to both “young” (60–69) and “elderly” (70+) patients.
Indeed, current clinical practice guidelines do not differentiate treatment strategies based on ages, and state that treatment decisions for older women with breast cancer, including whether to have surgery, should be individualized based on tumor biology, patient general health status (eg, life expectancy, comorbidity, functional status), and patient preferences.[8,9] However, older women often receive less aggressive treatments. In a large study performed in the Netherlands, elderly patients had decreased rates of surgery, less adjuvant radiation therapy, and increased use of primary endocrine therapy, compared with their younger counterparts, even after controlling of confounding factors.[10] Some have debated that surgery can be avoided in older patients,[11] despite a meta-analysis finding a poorer OS approaching significance, P = .06, and a significant poorer progression-free survival, P = .0001.[12] There is evidence that elderly breast cancer patients generally tolerate surgery well, with low complication rates. In a 2011 study by Chatzidaki of 120 women aged 80 years and older with breast cancer, 32% had a simple mastectomy, 27% had breast conservation, and 6% had axillary dissection, yet major complications were seen in only 6% and involved wound healing.[13] Hence, our findings supported a similar surgical treatment strategy in patients over 60 years old.
In our study, the majority of patients were Caucasian, with early disease stage (stages I and II), had positive hormonal receptor and negative HER2 status, which are typical presentations of elderly breast cancer patients in the US. Clinicopathological parameters that impacted clinical outcomes were ethnicity, age, stage, ER/PR status, HER2 status, tumor grade, and single status. These findings are consistent with the conclusions of previous studies.[14,15] Multiple clinicopathological parameters seemed to impact surgical decisions. There was a clear trend to withhold surgery with increasing age, with between 2% and 11.1% of patients being not recommended for surgery, depending on the age category. Additionally, patients of African American, advanced stage or high tumor grade, positive HER2 status and divorced/widowed/single (known independent negative prognostic factors) were more likely not to receive surgery.[14,15] Withholding surgery in such patients may lead to even worse clinical outcomes, because our study shows that surgery is the single most important factor associated with improved OS and CSS (no surgery vs surgery: HR, 7.39; 95% CI 6.98–7.83 for OS; and HR, 8.71; 95% CI 7.87–9.63 for CSS). Interestingly, ER/PR status was not found to be associated with surgical decision, which can be a bias from data capturing. On the other side, the percentage of ER/PR positivity can range from 1% to 100% with different clinical implications and impact on decision makings. Unfortunately, we cannot further divide ER/PR status by weak, moderate, or strong expression and determine their correlations with surgical decision.
SEER did not report the denial reasons for surgery. If one assumes that Group 1 (surgery performed) and Group 2 (surgery recommended but not performed) are medically fit patients, then Group 3 (surgery not recommended and not performed) are medically unfit patients. When compared with Group 1, patients in Groups 2 and Group 3 had worse OS and CSS, with the worst being Group 3. Hence, while surgery provides a survival benefit in medically fit elderly patients, a good general health status (medically fit) is associated with a longer survival in the elderly who do not receive surgery, regardless of patient characteristics. The results suggest that the optimal treatment approach to healthy elderly women with newly diagnosed non-metastatic breast cancer could be similar to that of younger women and should include surgery. For TNBC patients, surgery should be performed whenever possible because of limited treatment options and poor prognosis without surgery.
Our study has limitations. First, the number of variables collected in the SEER database is limited. We were not able to evaluate the functional impact of surgery because related variables are not available in SEER. We were not able to adjust the variable of “comorbidity” such as the Charlson comorbidity index in our analysis, which can be an important confounding factor affecting treatment decisions and clinical outcomes. Second, group 3 patients may include a small percentage of medically fit patients who were denied surgery for other reasons. We are unable to further dissect this population but do not expect a significant change in our conclusions by excluding those patients. Thirdly, our study did not differentiate the impact of the surgical techniques, least invasive lumpectomy or most invasive mastectomy with axillary lymph node dissection. Although the “fit” elderly patients seems to benefit from surgery, they may not need aggressive surgical interventions to derive the same benefit, especially for those with favorable risk features. Studies have found that elderly women prefer breast conservative surgery over mastectomy for a better quality of life. There are ongoing trials, for example, souND trial which looks to de-escalate axillary surgery in early-stage breast cancer. Fourth, the standard SEER incidence database does not provide data on systemic therapy, and therefore we could not ascertain the receipt of chemo- or hormone-therapy. It is possible that some patients did not receive adjuvant systemic treatment following surgery, while patients who did not receive surgery were very unlikely to withhold systemic treatment. Considering the substantial survival benefit associated with surgery group, these results are compelling to favor surgery.
In conclusion, this large population-based cohort study shows that front definitive surgery for medically-fit elderly patients with localized breast cancer has significant survival benefit. Prospective clinical trials that focus on the elderly patients with breast cancer should be designed to better understand the optimal treatment approach.
Author contributions
Conceptualization: Ming Yin, Cristina I. Truica.
Data curation: Ming Yin.
Formal analysis: Ming Yin, Cristina I. Truica.
Methodology: Cristina I. Truica.
Writing – original draft: Ming Yin, Claire Verschraegen, Cristina I. Truica.
Writing – review and editing: Claire Verschraegen, Vinh-Hung Vincent, Sandipkumar M. Patel, Tiffany George, Cristina I. Truica.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CSS = cancer-specific survival, ER = estrogen receptor, HER2 = human epidermal growth factor receptor 2, HR = hazard ratio, OS = overall survival, PR = progesterone receptor, SEER = surveillance, epidemiology, and end results.
How to cite this article: Yin M, Verschraegen C, Vincent VH, Patel SM, George T, Truica CI. Impact of lack of surgery on outcomes in elderly women with nonmetastatic breast cancer—A surveillance, epidemiology, and end results 18 population based study. Medicine. 2020;99:3(e18745).
This study was supported by a gift in memory of Kerry Taylor.
Presented in part at 42nd ESMO Congress (ESMO 2017) September 8–12, 2017, Madrid, Spain.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].GRAYSON K, VINCENT VAV. The Next Four Decades: The Older Population in the United States: 2010 to 2050; 2010. Available at: http://www.census.gov/prod/2010pubs/p25-1138.pdf Accessed November 28, 2018. [Google Scholar]
- [2].Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758–65. [DOI] [PubMed] [Google Scholar]
- [3].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [4].Bouchardy C, Rapiti E, Fioretta G, et al. Undertreatment strongly decreases prognosis of breast cancer in elderly women. J Clin Oncol 2003;21:3580–7. [DOI] [PubMed] [Google Scholar]
- [5].Tahir M, Robinson T, Stotter A. How not to neglect the care of elderly breast cancer patients? Breast 2011;20:293–6. [DOI] [PubMed] [Google Scholar]
- [6].Noone AM, Krapcho HN, Miller M, et al. National Cancer Institute, SEER Cancer Statistics Review, 1975–2015. Bethesda, MD: 2018. [Google Scholar]
- [7].American Cancer Society. Breast Cancer Facts & Figures 2017–2018. Atlanta: American Cancer Society, Inc; 2017. [Google Scholar]
- [8].Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26: Suppl 5: v8–30. [DOI] [PubMed] [Google Scholar]
- [9].Giordano SH, Elias AD, Gradishar WJ. NCCN guidelines updates: breast cancer. J Natl Compr Canc Netw 2018;16:605–10. [DOI] [PubMed] [Google Scholar]
- [10].Bastiaannet E, Liefers GJ, de Craen AJ, et al. Breast cancer in elderly compared to younger patients in the Netherlands: stage at diagnosis, treatment and survival in 127,805 unselected patients. Breast Cancer Res Treat 2010;124:801–7. [DOI] [PubMed] [Google Scholar]
- [11].Pepping RMC, Portielje JEA, van de Water W, et al. Primary endocrine therapy in older women with breast cancer. Curr Geriatr Rep 2017;6:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hind D, Wyld L, Reed MW. Surgery, with or without tamoxifen, vs tamoxifen alone for older women with operable breast cancer: cochrane review. Br J Cancer 2007;96:1025–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chatzidaki P, Mellos C, Briese V, et al. Perioperative complications of breast cancer surgery in elderly women (>/=80 years). Ann Surg Oncol 2011;18:923–31. [DOI] [PubMed] [Google Scholar]
- [14].Soerjomataram I, Louwman MW, Ribot JG, et al. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat 2008;107:309–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cao SS, Lu CT. Recent perspectives of breast cancer prognosis and predictive factors. Oncol Lett 2016;12:3674–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.