Abstract
To determine the levels of parathyroid hormone (PTH) in the fluids of various tissues for identification of parathyroid glands during thyroidectomy.
Our study comprised 31 patients with thyroid cancer who underwent lobectomy with central compartment dissection at our hospital from October 2014 to February 2015. A total of 186 tissue samples, including 28 from parathyroid glands and 158 from non-parathyroid tissues, were obtained during the operations. Tissue fluids were collected via fine-needle aspiration to measure PTH levels; the tissue was punctured 3 times with a 26-gauge syringe needle and washed with 0.5 mL normal saline. Tissues were also prepared for pathological examination.
PTH concentrations were significantly higher in parathyroid tissues than non-parathyroid tissues. None of the patients had irremediable parathyroid dysfunction after surgical resection.
Use of fine-needle aspiration for quantification of PTH levels in tissue fluids rapidly, safely, and effectively identifies the parathyroid glands during thyroidectomy.
Keywords: central compartment lymph node dissection, intraoperative parathyroid hormone levels, parathyroid preservation
1. Introduction
Hypoparathyroidism after thyroidectomy is likely and can be detrimental to the patient. Its occurrence can be prevented by protecting the parathyroid glands during surgery; hence, accurate identification of these glands is important. Herein we examine the practicability, accuracy, and safety of fine-needle aspiration as a means of rapidly quantifying parathyroid hormone (PTH), which aids in identifying the parathyroid glands during thyroidectomy.
2. Materials and methods
2.1. Data
Our study comprised 31 patients who underwent surgery for thyroid cancer at our hospital. The surgeries were performed by the same group of doctors between October 2016 and February 2017. Six patients were men and 25 were women, and the median age was 44 years (range, 28–67 years).
The operation plans were in accordance with the Guidelines on the Diagnosis and Treatment of Thyroid Nodules and Differentiated Thyroid Carcinomas.[1] The procedures performed were hemi-thyroidectomy with unilateral central compartment lymph node dissection (16 patients), total thyroidectomy with central compartment lymph node dissection (10 patients), and total thyroidectomy with bilateral neck central compartment lymph node dissection (5 patients). The postoperative pathology showed no evidence of thyroid papillary carcinoma. In the 6 months after surgery, none of the patients developed hypocalcemia, and serum levels of PTH, calcium, and phosphate (PHOS) were within normal ranges.[2]
Because the postoperative pathology was clear of thyroid papillary carcinoma, hemi-thyroidectomy with unilateral central compartment lymph node dissection was performed, reserving 1 or more parathyroid gland. Thirty-one patients met the following conditions: no previous neck surgery or thyroidectomy; normal kidney function; no metabolic bone disease; no previous use of agents that affect calcium or PHOS metabolism (eg, calcium supplements, vitamin D, thiamine diuretics, anti-epileptic drugs); normal serum levels of PTH (15–65 ng/L), calcium (2.0– 2.8 mmol/L), and PHOS (0.7–1.5 mmol/L); and test materials collected from the parathyroid glands, thyroid gland, thymus, lymph nodes, fatty tissue, and muscles.
2.2. Methods
Tissue samples were collected from the central compartment of the neck via fine-needle aspiration. We used a 26-gauge syringe needle to puncture the tissue 3 times and 0.5 mL normal saline to extract the fluid from the punctured tissue. It is noteworthy that all steps were performed by a single person.
Use of a PTH kit from the Jiangsu Institute of Nuclear Medicine allowed us to quickly measure PTH concentrations in the tissue fluids. To assess the accuracy of the PTH measurements, we identified the tissue of origin via paraffin-based pathology. The following items were recorded: blood levels of PTH, calcium, and PHOS before and 1 day after surgery; the amount of PTH in the tissue fluid; the results of paraffin-based pathology; and the occurrence of hypocalcemia and irremediable hypoparathyroidism, as well as their treatments and outcomes.
2.3. Ethical approval
The study was approved by the ethics committee of the Jiangsu Institute of Nuclear Medicine in China (No.201602004). Informed consent was obtained from all study participants during the medical examination.
2.4. Statistical analyses
SPSS 18.0 software was used to analyze the results and to determine the sensitivity, specificity, and biochemical index of PTH levels for pathological diagnosis. To determine whether the quantitative data were normally distributed, we used the formula “χ2 ± S”. The exact probabilities test was used to compare PTH levels in the various tissue fluids. P < .05 was considered statistically significant.
3. Results
3.1. Blood biochemical examination
The blood indexes for the 31 patients in our study were all in the normal range before surgery: PTH, 22.87 to 65 pg/mL; calcium, 2.18 to 2.74 mmol/L; and PHOS, 0.84 to 1.45 mmol/L. The day after surgery, the values were as follows: PTH, 6.4 to 61.39 pg/mL (< 3 pg/mL in 3 patients); calcium, 1.87 to 2.44 mmol/L (< 2.0 mmol/L in 4 patients); and PHOS, 0.93 to 1.88 mmol/L (> 1.5 mmol/L in 7 patients). All values were within the normal range 6 months after surgery.
3.2. PTH levels in parathyroid and non-parathyroid tissue
The mean PTH concentration was significantly higher in parathyroid tissue (1431.84 ± 1388.75 pg/mL; range, 214–5000 pg/mL) than in non-parathyroid tissue (21.54 ± 5.46 pg/mL; range, 10.23–31.60 pg/mL) (P < .01). There was no significant difference in PTH levels among the non-parathyroid tissues (P > .05). These results are shown in Table 1 and Fig. 1.
Table 1.
Pathological results of tissue and results of PTH.
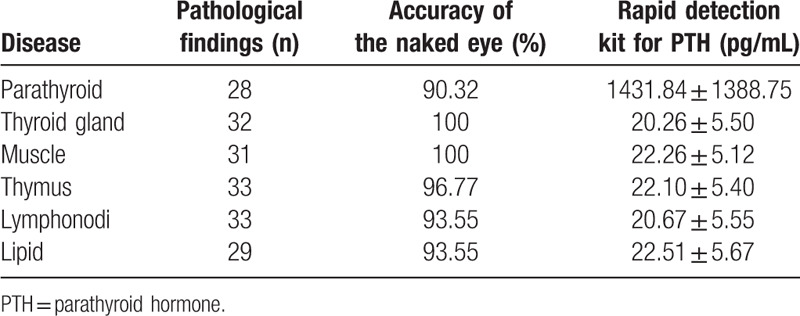
Figure 1.
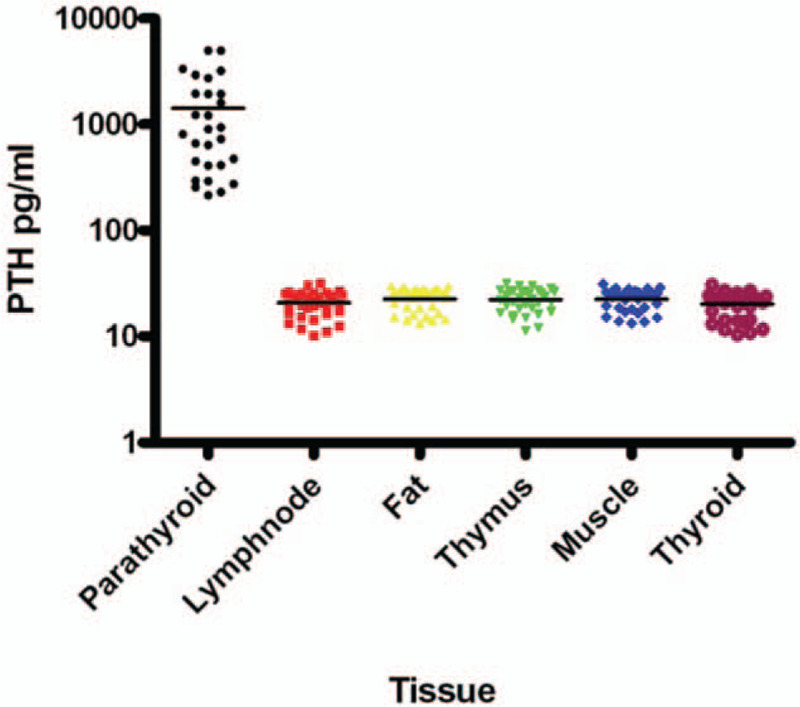
Results of PTH. PTH = parathyroid hormone.
3.3. Comparison of PTH levels in serum and tissues
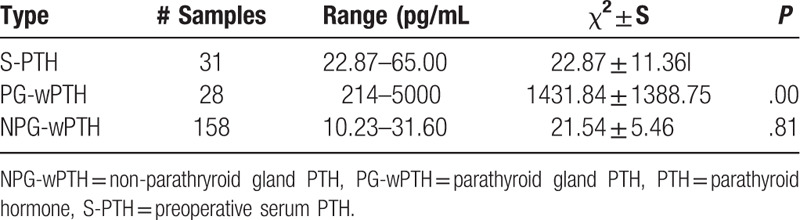
PTH levels were clearly lower in preoperatively collected serum than in the parathyroid tissue (P < .05) (Table 2). Although all PTH levels in non-parathyroid tissue were lower than the corresponding PTH levels in serum, the difference was not significant (P = .81).
Table 2.
Comparison of PTH levels in serum and tissue.
3.4. Sensitivity and specificity of tissue PTH levels for identification of the parathyroid glands
Among all 158 samples, the highest and lowest PTH levels in non-parathyroid tissue were 31.60 pg/mL and 214 pg/mL, respectively. Using an upper limit of 65 pg/mL as the cutoff value, the sensitivity of PTH for identifying parathyroid tissue was 28/28 (100%) and the specificity was 158/158 (100%).
4. Discussion
There has been a significant increase in the incidence of differentiated thyroid carcinoma in recent years. The current recommended thyroidectomy procedure is lobectomy with central compartment dissection,[1,22] and the most common postoperative complication is hypoparathyroidism. According to the literature, the postoperative rates of hypocalcemia and hypothyroidism are 19% to 38% and 0% to 3%, respectively.[3–5]
Protection of the parathyroid glands during surgery is the most effective means of preventing hypoparathyroidism, and for this, accurate quantification of PTH is key. However, the parathyroid glands, thyroid gland, muscles, lymph nodes, thymus, and fatty tissue can be difficult to distinguish. During thyroid surgery, surgeons can identify the parathyroid glands by their location, color, character, and vessels. In the presence of a large number of thyroid nodules, there is an increase in fatty infiltration of the central neck lymph nodes, and even experienced surgeons find it very difficult to distinguish the parathyroid glands.[7–9] Consequently, inadvertent parathyroidectomy can occur during thyroid surgery, with a reported incidence rate of 1% to 15%.[10] In our study, 28 of the 31 parathyroid glands examined were confirmed, whereas the remaining 3 were misidentified (the correct identities were lymph node, fatty, and thymus tissue).
Methods for identifying the parathyroid glands include indocyanine green-enhanced fluorescence,[11,12] use of a gamma probe,[13] use of carbon nanoparticles,[14,15] and intravenous injection of methylene blue.[16] However, these methods are still in the experimental stage or not ready for widespread use in clinical practice. Although use of frozen sections remains the gold standard, it has many problems: limited preoperative preparation time for tissue removal, a 20-minute intraoperative wait time, a 1% error rate, and technology- and equipment-related difficulties.[6,17,18] Obviously, we need a new method that is convenient, effective, and safe.
Monitoring hyperthyroidism by measuring PTH levels in intraoperatively obtained parathyroid gland aspirates was first reported in 2000.[19] Thereafter, multiple studies documented the rapidity, high diagnostic precision, and limited invasiveness of this method.[6,7,20,21] However, this method is rarely used to identify normal parathyroid glands during thyroid surgery because the normal parathyroid gland is too small to discern.[6] Haiyan et al[18] compared the results of intraoperative fine-needle aspiration with pathological findings in patients with normal parathyroid glands. A significant difference between PTH levels in parathyroid and non-parathyroid tissue was found.
In the present study, we used fine-needle aspiration to measure PTH levels in the parathyroid glands, thyroid gland, muscles, lymph nodes, thymus, and fatty tissue. The measurements were then compared with the pathology results. We found that PTH levels were much higher in parathyroid tissue than in non-parathyroid tissue (all P values < .05) and preoperatively collected serum (P > .05). There was no significant difference in PTH levels among the non-parathyroid tissues (P > .05) or between non-parathyroid tissue and preoperatively collected serum (P = .81). PTH levels were lower in non-parathyroid tissue than in the corresponding serum samples. Using an upper limit of 65 pg/mL as the cut-off PTH value, the sensitivity of PTH for identification of parathyroid tissue was 28/28 (100%) and the specificity was 158/158 (100%); these results accord with those reported by Abdelghani et al.[20]
Our study indicates that fine-needle aspiration is an effective method for measuring PTH levels during thyroidectomy. The combination of this rapidly performed procedure and the use of portable equipment will further facilitate the identification of the parathyroid glands in a convenient, accurate, and safe manner commensurate with the surgeon's needs. Moreover, it represents an entirely new approach to parathyroid gland identification. Using fluorescent microsphere immunochromatography, we can measure PTH concentrations in fine-needle tissue aspirates in 5 minutes. The benefits of this methodology include better protection of the parathyroid glands during surgery, reduced rates of hypothyroidism, shorter hospital stays, and lower medical costs.[11,12] Therefore, the method described herein is worth popularizing.
Author contributions
Conceptualization: Liguo Zhu, Jiandong Bao, Bin Zhou, Zhongwei Lv.
Project administration: Bin Zhou, Zhongwei Lv.
Resources: Guohua Zhu, Liguo Zhu, Jiandong Bao, Hong Yong Hu.
Software: Jun Fan.
Writing – original draft: Xian Zou, Zhongwei Lv.
Writing – review and editing: longshun shi.
longshun shi orcid: 0000-0002-3944-3146.
Footnotes
Abbreviations: PHOS = phosphate, PTH = parathyroid hormone.
How to cite this article: Zou X, Shi L, Zhu G, Zhu L, Bao J, Fan J, Hu Y, Zhou B, Lv Z. Fine-needle aspiration with rapid parathyroid hormone assay to identify parathyroid gland in thyroidectomy. Medicine. 2020;99:16(e19840).
XZ and LS contributed equally to this work.
This work was supported by the State Key Laboratory of Materials-Oriented Chemical Engineering (KL17-11) and Capital Project of Science and Technology Development in Wuxi (WX03-02B0105-071700-55).
The authors have no conflicts of interest to disclose.
References
- [1].Ming Gao, Weiping Teng, Yongfeng Liu. Guidelines on the diagnosis and treatment of thyroid nodules and differentiated thyroid carcinomas. Chin J Clin Oncol 2012;29:1249–72. [Google Scholar]
- [2].Carty SE, Cooper DS, Doherty GM, et al. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer: the American thyroid association surgery working group with participation from the American association of endocrine surgeons, American academy of otolaryngology-head and neck surgery, and American head and neck society. Thyroid 2009;19:1153–8. [DOI] [PubMed] [Google Scholar]
- [3].Edafe O, Antakia R, Laskar N, et al. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg 2014;101:307–20. [DOI] [PubMed] [Google Scholar]
- [4].Paek SH, Lee YM, Min SY, et al. Risk factors of hypoparathyroidism following total thyroidectomy for thyroid cancer. World J Surg 2013;37:94–101. [DOI] [PubMed] [Google Scholar]
- [5].Duclos A, Peix JL, Colin C, et al. Influence of experience on performance of individual surgeons in thyroid surgery: prospective cross sectional multicentre study. BMJ 2012;344:d8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pelizzo MR, Losi A, Boschin IM, et al. Rapid intraoperative parathyroid hormone assay in fine needle aspiration for differential diagnosis in thyroid and parathyroid surgery. Clin Chem Lab Med 2010;48:1313–7. [DOI] [PubMed] [Google Scholar]
- [7].Chan RK, Ibrahim SI, Pil P, et al. Validation of a method to replace frozen section during parathyroid exploration by using the rapid parathyroid hormone assay on parathyroid aspirates. Arch Surg 2005;140:371–3. [DOI] [PubMed] [Google Scholar]
- [8].Kiblut NK, Cussac JF, Soudan B, et al. Fine needle aspiration and intraparathyroid intact parathyroid hormone measurement for reoperative parathyroid surgery. World J Surg 2004;28:1143–7. [DOI] [PubMed] [Google Scholar]
- [9].Guerrero MA, Suh I, Vriens MR, et al. The number of needle passes affects the accuracy of parathyroid hormone assay with intraoperative parathyroid aspiration. Am J Surg 2010;200:701–6. [DOI] [PubMed] [Google Scholar]
- [10].Abboud B, Sleilaty G, Braidy C, et al. Careful examination of thyroid specimen intraoperatively to reduce incidence of inadvertent parathyroidectomy during thyroid surgery. Arch Otolaryngol Head Neck Surg 2007;133:1105–10. [DOI] [PubMed] [Google Scholar]
- [11].Suh YJ, Choi JY, Chai YJ, et al. Indocyanine green as a near-infrared fluorescent agent for identifying parathyroid glands during thyroid surgery in dogs [J]. Surg Endosc 2015;29:2811–7. [DOI] [PubMed] [Google Scholar]
- [12].Lavazza M, Liu X, Wu C, et al. Indocyanine green-enhanced fluorescence for assessing parathyroid perfusion during thyroidectomy[J]. Gland Surg 2016;5:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pederson LC, Shapiro SE, Fritsche HA, et al. Potential role for intraoperative gamma probe identification of normal parathyroid glands [J]. Am J Surg 2003;186:711–7. [DOI] [PubMed] [Google Scholar]
- [14].Yu W, Zhu L, Xu G, et al. Potential role of carbon nanoparticles in protection of parathyroid glands in patients with papillary thyroid cancer[J]. Medicine 2016;95:23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gu J, Wang J, Nie X, et al. Potential role for carbon nanoparticles identification and preservation in situ of parathyroid glands during total thyroidectomy and central compartment node dissection[J]. Int J Clin Exp Med 2015;8:9640. [PMC free article] [PubMed] [Google Scholar]
- [16].Patel HP, Chadwick DR, Harrison BJ, et al. Systematic review of intravenous methylene blue in parathyroid surgery. Br J Surg 2012;99:1345–51. [DOI] [PubMed] [Google Scholar]
- [17].Clark OH, Gooding GA, Ljung BM. Locating a parathyroid adenoma by ultrasonography and aspiration biopsy cytology [J]. West J Med 1981;135:154. [PMC free article] [PubMed] [Google Scholar]
- [18].Huang H, et al. [Fine-needle aspiration with measurement of parathyroid hormone levels in thyroidectomy]. Chinse Journal of Otorhinolaryngology-skull Base Surgery (GJOSS) 2013;48:934–8. [PubMed] [Google Scholar]
- [19].Perrier ND, Ituarte P, Kikuchi S, et al. Intraoperative parathyroid aspiration and parathyroid hormone assay as an alternative to frozen section for tissue identification[J]. World J Surg 2000;24:1319–22. [DOI] [PubMed] [Google Scholar]
- [20].Abdelghani R, Noureldine S, Abbas A, et al. The diagnostic value of parathyroid hormone washout after fine-needle aspiration of suspicious cervical lesions in patients with hyperparathyroidism [J]. The Laryngoscope 2013;123:1310–3. [DOI] [PubMed] [Google Scholar]
- [21].Horányi J, Duffek L, Szlávik R, et al. Intraoperative determination of PTH concentrations in fine needle tissue aspirates to identify parathyroid tissue during. [DOI] [PubMed] [Google Scholar]
- [22].National Comprehensive Cancer Network. (NCCN) Clinical Practice Guidelines in Oncology. Thyroid Cancer, Version 1 2016. [Google Scholar]


