Abstract
Background:
This meta-analysis focuses on the controversial efficacy and safety of platelet-rich plasma (PRP) as compared with hyaluronic acid (HA) in the clinical treatment of knee osteoarthritis. We have attempted to provide an evidence-based medicine protocol for the conservative treatment of knee osteoarthritis. In addition, we included the latest relevant literature in this meta-analysis, and a staging study was conducted to compare the therapeutic effects of PRP and HA for knee osteoarthritis over different time periods.
Methods:
An online computer search with “platelet-rich plasma” and “knee osteoarthritis” as search terms was conducted in the PubMed, EMBASE, and Cochrane Library databases. We conducted a quality assessment of the retrieved literature and extracted the following indicators: visual analog scale (VAS) score, subjective International Knee Documentation Committee (IKDC) score, Western Ontario and McMaster Universities (WOMAC) score, Knee Injury and Osteoarthritis Outcome Score (KOOS), and adverse events. RevMan5.3 software was used to determine the effect sizes, and indicators were compared across studies at three different time points from the administration of treatment.
Results:
A total of 14 randomized controlled trials (RCTs) involving 1350 patients were included. Long-term VAS, IKDC, WOMAC-Pain, WOMAC-Stiffness, WOMAC-Physical Function, and WOMAC-Total scores at each time point were higher in the PRP group than in the HA group. There were no significant differences in the remaining indicators between the two groups.
Conclusion:
Compared with HA, PRP offers obvious advantages in the conservative treatment of knee osteoarthritis. Treatment with PRP can reduce long-term pain and improve knee joint function with no additional risks. Therefore, PRP can be widely used for the conservative treatment of knee osteoarthritis.
Keywords: hyaluronic acid, knee osteoarthritis, meta-analysis, platelet-rich plasma
1. Introduction
Knee osteoarthritis is a common joint disease affecting middle-aged and older adults. Its symptoms include pain and limited range of motion in the knee and stiffness of the knee joint.[1,2] To date, there is no complete cure, and current treatment aims to delay symptoms, relieve pain, and improve motor function.[3] Total knee arthroplasty is generally used for treating advanced knee osteoarthritis. However, sometimes arthroplasty cannot be performed due to various co-morbidities, age restrictions and quality of the materials used. Moreover, the replacement joint has a certain service life, and may need to be renovated in the later stage, so it is necessary to avoid joint replacement as far as possible, or delay the time of joint replacement as much as possible.[4] Conservative treatment is preferred for early stage knee osteoarthritis, which can delay the need for arthroplasty. As a common conservative treatment, intra-articular injection of hyaluronic acid (HA) can regulate vascular permeability, lubricate the joints, reduce joint loading, and promote wound healing.[5,6]
In recent years, there has been increasing attention focused on the intra-articular injection of platelet-rich plasma (PRP). PRP is a concentrate of platelets derived from whole blood by centrifugation that contains a large amount of proteins and growth factors, including platelet-derived factors and transforming growth factor β. It is believed to support various important physiological functions such as anti-inflammation,[7] analgesia,[8] pro-proliferation of chondrocytes, and cartilage repair.[9–12]
In plastic surgery, PRP, which has been used extensively, has been shown to delay aging and enhance cell viability.[13–15] However, its role in the treatment of knee osteoarthritis has not yet been clarified. To this end, a large number of clinical trials and meta-analyses have been conducted, but a published meta-analysis showed high heterogeneity because of the concurrent combination of PRP with autologous PRP and plasma rich in growth factors.[16] Moreover, another meta-analysis had an error in the extracted data.[17] Therefore, we conducted a meta-analysis on the basis of studies related to PRP and multiple high-level randomized controlled trials (RCTs)[18–22] published recently. In this study, we have attempted to provide an evidence-based medicine protocol for the conservative treatment of knee osteoarthritis.
2. Methods
2.1. Study selection
Two investigators independently screened the literature and extracted and cross-checked the data according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.[23] Divergences of opinion between the two researchers were resolved by consulting a third researcher. All analyses were based on previously published studies; thus, no ethical approval and patient consent are required.
2.2. Search strategy
A search strategy was developed using “platelet-rich plasma” and “knee osteoarthritis” as keywords. We conducted a search of the PubMed, EMBASE, and Cochrane Library databases and manually searched relevant Chinese and English language journals and their references. The detailed search strategy for PubMed as an example was (((((Platelet-Rich Plasma) OR Plasma, Platelet-Rich) OR Platelet Rich Plasma) OR PRP)) AND ((((((((Osteoarthritis, Knee) OR Knee Osteoarthritides) OR Knee Osteoarthritis) OR Osteoarthritides, Knee) OR Osteoarthritis of Knee) OR Knee, Osteoarthritis of) OR Knees, Osteoarthritis of) OR Osteoarthritis of Knees).
2.3. Eligibility criteria
The inclusion criteria were as follows:
-
1.
patients with knee osteoarthritis;
-
2.
PRP used as the test group and HA used as control;
-
3.
RCTs;
-
4.
citing studies involving at least one of the following indicators: visual analog scale (VAS), subjective International Knee Documentation Committee (IKDC) score, Western Ontario and McMaster Universities (WOMAC) total and subscores, Knee Injury and Osteoarthritis Outcome Score (KOOS), and adverse events.
Studies were excluded if they
-
1.
included animals or cadavers as research objects;
-
2.
were unable to extract or convert valid data;
-
3.
were retrospective studies, literature reviews, or conference papers without full text.
2.4. Data extraction
Two researchers independently extracted data through a predesigned data sheet. In accordance with the Cochrane Handbook for Systematic Reviews of Interventions,[24] the researchers converted valid data if the standard deviation could not be obtained. The risk of bias was assessed for each RCT.
2.5. Outcome measures
Considering comparative results might be varied at different observational time points, the five indicators were compared at three observational time points after injection: short term (<12 weeks), medium term (≥12 weeks to <24 weeks), and long term (24 weeks; if there was no follow-up at 24 weeks, the last follow-up data were taken).
VAS is a scale that intuitively quantifies pain in the knee. A lower score indicates milder pain.
IKDC is a subjective scale for the evaluation of the knee joint. A higher score indicates better symptoms, functions, and physical activity.
WOMAC total and subscores is a rating scale for assessing the structure and function of the knee joint in terms of pain, stiffness, and joint function. A lower score indicates better function.
KOOS is a symptom or functional score for assessing patients with osteoarthritis consisting of five subdomains: symptoms, pain, activities of daily living (ADLs), sport, and quality of life (QoL).
Adverse events include pain, swelling, effusion, deep vein thrombosis, tissue hypertrophy, adhesions, hypertension, and proteinuria.
2.6. Statistical analysis
We conducted statistical analysis using the RevMan 5.3 software (Review Manager [RevMan] Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The chi-square test was used to assess heterogeneity. I2 > 50% indicates high heterogeneity, and a random-effects model was used; otherwise, a fixed-effects model was used. Relative risk (RR) was used for assessing dichotomous variables; standardized mean differences were used for continuous variables.[25] The 95% confidence interval (CI) estimates and hypothesis test results for each variable were displayed on a forest plot. For each outcome indicator with significant heterogeneity, we screened the sources of heterogeneity through a sensitivity analysis in which the included studies were removed one at a time. A publication bias assessment using funnel plots was conducted if no <10 studies were included.
3. Results
3.1. Literature search and data analysis groups
A total of 820 relevant studies were retrieved and screened, ultimately including 14 RCTs (Fig. 1) and 1350 patients in the analyses. Görmeli et al[26] reported three parallel groups: PRP1 (1 dose of PRP), PRP3 (3 doses of PRP), and HA. This was the only study that compared 1 dose with 3 doses, consisting of two RCTs. In the other studies, only one dose was stated. We performed statistical analyses in two controlled trials: PRP1 vs HA and PRP3 vs HA. The results of the quality evaluation are shown in Figures 2 and 3.
Figure 1.
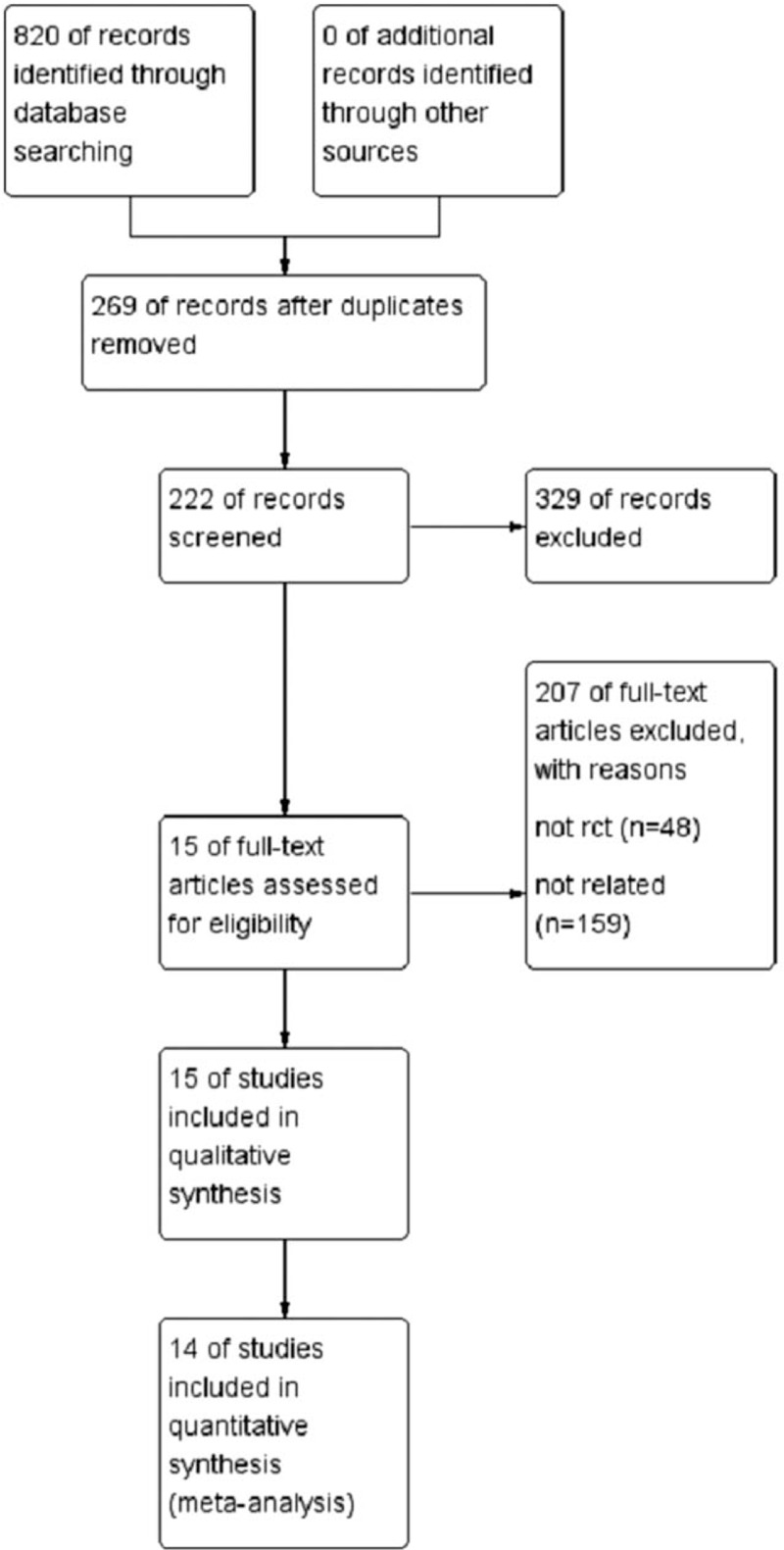
Flowchart of study selection.
Figure 2.
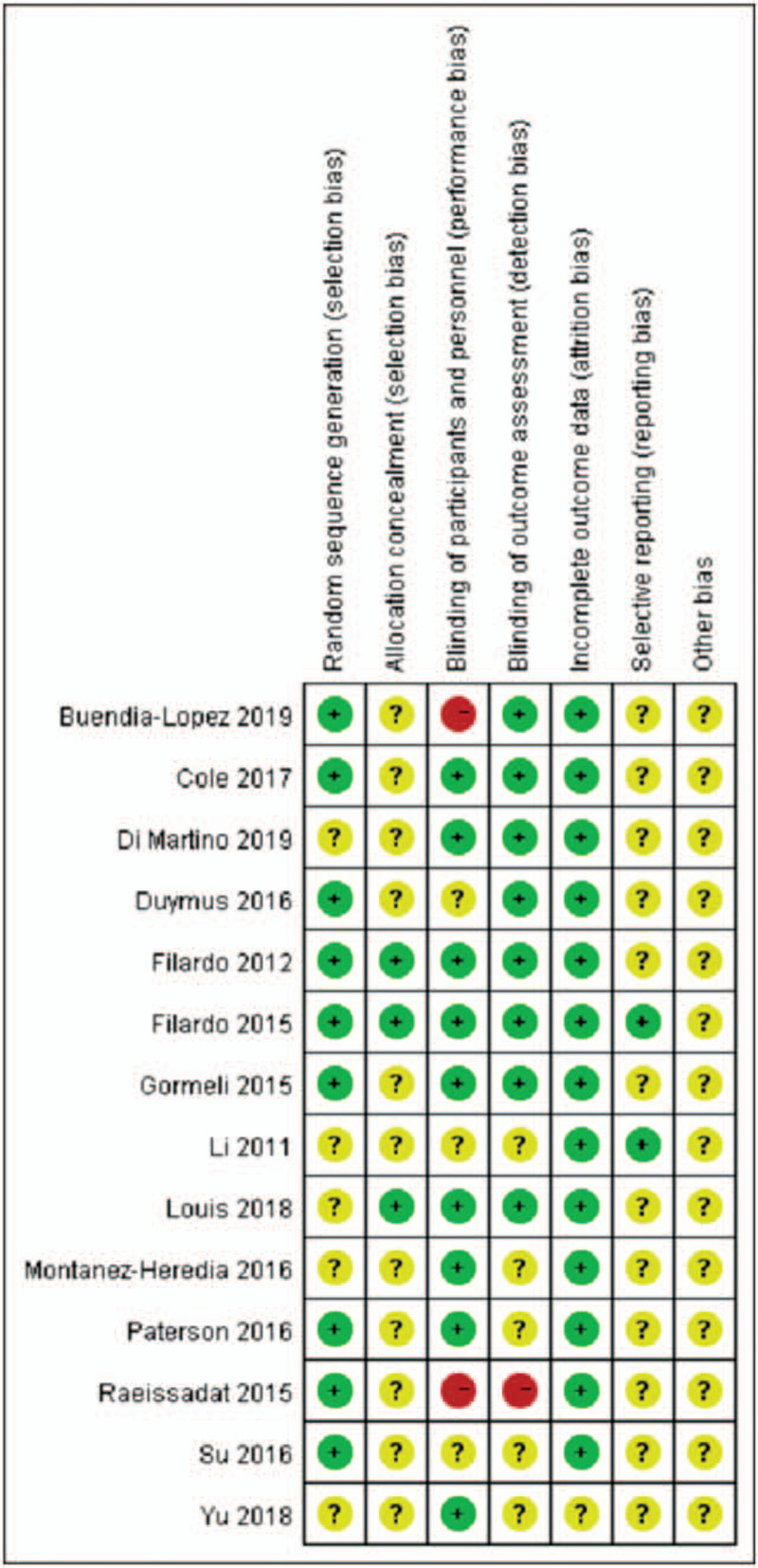
Methodological quality of the included studies.
Figure 3.
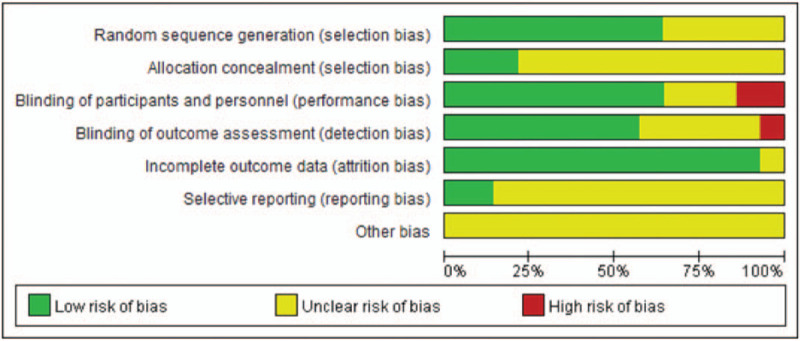
The methodological quality of the included studies.
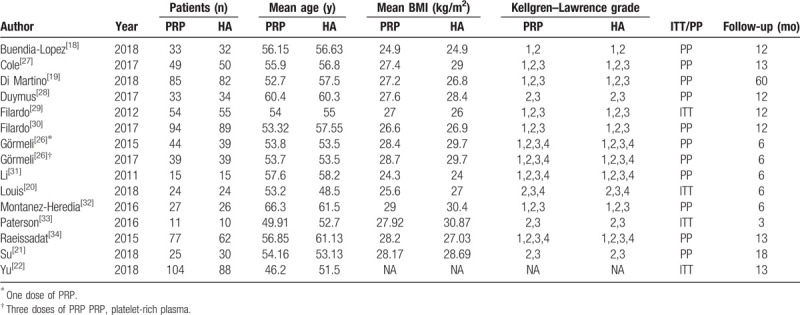
3.2. Study characteristics
There were 714 patients in the PRP group and 636 patients in the HA group. The follow-up period ranged from 3 to 60 months. Specific characteristics are shown in Table 1.
Table 1.
Main characteristics of all eligible studies included in the analysis.
3.3. Clinical outcomes
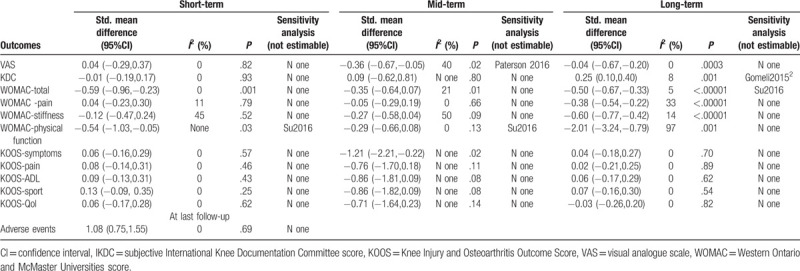
3.3.1. VAS
In the short-term period, 3 studies[21,28,33] were included, with 69 patients in the PRP group and 74 in the HA group. As I2 = 0%, indicating no heterogeneity, the fixed-effects model was used. There was no statistical difference in the VAS score between the groups.
In the mid-term period, 4 studies[20,21,28,33] were included, with 90 patients in the PRP group and 97 in the HA group. As I2 = 73%, indicating high heterogeneity, the study by Paterson et al[33] was removed for the sensitivity analysis, and the I2 value was reduced to 40%. The fixed-effects model was then used. The VAS score in the PRP group was significantly lower than that in the HA group.
In the long-term period, 4 studies[18,21,27,28] were included, with 140 patients in the PRP group and 146 in the HA group. As I2 = 0%, indicating no heterogeneity, the fixed-effects model was used. The VAS score in the PRP group was significantly lower than that in the HA group.
3.3.2. IKDC
In the short-term period, 3 studies[19,29,30] were included, with 233 patients in the PRP group and 226 patients in the HA group. As I2 = 0%, indicating no heterogeneity, the fixed-effects model was used. There was no statistical difference in the IKDC score between the groups.
In the mid-term period, 1 study[31] was included, with 15 patients in the PRP group and 15 patients in the HA group. There was no statistical difference in the IKDC score between the groups.
In the long-term period, 6 studies[19,26,27,29–31] were included, with 380 patients in the PRP group and 369 patients in the HA group. I2 = 78%, indicating high heterogeneity. The IKDC score in the PRP group was significantly higher than that in the HA group. The PRP3 group reported by Görmeli et al[26] was removed for the sensitivity analysis. As I2 = 8%, indicating low heterogeneity, the fixed-effects model was used. The IKDC score in the PRP group was still significantly higher than that in the HA group.
3.3.3. WOMAC-total
In the short-term period, 2 studies[21,28] were included, with 58 patients in the PRP group and 64 in the HA group. As I2 = 0%, indicating no heterogeneity, the fixed-effects model was used. There was a statistical difference in the WOMAC-Total score between the groups.
In the mid-term period, 4 studies[20,21,28,31] were included, with 58 patients in the PRP group and 64 patients in the HA group. As I2 = 21%, indicating low heterogeneity, the fixed-effects model was used. The WOMAC-total score in the PRP group was significantly lower than that in the HA group.
In the long-term period, 6 studies[18,21,22,28,31,33] were included, with 331 patients in the PRP group and 291 patients in the HA group. I2 = 88%, indicating high heterogeneity. The WOMAC-total score in the PRP group was significantly lower than that in the HA group. The study reported by Su et al[21] was removed for the sensitivity analysis. As I2 = 5%, indicating low heterogeneity, the fixed-effects model was used. The WOMAC-total score in the PRP group was significantly lower than that in the HA group.
3.3.4. WOMAC-pain
In the short-term period, 3 studies[21,27,28] were included, with 107 patients in the PRP group and 114 in the HA group. As I2 = 11%, indicating low heterogeneity, the fixed-effects model was used. There was no statistical difference in the WOMAC-Pain score between the groups.
In the mid-term period, 4 studies[20,21,27,28] were included, with 129 patients in the PRP group and 138 patients in the HA group. As I2 = 0%, indicating no heterogeneity, the fixed-effects model was used. There was no statistical difference in the WOMAC-Pain score between the groups.
In the long-term period, 6 studies[18,21,22,27,28,34] were included, with 321 patients in the PRP group and 296 patients in the HA group. As I2 = 33%, indicating low heterogeneity, the fixed-effects model was used. The WOMAC-pain score in the PRP group was significantly lower than that in the HA group.
3.3.5. WOMAC-stiffness
In the short-term period, 2 studies[21,28] were included, with 58 patients in the PRP group and 64 in the HA group. As I2 = 45%, indicating a mild heterogeneity, the fixed-effects model was used. There was no statistical difference in the WOMAC-Stiffness score between the groups.
In the mid-term period, 3 studies[20,21,28] were included, with 80 patients in the PRP group and 88 patients in the HA group. As I2 = 50%, indicating moderate heterogeneity, the fixed-effects model was used. There was no statistical difference in the WOMAC-stiffness score between the groups.
In the long-term period, 5 studies[18,21,22,28,34] were included, with 272 patients in the PRP group and 246 patients in the HA group. As I2 = 14%, indicating low heterogeneity, the fixed-effects model was used. The WOMAC-stiffness score in the PRP group was significantly lower than that in the HA group.
3.3.6. WOMAC-physical function
In the short-term period, 2 studies[21,28] were included, with 58 patients in the PRP group and 64 in the HA group. I2 = 57%, indicating moderate heterogeneity. The WOMAC-physical function score in the PRP group was significantly lower than that in the HA group. The study by Su et al[21] was removed for the sensitivity analysis. The WOMAC-physical function score in the PRP group was still significantly lower than that in the HA group.
In the mid-term period, 3 studies[20,21,28] were included, with 80 patients in the PRP group and 88 patients in the HA group. I2 = 84%, indicating high heterogeneity. The study by Su et al[21] was removed for the sensitivity analysis. There was no statistical difference in the WOMAC-physical function score between the groups.
In the long-term period, 5 studies[18,21,22,28,34] were included, with 272 patients in the PRP group and 246 patients in the HA group. I2 = 97%, indicating high heterogeneity, and the source of heterogeneity was not found. The random-effects model was then used. The WOMAC-physical function score in the PRP group was significantly lower than that in the HA group.
3.3.7. KOOS-symptoms
In the short-term period, 3 studies[29,30,33] were included, with 159 patients in the PRP group and 154 in the HA group. As I2 = 0%, indicating no heterogeneity, the fixed-effects model was used. There was no statistical difference in the KOOS-symptoms score between the groups.
In the mid-term period, 1 study[33] was included, with 10 patients in the PRP group and 9 patients in the HA group. The KOOS-symptoms score in the PRP group was significantly lower than that in the HA group.
In the long-term period, 2 studies[29,30] were included, with 148 patients in the PRP group and 144 patients in the HA group. As I2 = 0%, indicating no heterogeneity, the fixed-effects model was used. There was no statistical difference in the KOOS-symptoms score between the groups.
3.3.8. KOOS-pain
In the short-term period, 3 studies[29,30,33] were included, with 159 patients in the PRP group and 154 in the HA group. As I2 = 0%, indicating no heterogeneity, the fixed-effects model was used. There was no statistical difference in the KOOS-pain score between the groups.
In the mid-term period, 1 study[33] was included, with 10 patients in the PRP group and 9 patients in the HA group. There was no statistical difference in the KOOS-pain score between the groups.
In the long-term period, 2 studies[29,30] were included, with 148 patients in the PRP group and 144 patients in the HA group. As I2 = 0%, indicating no heterogeneity, the fixed-effects model was used. There was no statistical difference in the KOOS-pain score between the groups.
3.3.9. KOOS-ADL
In the short-term period, 3 studies[29,30,33] were included, with 159 patients in the PRP group and 154 in the HA group. As I2 = 0%, indicating no heterogeneity, the fixed-effects model was used. There was no statistical difference in the KOOS-ADL score between the groups.
In the mid-term period, 1 study[33] was included, with 10 patients in the PRP group and 9 patients in the HA group. There was no statistical difference in the KOOS-ADL score between the groups.
In the long-term period, 2 studies[29,30] were included, with 148 patients in the PRP group and 144 patients in the HA group. As I2 = 0%, indicating no heterogeneity, the fixed-effects model was used. There was no statistical difference in the KOOS-ADL score between the groups.
3.3.10. KOOS-sport
In the short-term period, 3 studies[29,30,33] were included, with 159 patients in the PRP group and 154 in the HA group. As I2 = 0%, indicating no heterogeneity, the fixed-effects model was used. There was no statistical difference in the KOOS-sport score between the groups.
In the mid-term period, 1 study[33] was included, with 10 patients in the PRP group and 9 patients in the HA group. There was no statistical difference in the KOOS-ADL score between the groups.
In the long-term period, 2 studies[29,30] were included, with 148 patients in the PRP group and 144 patients in the HA group. As I2 = 0%, indicating no heterogeneity, the fixed-effects model was used. There was no statistical difference in the KOOS-ADL score between the groups.
3.3.11. KOOS-QoL
In the short-term period, 3 studies[29,30,32] were included, with 159 patients in the PRP group and 154 in the HA group. As I2 = 0%, indicating no heterogeneity, the fixed-effects model was used. There was no statistical difference in the KOOS-QoL score between the groups.
In the mid-term period, 1 study[33] was included, with 10 patients in the PRP group and 9 patients in the HA group. There was no statistical difference in the KOOS-QoL score between the groups.
In the long-term period, 2 studies[29,30] were included, with 148 patients in the PRP group and 144 patients in the HA group. As I2 = 0%, indicating no heterogeneity, the fixed-effects model was used. There was no statistical difference in the KOOS-QoL score between the groups.
3.3.12. Adverse events
In a global assessment, 8 studies[18–22,31–33] were included, with 251 patients in the PRP group and 254 patients in the HA group. I2 = 0%, indicating no heterogeneity, and there was no statistical difference in terms of adverse events between the groups. The details are shown in Table 2.
Table 2.
Clinical outcomes.
4. Discussion
In this study, we analyzed the efficacy and safety of PRP and HA in the clinical treatment of knee osteoarthritis and conducted a staging study to compare the therapeutic effects of PRP and HA in different time periods, providing evidence-based medical options for the conservative treatment of knee osteoarthritis. The PRP group was superior to the HA group in terms of long-term VAS, IKDC score, WOMAC-pain score, WOMAC-stiffness score, and WOMAC-physical function score, as well as short-, mid-, and long-term WOMAC-total scores. There were no statistical differences in the other indicators.
In 2019, Han et al[17] conducted a meta-analysis, in which they miscalculated the standard error (SE) as a standard deviation (SD) for the statistical analysis, affecting the credibility of the relevant results. Their meta-analysis only included the literature published until April 2018 and did not include several high-level RCTs published later. Therefore, another meta-analysis needs to include updated data from these later trials.
Pain relief is the focus of treatment for knee osteoarthritis, and the VAS score is an important outcome measure. Cole et al[26] found that compared with HA, PRP significantly relieved pain in the long-term follow-up (24 and 52 weeks). This conclusion has been confirmed in our meta-analysis. We found that the long-term WOMAC-pain score and the mid- and long-term VAS scores of the PRP group were significantly reduced, but there was no statistical difference between the two groups in the short-term VAS score and the short- and mid-term WOMAC-pain scores. The aforementioned results are mainly due to the different mechanisms of PRP and HA. PRP can inhibit inflammatory factors such as tumor necrosis factor α and interleukin[35] and reduce the inflammatory response in knee osteoarthritis.[36–40] In addition, Asfaha et al[8] found that protease-activated receptor 4 in PRP has endogenous analgesic effects and alleviates inflammation-related pain. In contrast, HA can only increase the viscosity and elasticity of the joint fluid and thus reduce pain via lubrication.[28] With longer time after HA treatment, the lubrication effect decreases, and the pain usually reappears.
Functional improvement is the ultimate goal of knee joint treatment. To comprehensively evaluate the function of the knee joint, we adopted the IKDC, WOMAC, and KOOS scores. In the sensitivity analysis, the study by Su et al[21] is a source of heterogeneity in the WOMAC-total score, which may be due to the small number of Chinese patients in their study as well as the subjects’ insensitivity to the WOMAC-total scoring.[41] We conducted a statistical analysis and found that short- and mid-term IKDC, WOMAC-stiffness, and WOMAC-physical function scores showed no statistical difference between the PRP and HA groups, whereas the long-term scores were significantly improved in the PRP group, as demonstrated by Raeissadat et al.[34] In the study by Raeissadat et al,[34] the WOMAC-physical function score in the PRP group was superior to that in the HA group at 52 weeks of follow-up, indicating that patients are likely to increase their performance of rehabilitation exercises because of relief from pain. Patients are generally afraid of pain and therefore may neglect rehabilitation exercises and reduce joint activity, resulting in intra-articular adhesions, which in turn affect functional recovery.[15,42] In the PRP group, patients with pain relief could perform better rehabilitation training to improve their physical functions and mobility. The WOMAC-total score can better highlight the advantages of PRP in the treatment of knee osteoarthritis. We found that the WOMAC-total scores in the PRP group were superior to those in the HA group. However, the mid-term KOOS-symptoms score in the HA group was superior to that in the PRP group, but the data were only from 19 patients reported in the study by Paterson et al.[33] We did not find a statistical difference between the PRP and HA groups in terms of KOOS-pain, ADL, SPORT, and QoL scores in each period, as only Filardo et al[29,30] and Paterson et al[33] have used these indicators. Therefore, further exploration with a larger sample size is warranted.
There were no statistical differences in the adverse events between the PRP and HA groups. In other words, PRP injection is safe with no additional side effects.
4.1. Limitations
This study is limited by the differences in the original RCT protocols and insufficient representation of some of the outcome indicators. High-quality large-scale RCTs are required for verification. Another concern is that there is no uniform standard for the preparation and injection of PRP and HA, which may cause certain heterogeneity in each study.
5. Conclusion
Compared with HA, PRP offers more advantages in the conservative treatment of knee osteoarthritis, including reduced long-term pain and improved knee joint function. PRP has no evident additional risk and can be widely used as a conservative treatment for knee osteoarthritis.
Author contributions
Conceptualization: Shishun Zhao, Meng Xu.
Data curation: Zehan Chen.
Formal analysis: Chang Wang.
Investigation: Zehan Chen.
Methodology: Zhe Zhu.
Resources: Meng Xu.
Software: Zehan Chen.
Supervision: Chang Wang, Di You, Shishun Zhao.
Validation: Zehan Chen, Di You.
Writing – original draft: Zehan Chen.
Writing – review & editing: Shishun Zhao, Zhe Zhu, Meng Xu.
Footnotes
Abbreviations: CI = confidence interval, EMBASE = Excerpta Medica Database, HA = hyaluronic acid, IKDC = subjective International Knee Documentation Committee score, KOOS = Knee Injury and Osteoarthritis Outcome Score, PRP = platelet-rich plasma, RCTs = randomized controlled trials, RR = relative risk, VAS = visual analog scale, WOMAC = Western Ontario and McMaster Universities score.
How to cite this article: Chen Z, Wang C, You D, Zhao S, Zhu Z, Xu M. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: A meta-analysis. Medicine. 2020;99:11(e19388).
The authors have no conflicts of interest to disclose.
References
- [1].Deshpande BR, Katz JN, Solomon DH, et al. Number of persons with symptomatic knee osteoarthritis in the US: impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res 2016;68:1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Youm J, Chan V, Belkora J, et al. Impact of socioeconomic factors on informed decision making and treatment choice in patients with hip and knee OA. J Arthroplasty 2015;30:171–5. [DOI] [PubMed] [Google Scholar]
- [3].Harvey WF, Hunter DJ. The role of analgesics and intra-articular injections in disease management. Med Clin North Am 2009;34:777–88. [DOI] [PubMed] [Google Scholar]
- [4].Bourne RB, Chesworth BM, Davis AM, et al. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res 2010;468:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Faust HJ, Sommerfeld SD, Rathod S, et al. A hyaluronic acid binding peptide-polymer system for treating osteoarthritis. Biomaterials 2018;183:93–101. [DOI] [PubMed] [Google Scholar]
- [6].Arslan E, Ekiz MS, Cimenci CE, et al. Protective therapeutic effects of peptide nanofiber and hyaluronic acid hybrid membrane in in vivo osteoarthritis model. Acta biomaterialia 2018;73:263–74. [DOI] [PubMed] [Google Scholar]
- [7].Woodell-May JE, Pietrzak WS. Platelet-rich plasma in orthopedics. In: Musculoskeletal Tissue Regeneration. Springer 2008;547–68. [Google Scholar]
- [8].Asfaha S, Cenac N, Houle S, et al. Protease-activated receptor-4: a novel mechanism of inflammatory pain modulation. Br J Pharmacol 2010;150:176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Filardo G, Kon E, Roffi A, et al. Platelet-rich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc 2015;23:2459–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Spreafico A, Chellini F, Frediani B, et al. Biochemical investigation of the effects of human platelet releasates on human articular chondrocytes. J Cell Biochem 2010;108:1153–65. [DOI] [PubMed] [Google Scholar]
- [11].Frizziero A, Ferraro C, Masiero S. Platelet rich plasma intra-articular injections: a new therapeutic strategy for the treatment of knee osteoarthritis in sport rehabilitation. A systematic review. Sport Sci Health 2012;8:15–22. [Google Scholar]
- [12].Esra Pancar Y, Gokhan S, Fatma A, et al. Evaluation of effects of platelet-rich plasma on human facial skin. J Cosmet Laser Ther 2014;16:206–8. [DOI] [PubMed] [Google Scholar]
- [13].Frautschi RS, Hashem AM, Halasa B, et al. Current evidence for clinical efficacy of platelet rich plasma in aesthetic surgery: a systematic review. Aesthet Surg J 2017;37:353. [DOI] [PubMed] [Google Scholar]
- [14].Kamakura T, Kataoka J, Maeda K, et al. Platelet-rich plasma with basic fibroblast growth factor for treatment of wrinkles and depressed areas of the skin. Plastic and reconstructive surgery 2015;136:931–9. [DOI] [PubMed] [Google Scholar]
- [15].Pietro G, Barbara DA, Methap P, et al. Adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical evaluation for cell-based therapies in patients with scars on the face. J Craniofac Surg 2014;25:267–72. [DOI] [PubMed] [Google Scholar]
- [16].Zhang HF, Wang CG, Li H, et al. Intra-articular platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis. Drug Design Develop Ther 2018;12:445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Han Y, Huang H, Pan J, et al. Meta-analysis comparing platelet-rich plasma vs hyaluronic acid injection in patients with knee osteoarthritis. Pain Med 2019;20:1418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Buendia-Lopez D, Medina-Quiros M, Fernandez-Villacanas Marin MA. Clinical and radiographic comparison of a single LP-PRP injection, a single hyaluronic acid injection and daily NSAID administration with a 52-week follow-up: a randomized controlled trial. J Orthop Traumatol 2018;19:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Di Martino A, Di Matteo B, Papio T, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med 2018;doi: 10.1177/363546518814532. [DOI] [PubMed] [Google Scholar]
- [20].Louis M, Magalon J, Jouve E, et al. Growth factors levels determine efficacy of platelets rich plasma injection in knee osteoarthritis: a randomized double blind noninferiority trial compared with viscosupplementation. Arthrosc J Arthrosc Relat Surg 2018. [DOI] [PubMed] [Google Scholar]
- [21].Su K, Bai Y, Wang J, et al. Comparison of hyaluronic acid and PRP intra-articular injection with combined intra-articular and intraosseous PRP injections to treat patients with knee osteoarthritis. Clin Rheumatol 2018;37:1341–50. [DOI] [PubMed] [Google Scholar]
- [22].Yu W, Xu P, Huang G, et al. Clinical therapy of hyaluronic acid combined with platelet-rich plasma for the treatment of knee osteoarthritis. Exp Ther Med 2018;16:2119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Panic N, Leoncini E, Belvis GD, et al. Evaluation of the endorsement of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One 2013;8:e83138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Jüni P, Altman DG, Egger M. Assessing the quality of randomised controlled trials; 2001. [Google Scholar]
- [25]. Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta-analyses; 2008. [Google Scholar]
- [26].Görmeli G, Görmeli C, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc 2017;25:958–65. [DOI] [PubMed] [Google Scholar]
- [27].Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med 2017;45:339–46. [DOI] [PubMed] [Google Scholar]
- [28].Duymus T, Mutlu S, Dernek B, et al. Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg Sports Traumatol Arthrosc 2017;25:485–92. [DOI] [PubMed] [Google Scholar]
- [29].Filardo G, Kon E, Di MA, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord 2012;13:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med 2015;43:1575–82. [DOI] [PubMed] [Google Scholar]
- [31].Li M, Zhang C, Ai Z, et al. Therapeutic effectiveness of intra-knee-articular injection of platelet-rich plasma on knee articular cartilage degeneration. Zhongguo xiu fu chong jian wai ke za zhi 2011;25:1192–6. [PubMed] [Google Scholar]
- [32].Montanez-Heredia E, Irizar S, Huertas P, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritic knee pain: a randomized clinical trial in the context of the Spanish national health care system. Int J Mol Sci 2016;17: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Paterson K, Nicholls M, Bennell K, et al. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: a double-blind, randomized controlled pilot study. BMC Musculoskelet Disord 2016;17:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Raeissadat S, Rayegani S, Hassanabadi H, et al. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights Arthritis Musculoskelet Disord 2015;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hedbom E, Häuselmann HJ. Molecular aspects of pathogenesis in osteoarthritis: the role of inflammation. Cell Mol Life Sci 2002;59:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Badlani N, Inoue A, Healey R, et al. The protective effect of OP-1 on articular cartilage in the development of osteoarthritis 1. Osteoarthritis Cartil 2008;16:600–6. [DOI] [PubMed] [Google Scholar]
- [37].Chubinskaya S, Hurtig M, Rueger DC. OP-1/BMP-7 in cartilage repair. Int Orthop 2007;31:773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fortier LA, Barker JU, Strauss EJ, et al. The role of growth factors in cartilage repair. Clin Orthop Relat Res 2011;469:2706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lawrence JTR, James B, Toth AP. Emerging ideas: prevention of posttraumatic arthritis through interleukin-1 and tumor necrosis factor-alpha inhibition. Clin Orthop Relat Res 2011;469:3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Scanzello CR, Umoh E, Pessler F, et al. Local cytokine profiles in knee osteoarthritis: elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthritis Cartil 2009;17:1040–8. [DOI] [PubMed] [Google Scholar]
- [41].Allen KD, Oddone EZ, Coffman CJ, et al. 046 Racial differences in osteoarthritis pain and function: Potential explanatory factors. Osteoarthritis Cartil 2010;18:160–7. [DOI] [PubMed] [Google Scholar]
- [42].Béla S, Hollander AP, Paul D, et al. Associations between pain, function, and radiographic features in osteoarthritis of the knee. Arthritis Rheumatol 2014;54:230–5. [DOI] [PubMed] [Google Scholar]


