Abstract
Although proliferating cell nuclear antigen (PCNA) plays an important role in tumor proliferation and its expression level is closely related to the biological activity of tumor cells, PCNA expression in non-small cell lung cancer (NSCLC) has been seldom reported. In this study, we aimed to investigate the significance of PCNA expression in NSCLC tissues. PCNA expression in NSCLC and adjacent tissues were assessed by immunohistochemistry (IHC), western blotting, and reverse transcription polymerase chain reaction. Single factor analysis was used to study the relationship between the expression of PCNA and clinicopathological features of NSCLC. Multi-factor Cox survival analysis was used to evaluate the relationship between the expression of PCNA and overall survival of postoperative NSCLC patients. The areas under the receiver operating characteristics were calculated to evaluate the value of PCNA expression level in predicting the 3-year survival of NSCLC patients. IHC analysis showed that the positive expression rates of PCNA protein in NSCLC and adjacent tissues were 91.79% (257/280) and 25.83% (31/120), respectively. Western blotting confirmed that PCNA protein level was significantly higher in NSCLC tissues than in the adjacent tissues (P < .05). Reverse transcription polymerase chain reaction showed that the positive rate of PCNA mRNA in NSCLC was 88.93% (249/280), which was significantly higher than that in adjacent tissues 29.17% (35/120) (P < .05). Both PCNA mRNA and protein levels were correlated with tumor differentiation, size, metastasis, and stage in NSCLC. Patients exhibiting higher PCNA protein expression had a significantly shorter disease-specific survival rate than the other patients. PCNA protein level and tumor pathological type, metastasis, differentiation degree, and stage were independent factors affecting the overall survival of postoperative patients. The areas under the receiver operating characteristics of PCNA mRNA for predicting the 3-year survival of NSCLC patients was 0.89 (0.79–0.98), with a sensitivity and specificity of 0.84 and 0.76, respectively. In conclusion, high PCNA protein and mRNA levels may be associated with the occurrence, development, and prognosis of NSCLC.
Keywords: immunohistochemistry, non-small cell lung cancer, prognostic value, proliferating cell nuclear antigen
1. Introduction
Lung cancer is one of the most common malignancies in China, among which non-small cell lung cancer (NSCLC) accounts for approximately 85%.[1,2] In recent years, the incidence and mortality of lung cancer have been increasing in China.[3,4] Epidemiological studies have estimated that the average annual incidence of lung cancer can reach (283–593)/100,000.[5] Studies have shown that long-term smoking is closely related to the occurrence of lung cancer.[6,7] Environmental and genetic factors can influence the occurrence and progression of lung cancer and affect the clinical and pathological characteristics.[8,9] As a common malignant tumor of the respiratory system, the cancer-free survival period in lung cancer is <38 months. Even after a comprehensive treatment, including surgery, the 3-year mortality rate of lung cancer patients is still >35%.[10] Many studies have shown that surgery is still the most effective choice for the treatment of lung cancer, but by the time a diagnosis is made, most patients already have advanced cancer and have lost the opportunity for surgery.[11,12] Therefore, radiotherapy and chemotherapy have become indispensable in the treatment of lung cancer despite their toxicity, side effects, and long-term use of cancer cells to reduce their toxicity.[13,14]
Proliferating cell nuclear antigen (PCNA) is a nuclear protein that regulates cell cycle and participates in DNA synthesis.[15,16] The level of PCNA increases before DNA synthesis with the transition of the cell from the G1 phase to S phase and decreases back during the M phase.[17] Accordingly, PCNA largely reflects cell proliferation activity and is commonly used as a marker of cell proliferation in healthy and tumor tissues.[18] Furthermore, the expression level of PCNA is closely correlated with the biological activity of tumor cells, suggesting an active role of PCNA in tumorigenesis.[19,20] Since tumor cells with high proliferation activity are more likely to metastasize, PCNA levels in various malignant tumors, such as breast and duodenal cancers, are commonly found significantly higher than the levels in normal tissues.[21,22] PCNA expression is almost ubiquitous in mammals. Tumorigenesis results from unrestricted proliferation and suppression of apoptosis.[23,24] PCNA levels in proliferating cells cycle. PCNA is rarely expressed in stationary cells and begin to be synthesized in the G1 phase, peaking in the S phase, and significantly decreasing in the M and G2 phases.[25] Juríková et al have found that breast cancer patients with clinical stage III-IV and lymph node metastasis increased have increased levels of PCNA protein.[26] These observations all show that the expression of PCNA has a significant correlation with cell proliferation, and accordingly, PCNA can be used as an endogenous marker to determine the proliferative activities of cells.
Although PCNA is a good indicator of cell proliferation and is used in the prognoses of cancer patients, the correlation between PCNA expression and NSCLC remains unclear. Clarification of the relationship between PCNA and NSCLC can facilitate the prevention, diagnosis, and treatment of this debilitating disease. Accordingly, in this study, we aimed to evaluate the role and prognostic value of PCNA in NSCLC.
2. Materials and methods
2.1. Patients and controls
We retrospectively analyzed a cross-sectional group of 280 eligible NSCLC patients selected from a total of 348 patients from the Fifth People's Hospital of Wuxi, the First People's Hospital of Yancheng City, Linyi Traditional Hospital, Yancheng Hospital of Traditional Chinese Medicine, and the First Affiliated Hospital of Zhejiang Chinese Medical University between January, 2013 and January, 2017. Figure 1 shows the flow chart of the screening procedure for the NSCLC patients from these 5 hospitals. All patients underwent surgical resection. The inclusion and exclusion criteria in diagnosing NSCLC were as follows:
Figure 1.
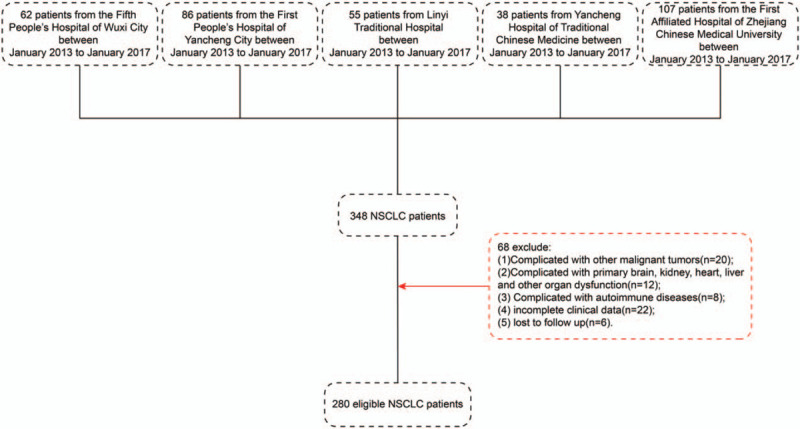
Screening flow chart for non-small cell lung cancer patients.
Inclusion criteria:
-
(1)
NSCLC had been diagnosed clinically and pathologically;
-
(2)
paraffin specimens were available;
-
(3)
the patients had received no preoperative radiotherapy or chemotherapy; and
-
(4)
the complete clinical data were available.
Exclusion criteria:
-
(1)
presence of other malignant tumors;
-
(2)
presence of primary organ dysfunction;
-
(3)
presence of an autoimmune disease;
-
(4)
incomplete clinical data; or
-
(5)
the patients lost during the follow-up.
Among the selected 280 NSCLC patients (aged 30–88 years, mean age of 59 years), 185 were males and 95 were females. The pathological types of the tumors were squamous cell carcinoma (125 cases), adenocarcinoma (91 cases), and adenosquamous carcinoma (64 cases). There were 106 and 174 cases with tumor size <5 cm and >5 cm, respectively. Poor, high-to-moderate, grade I–II, or grade III–IV differentiation was observed in 195, 85, 65, and 215 cases, respectively. Adjacent tissue specimens were collected from 120 paired adjacent tissues (aged 36–90 years, mean age of 55 years) to serve as a control group.
The follow-up results for the 280 patients enrolled in this study were obtained from the telephone interviews. Postoperative follow-ups were performed every 2, 3, and 6 months in the first, second, and third years, respectively, and then every 8 months until mortality. All the specimens were obtained under informed consent upon the approvals of the Ethics Committees of the 5 hospitals (Identification No. HMU (Ethics) 2017-k-133 and 2017003).
2.2. Immunohistochemical (IHC) staining
IHC was performed to analyze the cellular distribution of PCNA. The Envision and DAB chromogenic reagent kit (antibody diagnostic inc, ADI) were used to immunohistochemical staining. Paraffin specimens were de-waxed in xylene, and successively rehydrated with washes of 100%, 95%, 85%, and 75% ethanol. Antigen retrieval was performed with the citrate buffer. The samples were incubated in 10% goat serum for 30 minutes at about 25°C against non-specific antibody-binding. Subsequently, they were incubated with the rabbit anti-PCNA polyclonal antibodies (1:200) (Abcam Company) overnight and then with the secondary antibody solution for 1 hour at 4°C. Afterward, the samples were washed with the DAB solution (Jinqiao Company, China) for 5 minute and stained with hematoxylin for 20 seconds at about 25°C. Finally, they were washed with 1% hydrochloric acid in ammonia water and mounted with PBS buffer. The samples were examined under an optical microscope, and the proportion of positive area was calculated.
The IHC results were determined by 2 pathologists, who scored the NSCLC samples and adjacent tissues and considered the cells with stained nuclear granules positive. Each slice was randomly selected and 10 visual fields per slice were examined and 100 tumor cells were counted in each field under a light microscope. Staining score criteria were as follows: negative (–), no brown-yellow staining in tumors or glandular epithelial cells; weakly positive (+), the number of positive cells was <25%; positive (++), the number of positive cells ranged from 25% to 50%; highly positive (+++), the number of positive cells was >50%. For the convenience of statistical analyses, negative (-) or weakly positive (+) are defined as low expression, positive (+ +) or strongly positive (+ + +) are defined as high expression.
2.3. Western blotting
The tissue sample was homogenized and lysed by RIPA lysate (10:1) (Beyotime Biotech, Haimen, China), centrifuged, and the supernatant was harvested as the total protein sample. The protein concentration of each extract was determined by the BCA kit (Beyotime Biotech, China). Concentrated gum and separating gum were made and water-sealed for 30 minutes. Gel electrophoresis of twelve alkylbenzene sulfonate was carried out for 1 to 2 hours, and then wet to 30 to 50 minutes. Five percent skimmed milk powder was sealed for 1 hour, and the membranes were incubated with polyclonal antibodies (rabbit anti-human PCNA, dilution 1:100) overnight at 4°C. On the second day, the membrane was incubated with the secondary antibodies (dilution 1:100; Beyotime Biotech, Haimen, China)) at about 25°C for 1 to 2 hours. Exposure in gel imaging system.
2.4. Semi-quantity reverse transcription polymerase chain reaction
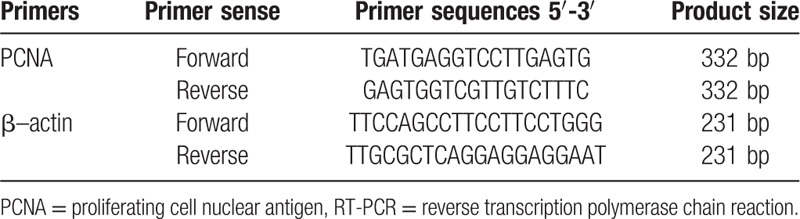
Total RNA was isolated from tissues using the TRIzol reagent (BioTeke, Beijing, China) and quantified by Nanodrop spectrophotometer. Total RNA (4 μg per sample) was isolated and used to generate the complementary DNA, which was then amplified by semi-quantitative PCR with β-actin as reference. The sequences of the primers used in this study are provided in Table 1. Thermal cycling conditions were as follows: pre-denaturation at 94°C for 30 seconds, amplification at 51°C for 35 seconds and 72°C for 65 seconds, totaling 30 cycles, and final extension at 72°C for 5 minutes. The amplicons were examined by agarose gel electrophoresis using a Quantity-One electrophoresis apparatus. The absorbance (a) values of the belt and the reference were determined, and the results were expressed by the ratio (sample value/reference value). If the ratio of NSCLC value and reference value was greater than the value, it was expressed positively. Otherwise, it was negative.
Table 1.
Primer sequences for RT-PCR analysis.
2.5. Statistical methods
SPSS13.0 statistical software was used for statistical analysis. The χ2 test was used to compare the association between the expression status of PCNA and clinicopathological parameters in cancer and adjacent tissues. The Kaplan-Meier survival analysis was performed to analyze disease-specific and -free survival rates. The log-rank test was performed to analyze the difference in survival curves. The multivariable regression analysis was performed to detect prognostic factors using the Cox proportional hazards model. A value of P < .05 was considered statistically significant. Areas under the receiver operating characteristics (AUROCs) were calculated to evaluate the value of PCNA mRNA for predicting the 3-year survival of NSCLC patients.
3. Results
3.1. PCNA protein expression in NSCLC and adjacent healthy tissues
IHC was used to evaluate the expression of PCNA in NSCLC and adjacent tissues. PCNA proteins were mainly found in the nucleus of NSCLC cells. The PCNA protein level in NSCLC tissues was significantly higher than in adjacent tissues (Fig. 2A-D). The positive expression rates of PCNA protein in NSCLC and adjacent tissues were 91.79% (257/280) and 25.83% (31/120), respectively (P < .05; Fig. 2E). Western blotting also revealed that PCNA protein level in NSCLC tissues was significantly higher than that in the adjacent tissues (P < .05; Fig. 3A).
Figure 2.

Immunohistochemical analysis of NSCLC and adjacent tissues for PCNA expression. ∗∗P < .05. Weak PCNA expression in adjacent tissues at 100× (A) and 400× (B) magnification. Strong PCNA expression in NSCLC tissues at 100× (C) and 400× (D). The expression rates of PCNA protein in NSCLC and adjacent tissues (E). NSCLC = non-small cell lung cancer, PCNA = proliferating cell nuclear antigen.
Figure 3.
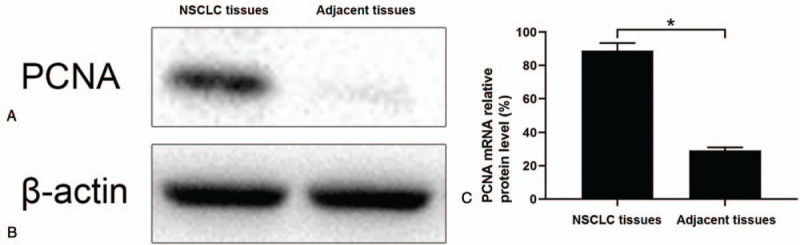
The expression of PCNA protein and mRNA in NSCLC and adjacent tissues. (A) Western blot showing the expression of PCNA protein in NSCLC and adjacent tissues. (B) Semi-quantity reverse transcription polymerase chain reaction results showing the expression of PCNA mRNA in NSCLC and adjacent tissues. NSCLC = non-small cell lung cancer, PCNA = proliferating cell nuclear antigen.
3.2. PCNA mRNA levels in NSCLC and adjacent healthy tissues
Next, we evaluated PCNA mRNA levels in NSCLC and adjacent tissues by reverse transcription polymerase chain reaction. The results displayed that the positive rate of PCNA mRNA in NSCLC tissues was 88.93% (249/280), which was significantly higher than that in adjacent tissues 29.17% (35/120) (P < .05; Fig. 3B).
3.3. Relationship between PCNA mRNA and protein levels and clinicopathological features in NSCLC
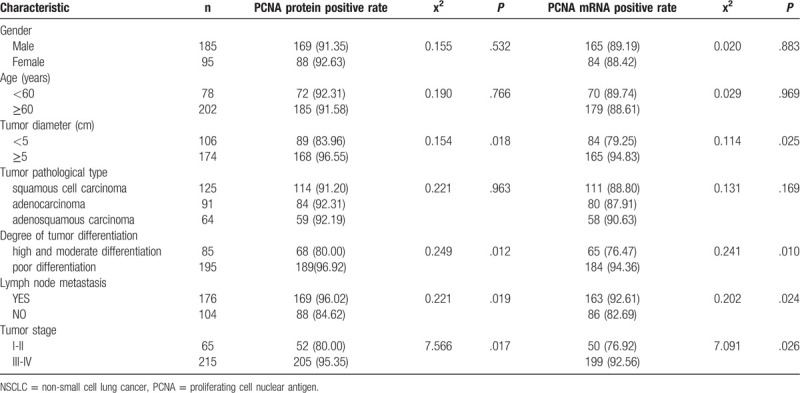
PCNA mRNA and protein levels in NSCLC tissues were consistently extremely high in NSCLC tissues. Single factor analysis showed both PCNA mRNA and protein are not correlated with gender, age, or tumor pathological type (all P > .05), but related to tumor size, stage, tumor metastasis, and the degree of tumor differentiation (all P < .05; Table 2).
Table 2.
Correlation of PCNA protein and gene expression with clinicopathological features in NSCLC [N(%)].
3.4. Prognostic values of the expression status of PCNA protein for the overall survival of postoperative NSCLC patients
We performed follow-up examinations up to 4 years. Patients with high PCNA protein levels in the NSCLC tissues had a significantly shorter disease-specific survival rate than the other patients. Kaplan-Meier survival analysis showed that there was statistically significant difference between these 2 groups of patients (P < .001) (Fig. 4). Among the 228 patients exhibiting high PCNA protein levels in the NSCLC tissues, 206 patients died, whereas among the 52 patients with low PCNA protein levels, 29 patients died. The median survival times of the patients with high and low PCNA levels were (12.0 + 1.2) and (24.6 + 6.8) months, respectively, indicating that high expression of PCNA affected the prognosis of NSCLC patients.
Figure 4.

Proliferating cell nuclear antigen expression and survival analyses of the non-small cell lung cancer patients.
Multi-factor Cox survival analysis showed that PCNA protein level, tumor pathological type, metastasis, stage, and differentiation degree were independent factors affecting the overall survival of postoperative patients. Additionally, gender, age, and tumor size were not independent factors affecting the overall survival of postoperative patients (Fig. 5).
Figure 5.
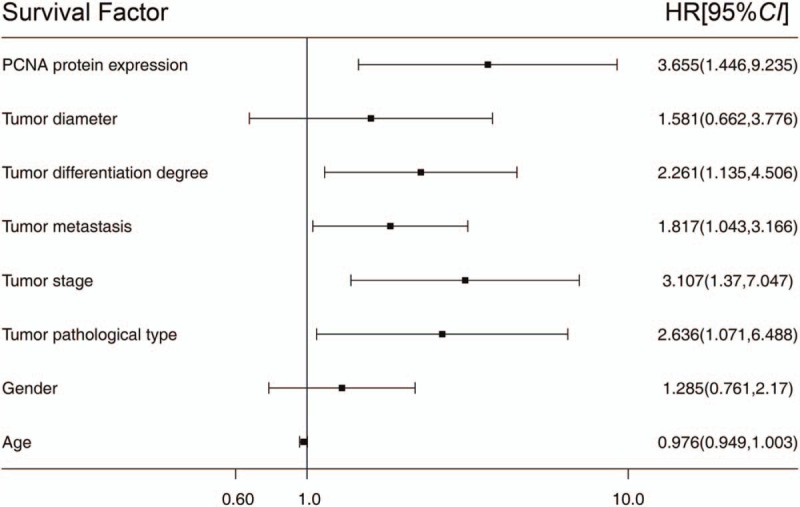
Correlation between the proliferating cell nuclear antigen expression and prognosis of the non-small cell lung cancer patients: Multi-factor Cox survival.
3.5. The accuracy of predicting the three-year survival of NSCLC patients via PCNA mRNA levels
To further clarify the role of PCNA in the overall survival of postoperative patients, AUROCs were calculated to evaluate the value of PCNA mRNA in predicting the 3-year survival of NSCLC patients. The AUROC of PCNA mRNA in predicting the 3-year survival was 0.89 (0.79–0.98), with a sensitivity of 0.84 and specificity of 0.76 (Fig. 6).
Figure 6.
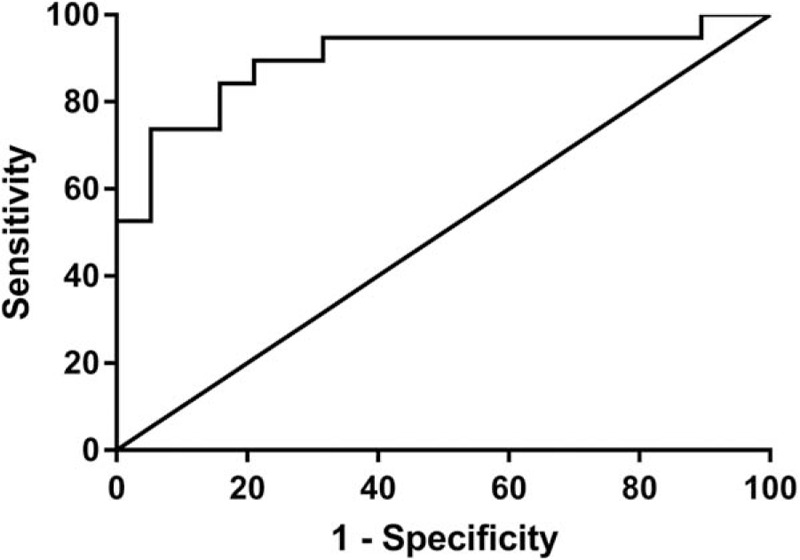
The areas under the receiver operating characteristics of proliferating cell nuclear antigen mRNA for predicting the 3-year survival of non-small cell lung cancer patients.
4. Discussion and conclusions
PCNA is considered as an important prognostic indicator of cancer. Its expression has been found to be significantly higher in various malignant tumors, such as breast and duodenal cancers than in normal tissues.[21] PCNA protein level increases as the pathological grade, TNM stage, and the number of lymph node metastases increase in breast cancer. In addition, PCNA expression has been shown to be related to the degree of cell proliferation in oral mucosal lesions and to the histological grade of oral precancerous lesions.[27,28] During oral mucosal epithelial tumorigenesis, PCNA level gradually increases up to 4- to 10-fold. Only the PCNA labeling index increased significantly, and the amount of PCNA per labeled cell also increased. Mumbuc et al[29] have also found that the PCNA protein levels in nasal and paranasal sinus cancer tissues were significantly higher than that in normal nasal mucosa tissues, and the proportion of PCNA-positive cells increases with the increase of the histological grade, indicating that the expression of PCNA might be related to the nasal and paranasal sinus tumorigenesis. All these changes are consistent with the temporal changes in DNA replication, confirming that the expression of PCNA is closely related to the cell cycle. Therefore, changes in PCNA levels can reflect the changes in cell dynamics and represent the proliferative potentials of cells, and it can be used as an alternative endpoint marker for the chemoprevention of cancer.
In our study, we investigated the significance of PCNA expression in NSCLC and adjacent healthy tissues. Our study confirmed that PCNA protein was localized in the nucleus of NSCLC cells. This study revealed that the levels of both PCNA mRNA and protein were higher in NSCLC than in adjacent tissues, presumably indicating a relationship between high PCNA expression and tumorigenesis in NSCLC. The relationship between PCNA expression and the clinical pathological features of NSCLC patients are consistent. Our study also found that the expression of PCNA in NSCLC was not related to gender, age, or tumor pathological type. Both PCNA protein and mRNA levels were related to tumor differentiation, size, stage, and metastasis in NSCLC. The levels of PCNA protein and mRNA in the poor differentiation group and lymph node metastasis group were significantly higher than those in the high and moderate differentiation groups and without lymph node metastasis group. The levels of PCNA protein and mRNA in stage III-IV group and tumor size ≥5 cm group were significantly higher than those in stage I-II group and tumor size <5 cm group. All of these indicate that PCNA may play an important role in the formation, invasion, and metastasis of NSCLC.
Moreover, we further studied the relationship between the expression of PCNA and overall survival of NSCLC patients. The median survival time of the patients with high PCNA expression was significantly shorter than those with low expression. Multi-factor Cox survival analysis showed that PCNA protein level and tumor stage, differentiation degree, pathological type, and metastasis were independent factors affecting the overall survival of NSCLC patients. All these results revealed that PCNA expression was related to the prognosis of NSCLC patients. Of note, PCNA mRNA had a good AUROC in predicting the three-year survival, with a sensitivity of 0.84 and specificity of 0.76, suggesting that PCNA mRNA can be used to predict the prognosis of NSCLC patients effectively.
The present study also had a few limitations that warrant consideration. First, all the patients were enrolled from 5 hospitals in different regions of China. Therefore, the study may suffer from selection bias or heterogeneity among these hospitals. Therefore, although this study is the largest among its peers that have addressed PCNA protein expression in NSCLC, validation in more patients is necessary. Next, the ability of PCNA mRNA as a clinical predictor for the 3-year survival of NSCLC patients needs to be further validated. In summary, our study revealed that high PCNA protein and mRNA levels were closely related to the occurrence, development, and prognosis of NSCLC. Therefore, the study results presented here may facilitate the development of new therapeutic and diagnostic strategies in NSCLC.
Author contributions
XY and BL contributed to study concept and design, acquisition of data, analysis, interpretation of data, and critical revision of the manuscript. HX contributed to study concept, design, and draft of the manuscript. GL and XZ contributed to acquisition of data, analysis and interpretation of data. JL and LL contributed to study concept and design and critical revision of the manuscript.
Conceptualization: Xiaolan Ye, Hanrong Xu, jun liu.
Data curation: Xinguo Zhao, Jiangyan Xu.
Formal analysis: Xiaolan Ye, Bai Ling, Liangeng Liu.
Funding acquisition: Jiangyan Xu, jun liu.
Investigation: Xiaolan Ye, Bai Ling, Hanrong Xu, Gongqi Li, Jiangyan Xu, jun liu, Liangeng Liu.
Methodology: Xiaolan Ye, Hanrong Xu.
Project administration: Hanrong Xu.
Resources: Xiaolan Ye, Bai Ling, Hanrong Xu, Gongqi Li, Xinguo Zhao, Liangeng Liu.
Validation: Gongqi Li, Xinguo Zhao, Jiangyan Xu.
Writing – review & editing: Hanrong Xu, Jiangyan Xu, jun liu.
Footnotes
Abbreviations: AUROC = areas under the receiver operating characteristics, IHC = immunohistochemistry, NSCLC = non-small cell lung cancer, PCNA = proliferating cell nuclear antigen.
How to cite this article: Ye X, Ling B, Xu H, Li G, Zhao X, Xu J, Liu J, Liu L. Clinical significance of high expression of proliferating cell nuclear antigen in non-small cell lung cancer. Medicine. 2020;99:16(e19755).
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
XY and BL contributed equally to this work.
This research was supported by the Youth Medical Talent of Jiangsu Province (grant no. QNRC2016163 to JL) and Zhejiang Provincial Natural Science Foundation (grant no. LY19H290005 to JX).
The present study was approved by The Ethics Committees of the First People's Hospital of Yancheng (Yancheng, China) and the Fifth People's Hospital of Wuxi (Wuxi, China), Linyi Traditional Hospital(Yancheng, China), Yancheng Hospital of Traditional Chinese Medicine (Linyi, China), and the First Affiliated Hospital of Zhejiang Chinese Medical University (Hangzhou, China) and was performed in accordance with the Declaration of Helsinki. Informed consent was obtained from each patient.
The authors have no conflicts of interests to disclose.
References
- [1].Herbst RS, Arkenau HT, Santana-Davila R, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol 2019;20:1109–23. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [2].Cao F, Gong YB, Kang XH, et al. Degradation of MCL-1 by bufalin reverses acquired resistance to osimertinib in EGFR-mutant lung cancer. Toxicol Appl Pharmacol 2019;379:114662.[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [3].Bruntz RC, Belshoff AC, Zhang Y, et al. Inhibition of anaplerotic glutaminolysis underlies selenite toxicity in human lung cancer. Proteomics 2019;19:e1800486.[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol 2019; doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang X, Li W, Zhang N, et al. Opportunities and challenges of co-targeting epidermal growth factor receptor and autophagy signaling in non-small cell lung cancer. Oncol Lett 2019;18:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Patro M, Gothi D, Vaidya S, et al. A “triple whammy” in adenocarcinoma lung. Lung India 2019;36:340–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Huemer F, Lang D, Westphal T, et al. Baseline absolute lymphocyte count and ECOG performance score are associated with survival in advanced non-small cell lung cancer undergoing PD-1/PD-L1 blockade. J Clin Med 2019;8:pii: E1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim YJ, Kim JH, Kim O, et al. Caveolin-1 enhances brain metastasis of non-small cell lung cancer, potentially in association with the epithelial-mesenchymal transition marker SNAIL. Cancer Cell Int 2019;19:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Qi MM, Ge F, Chen XJ, et al. MiR-124 changes the sensitivity of lung cancer cells to cisplatin through targeting STAT3. Eur Rev Med Pharmacol Sci 2019;23:5242–50. [DOI] [PubMed] [Google Scholar]
- [10].Wang T, Jing B, Sun B, et al. Stabilization of PTGES by deubiquitinase USP9X promotes metastatic features of lung cancer via PGE2 signaling. Am J Cancer Res 2019;9:1145–60. [PMC free article] [PubMed] [Google Scholar]
- [11].Hung MS, Wu YF, Chen YC. Efficacy of chemoradiotherapy versus radiation alone in patients with inoperable locally advanced non-small-cell lung cancer: a meta-analysis and systematic review. Medicine (Baltimore) 2019;98:e16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhai G, Li G, Xu B, et al. miRNA-148b regulates radioresistance in non-small lung cancer cells via regulation of MutL homologue 1. Biosci Rep 2016;36:pii: e00354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [13].Zhang X, Wei L, Li J, et al. Epithelial-mesenchymal transition phenotype of circulating tumor cells is associated with distant metastasis in patients with NSCLC. Mol Med Rep 2019;19:601–8. [DOI] [PubMed] [Google Scholar]
- [14].Missel M, Borregaard B, Schoenau MN, et al. A sense of understanding and belonging when life is at stake-Operable lung cancer patients’ lived experiences of participation in exercise. Eur J Cancer Care (Engl) 2019;28:e13126. [DOI] [PubMed] [Google Scholar]
- [15].Qiu X, Wang H, Wang Z, et al. Expression of PCNA, Ki-67 and COX-2 in breast cancer based on DCE-MRI image information. J Infect Public Health 2019;doi: 10.1016/j.jiph.2019.06.024. [DOI] [PubMed] [Google Scholar]
- [16].Sato F, Bhawal UK, Tojyo I, et al. Differential expression of claudin-4, occludin, SOX2 and proliferating cell nuclear antigen between basaloid squamous cell carcinoma and squamous cell carcinoma. Mol Med Rep 2019;20:1977–85. [DOI] [PubMed] [Google Scholar]
- [17].Jimenji T, Matsumura R, Kori S, et al. Structure of PCNA in complex with DNMT1 PIP box reveals the basis for the molecular mechanism of the interaction. Biochem Biophys Res Commun 2019;516:578–83. [DOI] [PubMed] [Google Scholar]
- [18].Tehseen M, Raducanu VS, Rashid F, et al. Proliferating cell nuclear antigen-agarose column: a tag-free and tag-dependent tool for protein purification affinity chromatography. J Chromatogr A 2019;1602:341–9. pii: S0021-9673(19)30607-7. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [19].Qian J, Chen Y, Xu Y, et al. Interactional similarities and differences in the protein complex of PCNA and DNA replication factor C between rice and Arabidopsis. BMC Plant Biol 2019;19:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zheng C, Yang R. RCD24, B7-H4 and PCNA expression and clinical significance in ovarian cancer. J BUON 2019;24:715–9. [PubMed] [Google Scholar]
- [21].Smith SJ, Gu L, Phipps EA, et al. A Peptide mimicking a region in proliferating cell nuclear antigen specific to key protein interactions is cytotoxic to breast cancer. Mol Pharmacol 2015;87:263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Imazu H, Kasahara M, Shirono K, et al. A study of DNA ploidy pattern, proliferation index and PCNA in duodenal carcinoma. Nihon Shokakibyo Gakkai Zasshi [Article in Japanese] 1992;89:1499–505. [PubMed] [Google Scholar]
- [23].Paul Solomon Devakumar LJ, Gaubitz C, Lundblad V, et al. Effective mismatch repair depends on timely control of PCNA retention on DNA by the Elg1 complex. Nucleic Acids Res 2019;47:6826–41. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yuan Y, Wang Y, Liu Z, et al. MAT2B promotes proliferation and inhibits apoptosis in osteosarcoma by targeting epidermal growth factor receptor and proliferating cell nuclear antigen. Int J Oncol 2019;54:2019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pradhan S, Kalia I, Roy SS, et al. Molecular characterization and expression profile of an alternate proliferating cell nuclear antigen homolog PbPCNA2 in Plasmodium berghei. IUBMB Life 2019;71:1293–301. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [26].Juríková M, Danihel Ľ, Polák Š, et al. Ki67, PCNA, and MCM proteins: markers of proliferation in the diagnosis of breast cancer. Acta Histochem 2016;118:544–52. [DOI] [PubMed] [Google Scholar]
- [27].Kaplan I, Hochstadt T, Dayan D. PCNA in palate and tongue mucosal dysplastic lesions induced by topically applied 4NQO in desalivated rat. Med Oral 2002;7:336–43. [PubMed] [Google Scholar]
- [28].da Silva Fonseca LM, do Carmo MA. Identification of the AgNORs, PCNA and ck16 proteins in oral lichen planus lesions. Oral Dis 2001;7:344–8. [DOI] [PubMed] [Google Scholar]
- [29].Mumbuc S, Karakok M, Baglam T, et al. Immunohistochemical analysis of PCNA, Ki67 and p53 in nasal polyposis and sinonasal inverted papillomas. J Int Med Res 2007;35:237–41. [DOI] [PubMed] [Google Scholar]


