Abstract
Background:
The Prostate Cancer Prevention Trial has shown a protective effect of finasteride on prostate cancer, but it also showed that finasteride can increase the risk of high-grade prostate cancer. Several studies have investigated the relationship between finasteride and prostate cancer, but these studies have shown inconsistent results.
Ethics:
The protocol was approved by the institutional review board of each study center. Written informed consent will be obtained from all patients before registration, in accordance with the Declaration of Helsinki.
Methods:
We performed a systematic literature review and meta-analysis to assess the association between finasteride and prostate cancer. Systematic literature searches were conducted using PubMed, EMBASE, Science Direct/Elsevier, MEDLINE, CNKI, and the Cochrane Library up to October 2018 to identify studies that involved the relationship between finasteride and prostate cancer. Meta-analysis was performed using Review Manager and Stata software. Combined ORs were identified with 95% confidence intervals (95% CI) in a random or fixed effects model.
Results:
Eight studies were identified, including 54,335 cases of patients that used finasteride and 9197 patients who served as placebo controls. Our results illustrate that there is a significant correlation between finasteride use and prostate cancer with combined ORs of 0.70 [0.51, 0.96]. A significant correlation between finasteride use and high-grade prostate cancer was also observed with combined ORs of 2.10 [1.85, 2.38].
Conclusions:
This study confirms that finasteride significantly reduced the risk of prostate cancer; however, the malignant degree of prostate cancer was increased. Studies with larger sample sizes are needed to better clarify the correlation between finasteride use and prostate cancer.
Keywords: finasteride, high-grade cancer, prostate cancer
1. Introduction
Finasteride is a 5-alpha reductase enzyme inhibitor that inhibits the conversion of testosterone into the active androgen metabolite dihydrotestosterone, thereby lowering the prostate volume and serum prostate-specific antigen (PSA) levels.[1] Finasteride was used for the treatment of benign prostatic hyperplasia (BPH) and male pattern baldness. As we all know, androgens play an important role in the development of prostate cancer. Finasteride inhibits the conversion of androgen in the prostate.2,3 Thus, some researchers hypothesized that finasteride could reduce prostate cancer development. Recent studies have shown a 25% decrease in prostate cancer incidence in men receiving finasteride compared to a placebo.4,5
Despite these recent findings, finasteride is not labeled with a prostate cancer indication and was rarely used for prostate cancer prevention.6,7 Finasteride, at the dose used in the Prostate Cancer Prevention Trial (PCPT), was instead most commonly used to treat symptoms of benign prostatic hyperplasia (BPH).[8] Although it was used by millions of men for these indications, little is known about its long-term effects on the prostate. The PCPT reported a decreased incidence of prostate cancer overall but an increase in the incidence of high-grade prostate cancer with finasteride use compared to a placebo.
Recent studies have investigated the correlation between finasteride use and prostate cancer; however, they have shown contradictory results,9,10,11,12,13,14,15,16 with some studies showing that finasteride may decrease the risk of prostate cancer and different studies showing otherwise. Moreover, some studies reported that finasteride can increase the risk of high-grade prostate cancer. Therefore, we systematically reviewed the available literature and performed a meta-analysis to evaluate the relationship between finasteride use and prostate cancer risk; this review and analysis might provide valuable insights on the biology of prostate cancer.
2. Materials and methods
2.1. Literature search
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidance and the Cochrane handbook for systematic reviews of interventions. The meta-analysis was restricted to published studies that investigated the correlation between prostate cancer and high-grade cancer rates in patients who took finasteride compared to a placebo. Two independent reviewers searched PubMed, EMBASE, Science Direct/Elsevier, MEDLINE, CNKI, and the Cochrane Library from inception to October 2018 with no language or study type restrictions. The search terms combined text words and MeSH terms. For example, the search terms for finasteride were “finasteride,” “finasteride tablets” and “Finasteride Capsules” while the search terms for prostatic cancer and BPH were “prostatic cancer”, “prostatic tumors”, “prostate malignancies”, “prostate carcinoma”, “Prostate malignant diseases”, “prostatic neoplasms”, “cancer of prostate”, “neoplasms prostate”, “neoplasms prostatic”, “prostate neoplasms” or “PCa” and “Benign Prostatic Hyperplasia”, “Benign Prostatic Hyperplasia”, “BPH” and “prostatic hyperplasia”, respectively. All related articles and abstracts were retrieved. In addition, references cited within relevant reviews were retrieved by hand, and only full articles were searched.
2.2. Eligibility criteria
2.2.1. Inclusion criteria
Studies were included if patients met the diagnostic criteria of having benign prostatic hyperplasia (BPH) with a normal digital rectal examination, no clinically significant coexisting conditions and an American Urological Association symptom score of at least 4 but less than 20. This study was approved by the institutional review boards at all sites. The case groups were assigned to finasteride and the control groups were assigned to placebo. The available data were extracted from the articles and included the number of patients in the finasteride and control groups, the number of patients with prostate cancer and the number of patients with high-grade cancer.
2.2.2. Exclusion criteria
Studies were excluded if they involved case reports; were only published as abstracts, reports from meetings, or review articles; lacked a control population; or duplicated previous publications.
2.3. Study selection and validity assessment
Two independent reviewers screened the titles and abstracts of all citations from the literature search. All relevant studies that appeared to meet the eligibility criteria were retrieved. If an ambiguous decision was made based on the title and abstract, then the full text was retrieved for further analysis. The final decision on eligible studies was made by reviewing articles. Disagreements were resolved by consensus or by a third reviewer. Two reviewers completed a quality assessment according to the primary criteria for nonrandomized and observational studies of the Newcastle-Ottawa Quality Assessment scale (NOS) in meta-analyses.
2.4. Data extraction and statistical analysis
Data were extracted from the studies by 3 reviewers and included demographic data (authors, year of publication, country, number, and mean age of participants), prostate cancer outcome data and the number of high-grade prostate cancer cases. Disagreements were resolved by consensus. Quantitative meta-analysis was performed by 2 reviewers using Review Manager (RevMan) software (version 5.3, The Nordic Cochrane Centre, The Cochrane Collaboration, 2012, Copenhagen) and Stata software (version 12.0, College Station, Texas, USA). The available data were analyzed in the meta-analysis.
To calculate the combined OR and its 95% confidence interval (CI), heterogeneity was assessed using P-values and the I-square statistic (I 2) in the pooled analyses which represents the percentage of total variation across the studies.[24] If the P value was less than .1 or the I 2-value was greater than 50%, then the summary estimate was analyzed in a random-effects model. Otherwise, a fixed-effects model was applied. In addition, publication bias was detected by visual examination of the funnel plot symmetry, with asymmetry suggesting possible publication bias. It was also assessed by the Begg and Egger test in the meta-analysis. If the P value was less than.05, then publication bias existed.
3. Results
3.1. Characteristics of the included studies
Figure 1 shows the detailed review process. A total of 1423 unduplicated studies were identified, and 8 studies were ultimately selected according to the eligibility criteria. After group discussion, all reviewers agreed to include all 8 papers.
Figure 1.
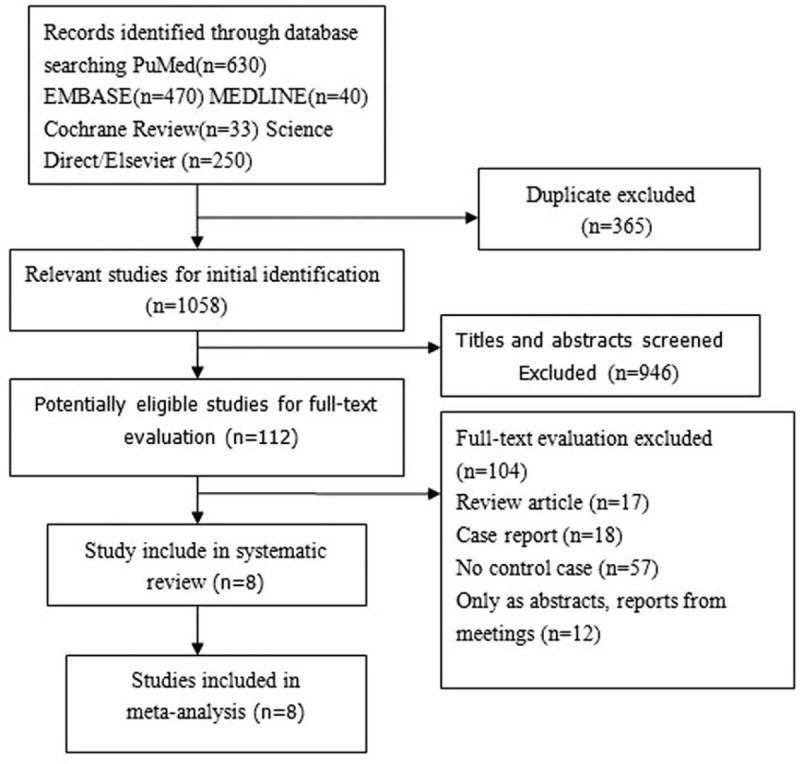
Flow diagram of the selection of eligible studies.
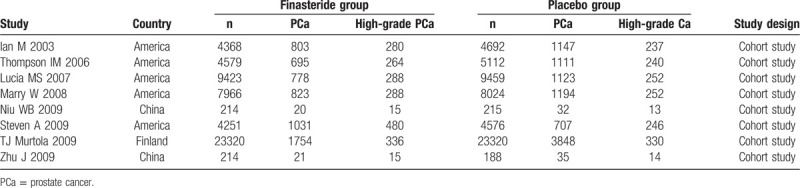
Table 1 summarizes the general data from the 8 studies. The retrieved studies included 54,335 patients who used finasteride and 9197 patients who served as placebo controls. The mean ages of the finasteride and placebo groups ranged from 55 to 75.6 years and 63 to 75.6 years, respectively. All studies reported exclusion/inclusion criteria.9,10,11,12,13,14,15,16
Table 1.
Characteristics of the included studies.
3.2. Meta-analysis
The heterogeneity test suggested a random effects model and the meta-analysis revealed that there is a significant correlation between the incidence of prostate cancer and finasteride use when comparing the finasteride and placebo groups, with an overall combined OR for the finasteride and placebo groups of 0.70 [0.51, 0.96] (Fig. 2). These results suggest that finasteride significantly reduces the risk of prostate cancer. Furthermore, a significant correlation between the rate of high-grade prostate cancer and finasteride use was observed when comparing the finasteride and placebo groups, with finasteride showing an increased risk of high-grade prostate cancer The overall combined OR for the finasteride and placebo groups was 2.10 [1.85, 2.38] (Fig. 3). Egger funnel plots (Figs. 4 and 5) suggested that there is no publication bias in the meta-analysis. Egger regression test also indicated little evidence of publication bias (P > .05) (Table 2). We also conducted a sensitivity analysis of the meta-analysis. We omitted 1 study at a time, and the calculated combined OR for the remaining studies yielded consistent results. In the overall meta-analysis, no single study significantly changed the combined results, a finding which indicates that the results are statistically stable and reliable (Figs. 6 and 7).
Figure 2.
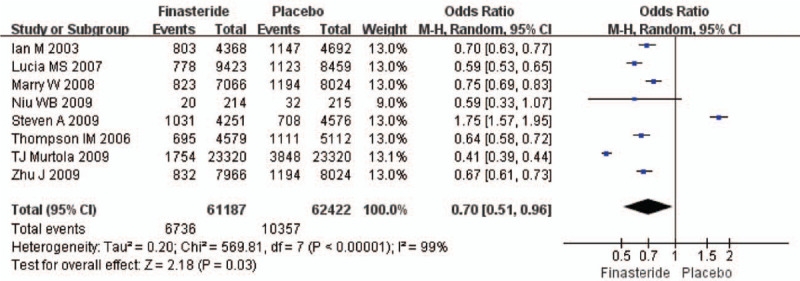
Forest plot showing the meta-analysis outcomes of the incidence of prostate cancer rate between finasteride and placebo group.
Figure 3.
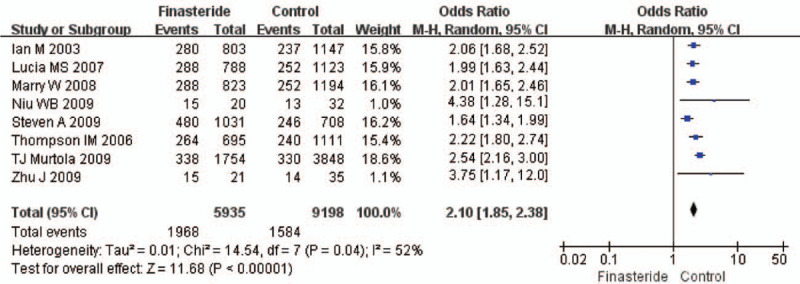
Forest plot of sub-analysis showing the meta-analysis outcomes of the high-grade prostate cancer rate between finasteride and placebo group.
Figure 4.
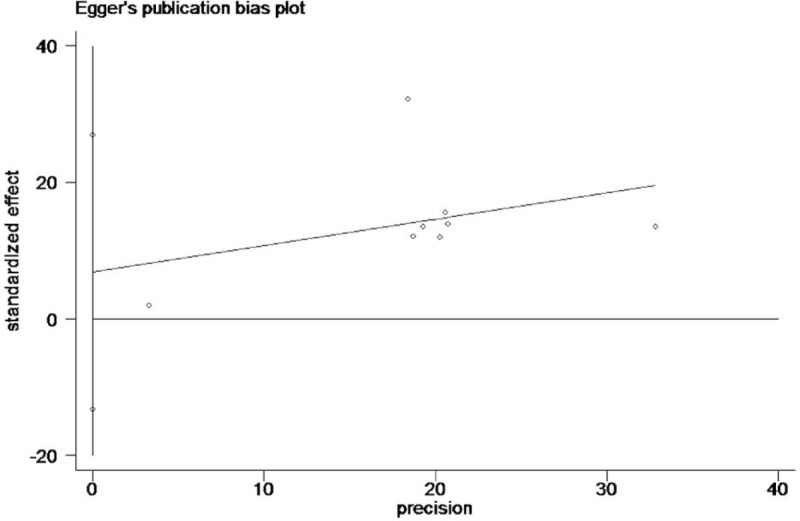
Egger publication bias plot of the incidence of prostate cancer rate between finasteride and placebo group.
Figure 5.
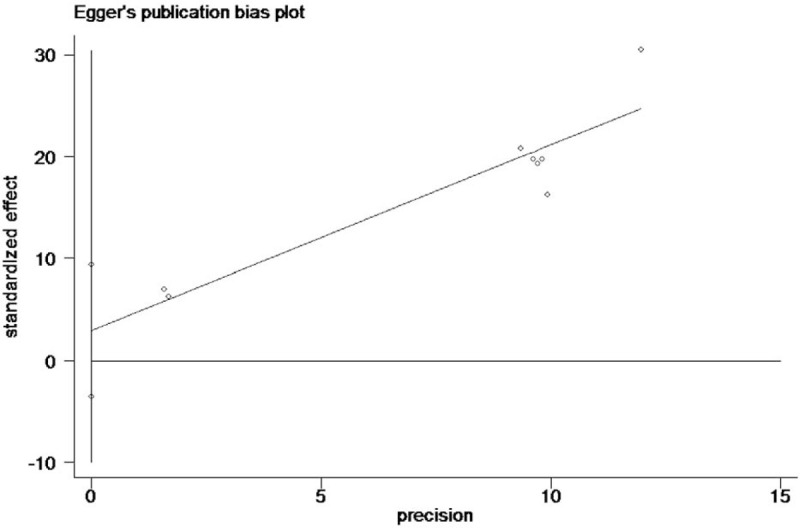
Egger publication bias plot of the incidence of high-grade prostate cancer rate between finasteride and placebo group.
Table 2.
The Egger test of publication bias.

Figure 6.
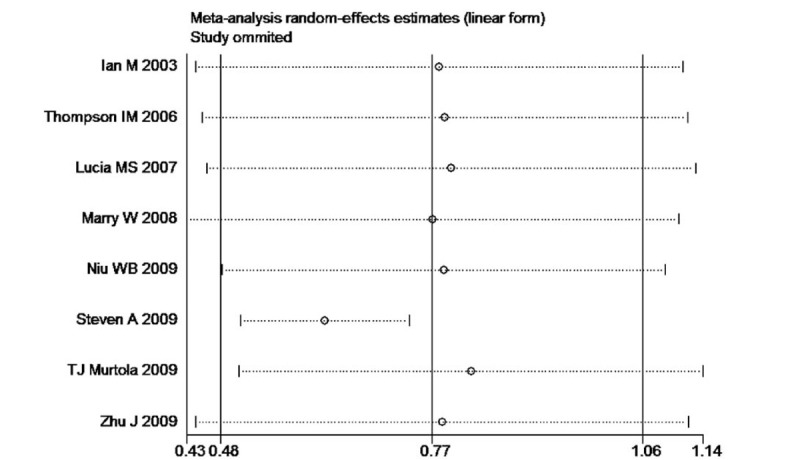
Sensitivity analysis plot of the incidence of prostate cancer rate between finasteride and placebo group.
Figure 7.
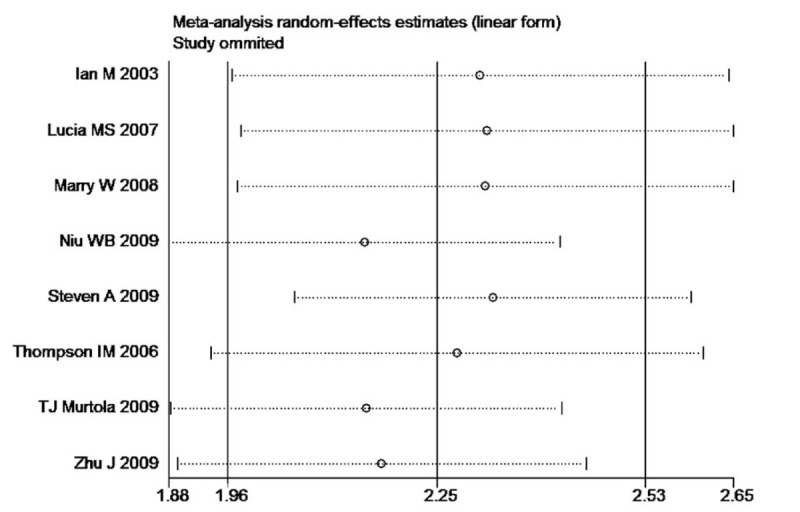
Sensitivity analysis plot of the incidence of high-grade prostate cancer rate between finasteride and placebo group.
4. Discussion
In our study, 8 literature papers were examined for differences in prostate cancer risk between the finasteride and placebo groups. Six studies9,11,12,14,16 reported a significant correlation, and 1 study showed no significant correlation between the incidence of prostate cancer and finasteride use compared to the placebo group.[10] One study showed that the incidence of prostate cancer in the finasteride group was higher than in the placebo group.[15] In our meta-analysis, there was a significant difference in the risk of developing prostate cancer between the finasteride and placebo groups (Fig. 2A). Eight literature reports studied high-grade cancer rates in the finasteride and placebo groups, and all studies showed that finasteride increases the risk of high-grade prostate cancer9,10,11,12,13,14,15,16 (Fig. 2B). These results are consistent with our meta-analysis.
Prostate cancer is one of the most frequently diagnosed malignancies in men and causes more than 250,000 deaths annually.17,18,19,20,16,17,18,19 Although prostate cancer is the most common noncutaneous tumor in developed countries, its etiology remains poorly understood.21,22 Androgen signaling is central to prostate cancer development and progression. A milestone in the progression of advanced prostate cancer is when it transitions from endocrine-driven to paracrine- or intracrine-driven androgen signaling, with progressive complexities in steroid hormone biosynthesis and alterations of the androgen receptor (AR).[23] There is abundant evidence that androgens influence the development of prostate cancer.3,24,25 AR is a ligand–activated intracellular transcription factor belonging to the family of steroid hormone receptors that also includes ER, glucocorticoid receptor, progesterone receptor, and mineralocorticoid receptor.[26] Finasteride can up-regulate the expression of androgen receptor (AR) in benign prostatic hyperplasia and prostate cancer LNCaP cells. It is believed that the conversion of testosterone to dihydrotestosterone decreases after the use of finasteride, and the low level of dihydrotestosterone environment leads to the transformation of androgen-dependent prostate cancer to androgen-independent prostate cancer, which leads to clonal growth of high-grade prostate cancer.[27] Finasteride reduces the production of dihydrotestosterone. In the short run, it reduces the stimulation of hormones to normal tissues and cancer cells, and inhibits the growth of cancer cells to some extent. However, with the prolongation of time, due to long-term low-level dihydrotestosterone stimulation of the original cancer tissue, AR in cancer tissue can be up-regulated and amplified, leading to the occurrence of higher-grade prostate cancer. AR is also subject to posttranscriptional modifications, including phosphorylation, acetylation, methylation, SUMOylation, and ubiquitination together with phosphorylation. These post-transcriptional modifications alter AR functional activity, including transcriptional activity, stability, and cellular localization.[28] The modification and translation of AR receptor affect the occurrence and development of prostatic hyperplasia and prostate cancer.29,30,31,32,33,34
Finasteride treatment induces both apoptosis and a reduction in cellular volume (hormonal atrophy) in the prostate,35,36,37 which leads to an overall reduction in the prostate volume. The development of the steroid 5a-reductase inhibitor finasteride, which inhibits the conversion of testosterone to the more potent androgen dihydrotestosterone, created an opportunity to test the possibility that lowering the androgen levels in the prostate would reduce the risk of prostate cancer. From the start of many studies, finasteride has been available for the treatment of benign prostatic hyperplasia and it has since been approved for the treatment of male pattern baldness. Although it is used by millions of men for these indications, little is known about its long-term effects on prostate health.38,39,40
Currently, many studies have reported that finasteride reduces the risk of prostate cancer in clinical trials marked by frequent disease monitoring, but finasteride is also associated with an increased risk of high-grade prostate cancer.9,10,11,12,13,14,15,16 One possible explanation for this difference is grading bias in which histologic changes that mimic those of high-grade disease are caused by androgen-deprivation therapy.41,42,43,44,45 There are, however, differences of opinion as to whether this effect occurs with finasteride use. It is possible that finasteride induces high-grade tumors by reducing the level of intracellular dihydrotestosterone within the prostate. There is evidence that the prostate tumors that develop in men with low testosterone levels have higher Gleason grades and worse outcomes than prostate cancers that develop in men with normal testosterone levels.46,47,48 It is also possible that finasteride selects for high-grade tumors by selectively inhibiting low-grade tumors. Kim et al reported that lower AR expression in prostate tumor areas after short-term finasteride exposure is in line with an emerging concept that reduced androgens in prostate tissues may over time lead to derepression of AR expression, which in turn deregulates AR function and downstream derepression of specific AR target genes normally repressed by androgens.49,50 These gene activities may lead to stochastic activation of oncogenic signaling that promotes the development of aggressive prostate cancer. However, long-term follow-up in these men and further laboratory research will be required to determine the reason for the association between finasteride use and high-grade prostate cancer.
There are some limitations in our study that need to be taken into consideration when interpreting the results of this meta-analysis. First, the sample size of each study was relatively small, and a total of 54,335 patients who used finasteride and 9197 placebo control patients were investigated in all 8 studies. Second, several studies related to the subject were excluded due to the lack of control data, especially some new studies, so the studies found in this meta-analysis was date until the year 2009. Third, in all 8 studies, the finasteride groups were assigned to 5 mg finasteride daily, and 6 studies followed for 7-year period prevalence of prostate cancer, 2 studies followed for 1-years. What's more, in this meta-analysis, 5 studies from America, 2 studies from china and 1 study from Finland, because of the limited amount of original research, a subgroup of finasteride and prostate cancer in different race was not conducted. Fourth, this meta-analysis did not have information on less established prostate cancer risk factors such as dietary patterns or nutrient intake (such as selenium or vitamin E). Medication users may be more health conscious than nonusers and follow a healthier diet, which could have reduced the incidence in medication users. As such, it is hard to make definitive conclusions about the long-term effects of finasteride use on the prostate.
In summary, the meta-analysis results in this paper add to the evidence of an association between finasteride use and prostate cancer. These results suggest that finasteride significantly reduces the risk of developing prostate cancer, but the malignant degree of prostate cancer might be increased by finasteride use. However, studies with larger sample sizes are needed to better elucidate the correlation between finasteride use and prostate cancer.
Author contributions
Conceptualization: Lei Wang, Yonghua Lei.
Data curation: Yanyao Gao, Juanhua Tian, Yonghua Lei.
Formal analysis: Qisheng Tang, Man Li, Lei Wang.
Funding acquisition: Ruixiao Li, Xiaojing Bai, Meiyu Wang, Na Zhang, Yuefeng Du.
Investigation: Dong Wang.
Methodology: Yu Chen, Dong Cui, Man Li.
Project administration: Bo Zhang, He Wang.
Software: Dong Cui, Xinwei Zhang, Lijun Mu, Yuefeng Du.
Supervision: Bo Zhang, He Wang.
Footnotes
Abbreviations: AR = androgen receptor, BPH = benign prostatic hyperplasia, CI = confidence interval, OR = odds ratio, PCPT = Prostate Cancer Prevention Trial, PSA = prostate-specific antigen.
How to cite this article: Wang L, Lei Y, Gao Y, Cui D, Tang Q, Li R, Wang D, Chen Y, Zhang B, Wang H. Association of finasteride with prostate cancer: a systematic review and meta-analysis. Medicine. 2020;99:15(e19486).
LW and Yl contributed equally to this work.
The authors have no funding and conflicts of interests to disclose.
References
- [1]. Nead KT, Sinha S, Yang DD, et al. Association of androgen deprivation therapy and depression in the treatment of prostate cancer: A systematic review and meta-analysis. Urol Oncol 2017;35:664.e1–9. [DOI] [PubMed] [Google Scholar]
- [2]. Parsons JK, Schenk JM, Arnold KB, et al. Finasteride reduces the risk of incident clinical benign prostatic hyperplasia. Eur Urol 2012;62:234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Ross RK, Bernstein L, Lobo RA. 5-Alpha-reductase activity and risk of prostate cancer among Japanese and US white and black males. Lancet 1992;339:887–9. [DOI] [PubMed] [Google Scholar]
- [4]. Scardino PT. The prevention of prostate cancer-the dilemma continues. N Engl J Med 2003;49:297–9. [DOI] [PubMed] [Google Scholar]
- [5]. Thompson IM, Klein EA, Lippman SM, et al. Prevention of prostate cancer with finasteride: US/European perspective. Eur Urol 2003;44:650–5. [DOI] [PubMed] [Google Scholar]
- [6]. Eastman P. ODAC Recommends against finasteride and dutasteride for prostate cancer chemoprevention. Oncol Times 2011;33:22–4. [Google Scholar]
- [7]. Thompson IM, Tangen CM, Goodman PJ. Chemoprevention of prostate cancer. J Urol 2009;182:499–507. [DOI] [PubMed] [Google Scholar]
- [8]. Tacklind J, Fink HA, Macdonald R. Finasteride for benign prostatic hyperplasia. Cochrane Database Sys Rev 2010;10:601–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Zhu J, Gao JP, Xu AX. The influence of finasteride on incidence and pathology grading of prostate cancer. Zhonghua Wai Ke Za Zhi 2010;48:761–3. [PubMed] [Google Scholar]
- [10]. Wenbin Niu, Chunling Wang, Huifeng Cao. The influence of finasteride on Pathological grading and vascularization of prostate cancer. Chin J Gerontol 2009;30:1594–6. [Google Scholar]
- [11]. Lucia MS, Epstein JI, Goodman PJ. Finasteride and high -grade prostate cancer in the prostate cancer prevention trial. J Natl Cancer Inst 2007;99:1375–83. [DOI] [PubMed] [Google Scholar]
- [12]. Thompson IM, Goodman PJ, Tangen CM. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003;349:215–24. [DOI] [PubMed] [Google Scholar]
- [13]. Mary W Redman, Catherine M, Tangen PH. Finasteride does not increase the risk of high-grade prost ate cancer: A bias -adjusted modeling approach. Cancer Prev Res (Phi la Pa) 2008;1:174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Murtola TJ, Tammela TJ. Prostate cancer incidence among finasteride and alpha-blocker users in the finnish prostate cancer screening trial. Br J Cancer 2009;101:843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Kaplan SA, Roehrborn CG, Meehan AG. PCPT: Evidence that finasteride reduces risk of most frequently detected intermediate and high-grade (Gleason score 6 and 7) cancer. Urology 2009;73:935–9. [DOI] [PubMed] [Google Scholar]
- [16]. Thompson IM, Tangen CM. Finasteride improves the sensitivity of Digital rectal examination for prostate cancer detection. J Urol 2007;177:1749–52. [DOI] [PubMed] [Google Scholar]
- [17]. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66. [DOI] [PubMed] [Google Scholar]
- [18]. Gohagan JK, Prorok PC, Hayes RB. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control Clin Trials 2000;21:251–72. [DOI] [PubMed] [Google Scholar]
- [19]. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- [20]. Crawford ED. Understanding the epidemiology, natural history, and key pathways involved in prostate cancer. Urology 2009;73:4–10. [DOI] [PubMed] [Google Scholar]
- [21]. Jemal A, Center MM, DeSantis C. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:189–907. [DOI] [PubMed] [Google Scholar]
- [22]. Horwich A, Parker C, Bangma C. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21:129–33. [DOI] [PubMed] [Google Scholar]
- [23]. Logothetis CJ, Gallick GE, Maity SN. Molecular classification of prostate cancer progression: foundation for marker driven treatment of prostate cancer. Cancer Discov 2013;3:849–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Hsing AW, Reichardt JK, Stanczyk FZ. Hormones and prostate cancer: current perspectives and future directions. Prostate 2002;52:213–35. [DOI] [PubMed] [Google Scholar]
- [25]. Giovannucci E, Stampfer MJ, Krithivas K. The CAG repeats within the androgen receptor gene and its relationship to prostate cancer. Proc Natl Acad Sci USA 1997;94:3320–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Kumar R. Steroid hormone receptors and prostate cancer: Role of structural dynamics in therapeutic targeting. Asian J Androl 2016;18:682–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Hsieh JT, Chen SC, Yu HJ, et al. Finasteride upregulates expression of androgen receptor in hyperplastic prostate and LNCaP cells: implications for chemoprevention of prostate cancer. Prostate 2011;71:1115–21. [DOI] [PubMed] [Google Scholar]
- [28]. van der Steen T, Tindall DJ, Huang H. Posttranslational modification of the androgen receptor in prostate cancer. Int J Mol Sci 2013;14:14833–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Boam T. Anti–androgenic effects of flavonols in prostate cancer. Ecancermedicalscience 2015;14:585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Nelles JL, Hu WY, Prins GS. Estrogen action and prostate cancer. Expert Rev Endocrinol Metab 2011;6:437–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Hu WY, et al. Estrogen-initiated transformation of prostate epithelium derived from normal human prostate stem-progenitor cells. Endocrinology 2011;152:2150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Bardin A, Boulle N, Lazennec G, et al. Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer 2004;11:537–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Levakov AF, et al. The expression and localization of estrogen receptor beta in hyperplastic and neoplastic prostate lesions. Vojnosanit Pregl 2015;72:906–13. [DOI] [PubMed] [Google Scholar]
- [34]. Mishra S, et al. Estrogen and estrogen receptor alpha promotes malignancy and osteoblastic tumorigenesis in prostate cancer. Oncotarget 2016;6:44388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Rittmaster RS, Norman RW, Thomas LN. Evidence for atrophy and apoptosis in the prostates of men given finasteride. J Clin Endocrinol Metab 1996;81:814–9. [DOI] [PubMed] [Google Scholar]
- [36]. Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Bio Sci 2007;12:4957–71. [DOI] [PubMed] [Google Scholar]
- [37]. Kaplan SA, Roehrborn CG, McConnell JD. Long-term treatment with finasteride results in a clinically significant reduction in total prostate volume compared to placebo over the full range of baseline prostate sizes in men enrolled in the MTOPS trial. J Urol 2008;180:1030–3. [DOI] [PubMed] [Google Scholar]
- [38]. Ishizuka O, Nishizawa O, Hirao Y, et al. Evidence-based meta-analysis of pharmaco therapy for benign prostatic hypertrophy. Int J Urol 2002;9:607–12. [DOI] [PubMed] [Google Scholar]
- [39]. Kyprianou N, Benning CM. Suppression of human prostate cancer cell growth by alpha1-adrenoceptor antagonists doxazosin and terazosin via induction of apoptosis. Cancer Res 2000;60:4550–5. [PubMed] [Google Scholar]
- [40]. Benning CM, Kyprianou N. Quinazoline-derived alpha1-adrenoceptor antagonists induce prostate cancer cell apoptosis via an alpha1-adrenoceptor-independent action. Cancer Res 2002;62:597–602. [PubMed] [Google Scholar]
- [41]. Epstein JI. Pathology of prostatic neoplasia in: Walsh PC, ed. Campbell's urology. Philadelphia 2002;8:3025–37. [Google Scholar]
- [42]. Smith DM, Murphy WM. Histologic changes in prostate carcinomas treated with leuprolide (luteinizing hormone-releasing hormone effect): distinction from poor tumor differentiation. Cancer 1994;73:1472–7. [DOI] [PubMed] [Google Scholar]
- [43]. Yang XJ, Lecksell K, Short K. Does long-term finasteride therapy affect the histologic features of benign prostatic tissue and rostate cancer on needle biopsy? Urology 1999;53:696–700. [DOI] [PubMed] [Google Scholar]
- [44]. Civantos F, Soloway MS, Pinto JE. Histopathological effects of androgen deprivation in prostatic cancer. Semin Urol Oncol 1996;2:22–31. [PubMed] [Google Scholar]
- [45]. Bostwick DG. Prostatic adenocarcinoma following androgen deprivation therapy: the new difficulty in histologic interpretation. Anat Pathol 1998;3:1–6. [PubMed] [Google Scholar]
- [46]. Ishikawa S, Soloway MS, Todd B. Prognostic factors in survival free of progression after androgen deprivation therapy for treatment of prostate cancer. J Urol 1989;141:1139–42. [DOI] [PubMed] [Google Scholar]
- [47]. Schatzl G, Madersbacher S, Haitel A. Associations of serum testosterone with microvessel density, androgen receptor density and androgen receptor gene polymorphism in prostate cancer. J Urol 2003;169:1312–5. [DOI] [PubMed] [Google Scholar]
- [48]. Prehn RT. On the prevention and therapy of prostate cancer by androgen administration. Cancer Res 1999;59:4161–4. [PubMed] [Google Scholar]
- [49]. Jeri Kima, John W, Eric A Klein. Tissue effects in a randomized controlled trial of short-term finasteride in early prostate cancer. EBioMedicine 2016;7:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Cai C, He HH, Chen S, et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell 2011;20:457–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


