Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) uses membrane-bound angiotensin-converting enzyme 2 (ACE2) to gain cell entry, leading to coronavirus disease 2019 (COVID-19). ACE2 counterbalances the effects of AT1 activation by angiotensin II as part of the renin–angiotensin–aldosterone system (RAAS). Lei Fang and colleagues1 hypothesised that cardiometabolic diseases (eg, hypertension, diabetes, and cardiac diseases) and RAAS inhibitors (eg, ACE inhibitors (ACEIs) and angiotensin II receptor blockers [ARBs]) might increase the risk of COVID-19 by upregulating ACE2. However, this provocative hypothesis and subsequent debate have occurred in the absence of any empirical evidence that cardiometabolic diseases or RAAS inhibitors affect ACE2 expression in human lungs.
To address this issue, we analysed the gene expression of ACE2 and two host cell proteases, TMPRSS2 and ADAM17, used as cofactors for virus entry in 1051 lung tissue samples from the Human Lung Tissue Expression Quantitative Trait Loci Study (Lung eQTL Study).2 Additionally, we analysed the expression of two other important RAAS genes: ACE (the protein product of which converts angiotensin I to angiotensin II) and AGTR1 (encodes for AT1). We related gene expression to each comorbidity or medication using a robust linear model. To determine relevant covariates and replicate our findings, we analysed two additional datasets: St Paul's Hospital cohort3 and the Lung Tissue Research Consortium (GEO #GSE47460) (appendix p 2).
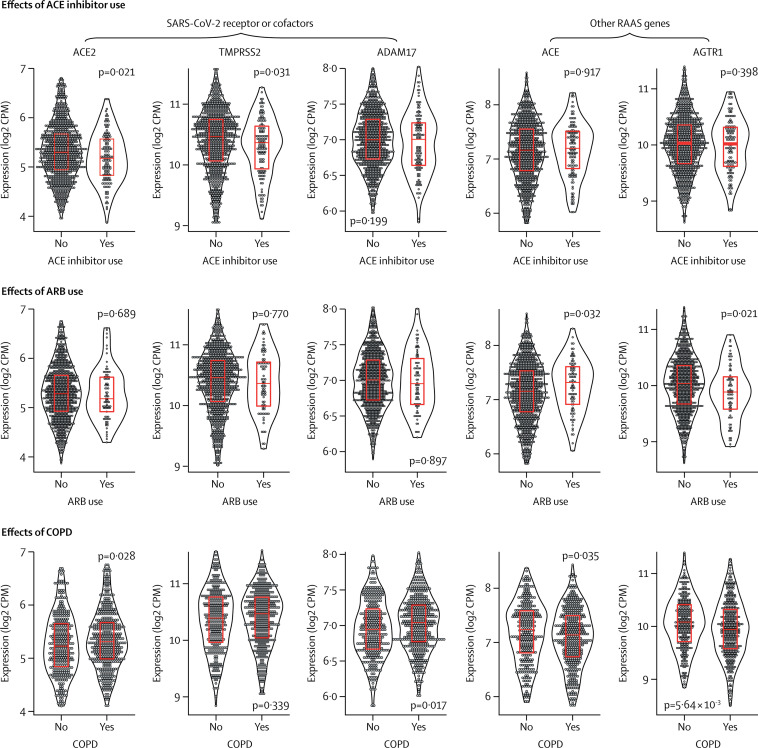
ACEI use was associated with significantly lower ACE2 and TMPRSS2 expression, but was not associated with ADAM17 expression. Neither cardiometabolic diseases (individually or in composite) nor ARBs were associated with altered expression of these genes (figure ; appendix pp 3–5), suggesting any increased risk of COVID-19 in these subpopulations is not related to upregulation of the SARS-CoV-2 receptor or cofactors in the lung; although, their expression in the rest of the respiratory tract still needs to be studied. In contrast to studies in other tissue types,4 we found that ACEIs reduce ACE2 expression in lung. It is possible that long-term ACEI use downregulates lung ACE2 expression by reducing substrate (ie, angiotensin II) availability, which might also explain why no effect of ARBs was seen. In theory, ACE2 downregulation might reduce the risk of SARS-CoV-2 infection because of reduced virus receptor availability. However, animal models suggest that ACE2 deficiency could exaggerate acute lung injury because of an imbalance in angiotensin II or AT1 signalling.5 The clinical significance of our finding is therefore unknown.
Figure.
Expression of SARS-CoV-2 receptor or cofactors and RAAS-related genes in human lung tissue
Lung tissue gene expression and phenotype data from 1051 participants in the Lung eQTL Study.2 Violin plots show the distribution of gene expression levels in log2 CPM (outliers have been removed). Superimposed box plots show median (IQR). p values are from robust linear models, adjusted for current smoking status. ARB=angiotensin II receptor blocker. COPD=chronic obstructive pulmonary disease. CPM=counts per million. eQTL=expression quantitative trait loci. RAAS=renin–angiotensin–aldosterone system. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Neither cardiometabolic diseases nor ACEI use were associated with ACE or AGTR1 expression, whereas use of ARBs was associated with increased ACE and decreased AGTR1 expression. The effect of these opposing changes on overall angiotensin II–AT1 signalling and risk of severe COVID-19 is uncertain. The amount of circulating angiotensin II protein might further modify the risk of lung injury, but was not measured in our cohort.
Chronic obstructive pulmonary disease (COPD) is also a proposed risk factor for severe COVID-19,6 which prompted us to examine its effects on the expression of these genes in the lung. COPD was associated with increased ACE2 expression in the Lung eQTL Study, a finding that was replicated in the Lung Tissue Research Consortium and St Paul's Hospital cohort. The association between COPD and ADAM17 and TMPRSS2 expression was inconsistent across the datasets (figure; appendix pp 3–5). Whether this increased expression translates to increased risk of SARS-CoV-2 infection is unknown because there is no in-vivo evidence that increased receptor availability increases viral entry. Additionally, COPD was associated with decreased expression of ACE and AGTR1 in the Lung eQTL Study, and its association with decreased AGTR1 expression was replicated in the Lung Tissue Research Consortium dataset (figure; appendix pp 3–5). The combination of increased ACE2 but decreased ACE or AGTR1 expression might be protective against acute lung injury,7 which could explain why there is no clear excess of patients with COPD among severe COVID-19 cases.6
We also examined the effects of smoking status on lung gene expression. Current smoking was associated with increased expression of ACE2, TMPRSS2, ADAM17, and ACE (appendix pp 3–6), which might represent a so-called perfect storm of excess viral receptor or cofactor availability and excess angiotensin II or AT1 activity, leading to severe COVID-19. The strong association between air pollution exposure and COVID-19 mortality8 suggests that inhaled noxious particles influence COVID-19 outcomes. Whether current cigarette smoking is an independent risk factor for severe COVID-19 is not yet clear.9
The limitations of our study are that we analysed gene rather than protein expression, although our recent work shows that the two are positively correlated,3 and that details such as duration of cardiometabolic diseases and RAAS inhibitor dose are not available.
Acknowledgments
SM reports personal fees from Novartis and Boehringer-Ingelheim and is supported by the MITACS Accelerate program. WT reports personal fees from Roche Diagnostics–Ventana, Merck Sharp Dohme, Bristol-Myers-Squibb, and AbbVie. DDS reports personal fees from Novartis and Boehringer-Ingelheim; grants and personal fees from AstraZeneca; and holds the De Lazzari Family Chair at Heart Lung Innovation and a Tier 1 Canada Research Chair in Chronic Obstructive Pulmonary Disease. CXY and YB declare no competing interests.
Supplementary Material
References
- 1.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hao K, Bosse Y, Nickle DC. Lung eQTLs to help reveal the molecular underpinnings of asthma. PLoS genetics. 2012;8 doi: 10.1371/journal.pgen.1003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung JM, Yang CX, Tam A. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020 doi: 10.1183/13993003.00688-2020. published online April 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. 2020 doi: 10.1001/jama.2020.4812. published online March 24. [DOI] [PubMed] [Google Scholar]
- 5.Imai Y, Kuba K, Rao S. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W-j, Liang W-h, Zhao Y. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020 doi: 10.1183/13993003.00547-2020. published online March 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, Baker A. Recombinant human ACE2: acing out angiotensin II in ARDS therapy. Critical Care. 2017;21:305. doi: 10.1186/s13054-017-1882-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X, Nethery RC, Sabath BM, Braun D, Dominici F. Exposure to air pollution and COVID-19 mortality in the United States. medRxiv. 2020 doi: 10.1101/2020.04.05.20054502. published online April 27. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 2020;8:e20. doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.