Abstract
Stereotactic body radiation therapy (SBRT) has emerged as a treatment option for unresectable hepatocellular carcinoma (HCC) patients. However, the treatment outcomes for patients with portal vein tumor thrombosis (PVTT) remain poor. In this study, we evaluate the efficacy of SBRT with and or without sorafenib for advanced HCC with PVTT.
Fifty four HCC patients with PVTT treated with SBRT using the Cyberknife system was retrospectively analyzed between January 2009 and June 2016. Of these, sorafenib combined with SBRT was administered to 18 patients and SBRT alone was administered to 36 patients. SBRT was designed to target the liver tumor and tumor thrombosis, with a radiation dose of 36 to 45 Gy (median 40 Gy) given in 3 to 5 fractions.
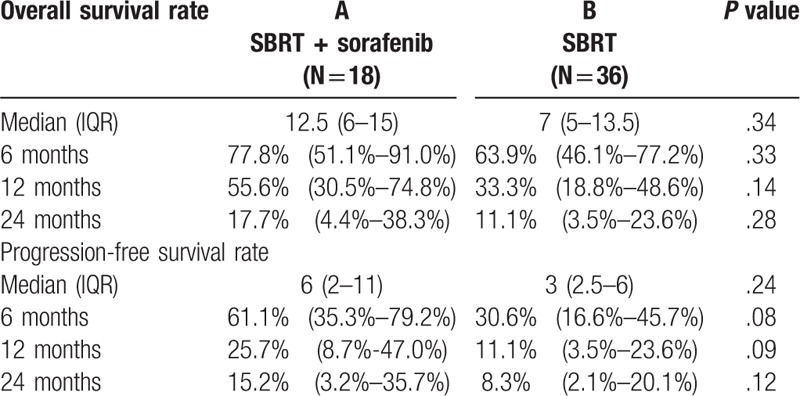
The mean follow-up period for SBRT with sorafenib and SBRT alone was 13.22 ± 10.07 months and 15.33 ± 22.01 months, respectively. The response rate was comparable in both groups. Complete response and partial response rates were 77.77% for SBRT with sorafenib and 75.00% without sorafenib (P = .43). The median progression-free survival rate was 6 months (2–11 months) versus 3 months (2–5.6 months) (P = .24) and the 1- and 2-year progression-free survival rates were 25.7% and 15.2% versus 11.1% and 8.3% (P = .1225). The median, 1- and 2-year overall survival rates (OSR) were 12.5 months, 55.6% and 17.7% versus 7 months (5–13.5 months), 33.3% and 11.1% (P = .28), for SBRT with sorafenib versus SBRT alone groups, respectively.
The result of our study shows that SBRT with sorafenib administered group resulted in a higher median, progression-free, and OSR for HCC patients with PVTT. However, the trends did not attain statistical significance. A large-scale randomized study is needed to assess the benefits of SBRT with sorafenib administration for patient with PVTT.
Keywords: portal vein tumor thrombosis, sorafenib, stereotactic body radiation therapy
1. Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and the fifth most common cancer worldwide, HCC predominantly occurs in patients with liver cirrhosis. Advanced HCC is characterized by its propensity to invade the vasculature within the liver. Portal vein tumor thrombosis (PVTT) is the most common form of macrovascular invasion of HCC and is prevalent in 10% to 60% of HCC cases at diagnosis. The presence of PVTT is associated with a poor prognosis as it is closely associated with intrahepatic metastasis and tumor recurrence. Patients only exhibit 2 to 4 months of overall survival with the best supportive care.[1,2]
In HCC patients with PVTT, management options are limited, and currently, no optimal treatment has been established. Surgical resection poses certain technical challenges, and standard treatment for advanced HCC such as transarterial chemoembolization (TACE), is generally contraindicated because it can induce hepatic necrosis and further deterioration of liver function. Therefore, TACE is limited to patients with adequate liver reserve; and patients with PVTT in areas other than the main or the first branch. In addition, it is limited to those with adequate collateral blood flow around the obstructed PVTT. Other treatment options include conventional radiation therapy and more recently hepatic arterial infusion chemotherapy. However, the overall survival rate (OSR) remains modest.[3–5]
Sorafenib (Nexavar, Bayer HealthCare, Leverkusen, Germany) is an oral multikinase inhibitor that suppresses tumor proliferation and aniogenesis. In 2 randomized trials, the SHARP and a multicenter study in the Asia-Pacific region, sorafenib was shown to be effective in patients with advance HCC. A subgroup analysis for macroscopic vascular invasions in both studies showed a survival benefit for sorafenib over placebo. The median survival rate was significant, 2 to 3 months longer in the sorafenib group than in the placebo group. Additionally, the median time to progression was significantly longer in the sorafenib group than in the placebo group.[6,7] Conventionally, the standard treatment algorithm proposed by the Barcelona Clinic Liver Cancer (BCLC) system considers the presence of PVTT, as a contraindication to surgery or TACE. Patients with PVTT are classified in the BCLC stage C category, with sorafenib as the standard of care. However, the survival benefits observed following sorafenib treatment are limited, and this underscores the need for better treatment strategies. Various attempts to develop alternative or combination treatments to improve the overall survival of patients with HCC and PVTT have been reported.[8,9]
With the recent advances in computer and imaging technologies, stereotactic body radiation therapy (SBRT) has become a safe and feasible option for HCC patients with radiation-induced liver disease (RILD) rates of ≤5%. SBRT has emerged as a treatment option for HCC patients who are ineligible for surgery, radiofrequency ablation (RFA), or liver transplant. Studies evaluating HCC patients treated with SBRT have reported high local control rates of 70% to 100%.[10–12] However, the outcomes for patients with PVTT remain poorer than the outcome for those without vascular tumor thrombosis. Huang et al,[13] reported that sorafenib sensitizes resistant HCC cells to radiation-induced apoptosis via downgrading phosphorylation of STAT3 in vitro and in vivo, thus, a combination of sorafenib and radiation therapy might demonstrate some clinical relevance. Yu et al,[14] investigated the in vitro and in vivo effects of sorafenib combined with radiation therapy on HCC. They found that sorafenib could potentiate irradiation in HCC cells and their xenografts by inhibiting radiation-induced proliferation and DNA repair, thereby promoting radiation-induced apoptosis and perhaps anti-angiogenesis in vivo. And in a study of a HCC cell line, Li et al,[15] concluded that sorafenib combined with irradiation exerted a schedule dependent effect in HCC cells in vitro. Sorafenib administered 30 minutes prior to irradiation reduced the anti-proliferative effects of irradiation against HCC whereas sorafenib administered 24 hours after irradiation increased the anti-tumor effects against HCC. Thus, with the findings of the above mention reports, there is a need to recognize the combined therapeutic effects of SBRT with sorafenib. Therefore, we retrospectively analyzed and evaluated the outcomes and the benefits of combining SBRT and sorafenib in clinical practice settings.
2. Methods and materials
2.1. Patient selection
Between January 2009 and June 2016, 54 patients were enrolled in this retrospective analysis at our institute. The inclusion criteria was based on the following: pathological confirmation of HCC or at least 1 radiological image with the classic HCC feature of enhancement accompanied by serum tumor marker alpha fetoprotein (AFP) level >200 ng/mL or at least 2 radiological images (computed tomography [CT]/magnetic resonance imaging [MRI]/Angiogram) with the classic imaging findings of HCC; presence of PVTT, confirmed using either tri-phase CT scans, MRI scans, or a hepatic angiogram. Patients with unresectable, medically inoperable HCC; an ECOG performance status of ≤2. A Child-Pugh score of ≤B. Any prior treatment was allowed except for previous liver irradiation. A normal liver volume minus gross tumor volume (GTV) must be ≥700 cm3.
Mandatory baseline examinations included dynamic MRI and/or triphasic CT of the liver; complete blood chemistry; liver function tests; hepatitis B and C antigens and viral titers; AFP level, and chest x-ray images. Patients with positive hepatitis B surface antigen (HbsAg) or elevated hepatitis B viral titers were given prophylactic anti-retroviral therapy from the initiation of SBRT to at least 6 months after treatment for prevention of post-RT reactivation of HBV.[16]
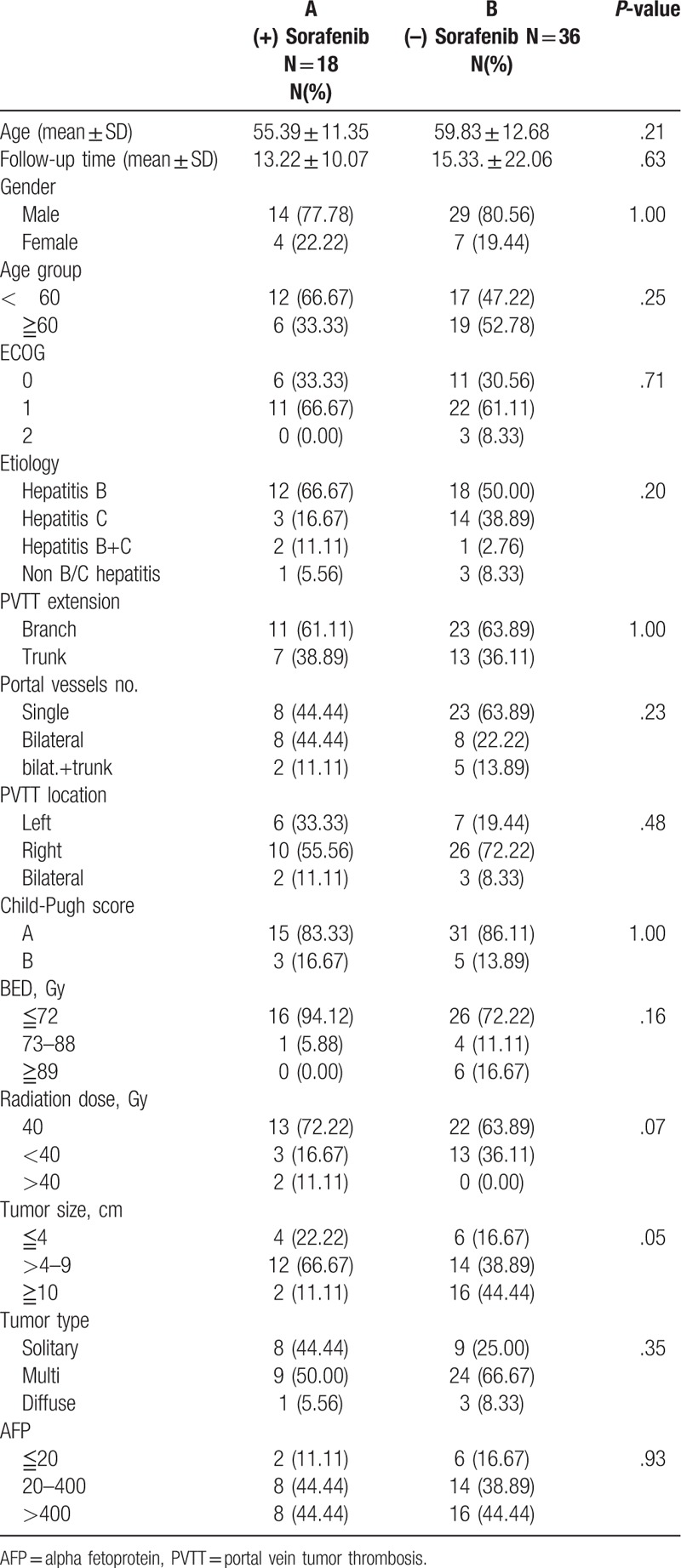
The characteristics and disease variables of the 54 patients in the study are summarized in Table 1. Sorafenib was approved by FDA, USA on November 2007 and was officially reimbursed by the National health insurance in Taiwan on August 2012 for the treatment of HCC with vascular or lymphatic invasion and distant metastases. We start prescribing sorafenib to SBRT on 2013 to every patient with macrovascular invasion and/or patient with limited lymphatic extension. Among the 18 patients with sorafenib medication, 13 patients was Child-Pugh A and 5 patients was Child-Pugh B, ECOG status range from 0 to 1, AJCC stage IIIB comprised 16 patients and 2 patients in stage IV, tumor thrombosis affecting only the portal vein branch was recorded in 13 patients with 5 patients extending to the main portal vein trunk. These patients are divided into 2 specific groups: those in group A (18 patients) were treated with SBRT combine with sorafenib and those in group B (36 patients) were treated with SBRT alone. The mean patients’ ages for those administered with sorafenib were 55.39 ± 11.35 years and those not administered sorafenib was 59.83 ± 12.68 years. The mean follow-up period for those treated with SBRT combine with sorafenib and those treated with SBRT alone group was 13.22 ± 10.07 months and 15.33. ± 22.01 months, respectively. Male predominance was found in both groups.
Table 1.
Demographic and clinical characteristic of study group.
Written informed consent was obtained from all patients before treatment and the study was approved by the Institutional Review Board of Chi Mei Medical Center.
2.2. Treatment details
2.2.1. SBRT
SBRT was performed using the CyberKnife, a robotic image-guided whole body radiosurgical system (Accuray Inc., Sunny vale, CA) equipped with the synchrony system, that is, a real-time respiratory tracking system for target volumes that move with respiration. Four gold fiducial markers were implanted percutaneously around the perimeter of the target volume using a sonographically-guided procedure 5 to 7 days before the acquisition of the CT-scan used for planning. Thin-slice CT-scan and MRI were performed with a slice thickness of 1 mm, the results of which were transferred to the CyberKnife planning system. Tumor contouring was performed on the planning CT with contrast. All patients were positioned on individually shaped vacuum pillows and wore a treatment jacket on which the optical markers were fixed. Any displacement of the patient during treatment was detected by either internal or external fiducial markers with sub-millimeter accuracy.[17]
The gross tumor volume (GTV) included visible tumor in the portal vein on CT scan or MRI with contrast. No clinical target volume (CTV) was further added. A part of hepatic tumor was included in the GTV only if the portal vein was directly invaded by the gross HCC tumor. The GTV was directly expanded 5 mm in all directions to create the planning target volume (PTV). Modification of PTV was performed if it extended into the dose-limiting organs, not including the normal liver. The median prescription dose was 40 Gy to the PTV given in 5 fractions over 5 to 10 days. The dose was prescribed to the isodose line covering 70% to 96% the PTV. Treatment was delivered with the real-time tracking system using the fiducial as a guide, and planning was performed with MultiPlan CyberKnife Treatment Planning System version 2.20 ( Accuray, Inc. Sunnyvale, CA).
The dose volume constraints have been described in detail in our previous reports.[11] The maximum permissible doses to spinal cord, stomach-duodenum, and bowel were 21, 35, and 38 Gy, respectively. The normal liver dose was limited by ≥700 mL, receiving ≤15–19 Gy in 3 to 5 fractions.
Sorafenib was administered orally at a 400 mg twice a day given after completion of SBRT for 12 patients, whereas 6 patients received sorafenib before and after SBRT. Dose reductions of sorafenib were allowed in cases of drug related grade 3 to 4 toxicities. Sorafenib was discontinued in case of intolerable toxicity despite dose reduction or follow-up image study taken every 2 months after the start of medication shows disease progression
2.3. Follow-up, response, and toxicity assessment
After completion of the treatment, the vital status evaluation, physical examination, liver function test, and complete blood tests were performed to assess acute toxicity. They were performed every 1 to 2 weeks in the first months and every 3 months thereafter. Imaging studies using 4-phase CT-scan or dynamic MRI of liver and AFP were performed 1 to 2 months and subsequently every 3- to 4-months after treatment. Toxicity grading was performed according to Common Toxicity Criteria Adverse Events version 4.0. Acute toxicities were defined as adverse events occurring within 3 months after SBRT, and late toxicities were those occurring after 3 months. Radiation-induced liver disease (RILD) was defined as either classic or non-classic RILD. Classic RILD was the presence of nonmalignant ascites and anicteric elevation of alkaline phosphatase level to twice the upper normal level or the baseline value occurring between 2 weeks and 3 months after the completion of irradiation. Non-classic RILD, typically occurring between 1 week and 3 months after therapy, involves elevation of transaminase to at least 5 times the upper limit of normal or pretreatment levels. For this classification, this should occur within 4 months of irradiation completion or a decline in liver function in the absence of classic RILD.[18] This endpoint was common in HCC patients of poor liver function (hepatitis B infection, Child-Pugh B and C) The diagnoses of both classification of RILD could be made only in the absence of evidence of tumor progression. Toxicity grading was recorded based on the worst toxicity recorded.
Tumor response was assessed using the modified response evaluation and criteriain solid tumor (mRECIST) in addition to portal vessels tumor thrombi response. Complete disappearance of the tumor contrast enhancement in the arterial phase and no radiographic evidence of tumor thrombi were defined as a complete response (CR); a decrease of >30% of an enhanced area in the arterial phase and tumor thrombi, considering the baseline diameter of the tumor as a reference was judged as a partial response (PR); a decrease of <30% of the longest diameter of the target tumor and tumor thrombi or no change was defined as stable disease (SD); and an increase of at least 20% of the diameter of the viable lesion, considering the smallest diameter of the lesion as reference, was judged as a progressive disease. Objective response was calculated for CR and PR, no response was calculated for SD and progressive disease. Local control was defined as no new lesion development or increase in tumor size within the PTV. Intrahepatic recurrence free was defined as no development of new lesion in the liver outside the PTV. Distant metastasis was defined as recurrence beyond the liver. Disease progression was defined as the development of intrahepatic recurrence and distant metastasis.
2.4. Statistical analysis
Patient characteristics in the 2 groups were compared using the Mann–Whitney U test or t tests and Fisher exact for continuous and categorical variables, respectively. All end points were measured from the day of SBRT initiation. Overall survival curves and local control rates were calculated using Kaplan–Meier analysis. Log-rank testing was used to compare outcomes between the subsets of the patients analyzed. For all tests, 2-sided P-values ≤.05 were considered significant. Data were analyzed using SPSS statistics
3. Results
3.1. Patient characteristics
Pretreatment characteristics of patients and tumors are summarized in Table 1. The general condition of most of the patients was good. Patients with ECOG scores 0 to 1 (92.59%) and with a Child-Pugh score A (85.19%) comprised the majority of cases in both groups. PVTT invading only a single vessel was found in 8 (44.44%) patients and tumor thrombosis involving a branch of the portal vein without extending to the major trunk was found in 11 (61.11%) patients in group A. Among group B patients, 23 (63.89%) had single vessel involvement and 23 (63.89%) had PVTT not extending to main trunk. A total of 18 (33.33%) patients in group A and 36 (66.66%) in group B received radiation dose of 40 Gy in 5 fractions.
3.2. Tumor response and local control
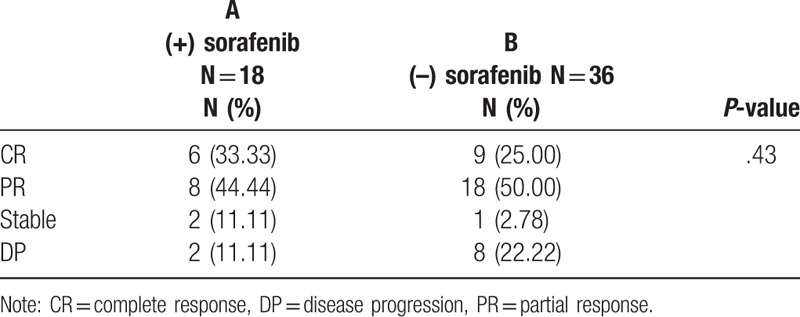
The results of radiotherapeutic response are summarized in Table 2. The overall response rate (CR plus PR) was 77.77% (14/18) in group A patients and 75% (27/36) in group B patients. Among the 54 patients, CR was achieved in 33.33% (6/18) of group A and 25% (9/36) of group B patients. PR was achieved in 44.44% (8/18) of group A and 50% (18/36) of group B. SD was achieved in 11.11% (2/18) of group A patients and 2.78% (1/36) of group B patients. Disease progression was relatively lower in group A patients 11.11% (2/18) than in group B patients 22.22% (8/36).
Table 2.
Tumor response after SBRT with or without sorafenib.
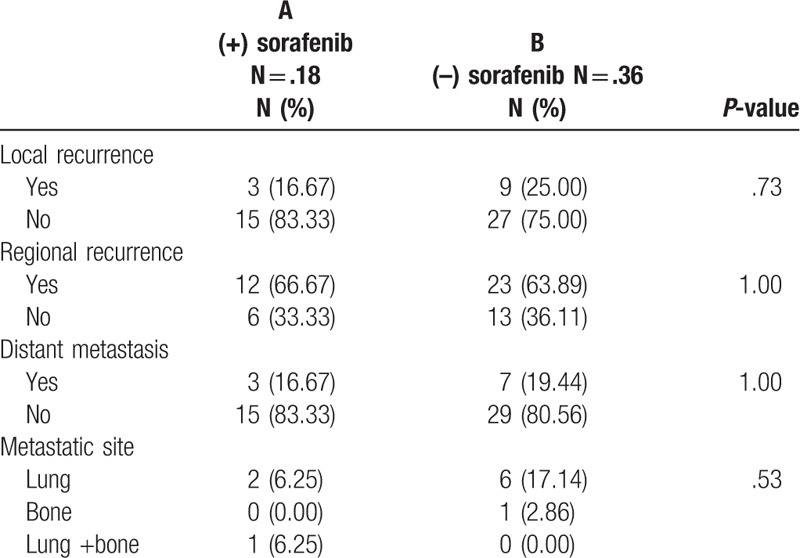
The major pattern of failure in both groups was regional recurrence (Table 3), which developed in 66.67% (12/18) of group A and in 63.89% (23/36) of group B. Although it does not attained statistical significance, patients receiving SBRT followed by sequential administration of sorafenib (group A) tended to have trend toward a better local control rate than those not receiving sorafenib (group B). Furthermore, 83.33% (15/18) of group A achieved local control rate, and 75% (22/36) of group B patients achieved the same. Distant metastases were found in 16.67% (3/18) and 19.44% (7/36) in group A and B patients, respectively. Lung was the major site of metastases for both groups
Table 3.
Pattern of failure.
3.3. Survival outcomes
Among patients receiving SBRT followed by sorafenib (group A), the median survival rate was 12.5 months (range, 6–15 months), and the 6 months and 1-yr, 2-yrs OSR was 77.8%, 55.6%, 17.7%, respectively. Among patients receiving SBRT alone (group B), the median survival rate was 7 months (range, 5–13.5 months), and 6 months, 1 and 2-years OSR was 63.9%, 33.3%, and 11.1%, respectively (Fig. 1). The progression-free survival rate (PFSR) for group A was 61.15%, 25.7%, 15.2% at 6 months, 1 and 2-years, respectively. And the median-progression free survival rate was 6 months (range, 2–11 months). For group B, the 6 months, 1-yr, 2-yrs OSR was 30.6%, 11.1%, 8.3% and the median was 3 months (range, 2.5–6 months) (Fig. 2, Table 4). There was a trend toward better outcome for OSR and DPFSR among group A patients than group B patients. Unfortunately, the P value did not reach statistical significance.
Figure 1.
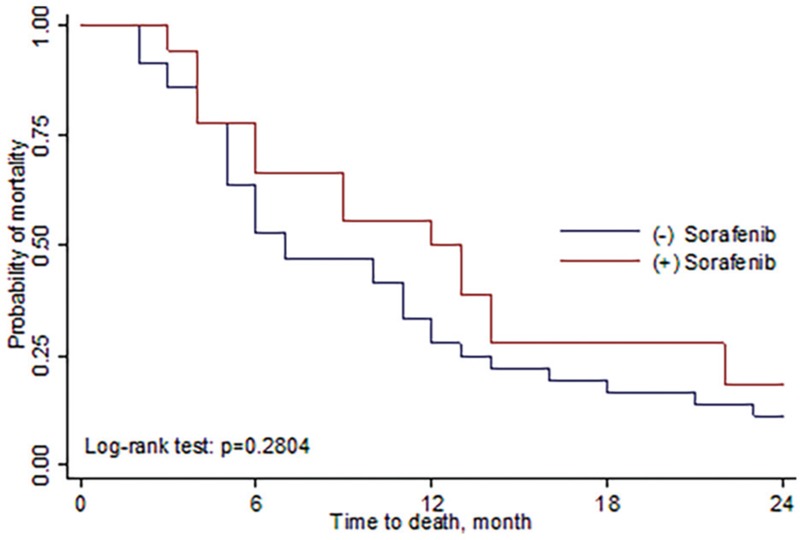
Overall survival rate comparing SBRT with sorafenib and SBRT alone. The 1 and 2 years OSR for SBRT combined with sorafenib was 55.6% and 17.7%. While for SBRT alone, the 1 and 1 year OSR was 33.3% and 11.1%, respectively. OSR = overall survival rate, SBRT = stereotactic body radiation therapy.
Figure 2.
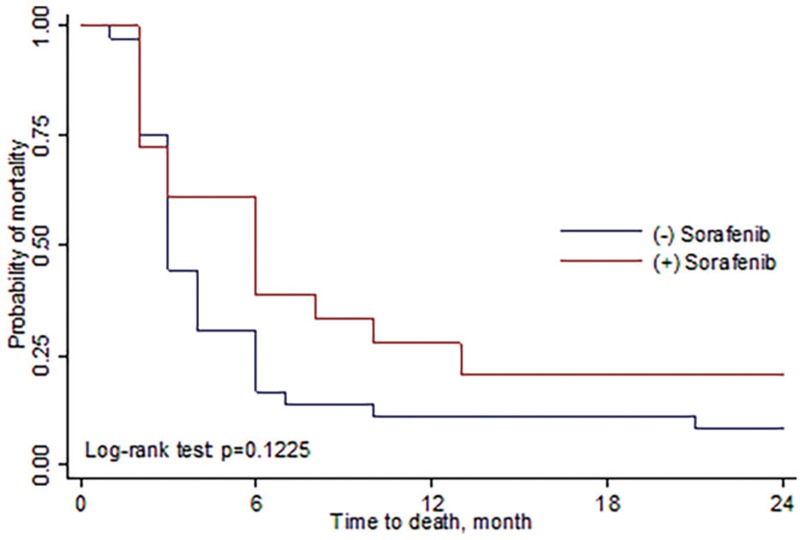
Progression-free survival rate comparing SBRT with sorafenib and SBRT alone. The 1 and 2 years PFS for SBRT combined with sorafenib was 25.7% and 15.2%. While for SBRT alone, the 1 and 2 years PFS was 11.1% and 8.3%, respectively. PFS = progression-free survival, SBRT = stereotactic body radiation therapy.
Table 4.
Life table.
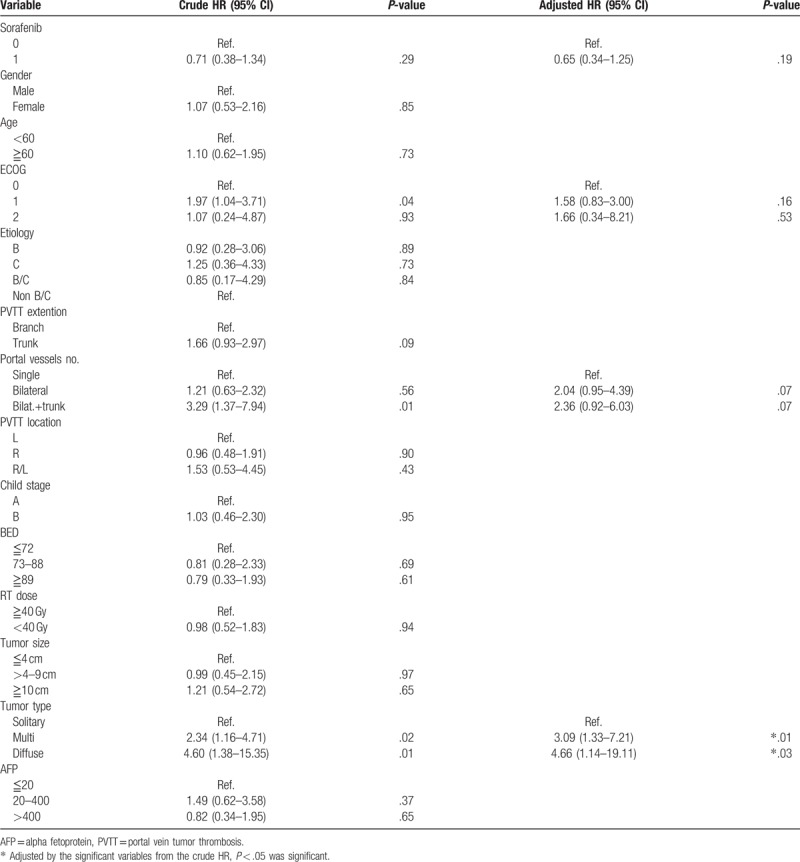
A Cox proportional hazards regression model demonstrated that the risk of death in both groups was lower in patients with a good performance status (ECOG 0 > 1–2), PVTT affecting a single vessel (single vessel > bilateral vessels and or bilateral vessels with main trunk), and those presence of a solitary tumor (solitary > multiple and diffuse). The factor that remained independently significant in the multivariate analysis was diffuse tumor type as the worst prognosis factor. (HR, 4.66, 95% CI, 1.14–19.11; P = .03). (Table 5).
Table 5.
Cox proportional hazard regressions analysis of factors related to overall survival.
3.4. Toxicity
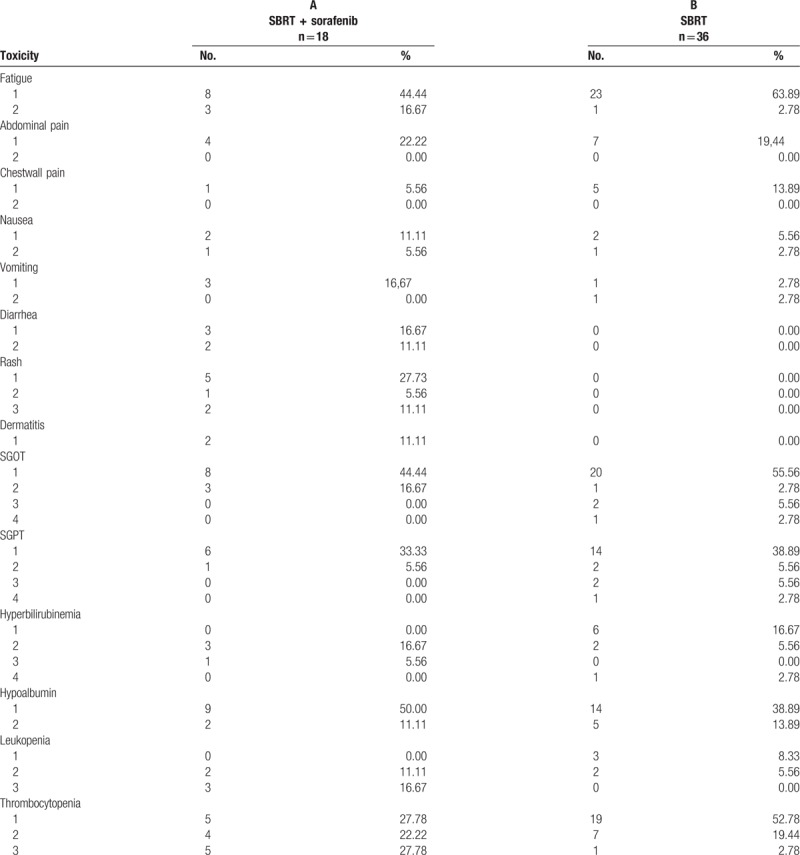
The frequency of treatment related toxicities in both groups is presented in Table 6. Grade 1–2 fatigue (group A 11/18, 61.11%, group B 24/36, 66.67%) was the most common adverse toxicity in both groups. Mild to moderate diarrhea (5/18, 27.78%), rash (7/18, 44.45%), radiation dermatitis (8/18%, 44.45%) were mainly seen in patients administered sorafenib, with 2 (11.11%) patients experiencing a grade 3 rash that was relieved after sorafenib was discontinued. Other than that, transient elevation of grade 1–2 biochemical changes such as SGOT, SGPT, bilirubin, and albumin were noted in both groups. Although, leukopenia and thrombocytopenia were found in both groups, grade 3 toxicity was more commonly found in those administered sorafenib. There were 3 cases of grade 3–4 liver enzyme adverse effect in patients treated with SBRT alone, 2 eventually recover 1 month after SBRT. However, 1 patient developed RILD due to reactivation of the hepatitis B virus, which eventually led to liver failure and death.
Table 6.
Acute toxicity (CTCAEv4.0).
4. Discussion
PVTT is a common phenomenon in HCC. Compared with HCC without PVTT, HCC with PVTT is characterized by an aggressive disease course, poorer hepatic function, less treatment tolerance, higher chance of complication related portal hypertension, and poorer prognosis than patients without PVTT. To date no credible evidence is available for establishing an optimal treatment strategy for HCC with tumor thrombus. Sorafenib, a potent multikinase inhibitor with antiangiogenic and antiproliferative properties that targets the Raf/MEK/ERK pathway has rapidly gain acceptance as a first-line treatment for locally advanced and metastatic HCC. Two phase 3 trials demonstrated that sorafenib was efficacious and well tolerated in patients with advanced HCC.[6,7] Conventionally, the treatment algorithm proposed by the BCLC system considers the presence of PVTT, regardless of the degree of invasion, as a contraindication to surgery TACE, and therefore recommends only palliative treatment with sorafenib or other systemic agents. However, several study results show that sorafenib monotherapy has limited or no clinical benefit compare with other treatment modalities. Jeong et al,[19] evaluated 143 patients with unresectable HCC and reported that 30 patients with advances HCC and PVTT were treated with sorafenib monotherapy. The overall survival time was only 3.1 months among those HCC with PVTT after sorafenib monotherapy, and the median progression-free survival time was only 2.0 months. Nakazawa et al[5] found that the median survival time of HCC patients with PVTT in the main trunk or the first branch was similar after sorafenib administration (4.3 months) or radiotherapy (5.9 months; P = .12). However, when propensity score-matched patients were compared, the median survival time was found to be significantly longer after radiotherapy (10.9 months vs 4.8 months; P = .03). Song et al[20] compared sorafenib and HIAC to treat HCC patients with PVTT reported that HIAC led to a significantly higher disease control rate (P = .001) and significantly longer median OSR (7.1 months vs 5.5 months; P = .01). Another study[21] which compared treatment with a combination of TACE and sorafenib versus sorafenib alone for HCC with PVTT patients found a similar disease control rate and median OSR in the 2 groups (7 months vs 6 months; P = .54). Evidence indicates that sorafenib monotherapy is inferior to other monotherapies or combination treatments.
Theoretically, sorafenib-mediated blockage of the Raf/MAPK and VEGF receptor pathways might enhance the efficacy of radiation therapy. Radiation followed sequentially by sorafenib was associated with the greatest delay in tumor growth. A phase 2 study by Chen et al,[22] analyzed 40 patients with unresectable HCC and 24 patients coexisting with PVTT who were treated with radiation therapy concurrent and sequential sorafenib. The radiation dose was conventional dose ranging from 40 to 60 Gy, achieved a CR and PR rate of 55% with a 2-years in-field progression free survival rate of 39%. The data from this concurrent approach did not appear to be particularly superior to the results from some retrospective studies investigating response rates with RT alone; because hepatoxicity is a major problem, concurrent treatment should be used with caution.
In recent years with the advances in radiotherapy technology, radiation therapy including proton beam, intensity-modulated radiotherapy, and SBRT has become one of the major modalities in the treatment of HCC. A large scale, multicenter study by Im et al[23] involving 985 HCC patients with PVTT in the main trunk and/or first branch treated with radiotherapy. Revealed a response rate of 51.8% with a median OSR of 10.2 months. From our previous study,[11] we retrospectively analyzed 115 unresectable HCC patients treated with CyberKnife SBRT. Achieving an encouraging outcome, however, HCC with PVTT remain one of the major poor prognostic factors, the median survival was 8 months versus 17.5 months for HCC without PVTT; the 1 and 2-year OSR was 35.3% and 12.50% versus 75.31% and 53.7% for HCC free from PVTT. Xi et al,[24] treated 41 HCC with PVTT/inferior vena cava tumot thrombosis (IVCTT) patients with SBRT. Radiation dose was 36 Gy in 6 fractions achieved a median survival rate of 13 months, with a 1-year OSR of 50.3%, the response rate was CR in 36.6% and PR in 17.1%. Kong et al,[25] retrospectively analyzed 80 HCC with PVTT patients using different radiotherapy techniques and reported that a higher dose and high biological effective palliative dose (≥58.9 Gy) could improve the long term outcomes. The available evidence indicates that RT should be an option for unresectable HCC patients with PVTT. And a higher radiation dose tends to achieve better outcomes. In our study, SBRT alone to HCC with PVTT demonstrated a similar result as other studies, median survival rate of 7 months, 1 and 2-year OSR was 33.3% and 11.1%, respectively. When sorafenib was administered after SBRT, the median survival rate increased to 12.5 months; a higher 1 and 2-years OSR of 55.6% and 17.7% respectively, was also achieved. The median progression free survival rate was 3 months longer than for patient treated with SBRT alone. The 1 and 2-years progression-free survival rate was also relatively longer compared with patients who received SBRT alone. Although, the results did not achieve statistical significance, the benefits of combining sorafenib remain to be determined.
Acute toxicity was the major concern in combining sorafenib with SBRT. A Phase 1 trial of sorafenib and SBRT for HCC was reported by Brade et al,[26] in which the combination of sorafenib delivered continuously within 2-week, 6 fraction courses of SBRT to patients with locally advanced HCC and with a Child-Pugh score A liver function resulting in unacceptably high rates of serious toxicity. Therefore, they strongly do not recommend concurrent sorafenib and SBRT in particular for patients in whom irradiation of large amounts of liver (veff of 40–60%) or luminal GI tissue is required. In the present study, among the 18 patients receiving sorafenib, 13 patients received sorafenib after SBRT, whereas 5 patients started the medication before SBRT but were temporarily halted while undergoing SBRT and resume after SBRT was completed. Mild–moderate fatigue was the major adverse event seen in both groups. Grade 1–2 transient liver dysfunction was also commonly recorded in both groups. However, skin rash (44.45%), radiation dermatitis (11.11%), and diarrhea (27.78%) were primarily noted in the SBRT with sorafenib group. Grade 3 leukopenia (16.67%) and thrombocytopenia (27.78%) were also recorded in those taking sorafenib. These patients tend to recover after tapering off sorafenib dose and/or discontinuing sorafenib intake. RILD did not occur in the patients taking sorafenib. The only death from RILD was recorded in a patient receiving SBRT alone. The primary cause of RILD was reactivation of HBV infection resulting in acute liver failure and death. Grade 3 leukopenia and thrombocytopenia were a major problem in the SBRT with sorafenib group. Most HCC patients have underlying liver cirrhosis; therefore, the combination treatment should be used with caution.
For HCC patients, death usually results from uncontrol intrahepatic tumor extension resulting in liver failure. However, in patients with PVTT or IVC tumor thrombosis, survival was not related to tumor type. Most died from thrombosis and not because of intrahepatic tumors. After radiation therapy of PVTT, patients experienced a longer survival time, and the failure pattern returned to intrahepatic tumors rather than that of tumor thrombosis.[27] In our study, one of the major factors affecting survival was tumor type (unifocal vs multi/diffuse). Large, diffuse tumor was the worst prognostic factor affecting both groups of patients. Other poor prognostic factors include poor ECOG status, and PVTT involving the bilateral branch and main trunk. Therefore, SBRT with or without sorafenib administration plays an important role in prolonging survival by relieving portal vein occlusion from tumor thrombosis.
SBRT with or without sorafenib administration is a promising therapeutic strategy for HCC with PVTT. Survival remains limited because of the high frequency of intra- and extra-hepatic recurrences. In theory, the combination of radiotherapy and systemic therapy may provide clinical benefits for patients with PVTT. In the current study, 18 patients (32.14%) received combination therapy of sorafenib and SBRT. The group that received this treatment demonstrated a favorable trend in median survival (12.5 months vs 7 months), 1 and 2-yrs OSR (55.6% vs 33.3% and 17.7% vs 11.1%), longer PFS, median PFS (6 months vs 3 months), 1 and 2-yrs PFS (25% vs 11.1% and 15.2% vs 8.3%). Moreover, disease progression tends to be lower in the combined treatment group (11.11% vs 22.22%). Unfortunately, all these data did not reach statistical significance. The limited number of patients who received sorafenib in our study may have affected the analysis of this therapeutic efficacy; thus, a prospective study addressing this combination are warranted. A retrospective, single-institution study was another limitation of this study. Nevertheless, this is one of the few studies to date that has reported clinical outcomes of HCC with PVTT patients treated with a combination of SBRT and sorafenib.
5. Conclusion
Our study shows that SBRT with or without the combination of sorafenib for HCC patients with PVTT are feasible and effective. The group treated with combined SBRT and sorafenib group demonstrated a better median, 1 and 2-yrs. OSR and PFS. However, this treatment strategy did not attain statistical significance. Randomized trials with a larger sample size are needed to test the efficacy of combining SBRT and sorafenib in advance HCC with PVTT patients.
Acknowledgments
The study was presented at the 59th Annual Meeting of the American Society for Radiation Oncology, San Diego, California, USA, Sep 24–27, 2017.
Author contributions
Conceptualization: Jenny Que.
Data curation: Jenny Que, Hung-Chang Wu, Chia-Hui Lin, Chung-I Huang, Li-Ching Li.
Formal analysis: Jenny Que, Chung-Han Ho.
Investigation: Jenny Que, Chung-I Huang.
Methodology: Jenny Que, Hung-Chang Wu.
Project administration: Jenny Que.
Resources: Jenny Que, Hung-Chang Wu, Chia-Hui Lin, Chung-I Huang, Li-Ching Li.
Software: Chung-Han Ho.
Supervision: Jenny Que, Hung-Chang Wu.
Validation: Jenny Que, Li-Ching Li, Chung-Han Ho.
Visualization: Jenny Que.
Writing – original draft: Jenny Que.
Writing – review & editing: Jenny Que.
Footnotes
Abbreviations: AFP = alpha fetoprotein, BED = Biological effective dose, BCLC = Barcelona Clinic Liver Cancer, CI = Confidence interval, CR = complete response, CT = computed tomography, CTV = clinical target volume, 3,ECOG = Eastern Cooperative Oncology Group, FDA= U.S Food and Drug Administration, GI = gastrointestinal, GTV = gross tumor volume, HbsAg = Hepatitis B surface antigen, HBV = Hepatitis B virus, HCC = hepatocellular carcinoma, HR = Hazard ratio, IVCTT = inferior vena cava tumot thrombosis, MRI = magnetic resonance imaging, OSR = overall survival rate, PD = progressive disease, PFS = progression-free survival, PR = partial response, PTV = planning target volume, PVTT = portal vein tumor thrombosis, RFA = radiofrequency ablation, RILD = radiation-induced liver disease, RT = radiation therapy, SBRT = stereotactic body radiation therapy, SD = stable disease, SHARP = Sorafenib HCC Assessment Randomized Protocol, SPSS = Statistical Product and Service Solutions, SGOT = Serum Aspartate Aminotransferase, SGPT = Serum Alanine Aminotransferase, STAT3 = Signal transducer and activator of transcription, TACE = transarterial chemoembolization, VEGF= Vascular endothelial Growth factor.
How to cite this article: Que J, Wu HC, Lin CH, Huang CI, Li LC, Ho CH. Comparison of stereotactic body radiation therapy with and without sorafenib as treatment for hepatocellular carcinoma with portal vein tumor thrombosis. Medicine. 2020;99:13(e19660).
Ethics approval and consent to participate: Written informed consent was obtained from all patients before treatment, and the study was approved by the institutional review board of Chi Mei Medical Center.
Funding: Self-funding.
The authors declare that they have no competing interests.
The authors have no conflicts of interest to disclose.
References
- [1].Chan S, Chong C, Chan A, et al. Management of hepatocellular carcinoma with portal vein tumor thrombosis: review and update at 2016. World J Gastroenterol 2016;22:7289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jiang JF, Lao YC, Yuan BH, et al. Treatment of hepatocellular carcinoma with portal vein tumor thrombus: advances and challenges. Oncotarget 2017;8:33911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lau WY, Sangro B, Chen PJ, et al. Treatment for hepatocellular carcinoma with portal vein tumor thrombosis: the emerging role for radioembolization using yttrium-90. Oncology 2013;84:311–8. [DOI] [PubMed] [Google Scholar]
- [4].Ando E, Tanaka M, Yamashita F, et al. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer 2002;95:588–95. [DOI] [PubMed] [Google Scholar]
- [5].Nakazawa T, Hidaka H, Shibuya A, et al. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol 2014;14:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Llovet JM, Ricci S, Mazzaferro V, et al. SHARP Investigators Study Group: Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–90. [DOI] [PubMed] [Google Scholar]
- [7].Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- [8].Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Han K, Kim JH, Ko G, et al. Treatment of hepatocellular carcinoma with portal venous tumor thrombosis: a comprehensive review. World J Gastroenterol 2016;22:407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2008;20:657–64. [DOI] [PubMed] [Google Scholar]
- [11].Que J, Kuo HT, Lin LC, et al. Clinical outcomes and prognostic factors of cyberknife stereotactic body radiation therapy for unresectable hepatocellular carcinoma. BMC Cancer 2016;16:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kuo HT, Que J, Lin LC, et al. Impact of tumor size after stereotactic body radiation therapy for inoperable hepatocellular carcinoma. Medicine (Baltimore) 2017;96:e9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huang CY, Lin CS, Tai WT, et al. Sorafenib enhances radiation-induced apoptosis in hepatocellular carcinoma by inhibitin STAT3. Int J Radiat Oncol Biol Phys 2013;86:456–62. [DOI] [PubMed] [Google Scholar]
- [14].Yu W, Gu K, Zhan Y, et al. Sorafenib potentiates irradiation effect in hepatocellular carcinoma in vitro and in vivo. Cancer Lett 2013;329:109–17. [DOI] [PubMed] [Google Scholar]
- [15].Li Q, Hu Y, Xi M, et al. Sorafenib modulates the radio sensitivity of hepatocellular carcinoma cells in vitro in a schedule-dependent manner. BMC Cancer 2012;12:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim JH, Park JW, Kim TH, et al. Hepatitis B virus reactivation after three-dimensional conformal radiotherapy in patients with hepatitis B virus-related hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2007;69:813–9. [DOI] [PubMed] [Google Scholar]
- [17].Hoogeman M, Prevost JB, Nuyttens J, et al. Clinical accuracy of the respiratory tumor tracking system of the cyberknife: assessment by analysis of log files. Int J Radiat Oncol Biol Phys 2009;74:297–303. [DOI] [PubMed] [Google Scholar]
- [18].Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys 2010;76: suppl: S94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jeong SW, Jang JY, Shim KY, et al. Practical effect of sorafenib monotherapy on advanced hepatocellular carcinoma and portal vein thrombosis. Gut Liver 2013;7:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Song DS, Song MJ, Bae SH, et al. A comparative study between sorafenib and hepatic arterial inclusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol 2015;50:445–54. [DOI] [PubMed] [Google Scholar]
- [21].Zhang Y, Fan W, Wang Y, et al. Sorafenib with and without transarterial chemoembolization for advance hepatocellular carcinoma with main portal vein tumor thrombosis: a retrospective analysis. Oncologist 2015;20:1417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen SW, Lin LC, Kuo YC, et al. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2014;88:1041–7. [DOI] [PubMed] [Google Scholar]
- [23].Im JH, Yoon SM, Park HC, et al. Radiotherapeutic strategies for hepatocellular carcinoma with portal vein tumor thrombosis in a hepatitis B endemic area. Liver Int 2017;37:90–100. [DOI] [PubMed] [Google Scholar]
- [24].Xi M, Zhang L, Zhao L, et al. Effectiveness of stereotactic body radiation therapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PLoS One 2013;8:e63864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kong XG, Dong YP, Wu JX, et al. High-biologically effective dose palliative radiotherapy for a tumor thrombus might improve the long term prognosis of hepatocellular carcinoma: a retrospective study. Radiat Oncol 2017;12:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brade A, Ng S, Brierley J, et al. Phase1 trial of sorafenib and stereotactic body radiation therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2016;94:580–7. [DOI] [PubMed] [Google Scholar]
- [27].Zheng ZC, Fan J, Tang ZY, et al. A comparison of treatment combination with or without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thombus. Int J Radiat Oncol Biol Phys 2005;61:432–43. Int J Radiat Oncol Biol Phys 2016, 94(3): 580-587. [DOI] [PubMed] [Google Scholar]


