Abstract
Background:
To evaluate the association between risperidone use and interleukin-6 (IL-6) levels by conducting a meta-analysis of controlled before-and-after studies.
Methods:
Studies were identified through a systematic search of PubMed and Embase. The mean and standardized differences were extracted to calculate the standardized mean differences. IL-6 levels were compared in patients with schizophrenia before and after risperidone treatment.
Results:
Ten studies were included in the final meta-analysis. The primary findings from our study suggest that there was a significant decrease in serum IL-6 levels after risperidone treatment (P = .021). A subgroup analysis revealed the sources of heterogeneity. The sensitivity analysis indicated that the results were stable, and no publication bias was observed.
Conclusions:
The present meta-analysis provides evidence that risperidone can significantly reduce IL-6 levels in schizophrenia. IL-6 is a potential biomarker of the pathophysiology and clinical processes of schizophrenia.
Keywords: interleukin-6, meta-analysis, risperidone, schizophrenia
1. Introduction
Schizophrenia is a chronic mental disorder with heterogeneous genetic and neurobiological backgrounds; this disorder has an average lifetime prevalence of less than 1%.[1] The physiological and pathological mechanisms of schizophrenia are unclear, but many factors may influence the occurrence of schizophrenia, such as genetic predisposition,[2] maternal infection during pregnancy,[3] and abnormal brain function. Gene-environment interactions play a key role in the risk of schizophrenia. Different environmental factors and inflammatory immune processes have been shown to be involved in the etiology and pathology of schizophrenia.[4] There is evidence that cytokine elevation plays an important role in the pathogenesis of schizophrenia and is closely related to its etiology and pathogenesis. Interleukin-6 (IL-6), a major cytokine, plays several important roles in the central nervous system (CNS), such as regulating brain development and synaptic plasticity, and is also related to sleep and stress.[5] Increased levels of IL-6 in the blood are 1 of the most commonly observed pieces of evidence linking schizophrenia to immunological characteristics.
Antipsychotic medication is the preferred treatment for schizophrenia. Risperidone, the representative second-generation antipsychotic drug, is effective against both positive and negative symptoms of schizophrenia. Adverse reactions to the drug are mild and generally do not include an effect on cognitive function. With these features, risperidone has become a first-line drug for the treatment of schizophrenia. Recent studies have suggested that antipsychotics, including risperidone, may have a direct effect on inflammatory status, such as significant changes in cytokine levels during antipsychotic treatment.[6] However, observations regarding cytokine changes after antipsychotic treatment have been inconsistent among studies, and the effects of risperidone on immune system regulation and cytokines remain unclear.
Therefore, this meta-analysis was designed to assess the effect of risperidone on IL-6 levels in patients with schizophrenia, with the goal of determining whether IL-6 is a suitable biomarker for evaluating the efficacy of schizophrenia treatments.
2. Materials and methods
We followed the PRISMA guidelines7,8 for reporting of inclusion criteria, assessment of publication bias, and synthesis of results.
2.1. Literature-search strategy
Ten Relevant studies were identified from PubMed and EMBASE electronic databases until October 2019 without restrictions on geography, type of publication or language. The authors performed a systematic literature review using the Medical Subject Headings terms. The keywords used for the search were antipsychotics, risperidone, schizophrenia, and IL-6. In addition, a manual search was carried out to identify references in the identified studies to identify possible other studies. Finally, we contacted the corresponding author to obtain the original data if the data in the research report is insufficient.
It is important to mention that when we searched literatures with these keywords
risperidone, schizophrenia and IL-6, we only got very limited number of articles. So, we expanded the search scope, add antipsychotic as keyword and got more literatures on the area. However, we only included papers with risperidone, not other antipsychotics as treatment drug in the meta-analysis.
2.2. Inclusion and exclusion criteria
Studies were included if
-
(1)
they involved subjects with schizophrenia-spectrum disorders as defined by the Diagnostic and Statistical Manual of Mental Disorders/International Classification of Diseases;
-
(2)
they employed a pre–post design that involved the administration of antipsychotics (typical, atypical, or mixed); and
-
(3)
they measured the plasma or serum levels of IL-6 in vivo before and after the treatment. When several articles dealt with the same population, we selected the article with the largest sample.
Studies were excluded if
-
(1)
the study design was cross-sectional;
-
(2)
the sample of patients comprised patients with Diagnostic and Statistical Manual of Mental Disorders Axis I disorders other than schizophrenia-spectrum disorders;
-
(3)
cytokine levels were measured using cerebrospinal fluid (CSF); or
-
(4)
cytokine levels were measured using supernatants of leucocytes stimulated in vitro, as such studies vary widely in the conditions of in vitro incubation. If the data were duplicated or the population was examined in more than 1 study, we included only the study with the largest sample size and the most comprehensive outcome evaluation.
2.3. Data extraction and quality assessment
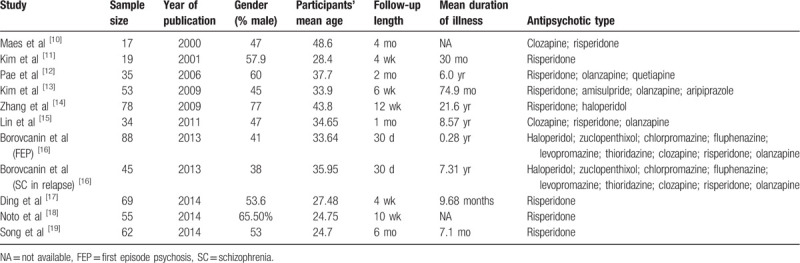
Two investigators independently evaluated the eligibility of studies retrieved from the database based on predetermined selection criteria. In addition, the references of the eligible articles were examined to locate studies not found in the computer search. These 2 authors independently extracted the following data from each study: sample size, year of publication, gender ratio of subjects (proportion of males), mean age of subjects, follow-up length, duration of illness, and antipsychotic type.
Articles were screened based on their titles, abstracts, and full texts prior to inclusion in the meta-analysis. The risk of bias was assessed by 2 reviewers working independently using a modified Newcastle-Ottawa classification for observational studies.[9]
2.4. Statistical analyses
Data were entered into Microsoft Excel, and the meta-analysis was conducted using STATA software. Study heterogeneity was estimated with the Cochran Q and I 2 statistics.[10] When the P value was < .1 and the I 2 value was > 50%, the data were considered heterogeneous, and a random-effects model was used[11]; these conditions were satisfied in all cases. Effect sizes consisted of the standardized mean difference (SMD) between IL-6 levels before and after treatment. Using Cohen method, the SMD was calculated as the difference between group means divided by the pooled standardized differences.
2.5. Ethics statement
All data sources and statistical analyses were based on previously published studies. Therefore, it did not require patient consent or ethical approval.
3. Results
3.1. Literature search and study characteristics
After preliminary screening, 388 relevant articles were obtained; 318 of those articles were eliminated based on their abstracts. After the full text of each work was read, another 60 studies were excluded for failure to meet the inclusion criteria. A total of 10 controlled before-and-after studies were included.12,13,14,15,16,17,18,19,20,21 Figure 1 shows the article-selection process, and Table 1 shows details on the included studies.
Figure 1.
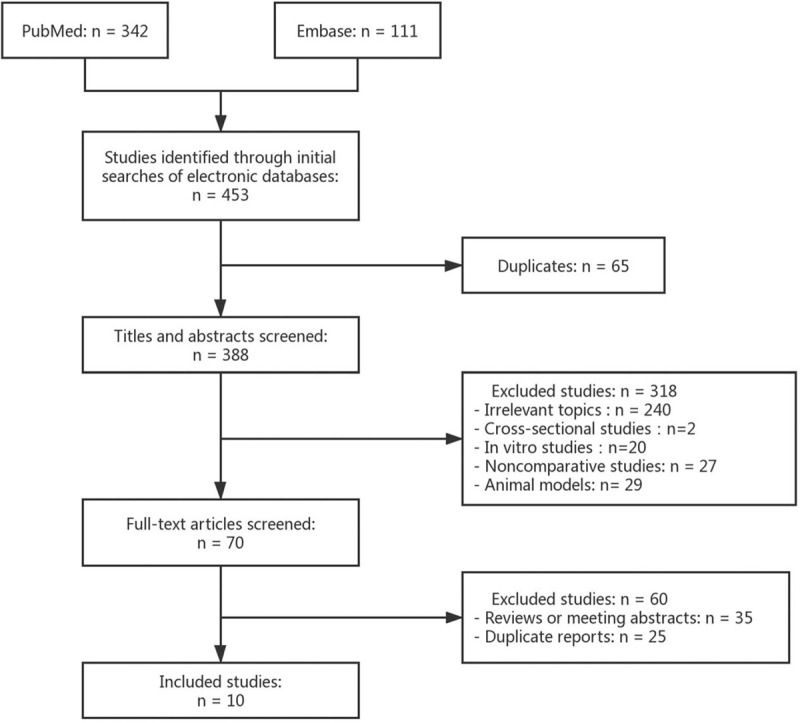
Study selection diagram.
Table 1.
Summarized characteristics of the included studies.
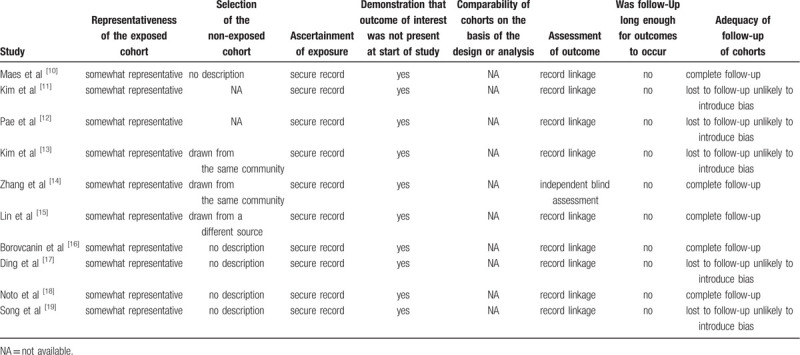
3.2. Risk of bias of selected studies
Overall, the risk of bias in the observational studies was moderate (Table 2) due to missing or unclear descriptions of follow-up time and loss to follow-up.
Table 2.
Risk of bias of observational studies.
3.3. Main analysis
The meta-analysis of the 10 controlled before-and-after studies, which included 546 individuals, indicated an association between risperidone use and levels of IL-6 (n = 10 studies; test for overall effect: SMD = -0.506, 95% confidence interval: -0.938 to 0.075, P = .021; test for heterogeneity: χ 2 = 116.50, P < .0001, I 2 = 91.4%) (Fig. 2).
Figure 2.
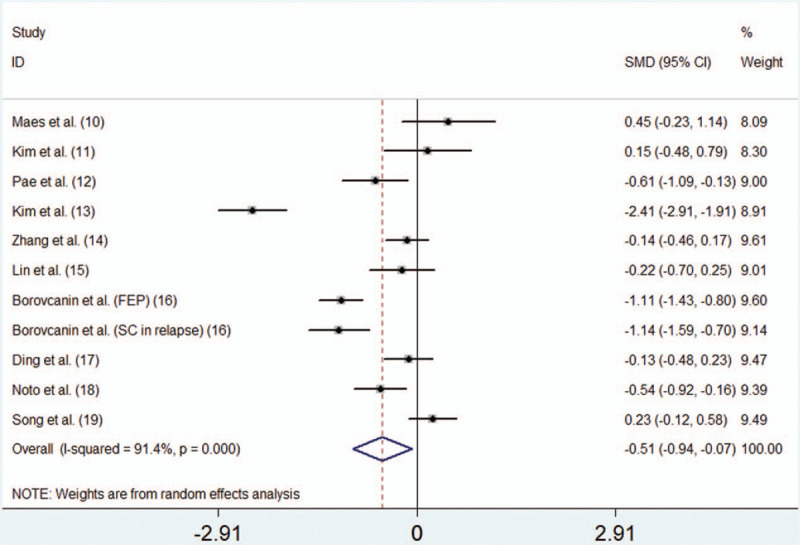
Forest plot of SMDs with CIs for risperidone use and levels of IL-6. CIs = confidence intervals, IL-6 = interleukin-6, SMD = standardized mean difference.
3.4. Subgroup meta-analysis
A subgroup meta-analysis was performed by region. We found that risperidone reduced IL-6 levels in European patients. In the Asian population, however, risperidone use was not associated with a decrease in IL-6 (Table 3).
Table 3.
Subgroup analyses of the association between risperidone use and IL-6 levels.
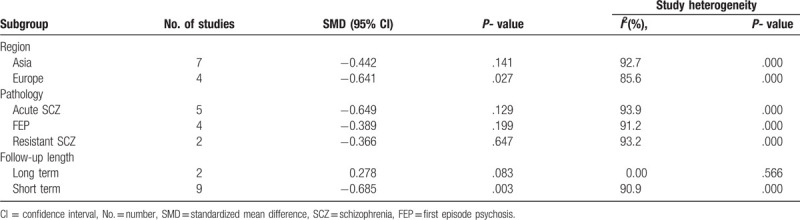
When we performed subgroup analysis based on patients’ conditions, we observed that risperidone was not associated with decreased IL-6 in patients with acute schizophrenia, refractory schizophrenia, or first-episode schizophrenia (Table 3).
Another subgroup meta-analysis was performed by follow-up length. We found that psychiatric patients who took risperidone had decreased levels of IL-6 in the short term. No statistical heterogeneity was observed in the long-term group (Fig. 3, Table 3).
Figure 3.
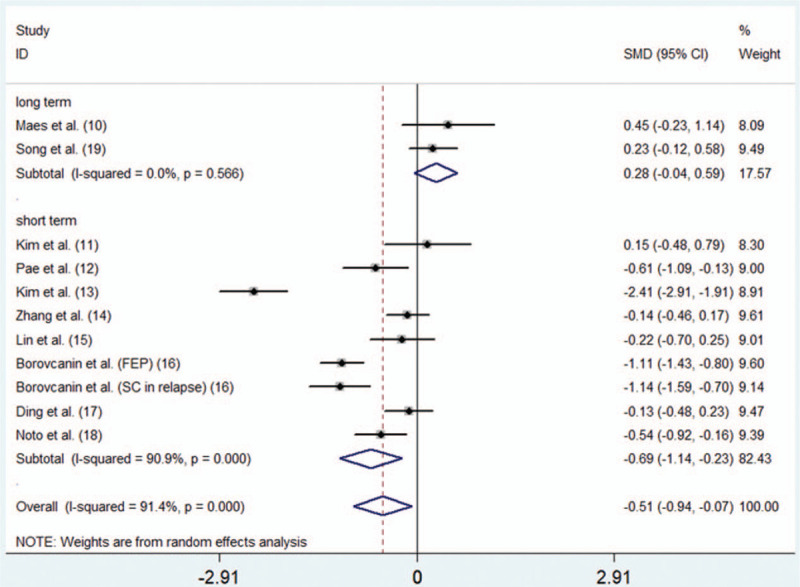
Forest plot for subgroup meta-analysis by follow-up length.
3.5. Sensitivity analysis
To assess the robustness of our analysis, we performed a sensitivity analysis by recalculating the combined results of the original analysis, excluding 1 study per iteration. The results showed that excluding any 1 study did not change the overall results (Fig. 4).
Figure 4.
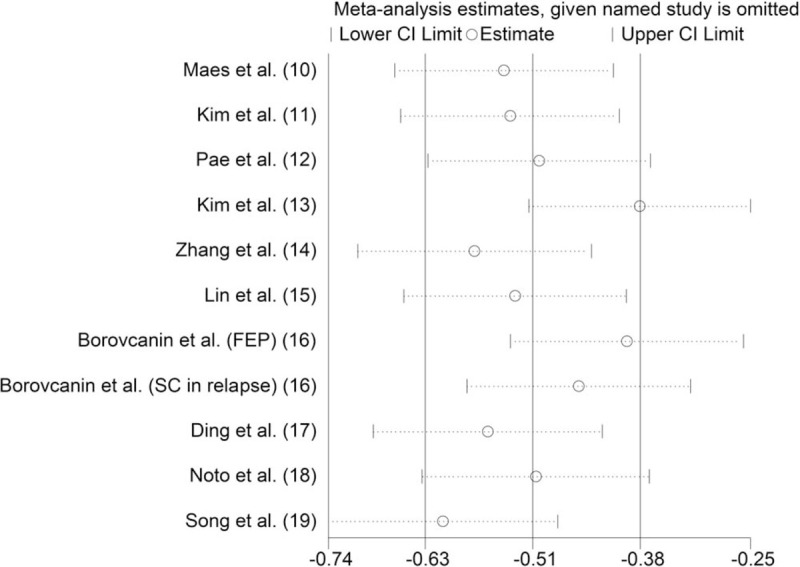
Sensitivity analysis of the association between risperidone use and IL-6 levels. IL-6 = interleukin-6.
3.6. Publication bias
Begg rank correlation test and Egger linear regression test indicated no evidence of publication bias among the studies [Begg, P > z = .755; Egger, P = .939, 95% confidence interval -10.96–10.22] (Figs. 5 and 6).
Figure 5.
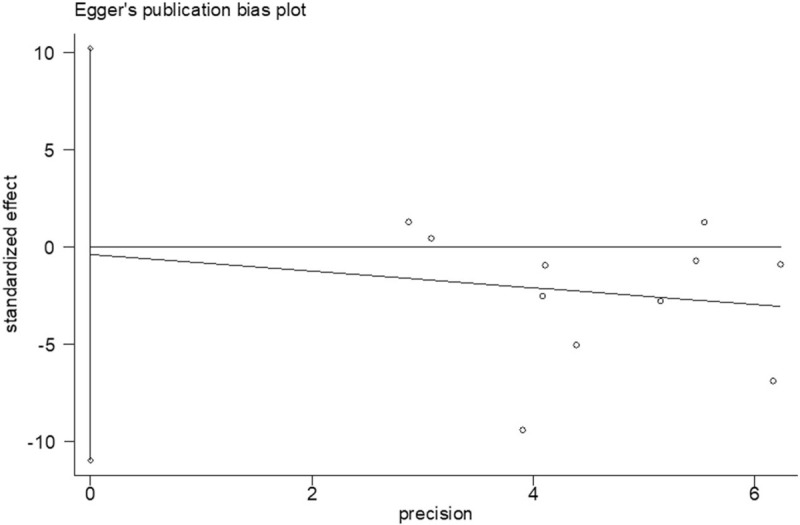
Egger publication bias plot.
Figure 6.
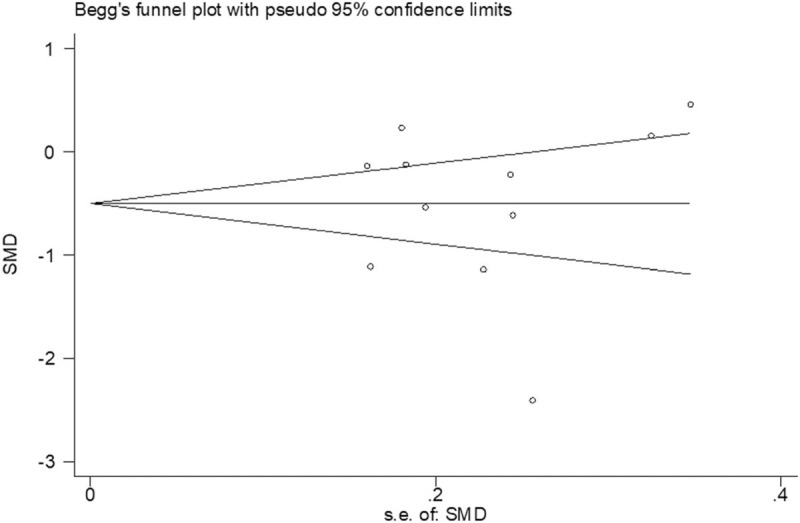
Begg publication bias plot.
4. Discussion
To the best of our knowledge, this is the first meta-analysis performed to describe the effects of risperidone on IL-6 levels in controlled before-and-after studies. The primary findings from our study consistently suggest a significant decrease in serum IL-6 after risperidone treatment. We also found that, in some cases, the changes in IL-6 levels could be impacted by region or follow-up length.
Our findings are also consistent with current research on risperidone. Noto et al[22] compared antipsychotic-naive first-episode psychosis patients with healthy people and found that treatment with risperidone significantly suppressed the immune inflammatory response system and compensatory immune-regulatory reflex system; in particular, the inflammation marker IL-6 was significantly reduced. In the present study, although heterogeneity was observed, the findings were stable and robust based on our sensitivity analysis. There are several plausible mechanisms by which risperidone may decrease IL-6 levels. Accumulating evidence has shown that astrocytes can amplify inflammatory responses in the CNS, a phenomenon that is closely related to the neurobiology and progression of neuropsychiatric disorders.23,24 Bobermin et al[25] observed that risperidone had an anti-inflammatory effect on C6 astroglia, decreasing the release of IL-6. Moreover, De Souza et al[26] found that risperidone inhibited IL-6-induced S100B secretion, reducing the secretion rate below the basal level. Noto et al[27] suggest that circulating levels of IL-6 and IL-10 might regulate the expression of the AKT1, DROSHA, NDEL1, DISC1, and MBP genes. Thus, risperidone treatment may modulate gene expression in the course of schizophrenia treatment. In recent years, scientists have found in biochemical studies of the central nervous system that IL-6 is produced by neurons, astrocytes and microglia and acts as a neurotrophic factor in the central nervous system.[28] However, a recent study of Mamun et al[29] shows that risperidone can attenuate the activation of microglia in the brain, which may reduce the levels of IL-6, and proposes that risperidone may contribute to the improvement in brain diseases.
Our overall study shows that treatment with risperidone can lead to a decrease in IL-6 levels; although a high I 2 (85.6%) was present for overall risperidone use, we identified the sources of heterogeneity by performing subgroup analyses. In the subgroup analysis by follow-up length, we found that no heterogeneity was observed in the long-term follow-up studies; all of the heterogeneity was derived from the short-term studies. One study in the meta-analysis found a significant decrease in IL-6 levels after short-term risperidone treatment: Song et al[30] found significant changes in cytokine levels after a mean of 4 weeks of antipsychotic treatment. Other researchers have reported similar findings; for example, 1 study observed decreased IL-6 levels within 9 days of antipsychotic treatment, and by 8 weeks, there was no significant difference between patients and control subjects.[31] Taken together, these findings suggest that IL-6 levels may be normalized to some extent shortly after risperidone treatment. Through a subgroup analysis by study region, we found that studies conducted in Europe showed significant decreases in IL-6 levels. In the Asian subgroup, however, it was not clear whether risperidone use had any effect on IL-6 levels. To the best of our knowledge, there are no studies compare Asian and Europe schizophrenia patients about serum IL-6 after risperidone treatment currently, and we hope to have opportunity to do this work in the future. Overall, this analysis suggested that risperidone could significantly reduce the level of IL-6 in the European population, which could contribute to its effectiveness in treating schizophrenia. In the subgroup analysis by patient pathology, there was still considerable heterogeneity among acute schizophrenia, resistant schizophrenia and first-episode schizophrenia. It is not clear whether patients’ condition can affect changes in IL-6 levels. A previous meta-analysis found evidence for cytokine alterations in schizophrenia but did not find an effect of clinical status, which was classified as acute, nonacute, or mixed.[32] A study by Miller et al[33] considered the effects of antipsychotic therapy on disease progression and the correlation between cytokine levels and clinical characteristics; the results of that study suggested that cytokine alterations in schizophrenia might vary with clinical status. Of course, many other factors may have made additional contributions. We will evaluate these factors in the future when the necessary data are available.
Our meta-analysis has several limitations. First, although all studies describing risperidone treatment and IL-6 levels were searched, the search was performed in databases that mainly index English-language material. The number of studies was relatively small, which means some may have been missed because they were published in non-English-language journals or in books or journals not included in computer databases. Second, studies with nonsignificant results, especially those that show a lack of effectiveness, may go unpublished because they are rejected by journals. Although publication bias was controlled by statistical methods, the possibility of publication bias could not be completely excluded. As a result, measures of pooled effects may be overestimated. Third, most studies did not control for known potential confounders that affect cytokine levels, such as age, gender, body mass index, and smoking, and these factors also varied between studies. Fourth, most studies in this meta-analysis did not standardize medication regimens, and different doses of risperidone may have different effects on IL-6 levels. Finally, this analysis was limited to studies conducted in blood rather than CSF, although the latter may have a more direct connection to the CNS. Therefore, further study of the effect of risperidone therapy on IL-6 levels in CSF may help confirm our results.
5. Conclusions
In summary, this meta-analysis provides evidence that risperidone use can significantly reduce IL-6 levels in the body. In addition, the correlation between cytokine levels and psychopathology may provide new immunoregulatory strategies for the treatment of schizophrenia. In summary, IL-6 is a potential biomarker and therapeutic target involved in the pathophysiology and clinical processes of schizophrenia.
In future studies, we should take into account the clinical status, disease duration, and risperidone dose of schizophrenia patients to better assess the impact of changes. Further research is needed on refractory psychiatric patients and CSF biochemistry.
Author contributions
Conceptualization: Ziqiao Feng, Yunqiao Zhang, Zhaowei Teng.
Data curation: Xu You, Yong Zeng.
Investigation: Yuhan Ma, Wenyu Zhang.
Methodology: Qing Long, Zijun Liu.
Resources: Yunqiao Zhang, Yong Zeng.
Software: Ziqiao Feng, Zhaowei Teng.
Supervision: Yong Zeng.
Writing – original draft: Ziqiao Feng.
Writing – review and editing: Yunqiao Zhang, Zhaowei Teng, Wei Hao.
Footnotes
Abbreviations: CNS = central nervous system, CSF = cerebrospinal fluid, IL-6 = interleukin-6, SMD = standardized mean difference.
How to cite this article: Feng Z, Zhang Y, You X, Zhang W, Ma Y, Long Q, Liu Z, Hao W, Zeng Y, Teng Z. Effects of risperidone on blood levels of interleukin-6 in schizophrenia: a meta-analysis. Medicine. 2020;99:15(e19694).
The datasets generated during and/or analyzed during the current study are publicly available.
Funding for this study was provided by the National Natural Science Foundation of China (No. 81760253; No. 81960254; No. 81660156; No. 31960136). Yunnan Science and Technology Department – Kunming Medical University Applied Basic Research Joint Special Fund Project (No. 2018FE001(-009); No. 2018FE001(-174)). Yunnan province philosophy and social science planning project (No. YB2017037). Yunnan health training project of high level talents (NO. L-2017021; H-2017064).
The authors have no conflicts of interest to disclose.
References
- [1]. Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primers 2015;1:15067. [DOI] [PubMed] [Google Scholar]
- [2]. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014;511:421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 2010;167:261–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry Clin Neurosci 2010;64:217–30. [DOI] [PubMed] [Google Scholar]
- [5]. Bauer S, Kerr BJ, Patterson PH. The neuropoietic cytokine family in development, plasticity, disease and injury. Nat Rev Neurosci 2007;8:221–32. [DOI] [PubMed] [Google Scholar]
- [6]. Zajkowska Z, Mondelli V. First-episode psychosis: an inflammatory state? Neuroimmunomodulation 2014;21:102–8. [DOI] [PubMed] [Google Scholar]
- [7]. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed) 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Eur J Epidemiol 2013;25:603–5. [DOI] [PubMed] [Google Scholar]
- [10]. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [12]. Maes M, Chiavetto LB, Bignotti S, et al. Effects of atypical antipsychotics on the inflammatory response system in schizophrenic patients resistant to treatment with typical neuroleptics. Eur Neuropsychopharmacol 2000;10:119–24. [DOI] [PubMed] [Google Scholar]
- [13]. Kim DJ, Kim W, Yoon SJ, et al. Effect of risperidone on serum cytokines. The International journal of neuroscience 2001;111:11–9. [DOI] [PubMed] [Google Scholar]
- [14]. Pae CU, Yoon CH, Kim TS, et al. Antipsychotic treatment may alter T-helper (TH) 2 arm cytokines. Int Immunopharmacol 2006;6:666–71. [DOI] [PubMed] [Google Scholar]
- [15]. Kim YK, Myint AM, Verkerk R, et al. Cytokine changes and tryptophan metabolites in medication-naive and medication-free schizophrenic patients. Neuropsychobiology 2009;59:123–9. [DOI] [PubMed] [Google Scholar]
- [16]. Zhang XY, Zhou DF, Qi LY, et al. Superoxide dismutase and cytokines in chronic patients with schizophrenia: association with psychopathology and response to antipsychotics. Psychopharmacology 2009;204:177–84. [DOI] [PubMed] [Google Scholar]
- [17]. Lin CC, Chang CM, Chang PY, et al. Increased interleukin-6 level in Taiwanese schizophrenic patients. Chang Gung medical journal 2011;34:375–81. [PubMed] [Google Scholar]
- [18]. Borovcanin M, Jovanovic I, Radosavljevic G, et al. Antipsychotics can modulate the cytokine profile in schizophrenia: attenuation of the type-2 inflammatory response. Schizophrenia research 2013;147:103–9. [DOI] [PubMed] [Google Scholar]
- [19]. Ding M, Song X, Zhao J, et al. Activation of Th17 cells in drug naive, first episode schizophrenia. Progress in neuro-psychopharmacology and biological psychiatry 2014;51:78–82. [DOI] [PubMed] [Google Scholar]
- [20]. Noto C, Ota VK, Gouvea ES, et al. Effects of risperidone on cytokine profile in drug-naive first-episode psychosis. Int J Neuropsychopharmacol 2014;18:yu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Song X, Fan X, Li X, et al. Changes in pro-inflammatory cytokines and body weight during 6-month risperidone treatment in drug naive, first-episode schizophrenia. Psychopharmacology 2014;231:319–25. [DOI] [PubMed] [Google Scholar]
- [22]. Noto MN, Maes M, Nunes SOV, et al. Activation of the immune-inflammatory response system and the compensatory immune-regulatory system in antipsychotic naive first episode psychosis. Eur Neuropsychopharmacol 2019;29:416–31. [DOI] [PubMed] [Google Scholar]
- [23]. Pinto JV, Passos IC, Librenza-Garcia D, et al. Neuron-glia interaction as a possible pathophysiological mechanism of bipolar disorder. Curr Neuropharmacol 2018;16:519–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Verkhratsky A, Parpura V. Astrogliopathology in neurological, neurodevelopmental and psychiatric disorders. Neurobiol Dis 2016;85:254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Bobermin LD, Da Silva A, Souza DO, et al. Differential effects of typical and atypical antipsychotics on astroglial cells in vitro. Int J Dev Neurosci 2018;69:1–9. [DOI] [PubMed] [Google Scholar]
- [26]. De Souza DF, Wartchow K, Hansen F, et al. Interleukin-6-induced S100B secretion is inhibited by haloperidol and risperidone. Prog Neuropsychopharmacol Biol Psychiatry 2013;43:14–22. [DOI] [PubMed] [Google Scholar]
- [27]. Noto C, Ota VK, Santoro ML, et al. Depression, cytokine, and cytokine by treatment interactions modulate gene expression in antipsychotic naive first episode psychosis. Mol Neurobiol 2016;53:5701–9. [DOI] [PubMed] [Google Scholar]
- [28]. Xiang YZ, Zhou DF, Zhang PY, et al. Elevated interleukin-2, interleukin-6 and interleukin-8 serum levels in neuroleptic-free schizophrenia: association with psychopathology. Schizophrenia Research 2002;57:247–58. [DOI] [PubMed] [Google Scholar]
- [29]. Mamun AAM, Rahman CMF, Saad CA, et al. Pretreatment with risperidone ameliorates systemic LPS-induced oxidative stress in the cortex and hippocampus. Front Neurosci 2018;12:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Song XQ, Lv LX, Li WQ, et al. The interaction of nuclear factor-kappa B and cytokines is associated with schizophrenia. Biol Psychiatry 2009;65:481–8. [DOI] [PubMed] [Google Scholar]
- [31]. Frommberger UH, Bauer J, Haselbauer P, et al. Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci 1997;247:228–33. [DOI] [PubMed] [Google Scholar]
- [32]. Potvin S, Stip E, Sepehry AA, et al. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry 2008;63:801–8. [DOI] [PubMed] [Google Scholar]
- [33]. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 2011;70:663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


