Supplemental Digital Content is available in the text
Keywords: Alzheimer's disease, clinical dementia rating, Korea, mini-mental state examination, Taiwan
Abstract
Both Taiwan and Korea are developed countries with different cultures. When encountering the issue of dementia, such sociobehavioral factors have various and different impacts on dementia. We aim to assess the cross-national difference of sociobehavioral impact on cognitive preservation in Alzheimer's disease (AD) between Taiwan and Korea.
A uniformed data set was administered regarding AD. We evaluated annual cognitive function using the Mini-Mental State Examination (MMSE), Clinical Dementia Rating sum of box (CDR-SB), and CDR for 2 continuous years. Annual change of scores compared with the baseline indicated cognitive change as preservation or decline. We recorded the sociodemographic variables of interest, including education duration, level of independence, living situation, and marital status. Step-wise regression analyses were performed to determine the independent factors for cognitive preservation.
In total, 503 participants in Taiwan and 77 participants in Korea were recruited from 2011 to 2014. The baseline demographic characteristics were different in levels of education, living situation, level of independence, and dementia severity between the 2 countries. With follow-up for 2 years, cognitive preservation was associated with CDR staging at baseline and independence [adjusted odds ratio (OR) = 1.657, 95% confidence interval (95% CI) = 1.109–2.477, P = .014] in the Taiwanese population, whereas cognitive preservation was related to living alone (adjusted OR = 3.316, 95% CI = 1.135–9.687, P = .028) in the Korean population. The levels of education showed inconsistency in cognitive preservation in both countries.
Cognitive preservation was associated with independence in the Taiwanese population, whereas cognitive preservation was related to living alone in the Korean population. By practicing relevant socioeconomic support, this might contribute to lessening the negative impact of dementia and preserving cognition in different countries.
1. Introduction
According to the World Health Report in 2003, there is increasing prevalence of dementia and its global burden.[1] Dementia contributes to health status in people aged over 60 and living with a disability other than stroke and cancer.[1] The Delphi consensus estimated that 24.3 million people had dementia in 2005, with 4.6 million new dementia cases reported every year, with numbers projected to double every 20 years to 81.1 million by 2040.[2] Although there are different increasing rates of dementia between countries, 60% of people with dementia lived in developing countries in 2001 and this will increase to 71% by 2040. It will increase by 100% between 2001 and 2040 in developed countries, but by more than 300% in India, China, and South Asian and Western Pacific regions.[2] Globally, Asia contains 48% of the patients with dementia and the estimated percentage will grow to 59% by 2050.[3] In Asia, the majority of people with dementia live in China and the developing western Pacific area, and these areas are where the majority of this population live globally.[2]
Taiwan and Korea are developed countries located in the western Pacific area. Both countries share several similarities in history, religions, politics, “Confucianism” philosophy, 4,5 economy,[6] and education.[7] As socioeconomics has rapidly developed, Taiwan and Korea have both faced the issue of dementia. Treatment of patients with dementia and distress from caregivers are great burdens of dementia, and as the majority of patients with dementia are living at home,[8] relatives or family members are commonly the caregivers, suffering from substantial practical, psychological, and economic strains. 9,10,11 Currently, both dementia disease course and caregiver support have common features in clinical practice. Furthermore, cultural background and socioeconomic conditions are considered to have impact on dementia and caregiver support. 12,13,14 Asian countries, especially in western Pacific areas, might have the majority of such patients globally with a heterogeneous clinical status, so to address this heterogeneity, Yang et al[13] conducted studies using a uniform data set (UDS) to enhance collaboration and study cohesiveness, demonstrating differences in dementia assessment and care in developing versus developed countries.12,15
As for the impact of socio-behavioral factors on cognitive function in Alzheimer's disease (AD) in systematic review and meta-analysis of observational studies, Williams et al[16] in 2010 determined that depressive disorders and unmarried status with less social support increased risk for AD, whereas higher levels of education and physical activity decreased risk for AD. They also found that depressive disorders increased risk of cognitive decline while physical activity decreased risk of cognitive decline, whereas higher levels of education, social network, or social support systems were not associated with risk of cognitive decline.[16] Baumgart et al[17] in 2015 concluded presence of depressive history increased risk of cognitive decline, whereas years of formal education and physical activity decreased risk of cognitive decline and dementia. Livingston et al[18] in 2017 identified sociobehavioral risk factors for dementia in late life, including depression, physical inactivity, and social isolation. By far, risk factors for cognitive decline in patients with AD regarding sociobehavioral factors under different cultural backgrounds are still inconclusive due to inadequate evidence and heterogeneity of studies.16,17,18
In our study, a uniformed data set (UDS) was administered regarding AD, in association with the applications of service composition and Internet of Medical Things. Service composition is a technology capable of combining a collection of existing services where many smaller services are coordinated together to form a larger one.[19] The Internet of Medical Things is a building block for modern health care having enormously stringent resource constraints; thus, lightweight health data security and privacy are crucial requirements.[20] Sociobehavioral factors have impacts on dementia in different nations and cultures, so by practicing relevant socioeconomic support behaviors, these might contribute to lessening the negative impact of dementia and preserving cognition in different country populations.[21] Our study focused on the cross-national difference in AD patients between Taiwan and Korea, and extended this to the issue of cognitive preservation in association with sociobehavioral factors with a 2-year follow-up.
2. Methods
2.1. Countries
The study was conducted in Taiwan and Korea at different times. The study was conducted from June 30, 2011, to December 31, 2012, in Taiwan by Yuan-Han Yang at Kaohsiung Medical University Hospital and his colleagues at other medical centers in Taiwan. The study was then extended to Korea, mainly Seoul Special City, from January 1, 2013, to June 30, 2014, to recruit AD patients by Sang-Yun Kim.12,13,15 It took 18 months to recruit the participants in each country.
2.2. Participants
All of the participants received a comprehensive medical evaluation, including clinical history, physical and neurologic examinations, brain-computed tomography or magnetic resonance imaging, and blood chemistry examinations to exclude other possible causes for the current cognitive status. For all the recruited participants, the diagnosis of AD was based on the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDSADRDA) criteria,[14] and the Diagnostic and Statistical Manual of Mental Disorder 4th edition (DSM-IV) criteria.[22] Patients with other conditions possibly contributing to the diagnosis of AD were excluded.
2.3. Ethics
All of the procedures were approved by the respective Institutional Review Boards or Ethic Committees from each country involved. All of the participants or their legal representatives provided written informed consent. Each of the patients was administered the examinations by a neuropsychologist and an experienced physician based on information from a knowledgeable source, usually a spouse or an adult child.
2.4. Evaluation of sociobehavioral factors
National Institute on Aging in United States convened a Clinical Task Force, composed of clinical leaders from Alzheimer's Disease Centers, to develop a uniform set of assessment procedures to characterize individuals with mild AD and mild cognitive impairment (MCI) compared with nondemented aging. The UDS[23] defines a common set of clinical observations to be collected longitudinally on ADC participants in accordance with standard methods. Data obtained with the UDS are submitted to the National Alzheimer's Coordinating Center and represent a unique and valuable source of data to support and stimulate collaborative research. In our study, we used the UDS as a tool to evaluate the demographic factors and related sociobehavioral factors. The UDS containing several individual forms, Form A–D, was originally designed to provide clinical and cognitive data from persons with AD dementia and cognitively healthy control individuals in the United States to support collaborative research initiatives.[23] In our study, Form A (A1–A5) was administered to collect the basic demographic characteristics of AD patients and their informants to outline the current status of AD and its related issues in Taiwan and Korea. The A1 component was the patient's demographic data, including information on sex, race, ethnicity, and marital status. The A2 component was the informant's demographic data, including the relationship with the patient and the number and frequency of visits made by the informant to the patient. Form A3 was the patient's family history related to dementia or AD, with a focus on the illness as experienced by the patient's first-degree relatives. Forms A4 and A5 were the patient's medications and health history, respectively.
2.5. Evaluation of cognitive preservation
For all of the recruited participants of AD, the corresponding evaluations, including the Traditional Chinese version Mini-Mental Status Examination (MMSE),[24] Clinical Dementia Rating (CDR),[25] and CDR scale Sum of Boxes (CDR-SB),[25] were collected at the recruited time and for 2 continuous years. Patients were excluded if not fulfilling the 2-year follow-up. The MMSE is a 30-point questionnaire that is used extensively in clinical and research settings to measure cognitive impairment. MMSE contains 8 categories, namely orientation to time (5 points), orientation to place (5 points), registration (3 points), attention and calculation (5 points), recall (3 points), language (2 points), repetition (1 point), and complex commands (6 points). MMSE is the summation of the scores of 8 categories. The higher scores of MMSE indicate better cognitive function. The CDR Dementia Staging Instrument in 1 aspect is a 5-point scale used to characterize 6 domains of cognitive and functional performance applicable to AD and related dementias, namely memory, orientation, judgment, and problem solving, community affairs, home and hobbies, and personal care. Each domain has a 5-point scale as follows, 0 = normal; 0.5= very mild dementia; 1 = mild dementia; 2 = moderate dementia; 3 = severe dementia. The summation of scores of each domain is calculated as CDR-SB. CDR Scoring Algorithm was developed to interpret the scores of each domain to the total CDR, where higher scores of CDR-SB and CDR indicate worse cognitive function. The necessary information to make each rating is obtained through a semi-structured interview of the patient and a reliable informant or collateral source, such as family members or caregivers, referred to as the CDR Assessment Protocol.
The change of MMSE scores in 1-year follow-up (1YΔMMSE) means the difference of MMSE scores between the second year and the first year (baseline). The definitions are the same as the 2-year follow-up and the change of CDR-SB scores (ΔCDR-SB). Cognitive preservation is defined as ΔMMSE more than or equal to zero or ΔCDR-SB less than or equal to zero. In brief, the definition for cognitive preservation and cognitive decline are summarized as follows:
1YΔMMSE= (MMSE-2nd year) – (MMSE-1st year)
2YΔMMSE= (MMSE-3rd year) – (MMSE-1st year)
ΔMMSE≧0, cognitive preservation
ΔMMSE < 0, cognitive decline
1YΔCDR-SB= (CDR-SB -2nd year) – (CDR-SB -1st year)
2YΔCDR-SB= (CDR-SB -3rd year) – (CDR-SB -1st year)
ΔCDR-SB≦0, cognitive preservation
ΔCDR-SB>0, cognitive decline
2.6. Statistical analysis
Descriptive statistical analyses were conducted for all continuous variables by mean (standard deviation) and categorical variables by numbers (percentage). We elucidated the demographic characteristics, including age (grouped as < 75 years of age and ≧75 years of age), gender and dementia severity by MMSE, CDR (grouped as 0.5, 1.2, and 3), and CDR-SB, as well as sociobehavioral factors, including education duration (grouped as <6 years and ≧6 years), living status, marital status, and level of independence. The difference of each factor for cognitive preservation in 1-year and 2-year follow-up were then compared, then the independent factors for cognitive preservation by step-wise regression analysis were identified and presented with adjusted odds ratio and 95% confidence intervals. All statistical tests were 2-tailed, and a P value of .05 was considered to show significance with 95% confidence interval. The data analysis was performed using SPSS (version 12.0.1 for Windows; SPSS Inc., Chicago, IL).
3. Results
3.1. Demographic characteristics
In total, 503 AD patients from Taiwan and 77 AD patients in Korea were recruited to participate in the study. The demographic characteristics of the participants and informants are shown in Figure 1 (supplementary Table 1). The mean age was 77.6 ± 7.8 years in Taiwan and 77.2 ± 5.7 years in Korea. As for the gender distribution in AD, females were consistently predominant in both Taiwan (69.4%) and Korea (75.3%). There was no significant difference in age and gender between the 2 countries. The levels of education in Taiwan were significantly higher than Korea (7.4 ± 5.3 vs 5.5 ± 4.6 years, P = .003). As for social-behavioral factors, Korea had significantly higher proportions in living alone (24.7% vs 9.0%, P < .001) and independence than did Taiwan (80.5% vs 66.7%, P = .008). There was no difference in marital status between the 2 countries. As for informants, there was no difference between the 2 countries, except for living together, which showed a higher proportion of living together of informants and AD patients in Taiwan than in Korea (74.0% vs 54.5%, P < .001).
Figure 1.
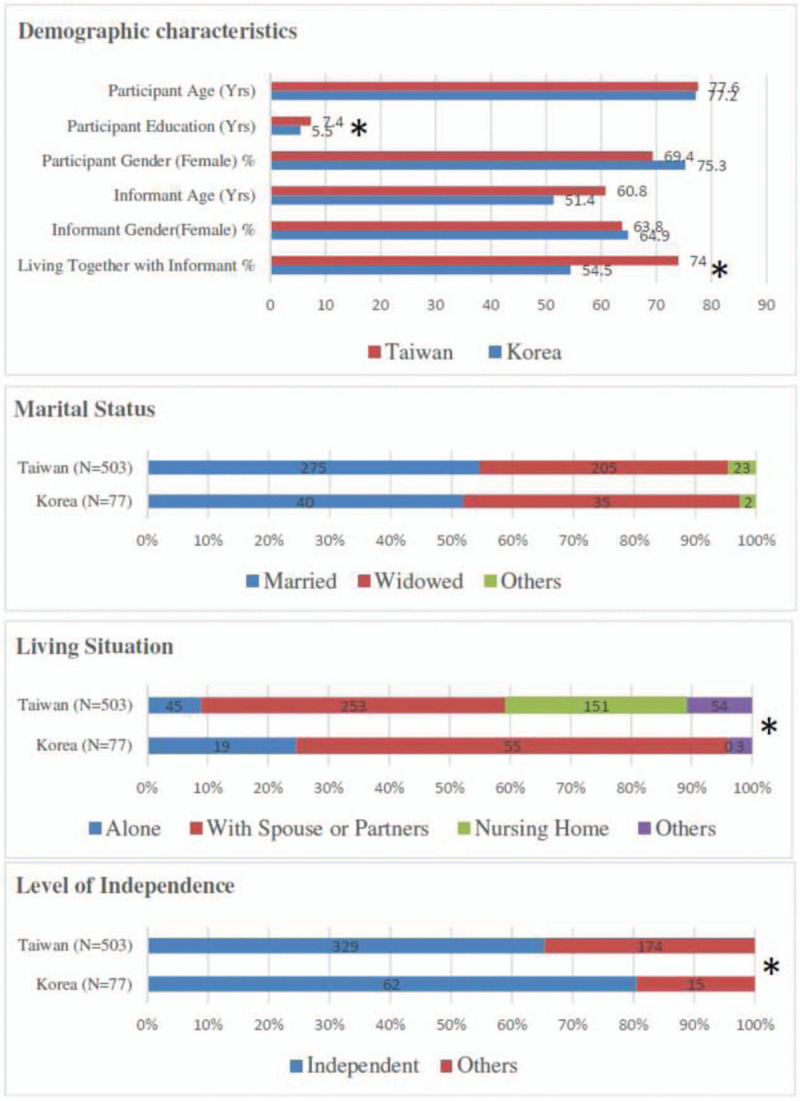
Demographic characteristics of the participants and informants in Taiwan and Korea. ∗ P < .05, statistically significant.
3.2. Baseline and follow-up cognitive function
The baseline dementia severity and follow-up cognitive function by CDR, CDR-SB, and MMSE between the 2 countries is illustrated in Figure 2 (supplementary Table 2). The baseline dementia severity and follow-up cognitive function in CDR in the recruited participants revealed difference between the 2 countries. The proportion of CDR 0.5 in baseline was higher in Korea (35.0% vs 18.7%), whereas the proportion of CDR 1 in baseline was higher in Taiwan (56.6% vs 33.8%). During the follow-up, the proportion of CDR2 and CDR3 increased in both countries, indicating the progressive disease course of dementia. The overall mean MMSE measures in baseline and follow-up in our study were not significantly different between the 2 countries. The overall CDR-SB in baseline showed no difference, whereas Korea had higher dementia severity scoring by CDR-SB in the follow-up than did Taiwan (for 1-year follow-up, 7.9 ± 4.3 vs 6.6 ± 3.2, P = .002, for 2-year follow-up, 9.3 ± 4.7 vs 7.3 ± 3.6, P < .001).
Figure 2.
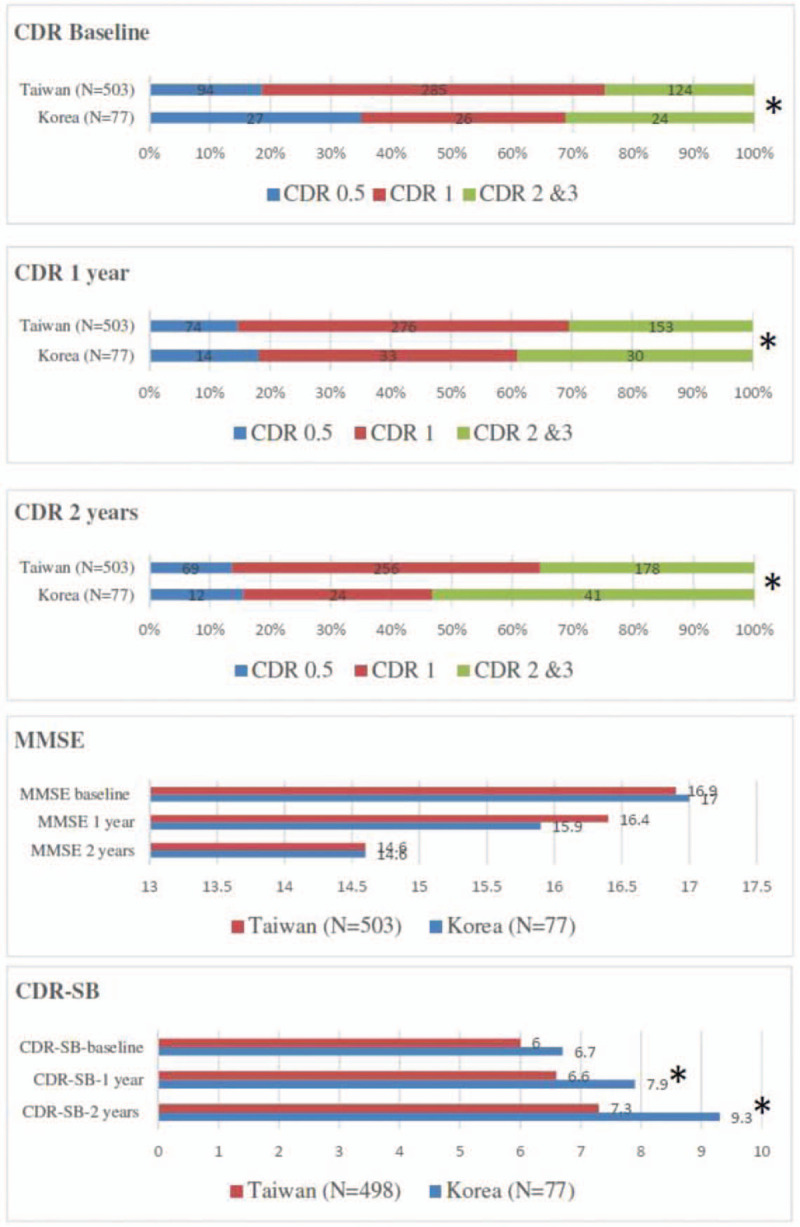
Baseline and follow-up cognitive function by CDR, MMSE, and CDR-SB in participants in Taiwan and Korea. CDR = Clinical Dementia Rating; CDR-SB = CDR scale Sum of Boxes; MMSE = Mini-Mental State Examination. ∗ P < .05, statistically significant.
3.3. Association of global status and follow-up cognitive change
Association of global status and follow-up cognitive change by MMSE in participants in Taiwan and Korea is shown in Figure 3 (supplementary Table 3). It shows that independence was associated with higher cognitive preservation in 2-year follow-up (72.8% vs 63.4%, P = .035), while lower levels of education and baseline dementia severity in CDR were associated with higher cognitive decline in 2-year follow-up in the Taiwanese population. In the Korean population, only living alone was the factor associated with cognitive preservation in 2-year follow-up (39.3% vs 16.3%, P = .025). Association of global status and follow-up cognitive change by CDR-SB in participants in Taiwan and Korea is shown in Figure 4 (supplementary Table 4). It shows that younger age, higher levels of education, and independence were associated with cognitive preservation in 2-year follow-up in the Taiwanese population. The role of independence was also noted in 1-year follow-up in Taiwan. In the Korean population, there was no factor associated with cognitive preservation in CDR-SB. The progressive disease course was not correlated with gender in both Taiwan and Korea in our study.
Figure 3.
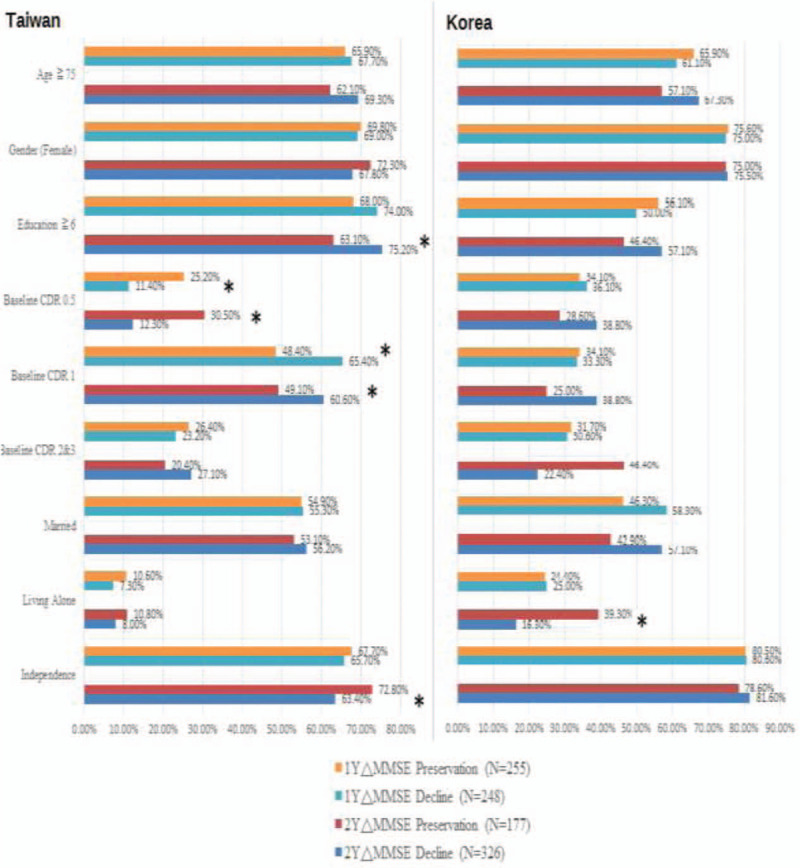
Association of global status and follow-up cognitive change by MMSE in participants in Taiwan (n = 503) and Korea (n = 77). ΔMMSE = change of MMSE scores in follow-up; CDR = Clinical Dementia Rating; MMSE = Mini-Mental State Examination. ∗ P < .05, statistically significant.
Figure 4.
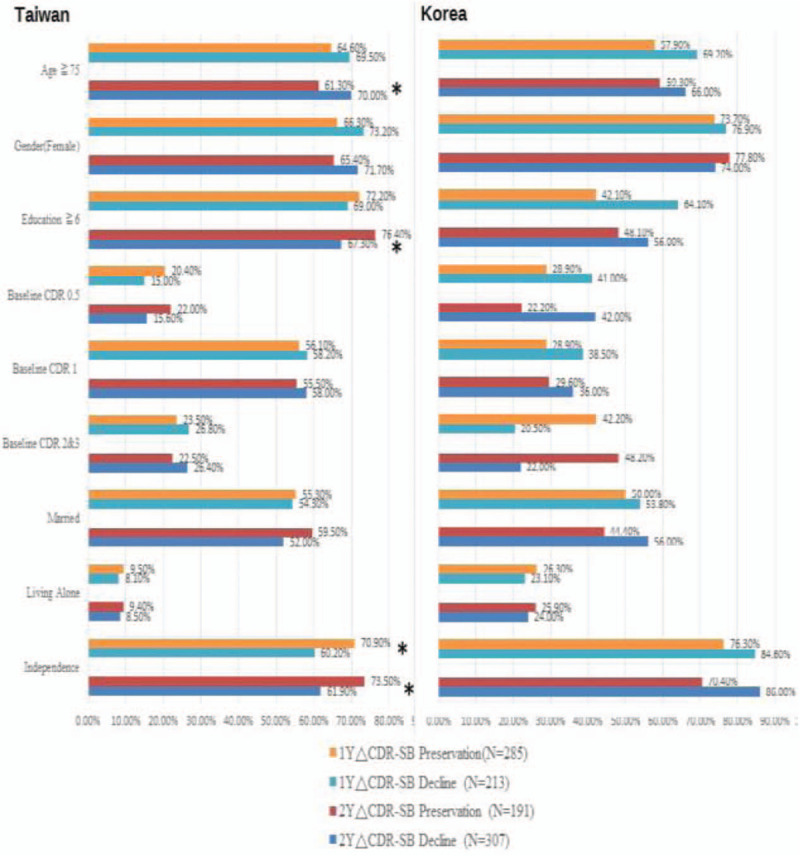
Association of global status and follow-up cognitive change by CDR-SB in participants in Taiwan (n = 498) and Korea (n = 77). ΔCDR-SB = change of CDR-SB scores in follow-up; CDR = Clinical Dementia Rating; CDR-SB = CDR scale Sum of Boxes. ∗ P < .05, statistically significant.
3.4. Factors for cognitive preservation
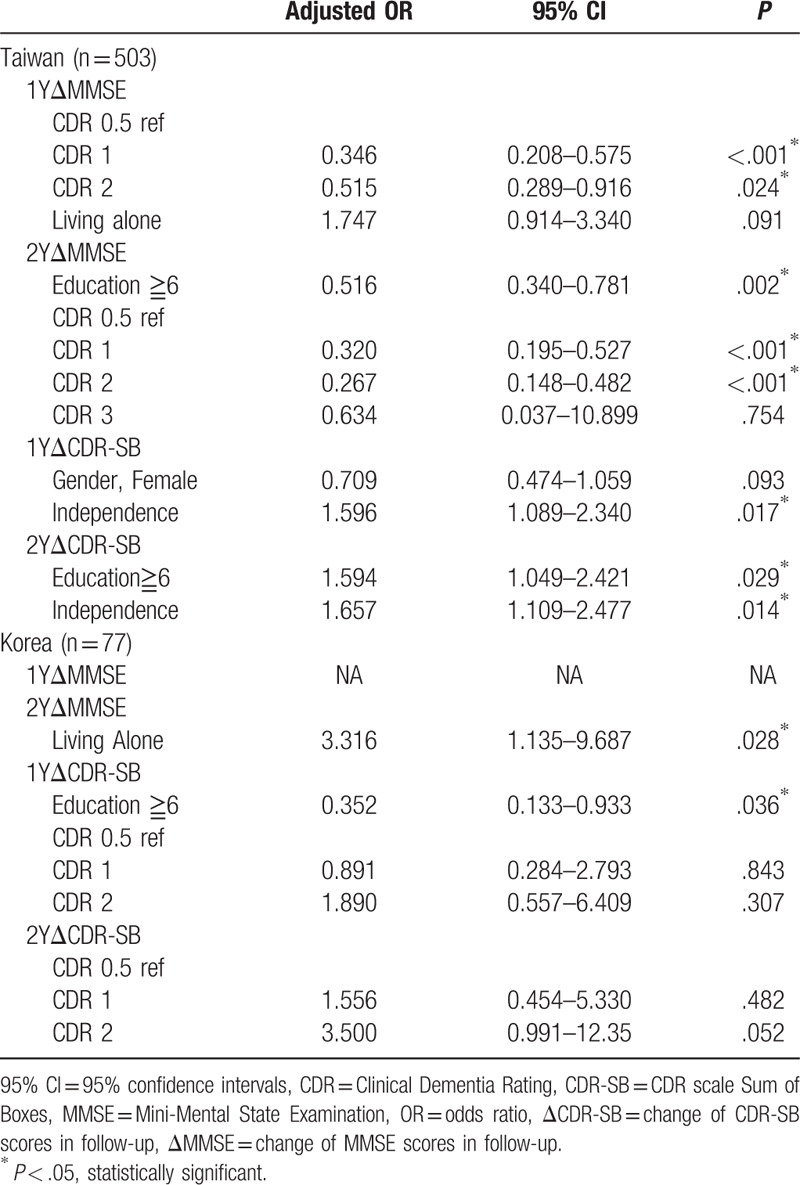
Table 1 reveals the result of follow-up cognitive preservation by MMSE and CDR-SB in Taiwan and Korea using step-wise regression analysis. By MMSE, the lower level of education and less severity of dementia were independent factors for cognitive preservation in Taiwan. By CDR-SB, the higher levels of education (adjusted OR = 1.594, 95% CI = 1.049–2.421, P = .029) and independence (adjusted OR = 1.657, 95% CI = 1.109–2.477, P = .014) were independent factors for cognitive preservation in 2-year follow-up in Taiwan. The role of levels of education in cognitive preservation was discrepant by MMSE and CDR-SB in Taiwan. By MMSE, living alone was the only factor for cognitive preservation (adjusted OR = 3.316, 95% CI = 1.135–9.687, P = .028) in 2-year follow-up in Korea. By CDR-SB, lower level of education was associated with cognitive preservation in 1-year follow-up in Korea.
Table 1.
Stepwise regression analysis for follow-up cognitive preservation by MMSE and CDR-SB in participants in Taiwan and Korea.
4. Discussion
We report on the cross-national difference of sociobehavioral factors for cognitive preservation in AD between Taiwan and Korean populations in a uniform manner. It demonstrated that cognitive preservation was associated with independence in the Taiwanese population, whereas cognitive preservation was related to living alone in the Korean population.
For age, there was no difference between participants in both country samples. For gender, females were predominant among both country patients (69.4% in Taiwan and 75.3% in Korea). This reflected the fact that AD was predominant in females, which is consistent with previous studies.26,27,28 Sociobehavioral factors, including levels of education, living situation, and level of independence, as well as baseline CDR staging were different between the 2 countries, and this might be due to the existing selection bias in recruiting the participants of AD and the limited case numbers in Korea.
The MMSE and CDR-SB in the recruited AD participants for the cognitive function evaluation in continuous follow-up for 2 years revealed progressive disease course in AD, with the MMSE scores being grossly proportional to the CDR-SB in the 2 countries. As far as the role of levels of education is concerned, it revealed inconsistent effect in cognitive preservation by MMSE and CDR-SB in the Taiwan population and by CDR-SB in that of Korea, so the role of levels of education in cognitive preservation remains inconclusive. Williams et al[16] concluded that higher levels of education decreased the risk of AD and had no association with cognitive decline in the literature review. Baumgart et al[17] identified years of formal education decreased the risk of dementia and cognitive function with a strong level of evidence.29,30 Furthermore, the discrepancies of role of levels of education in our study might result from MMSE being influenced by cultural background, education, and age31,32,33 therefore, the comparison of the impact of education duration in cognitive preservation by CDR-SB and MMSE could be disproportional in our study.
As for marital status, it is important for the clinical course of dementia, as it affects the survival condition of patients with dementia.[34] Previous studies found that AD patients will become institutionalized more rapidly if the caregiver is not the spouse.[35] In our study, we found that only 55.1% of AD participants in Taiwan and 51.9% of the AD participants in Korea were currently married. These findings emphasize the burden for both countries in caring for AD patients due to marital status. Sommerlad et al[36] conducted a systematic review and meta-analysis of observational studies, concluding being married is associated with a reduced risk of dementia than lifelong single people or being widowed, and this is underdiagnosed in routine clinical practice. Social isolation was identified as a risk factor for dementia by Livingston et al[18]; therefore, social isolation due to unmarried status might contribute to the development of dementia.[37] Sjöberg et al[38] concluded marital status and living situation both had the potential to buffer the detrimental effects of low mood on dementia onset.
Despite this, there appears to be no direct evidence so far pointing out marital status in association with cognitive decline in AD.16,17 Nevertheless, education, physical health, and enhancing social engagement as modifiable risk factors should be highlighted for dementia prevention in unmarried people. Overall, marital status showed no association with cognitive preservation in 2-year follow-up in our study. Further studies are needed to clarify the correlation.
Our finding identified independence was associated with cognitive preservation for AD participants in Taiwan, whereas living alone was associated with cognitive preservation for AD participants in Korea. The level of independence could reflect the disease severity and capacity of physical activity. In our study, a high majority of patients (66.7% in Taiwan and 80.5% in Korea) were independent, and a smaller part of them (13% in Taiwan and 13% in Korea) needed assistance for complex activities. It could be explained partly by most of our recruited AD participants being classified as mild to moderate stages (for CDR 1 and CDR 2, 80.9% in Taiwan and 59.8% in Korea). Most of our participants were hospital-based, so that participants at a severe stage would be less likely to be recruited in our study. The higher level of independence reflected less severity of dementia and higher capacity of physical activity.[36] The exercise and physical activity had beneficial effects on depression symptoms, and lessened the impact of depression on cognitive decline and AD. Thus, the finding of independence in association with cognitive preservation is predictable for AD participants in Taiwan in the study. Similar findings were determined for living status, where the family home was the most popular residence type for AD participants in Taiwan (91.0%), including living with a spouse or partner, with friends, relatives, or children, with a group, or at a nursing home with a caregiver. On the contrary, 24.7% of AD participants in Korea had residence type as living alone. Sjöberg et al[39] concluded marital status and living situation have the potential to buffer the detrimental effects of low mood on dementia onset, whereas our study showed living alone was associated with cognitive preservation for AD patients in Korea population. Living alone, on the contrary, might represent adequate available physical activities and capability of self-caring.
To some extent, it promoted cognitive preservation in the long-term follow-up in our study. Although living alone might lead to some kind of social isolation and depression, the cultural and economic effects should be considered to evaluate their effects in cognitive function. The study in Korea showed that living alone was susceptible to cognitive impairment and depression, influenced by factors, including the number of daily meals, social contact, and self-perceived health status.[40] If ongoing community interest and support was practiced, these were helpful in lessening the negative impact of living alone. Another study in Korea with participants mainly recruited from Seoul Special City concluded that economic resources were more important than health and social ties for alleviating the negative impact of living alone on the development of depressive symptoms in older widows.[41] With adequate socioeconomic support, it is explainable that living alone is associated with cognitive preservation for AD patients in Korea.
In our study, we evaluated the cognitive function using MMSE, CDR, and CDR-SB. MMSE has both validity and reliability for the diagnosis and longitudinal assessment of AD. The advantages for the MMSE include requiring no specialized equipment, short administration period, and ease of use,[42] while the limitations include being affected by demographic factors such as age, education, and cultural background; besides, MMSE lacks adequate sensitivity to discriminate MCI and mild AD from normal individuals.[43] CDR is credited with being able to discern very mild impairments, provides early and accurate diagnosis of dementia, and appears to be a reliable and valid tool for assessing and staging dementia, which is essential to proper clinical care of AD.[44] The disadvantages of CDR include taking a large amount of time, reliance on subjective assessment, and inability to capture changes over time.[45] The CDR-SB is extended from the CDR score for dementia staging, and owing to the increased range of values, the CDR-SB offers several advantages over the CDR score, including increased utility in accurately staging AD patients and tracking changes within and between stages of dementia severity. CDR-SB did not analyze other forms of mild dementia syndromes such as frontotemporal dementia or Parkinson disease, and although CDR-SB can discriminate between patients with very early AD and those with MCI, it is impossible to use CDR due to the nature of the scale.[46]
Our study has several strengths in providing useful information for cognitive preservation in AD patients. First, this is the first cross-national study to report the sociobehavioral factors for cognitive preservation between 2 countries with similar current social developments with continuous 2- year follow-up. Second, we used the UDS, which can connect and collaborate with Western society so that the data could be compared, and it would be helpful in upcoming dementia care in enhancing cognitive preservation for AD patients.[23]
Although our finding is encouraging, it has some limitations. First, sampling selection bias is present in the study and it is difficult to perform a randomized sampling study to register AD patients in both countries. Besides, there was a great difference in the numbers of participants (503 participants in Taiwan and 77 participants in Korea), which may have led to results bias. Second, not all of the recruited subjects were randomized to represent the typical dementia profiles in 2 countries. Country or society-specific characteristics of caregivers or informants in relation to disease severity could be related to many other socioeconomic statuses, which could be examined in another comprehensively randomized sampling study. Besides, medical services are affordable to people in higher socioeconomic societies and thus have an influence in cognitive preservation. Third, we only focused on the sociobehavioral factors, and did not address possible risk factors for cognitive decline and AD for analysis such as Apolipoprotein E gene with allele ε4 genotyping (APOEε4), traumatic brain injuries, lifestyle, depression, medication history, and family history.16,17 Besides, vascular risk factors possibly resulting in vascular dementia such as diabetes, hypertension, or previous stroke events were not presented in our study.[47] If possible, more large-scale studies using UDS to provide more updated information with multi-country involvement should be encouraged to explore the prevention of cognitive decline for AD in the Asian area.
5. Conclusion
Our study demonstrated that cognitive preservation was associated with independence in the Taiwanese population, whereas cognitive preservation was related to living alone in the Korean population. The levels of education showed inconsistency in cognitive preservation in both countries. By practicing relevant socioeconomic support systems, this might contribute in lessening the negative impact of dementia and preserving population cognition in different countries.
Acknowledgments
We acknowledge that the Asian Society Against Dementia provided the platform for this international study. We also greatly appreciate the support of Dr. John Morris, the Alzheimer's Disease Research Center, Washington University in St. Louis, in his role as an advisor for this study. This study was supported by grants from Kaohsiung Municipal Ta-Tung Hospital (Kmtth-107–004) and Kaohsiung Medical University Research Center Grant (KMU-TC108B01).
Author contributions
Conceptualization: Sun-Wung Hsieh, Yuan-Han Yang.
Data curation: Sang-Yun Kim, Yuan-Han Yang.
Formal analysis: Ling-Chun Huang.
Investigation: Yong-Soo Shim, Yuan-Han Yang.
Methodology: Sang-Yun Kim, Yuan-Han Yang.
Project administration: Ling-Chun Huang
Supervision: Yuan-Han Yang.
Writing – original draft: Sun-Wung Hsieh.
Sun-Wung Hsieh orcid: 0000-0003-3952-7718.
Supplementary Material
Footnotes
Abbreviations: AD = Alzheimer's disease, APOEε4 = Apolipoprotein E gene with allele ε4 genotyping, CDR = Clinical Dementia Rating, CDR-SB = CDR scale Sum of Boxes, DSM-IV = Diagnostic and Statistical Manual of Mental Disorder 4th edition, MMSE = Mini-Mental Status Examination, NINCDSADRDA = National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association, UDS = uniform data set.
How to cite this article: Hsieh SW, Kim SY, Shim YS, Huang LC, Yang YH. A comparison of socio-behavioral impact on cognitive preservation in Alzheimer's Disease between Taiwan and Korea: a cross-national study. Medicine. 2020;99:15(e19690).
All authors read and approved the final manuscript.
The authors report no conflicts of interest in this work.
Supplemental Digital Content is available for this article.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1]. World Health Organization. 2003 World Health Report. 2003 Shaping the Future. 20 Avenue Appia, 1211 Geneva 27, Switzerland. 2003/15424-Sadag-4500. [Google Scholar]
- [2]. Brookmeyer R, Johnson E, Ziegler-Graham K, et al. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement 2007;3:186–91. [DOI] [PubMed] [Google Scholar]
- [3]. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Kim HS, Plester BA, Harmony, et al. Psychological well-being in South Korean organizations. Front Psychol 2019;9:2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Hwang KK, Shiah YJ, Yit KT. Editorial: eastern philosophies and psychology: towards psychology of self-cultivation. Front Psychol 2017;8:1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Vogel EF. The Four Little Dragon: The Spread of Industrialisation in East Asia. London, England: Harvard University Press; 1991. [Google Scholar]
- [7]. Hapgood D. Diplomaism. New York: Donald W. Brown, Inc; 1971. [Google Scholar]
- [8]. Prince M. Care arrangements for people with dementia in developing countries. Int J Geriatr Psychiatry 2004;19:170–7. [DOI] [PubMed] [Google Scholar]
- [9]. Langa KM, Chernew ME, Kabeto MU, et al. National estimates of the quantity and cost of informal caregiving for the elderly with dementia. J Gen Intern Med 2001;16:770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Prince M. The need for research on dementia in developing countries. Trop Med Int Health 1997;2:993–1000. [DOI] [PubMed] [Google Scholar]
- [11]. Chandra V, Ganguli M, Pandav R, et al. Prevalence of Alzheimer's disease and other dementias in rural India: the Indo-US study. Neurology 1998;51:1000–8. [DOI] [PubMed] [Google Scholar]
- [12]. Wang WF, Chiu PY, Lin YT, et al. Registration of Alzheimer's disease in Taiwan: patient and informant. Am J Alzheimers Dis Other Demen 2014;29:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Yang YH, Wang H, Lam L, et al. Characteristics of Alzheimer's disease among patients in Taiwan, Hong Kong, and Beijing. J Alzheimers Dis 2014;42:193–200. [DOI] [PubMed] [Google Scholar]
- [14]. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–44. [DOI] [PubMed] [Google Scholar]
- [15]. Yang YH, Meguro K, Kim SY, et al. Impact of Alzheimer's disease in nine Asian countries. Gerontology 2016;62:425–33. [DOI] [PubMed] [Google Scholar]
- [16]. Williams JW, Plassman BL, Burke J, et al. Preventing Alzheimer's disease and cognitive decline. Evid Rep Technol Assess (Full Rep) 2010;193:1–727. [PMC free article] [PubMed] [Google Scholar]
- [17]. Baumgart M, Snyder HM, Carrillo MC, et al. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement 2015;11:718–26. [DOI] [PubMed] [Google Scholar]
- [18]. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673–734. [DOI] [PubMed] [Google Scholar]
- [19]. Sun X, Wang S, Xia Y, et al. Predictive-Trend-Aware Composition of Web Services with Time-Varying Quality-of-Service. IEEE Access 2019;8:1910–21. [Google Scholar]
- [20]. Pirbhulal S, Samuel OW, Wu W, et al. A joint resource-aware and medical data security framework for wearable healthcare systems. Future Gener Comput Syst 2019;95:382–91. [Google Scholar]
- [21]. Yang YH, Liscic R, Dominguez J. Framework of treating Alzheimer's dementia. Brain Sci Adv 5 2019;82-93: [Google Scholar]
- [22]. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). Washington. 1994;Washington, DC, USA: American Psychiatric Association, 143-147. [Google Scholar]
- [23]. Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 2006;20:210–6. [DOI] [PubMed] [Google Scholar]
- [24]. Folstein MF, Folstein SE, McHugh PR. ‘Minimental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [25]. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–4. [DOI] [PubMed] [Google Scholar]
- [26]. Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta-Analysis Consortium. JAMA 1997;278:1349–56. [PubMed] [Google Scholar]
- [27]. Craig MC, Murphy DG. Estrogen therapy and Alzheimer's dementia. Ann N Y Acad Sci 2010;1205:245–53. [DOI] [PubMed] [Google Scholar]
- [28]. Zandi PP, Carlson MC, Plassman BL, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA 2002;288:2123–9. [DOI] [PubMed] [Google Scholar]
- [29]. Meng X, D’Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One 2012;7:e38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Stern Y, Gurland B, Tatemichi TK, et al. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA 1994;271:1004–10. [PubMed] [Google Scholar]
- [31]. Anthony JC, LeResche L, Niaz U, et al. Limits of the ‘Mini-Mental State’ as a screening test for dementia and delirium among hospital patients. Psychol Med 1982;12:397–408. [DOI] [PubMed] [Google Scholar]
- [32]. Brayne C, Calloway P. The association of education and socioeconomic status with the Mini Mental State Examination and the clinical diagnosis of dementia in elderly people. Age Ageing 1990;19:91–6. [DOI] [PubMed] [Google Scholar]
- [33]. Murden RA, McRae TD, Kaner S, et al. Mini-Mental State exam scores vary with education in blacks and whites. J Am Geriatr Soc 1991;39:149–55. [DOI] [PubMed] [Google Scholar]
- [34]. Mittelman MS, Ferris SH, Shulman E, et al. A family intervention to delay nursing home placement of patients with Alzheimer disease. A randomized controlled trial. JAMA 1996;276:1725–31. [PubMed] [Google Scholar]
- [35]. Hebert R, Dubois MF, Wolfson C, et al. Factors associated with longterm institutionalization of older people with dementia: data from the Canadian Study of Health and Aging. J Gerontol A Biol Sci Med Sci 2001;56:M693–9. [DOI] [PubMed] [Google Scholar]
- [36]. Sommerlad A, Ruegger J, Singh-Manoux A, et al. Marriage and risk of dementia: systematic review and meta-analysis of observational studies. J Neurol Neurosurg Psychiatry 2018;89:231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Chen CPLH, Mok VCT. Marriage and risk of dementia: systematic review and meta-analysis of observational studies. J Neurol Neurosurg Psychiatry 2018;89:227. [DOI] [PubMed] [Google Scholar]
- [38]. Sjöberg L, Fratiglioni L, Lövdén M, et al. Low mood and risk of dementia: the role of marital status and living situation. Am J Geriatr Psychiatry 2019;28:33–44. [DOI] [PubMed] [Google Scholar]
- [39]. Dinas PC, Koutedakis Y, Flouris AD. Effects of exercise and physical activity on depression. Ir J Med Sci 2011;180:319–25. [DOI] [PubMed] [Google Scholar]
- [40]. Lee J, Ham MJ, Pyeon JY, et al. Factors affecting cognitive impairment and depression in the elderly who live alone: cases in Daejeon Metropolitan city. Dement Neurocogn Disord 2017;16:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Jeon GS, Choi K, Cho SI. Impact of living alone on depressive symptoms in older Korean widows. Int J Environ Res Public Health 2017;14:E1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Harrell LE, Marson D, Chatterjee A, et al. The Severe Mini-Mental State Examination: a new neuropsychologic instrument for the bedside assessment of severely impaired patients with Alzheimer disease. Alzheimer Dis Assoc Disord 2000;14:168–75. [DOI] [PubMed] [Google Scholar]
- [43]. Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 1992;40:922–35. [DOI] [PubMed] [Google Scholar]
- [44]. Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566–72. [DOI] [PubMed] [Google Scholar]
- [45]. Lim WS, Chong MS, Sahadevan S. Utility of the clinical dementia rating in Asian populations. Clin Med Res 2007;5:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. O’Bryant SE, Waring SC, Cullum CM, et al. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer's research consortium study. Arch Neurol 2008;65:1091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Yang YH, Fuh JL, Mok VC. Vascular contribution to cognition in stroke and Alzheimer‘s disease. Brain Sci Adv 2018;4:39–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


